Abstract
Scope
Peanut allergy stems from a Th2-biased immune response to peanut allergens leading to IgE production and allergic reactions upon ingestion.
Methods and Results
A model of peanut allergy in C3H/HeJ mice was used to assess whether Type A, B, or C CpG oligodeoxynucleotide (ODN) molecules would be effective in: (1) a prophylactic approach to prevent peanut allergy when administered simultaneously with a Th2-skewing adjuvant, and (2) a therapeutic model to allow for shortened immunotherapy. Type B ODNs were extremely effective in inhibiting anaphylaxis in the sensitization protocol as evidenced by differences in symptom scores, body temperature, and MMCP-1 release compared to sham treatment. In the therapeutic model, co-administration of Type B ODN plus peanut proteins was highly effective in reducing anaphylactic reactions in mice with established peanut allergy. The therapeutic effect was accompanied by an increase in IFN-γ and peanut-IgG2a, without a significant decrease in peanut-IgE or IL-4 responses.
Conclusions
CpG ODNs, especially Type B, were highly effective in inducing Th1-responses in mice undergoing induction of peanut allergy, as well as in mice undergoing therapy for established peanut allergy. Interestingly, the IgE response was not significantly altered, suggesting that IgG antibodies may be enough to prevent peanut-induced anaphylaxis.
Keywords: food allergy, peanut allergy, TLR9, CpG, mouse model
Introduction
Peanut allergy is a potentially life-threatening immunologic disease that affects millions of people worldwide. At present, there is no cure for peanut allergy and the standard-of-care is strict avoidance of trace amounts of peanut proteins and having access to emergency medicine, including epinephrine, at all times [1]. Recently, clinical trials have shown that desensitization of peanut allergic patients is possible with various forms of immunotherapy. Oral and sublingual immunotherapies for peanut [2–5], as well as other food allergens [6–8], have demonstrated large increases in threshold doses required to cause allergic reactions, however, further studies are needed before these therapies become standard practice [9]. An alternative to treating patients that have already developed peanut allergy is to prevent the allergy from developing in high-risk patients. This prophylactic approach is being investigated in a clinical trial in the U.K. entitled LEAP (Learning Early About Peanut allergy) in which atopic children deemed high-risk for developing peanut allergy are exposed by oral feeding of peanut-containing snacks (www.leapstudy.co.uk). It is hypothesized that feeding at a young age may induce oral tolerance to peanut, as has been shown to occur in mice [10, 11], although outcome data have not yet been published.
Patients with peanut allergy have IgE against peanut proteins that stem from a Th2-biased immune response to one or more of the protein allergens. The cause of a Th2-biased immune response to peanut proteins is not well understood. Some studies indicate an innate ability of peanut or other food allergens themselves to direct the T cell response toward an allergic phenotype [12, 13], while others have shown that a lack of suitable Toll-like receptor or other immune-educating signals may be the culprit [14–17]. Immunotherapy for aeroallergies, hymenoptera allergy, and more recently food allergies has repeatedly been demonstrated to induce large increases in allergen-specific IgG [18, 19]. IgG antibodies are often thought of as “blocking antibodies” [20, 21], although the functional ability of these antibodies in vivo has not been conclusively demonstrated. Regardless, immunotherapy mechanisms act at the T cell level, causing a shift away from a Th2-biased, high-IgE phenotype towards a Th1- and/or Treg-dominated, high IgG phenotype that likely plays a role in the persistence of protection from allergic reactions.
The use of a Th1-directing adjuvant may be a desirable candidate to both prevent and treat peanut allergy by inducing high levels of antigen-specific IgG. A well-known Th1-type adjuvant is CpG oligodeoxynucleotide (ODN), a TLR9 agonist [22]. TLR9 detects unmethylated CpG dinucleotides, which are common in bacterial and viral genomes, but often methylated in vertebrate genomoes preventing unwanted stimulation to self-antigens [23]. CpG ODNs are thus considered a pathogen-associated molecular pattern used by the immune system to stimulate responses directed at microbial invaders [23]. There are several types of CpG molecules, including Types A, B, and C [23, 24]. These vary slightly in the arrangement of CpG motifs in the molecule and can exert their effects on distinct cell types. Specifically, Type A CpG ODNs are strong inducers of IFN-α from plasmacytoid dendritic cells (pDC), but have weak effects on B cells, while Type B CpG ODNs exert potent effects on B cells such as proliferation and antibody secretion. Type C CpG-ODNs combine the characteristics of Types A and B ODNs, and thus act strongly on both B cells and pDCs. Some studies have been performed with peanut allergens and CpG in mice indicating the potential as an oral therapeutic [25] and possible use in preventing sensitization through the skin [26], however, important questions remain. We hypothesized that TLR9 agonist ODNs would prevent allergic sensitization in mice when administered simultaneously with peanut proteins in aluminum hydroxide, a well-known Th2-skewing adjuvant. Additionally, we hypothesized that ODNs would function as a therapeutic adjuvant by allowing for shorter duration with decreased antigen dosage in mice with established hypersensitivity to peanut.
Material and Methods
Mice
3 week old C3H/HeJ female mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were allowed to acclimate to their new environment for 2 weeks before being used in our protocols. Mice were housed under pathogen-free conditions with free access to water and food, while being kept on a peanut-free diet the entire study. All studies were approved by the IACUC at Duke University Medical Center.
Reagents
Peanut proteins were extracted from roasted peanut flour (Golden Peanut Company; Alpharetta, GA, USA) as previously described [27]. CpG ODN molecules were purchased from Invivogen (San Diego, CA). ODNs used in these studies were # 1585 (Type A), 1826 (Type B), 1668 (Type B), and M362 (Type C). Additionally, control ODNs containing GpC motifs for ODN 1826 and 1585 were used in the prophylactic model.
Dendritic cell assays
Dendritic cells (DCs) were enriched from C3H/HeJ mouse splenocytes using magnetic-assisted cell sorting (MACS) technology via an anti-CD11c kit (Miltenyi, Bergisch Gladbach, Germany). DCs were enriched to >80% purity as determined by FACS analysis (cells accounting for impurity were not assessed). DCs were cultured at 2 × 105 cells per well in 200 μL culture media for 24h with various ODNs (Fig 1). Supernatants were collected and IL-12 (p40) and TNF-α were measured by ELISA (R&D systems, Minneapolis, MN, USA).
Figure 1.
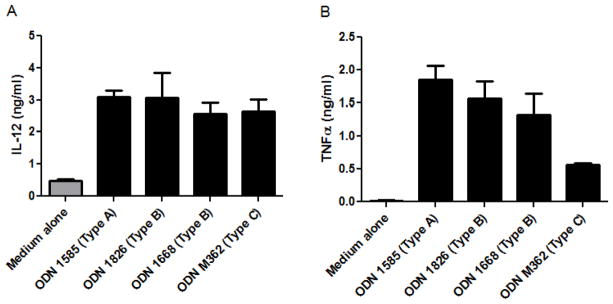
Secreted Th1-type cytokines from C3H/HeJ mouse dendritic cell cultures. DCs were enriched from splenocytes and cultured for 24 h in the presence of TLR9 agonist CpG ODNs. (A) IL-12 (p40) and (B) TNF-α were measured in the culture supernatant by ELISA. Bars represent mean values with standard deviation shown. Data are from duplicates with two mice each.
Prophylactic model
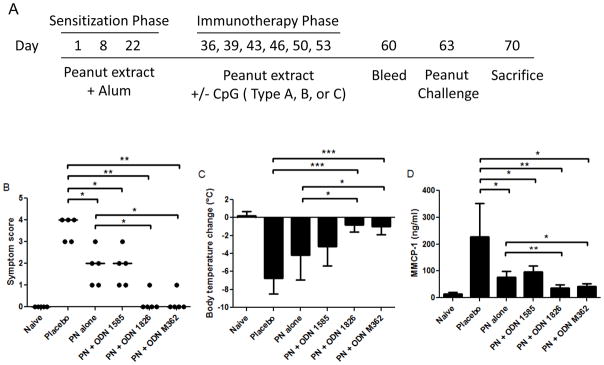
Mice received 0.5 mg peanut protein extract (described above) adsorbed to 2 mg aluminum hydroxide (Imject Alum, Fisher Scientific, Rockford, IL, USA) in the absence (i.e. sham-treated mice) or presence of 30 μg of either Type A, B, or C CpG ODN molecules on days 1, 8, and 22 via i.p. injection (Fig 2A). Solutions of peanut proteins plus the ODNs in PBS buffer were prepared in one tube, aluminum hydroxide was added, then placed on a shaker for 30 minutes prior to injection. Naïve mice were injected with PBS plus 2 mg Alum and were not exposed to peanut during the sensitization period. Mice were bled on day 30 and serum collected and frozen for later analyses. On Day 36, mice were challenged with peanut proteins in PBS by i.p. injection of 1 mg protein contained in 0.2 mL total volume per injection. Challenges were assessed for symptom scores graded on a 0–5 scale [28] with the following system: 0-no symptoms; 1- scratching, rubbing of face and snout; 2-puffy features around the eyes and face with reduction in normal activity; 3-respiratory distress and/or cyanosis of the tail and feet; 4-no movement following prodding; 5- death. Body temperatures were measured at baseline then 30 and 60 minutes post-challenge with a rectal thermal probe. Blood was collected following the 60 minute temperature reading for serum analysis of MMCP-1. MMCP-1 was quantified from sera diluted 1:50 using a kit according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA).
Figure 2.
Prevention of anaphylaxis through CpG ODN co-administration during sensitization. (A) Mice were sensitized to peanut proteins using aluminum hydroxide with or without CpG ODNs then challenged with peanut. (B) Symptom scores, (C) body temperature change, and (D) MMCP-1 were assessed for mice following peanut challenge on day 36. Closed circles represent individual mice with the median shown as a line; bars represent mean values with standard deviation shown. *, **, and *** indicate p<0.05, 0.01, and 0.001, respectively. Data is combined from two separate experiments.
Therapeutic model
Mice were sensitized to peanut by administration of 0.5 mg peanut proteins in 2 mg Alum on days 1, 8, and 22. Peanut proteins in PBS were mixed with Alum for 30 minutes on a shaker prior to injection. Immunotherapy was administered on days 36, 39, 43, 46, 50, and 53 (Fig 4A). Mice were separated into treatment groups as placebo, peanut alone, or peanut plus Type A, B, or C ODN. Placebo mice received only PBS during immunotherapy. The peanut alone group received 0.1 mg peanut protein on days 36 and 39; 0.25 mg peanut on days 43 and 46; and 0.5 mg peanut on days 50 and 53. The treatment groups receiving ODN-adjuvanted immunotherapy were administered peanut (at the same dosing schedule for the peanut alone group) plus CpG ODN at 30 μg per injection. Mice were bled on day 60 for serum collection. A peanut challenge was conducted and assessed on day 63 as described above for the prophylactic model.
Figure 4.
Therapeutic effects of CpG ODN-adjuvanted immunotherapy. (A) Mice were sensitized to peanut then underwent 3 weeks of immunotherapy with or without CpG ODNs and were challenged 10 days later. (B) Symptom scores, (C) body temperature change, and (D) MMCP-1 were assessed for mice following peanut challenge on day 63. Closed circles represent individual mice with the median shown as a line; bars represent mean values with standard deviation shown. *, **, and *** indicate p<0.05, 0.01, and 0.001, respectively. Data is representative of two separate experiments.
Peanut protein-specific IgE, IgG2a, and IgG1
Peanut protein-specific antibodies were measured from serum samples by an ELISA method as previously described [29].
Cytokine measurements from splenocytes
Spleens were removed from mice following sacrifices as indicated in Figures 2A and 4A. Single cell suspensions were prepared and placed in culture media as previously described [30]. Cells were cultured with or without peanut proteins (200 μg/ml) for 96h then supernatants were collected and cytokines quantified by ELISA (R&D Systems).
Statistical analyses
Data were plotted and analyzed for differences using Prism 5/GraphPad software. Symptom scores were compared using a Mann-Whitney test, while all other comparisons were made using unpaired t-tests. P-values less than 0.05 were considered significant.
Results
Type A and B CpG ODNs stimulate DCs from C3H/HeJ mice to produce IL-12 and TNF-α
In order to determine the effects of TLR9 ligands on C3H/HeJ mouse dendritic cells, we enriched DCs from mouse spleens and stimulated with Type A, B, and C CpG ODNs for 24 hours. After stimulation, supernatants were collected and assayed for IL-12 p40 and TNF-α. All 3 types of CpG were able to produce equal levels of IL-12 p40 (Fig 1A). TNF-α was also produced following stimulation with Types A and B CpG ODNs, however, lower levels were measured when Type C was used, although the statistical significance could not be determined since DCs from only two mice were used (Fig 1B). These findings indicated that all 3 types of CpG ODNs could induce IL-12 in cultured DCs, but only Type A and B produce robust secretion of TNF-α.
Co-administration of Type B, but not Type A or C, CpG ODNs during allergic sensitization prevents subsequent anaphylactic reactions
To test the effects of CpG ODNs in vivo, we used our well-established mouse model of peanut allergy [27, 29]. Mice were given peanut antigen plus aluminum hydroxide, a known Th2-skewing adjuvant, in the presence or absence of CpG molecules (Fig 2A). Following the sensitization period, mice underwent a provocation challenge with peanut extract. Symptom scores indicate that Type B CpG ODNs (i.e. 1826 and 1668) were highly effective in preventing allergic symptoms during peanut challenge, as median symptom scores were zero for both Type B ODNs. Type A ODN 1585 was no more effective than sham (PBS) treatment given during sensitization, whereas Type C ODN M362 showed some efficacy but was significantly less effective compared to Type B ODNs (Fig 2B). Decrease in core body temperature is an objective measure of anaphylaxis in mice, and these data also demonstrated that CpG ODN 1826 and 1668 were able to prevent decreased body temperature during challenge much more effectively than either sham, ODN 1585, or ODN M362. Mice had a mean body temperature decrease of approximately 1.0°C when sensitized in the presence of Type B ODNs, whereas mice given ODN 1585 or M362 during sensitization experienced a mean body temperature decrease of 5–10°C (Fig 2C). A third parameter used in our mouse model to measure anaphylaxis is mouse mast cell protease 1 (MMCP-1), which becomes highly elevated in serum following mast cell degranulation [31]. MMCP-1 data also indicate that Type B ODNs prevent mast cell degranulation upon peanut challenge, which is significantly suppressed relative to the sham, ODN 1585, and ODN M362 groups (Fig 2D). ODN 1826 Control (i.e. GpC dinucleotides present; Invivogen) showed no modulation of the allergic response, as was expected (data not shown). These data indicate that mice sensitized in the presence of Type B CpG ODNs 1826 and 1668, but not ODN 1585 or M362, experience significantly less severe allergic reactions.
Prophylactic administration of Type B CpG ODN is associated with increased peanut protein-specific Th1 responses
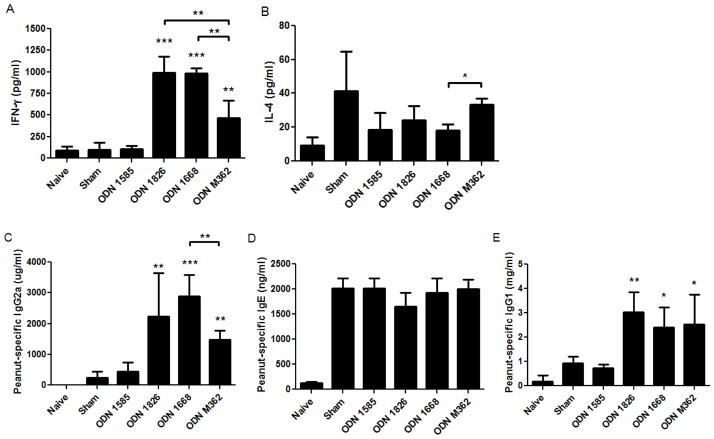
Our hypothesis was that CpG ODN molecules would direct T and B cells toward a Th1-dominated immune response via modulating DC function. T cell responses were measured by stimulating splenocytes from individual mice following sacrifice (Fig 2A) with peanut proteins for 96 hours then quantifying cytokine levels in the supernatants. IFN-γ was measured as the prototypical Th1-type cytokine, while IL-4 was used to represent Th2-type responses. IFN-γ was significantly increased in the mice given CpG ODN 1826 and 1668 compared to mice given ODN 1585, ODN M362, or sham treatment (Fig 3A). IL-4 was not significantly altered by any of the CpG ODN treatments, although a decreased trend was noted for some groups of mice compared to sham treatment (Fig 3B). Peanut protein-specific IgE and IgG2a, Th2- and Th1-driven antibodies, respectively, were measured in post-sensitization sera.
Figure 3.
Th1- and Th2-responses in the prophylactic model. (A) IFN-γ and (B) IL-4 were measured from splenocytes cultured with peanut proteins for 96 hours. (C) Peanut protein-specific IgG2a, (D) IgE, and (E) IgG1 were measured from serum collected following the sensitization period. Bars represent means with standard deviation shown. *, **, and *** indicate p<0.05, 0.01, and 0.001, respectively as compared to the sham and ODN 1585 groups. Other statistically significant differences are indicated by brackets. Data is combined from two separate experiments from the mice shown in Figure 2.
Again, the Th1-response was highly increased in mice given Type B ODNs 1826 and 1668 (p<0.01), but not mice given Type A ODN 1585, as indicated by peanut protein-specific IgG2a levels (Fig. 3C). The peanut protein-specific IgE levels were elevated in all groups of mice except the naïve mice (Fig. 3D). The Type C ODN M362-treated mice had significantly increased Th1-responses compared to sham and ODN 1585-treated mice, however, the IFN-γ and IgG2a levels were significantly lower than the Type B ODN-treated mice. Peanut protein-specific IgG1 was also quantified and was found to be significantly elevated for the groups that received ODN 1826, 1668, and M362 as compared to the sham and ODN 1585-treated mice (Fig. 3E). These mechanistic data demonstrate that Type B CpG ODNs cause the most profound Th1-biased responses to peanut antigens and are associated with decreased symptom scores, reduction in body temperature, and release of MMCP-1 following in vivo peanut challenges.
A truncated immunotherapy regimen with Type B CpG ODN-adjuvanted immunotherapy blocks peanut-induced anaphylaxis
Since CpG ODNs effectively interfered with allergy induction, we next tested whether CpG ODNs could be useful as an adjuvant for immunotherapy in mice with established peanut hypersensitivity. Mice were made allergic to peanut proteins then treated for 3 weeks with 2 injections per week of peanut proteins either with or without the various Types of CpG ODNs (Fig 4A). Typically, immunotherapy in this mouse model is given for 4 weeks with 3 injections per week in the absence of a Th1-directing adjuvant [27, 30]. Importantly, in this truncated immunotherapy regimen no mice had allergic reactions during the course of immunotherapy, as monitored by symptoms and body temperature (data not shown). Mice treated with peanut proteins alone had reduced symptoms (median score of 2) and MMCP-1 release (p<0.05 for both parameters) compared to placebo, although the mice did experience allergic reactions and a rather large decrease in body temperature (Fig 4). Mice given immunotherapy with peanut proteins plus ODN 1585 also had reduced allergic reactions compared to placebo, however, these mice also exhibited decreased body temperature and clearly exhibited signs of allergic reactions. No significant differences were found between mice given immunotherapy with peanut proteins alone and those given peanut plus ODN 1585.
Mice treated with CpG ODN 1826 or M362 and peanut proteins had median symptom scores of zero, which was significantly different compared to placebo-treated mice (p<0.01) and mice given peanut protein immunotherapy alone (p<0.05) (Fig 4B). Body temperatures also demonstrated significant differences between treatment groups. The groups given ODN 1826 and M362 had mean body temperature decreases of approximately 1.0°C, while mice given ODN 1585 or peanut alone experienced mean decreases of 3.2°C and 4.2°C, respectively (Fig 4C). The only treatment groups that were significantly different in terms of body temperature as compared to the peanut alone immunotherapy group were the mice given ODN 1826 and M362 (p<0.05). The MMCP-1 levels post-challenge indicated that all treatment groups had significantly less mast cell degranulation occur during the peanut challenge compared to placebo (Fig 4D). The mice given ODN 1826 and M362 had decreased serum MMCP-1 compared to the peanut alone group (p<0.01 and 0.05, respectively). Although peanut proteins alone had a protective effect in this truncated immunotherapy protocol compared to placebo, the mice given peanut plus ODN 1826 or M362 had substantially weaker allergic reactions than the group given peanut immunotherapy without adjuvant.
Type B CpG ODN-adjuvanted immunotherapy is associated with increased peanut-specific Th1 responses
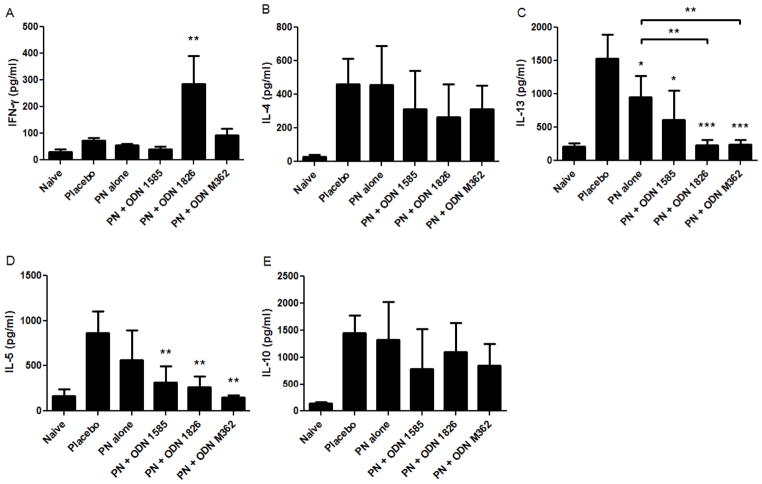
To understand immunologic differences in the immunotherapy groups, we measured Th1-, Th2-, and regulatory-type cytokines, as well as peanut protein-specific antibodies. Mice treated with CpG ODN 1826 and peanut proteins had increased IFN-γ responses from splenocytes cultured with peanut proteins compared to placebo and peanut alone groups (p<0.01), whereas none of the other treatment groups had significantly increased IFN-γ levels (Fig 5A). Three Th2-type cytokines, IL-4, IL-13, and IL-5 were quantified from splenocyte cultures. IL-4 was not different between groups (Fig. 5B), whereas IL-13 and IL-5 were found to be decreased in some groups relative to placebo (Fig. 5C and 5D). Importantly, IL-13, was significantly lower in the groups receiving peanut proteins plus ODN 1826 or ODN M362 as compared to the group receiving peanut proteins alone (Fig. 5C). Levels of IL-10 were not different among the groups (Fig. 5E).
Figure 5.
Th1-, Th2, and regulatory-cytokine responses in the therapeutic model. (A) IFN-γ, (B) IL-4, (C) IL-13, (D) IL-5, and (E) IL-10 were measured from splenocytes (from mice depicted in Figure 4) cultured with peanut proteins for 96 hours. Bars represent means with standard deviation shown. *, **, and *** indicate p<0.05, 0.01, and 0.001, respectively as compared to the placebo and peanut alone groups. Other statistically significant differences are indicated by brackets. Data is representative of two separate experiments.
Serum peanut protein-specific IgE quantities were not significantly different between any of the immunotherapy groups and placebo-treated mice (Fig. 6B). Peanut protein-specific IgG2a levels in serum were highly elevated in the ODN 1826 and M362 treatment groups compared to placebo (p<0.001) or peanut alone treated mice (p<0.001) (Fig 6A). All groups of mice receiving peanut proteins with or without ODNs had significantly elevated serum peanut protein-specific IgG1 compared to placebo (Fig. 6C). The in vitro findings indicate that the mice treated with Type B CpG ODN 1826 experienced less severe allergic reactions due to increased Th1-type responses.
Figure 6.
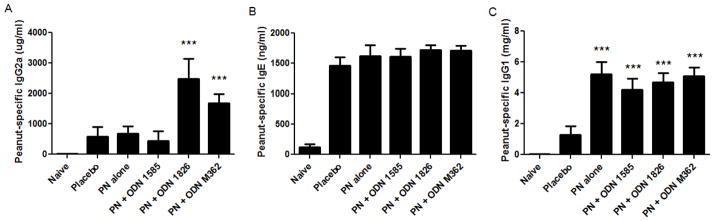
Peanut protein-specific antibody responses in the therapeutic model. (A) Peanut protein- specific IgG2a, (B) IgE, and (C) IgG1 were measured from serum samples collected following the immunotherapy phase. Bars represent means with standard deviation shown. *** indicate p< 0.001 as compared to the placebo group.
Discussion
Peanut allergy affects approximately 1% of the population in the United States, the United Kingdom, and Canada [32]. The allergy is life-long for at least 80% of patients and has been shown to decrease quality-of-life for patients and their families. The severity of allergy to peanuts is often linked to its implication in the large majority of fatal and life-threatening reactions [33]. There is no treatment currently practiced although some forms of immunotherapy are being intensely studied in clinical trials, including oral and sublingual immunotherapy [34]. Additionally, there are no established methods to prevent the allergy from developing, although some studies point towards early exposure as possibly decreasing the likelihood of developing peanut allergy [35]. In the study presented here, we examined the potential uses of a Th1-skewing molecule, the TLR9 agonist CpG ODN, in a prevention and a therapeutic model of peanut allergy in mice. Since allergies are driven by a Th2-biased immune response leading to the production of IgE, we hypothesized that CpG ODNs would be useful to both prevent and treat established allergy to peanuts.
As a prevention approach, we found that Type B CpG ODNs were highly effective in preventing anaphylactic reactions following a standard sensitization protocol. Interestingly, Types A and C CpG ODNs did not have appreciable effects, as mice still had allergic reactions and decreases in body temperatures upon challenge. In our model, the ODNs were given at the same time as the sensitizing (Th2-driving) adjuvant aluminum hydroxide along with peanut protein antigens. We envision the Type B CpG ODN driving a Th1-response even while the Th2 response is being established in vivo. This is evident from the humoral and cellular markers that we investigated. Peanut protein-specific IgE was elevated in all groups of mice, however, peanut-IgG2a was highly elevated in the mice receiving Type B ODNs. The IgG2a was also increased in the Type C group although these mice did experience allergic reactions during challenge, indicating that a certain threshold of peanut protein-specific IgG2a may be needed to prevent anaphylaxis, or other factors may be involved such as antibody affinity. In future experiments, it would be interesting to administer peanut antigen plus ODNs prior to sensitization. Once the immune system is educated toward a Th1 response to peanut antigens, it should be very difficult to later induce a Th2-response.
The differences in the effects exerted on humoral and cellular responses by the various types of ODNs do not appear to be a consequence of not stimulating DCs to produce IL-12, as all 4 ODNs used in the study produced similarly high levels of IL-12 from DCs (Fig 1). However, differences in TNF-α secretion were measured indicating that Type C is less effective in stimulating TNF-α production than either Type A or B (Fig 1B). It is plausible that effects we did not measure, such as cytokine responses from tissue-specific DCs, could vary among the three types of ODNs. For instance, while splenocytic DCs were found to contain high levels of TLR9, DCs found in the lung did not [36]. Additionally, Type B molecules may have direct effects on B cells to stimulate IgG2a production [37] while types A and C may have limited direct effects on B cells. Further investigations are warranted into the mechanisms by which the 3 types of ODNs function in vivo in our mouse model of peanut allergy.
In the therapeutic model, we administered peanut proteins as immunotherapy with or without CpG ODNs. The immunotherapy with peanut proteins alone (or other food protein antigens) is typically given over 4 weeks with 3 injections per week which renders mice non-reactive during provocation challenges [27, 30]. Here, we used a truncated immunotherapy regimen administering antigen with or without ODNs for only 3 weeks with 2 injections per week. In total, 6 immunotherapy injections were given under this regimen versus the typical 12 that are administered when antigen without adjuvant is used. The results demonstrate that while peanut proteins alone can provide a protective effect compared to placebo after 2 weeks of treatment (p<0.05 for symptom scores), using Type B or C ODNs results in improved efficacy and a significant improvement over peanut proteins alone. It should be noted that while Type C ODN provided protection against anaphylaxis in the therapeutic model, the same effect was not seen in the prophylactic model. These findings appear to indicate that the timing of administration of ODNs may be critical to their function in modulating allergies. Importantly, the ability to shorten the course of immunotherapy, decrease the total amount of antigen exposure by half in the sensitized mice, and improve efficacy is exciting and can potentially lead to improved therapeutics for peanut and other food allergies.
The mechanistic analyses for the therapeutic model demonstrated highly elevated peanut protein-specific IgG2a in the Type B and C therapy groups, with no reduction in peanut protein-specific IgE in any of the groups compared to placebo mice. Peanut protein-specific IgG1 was significantly elevated in all treatment groups relative to the placebo group. We hypothesize that the IgG2a is providing protection from allergic reactions during challenge by either neutralizing the peanut allergens before they reach the effector cells pre-loaded with IgE and/or these molecules may also be bound to effector cells and provide inhibitory signals through Fcγ receptors [38]. Since mice treated with peanut proteins alone without ODNs also have increased peanut-IgG1, we don’t believe IgG1 is critical to the in vivo challenge outcomes. Clinical immunotherapy for allergies has long been known to induce antigen-specific IgG and IgG4 responses which are often thought of as “blocking antibodies”. Antigen-specific IgG has been demonstrated to interfere with antigen binding to IgE molecules in serum-based assays using pre- and post-immunotherapy sera [4, 39, 40]. Although our protocol is fairly short (3 weeks of therapy injections, 1 week rest, then challenge), it appears as though peanut protein-specific IgG2a may be all that is required to provide highly effective immunotherapy even in the presence of elevated antigen-specific IgE. We do not feel that the abrogated anaphylactic reactions are the result of desensitization as there is a one week abstinence from therapy and the peanut protein immunotherapy without adjuvant did not provide as strong of a protection. We instead believe that the IgG2a generated by immunotherapy in the Type B and C ODN groups allows a more permanent protection, although further experiments will be needed to validate this hypothesis.
Since these experiments were conducted in a mouse model, it is difficult to ascertain how exactly these findings would translate into clinical peanut allergy. For instance, mice differ from humans in that TLR9 is expressed in monocytes and myeloid DCs, in addition to plasmacytoid DCs and B cells in which human TLR9 is found [22]. We are, however, encouraged that Th1-skewing adjuvants may someday be useful to clinicians as both a method to prevent peanut allergy and treat existing cases of peanut allergy. A clinical trial using CpG ODNs conjugated to a ragweed allergen was successful in inducing IgG, suppressing IgE increases during the pollen season, and provided a reduction in allergic symptoms demonstrating that this type of therapy could be clinically beneficial [41]. One can envision adjuvanted immunotherapy for food allergy being given subcutaneously if lower doses of antigen are achieved to minimize side effects. Adjuvants may also be extremely useful in SLIT protocols in which food challenge outcomes were highly variable among subjects in the treatment group [2]. Oral administration of ODNs may also be plausible as evidenced by a study in mice [25]. A chemically-modified ODN that has enhanced stability and bioavailability over conventional ODNs was given orally in mice sensitized to peanut proteins. The results demonstrated decreased allergic symptoms following a peanut challenge, along with decreased peanut-IgE and increased peanut-IgG2a. It is difficult to explain why peanut-IgE was decreased in this study but not in our therapeutic model, and could be a result of the modifications to the ODN molecule itself or the route of administration. It is also highly plausible that peanut-IgE levels would decrease in our model at later time points, as peanut immunotherapy in humans has shown an early increase in peanut-specific IgG4, whereas IgE takes longer than 12 months to decrease below baseline levels [4, 5].
In conclusion, we have demonstrated that CpG ODNs, particularly Type B ODNs, have the ability to induce potent Th1-responses to peanut antigens in mice. Type B ODNs proved beneficial in reducing anaphylactic reactions when administered during a sensitization protocol as well as when used as an adjuvant in a truncated immunotherapy protocol. These findings may someday be useful in prevention and treatment of peanut allergy in humans.
Acknowledgments
Funding: This work was supported by NIH (R01AI076357, R01AI079088, R21AI079873) and Food Allergy and Anaphylaxis Network (FAAN) grants to XPZ; MK was supported by an NIH NRSA Fellowship (1F32AI084332-01).
Footnotes
All work was conducted at Duke University Medical Center, Durham, NC USA.
Conflict of Interest
Besides being grantees from the funding sources listed above, none of the authors declare a conflict of interest with the work submitted in this manuscript.
References
- 1.Burks AW. Peanut allergy. Lancet. 2008;371:1538–46. doi: 10.1016/S0140-6736(08)60659-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6. e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. e5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105:444–50. doi: 10.1016/j.anai.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–2. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–66. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 11.Strid J, Thomson M, Hourihane J, Kimber I, Strobel S. A novel model of sensitization and oral tolerance to peanut protein. Immunology. 2004;113:293–303. doi: 10.1111/j.1365-2567.2004.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–85. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 13.Kean DE, Goodridge HS, McGuinness S, Harnett MM, Alcocer MJ, Harnett W. Differential polarization of immune responses by plant 2S seed albumins, Ber e 1, and SFA8. J Immunol. 2006;177:1561–6. doi: 10.4049/jimmunol.177.3.1561. [DOI] [PubMed] [Google Scholar]
- 14.Kulis M, Wan CK, Gorentla BK, Burks AW, Zhong XP. Diacylglycerol kinase zeta deficiency in a non-CD4(+) T-cell compartment leads to increased peanut hypersensitivity. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 16.Pochard P, Vickery B, Berin MC, Grishin A, Sampson HA, Caplan M, et al. Targeting Toll-like receptors on dendritic cells modifies the T(H)2 response to peanut allergens in vitro. J Allergy Clin Immunol. 2010;126:92–7. e5. doi: 10.1016/j.jaci.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006;61:64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 18.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. quiz 8–9. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clin Exp Allergy. 2011;41:1226–34. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 20.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scadding GW, Shamji MH, Jacobson MR, Lee DI, Wilson D, Lima MT, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 23.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–62. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 25.Zhu FG, Kandimalla ER, Yu D, Agrawal S. Oral administration of a synthetic agonist of Toll-like receptor 9 potently modulates peanut-induced allergy in mice. J Allergy Clin Immunol. 2007;120:631–7. doi: 10.1016/j.jaci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Adel-Patient K, Ah-Leung S, Bernard H, Durieux-Alexandrenne C, Creminon C, Wal JM. Oral sensitization to peanut is highly enhanced by application of peanut extracts to intact skin, but is prevented when CpG and cholera toxin are added. Int Arch Allergy Immunol. 2007;143:10–20. doi: 10.1159/000098221. [DOI] [PubMed] [Google Scholar]
- 27.Pons L, Ponnappan U, Hall RA, Simpson P, Cockrell G, West CM, et al. Soy immunotherapy for peanut-allergic mice: modulation of the peanut-allergic response. J Allergy Clin Immunol. 2004;114:915–21. doi: 10.1016/j.jaci.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 29.Kulis M, Pons L, Burks AW. In vivo and T cell cross-reactivity between walnut, cashew and peanut. Int Arch Allergy Immunol. 2009;148:109–17. doi: 10.1159/000155741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulis M, Li Y, Lane H, Pons L, Burks W. Single-tree nut immunotherapy attenuates allergic reactions in mice with hypersensitivity to multiple tree nuts. J Allergy Clin Immunol. 2011;127:81–8. doi: 10.1016/j.jaci.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Trong HL, Newlands GF, Miller HR, Charbonneau H, Neurath H, Woodbury RG. Amino acid sequence of a mouse mucosal mast cell protease. Biochemistry. 1989;28:391–5. doi: 10.1021/bi00427a054. [DOI] [PubMed] [Google Scholar]
- 32.Otsu K, Dreskin SC. Peanut allergy: an evolving clinical challenge. Discov Med. 2011;12:319–28. [PubMed] [Google Scholar]
- 33.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119:1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 34.Kulis M, Vickery BP, Burks AW. Pioneering immunotherapy for food allergy: clinical outcomes and modulation of the immune response. Immunol Res. 2011;49:216–26. doi: 10.1007/s12026-010-8183-9. [DOI] [PubMed] [Google Scholar]
- 35.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Arora M, Yarlagadda M, Oriss TB, Krishnamoorthy N, Ray A, et al. Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide. J Immunol. 2006;177:2373–83. doi: 10.4049/jimmunol.177.4.2373. [DOI] [PubMed] [Google Scholar]
- 37.Xu W, Tamura T, Takatsu K. CpG ODN mediated prevention from ovalbumin-induced anaphylaxis in mouse through B cell pathway. Int Immunopharmacol. 2008;8:351–61. doi: 10.1016/j.intimp.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–55. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 40.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, et al. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–9. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]