Abstract
Type 2 diabetes is well recognized as a noninsulin-dependent diabetic disease. Clinical evidence indicates that the level of circulating insulin may be normal, subnormal, and even elevated in type 2 diabetic patients. Unlike type 1 diabetes, the key problem for type 2 diabetes is not due to the absolute deficiency of insulin secretion, but because the body are no longer sensitive to insulin. Thus insulin resistance is increased and the sensitivity to insulin is reset, so increasing levels of insulin are required to maintain body glucose and metabolic homeostasis. How insulin resistance is increased and what factors contribute to its development in type 2 diabetes remain incompletely understood. Overemphasis of insulin deficiency alone may be too simplistic for us to understand how type 2 diabetes is developed and should be treated, since glucose metabolism and homeostasis are tightly controlled by both insulin and glucagon. Insulin acts as a YIN factor to lower blood glucose level by increasing cellular glucose uptake, whereas glucagon acts as a YANG factor to counter the action of insulin by increasing glucose production. Furthermore, other humoral factors other than insulin and glucagon may also directly or indirectly contribute to increased insulin resistance and the development of hyperglycemia. The purpose of this article is to briefly review recently published animal and human studies in this field, and provide new insights and perspectives on recent debates on whether hyperglucagonemia and/or glucagon receptors should be targeted to treat insulin resistance and target organ injury in type 2 diabetes.
Keywords: Glucagon, Glucagon receptor, Hyperglycemia, Hyperglucagonemia, Hypertension, Insulin, Type 2 diabetes
Introduction
According to the latest National Diabetes Statistics, 2011, from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), diabetes mellitus affect 25.8 million Americans of all ages, accounting for 8.3% of the U.S. population. For Americans of ages 65 years and older, nearly 27% had diabetes and 50% have prediabetes. Diabetes now ranks as the 7th leading cause of deaths in the United States, directly and indirectly costing the U.S. economy $174 billion a year. Over 1.6 million new cases will be diagnosed each year, with type 2 diabetes accounting for 90% to 95% of existing and new diabetic patients. Type 2 diabetes, previously called non-insulin-dependent diabetes mellitus (NIDDM), is characterized by the development of insulin resistance, impaired glucose tolerance, hyperglycemia, and cardiovascular, renal and neural complications [1–6••]. Unlike type 1 diabetes, the pathogenesis and therapeutic approaches for type 2 diabetes remain incompletely understood [1, 3••,5••–7]. It is well-recognized that circulating insulin levels may be normal, subnormal, and even elevated in type 2 diabetic patients [1,7]. Thus the key defect in type 2 diabetes is not due to insulin difficiency at least until the late stage of the disease, rather is whether the cells of the body respond properly to insulin [1,7]. Type 2 diabetes typically begins with the development of insulin resistance, which is followed by resetting the cellular sensitivity to insulin to higher levels of insulin in order to maintain body glucose and metabolic homeostasis. As insulin resistance further increases, pancreatic β cells would have to produce more insulin to meet the demand, which eventually leads the loss of β cellular function. How insulin resistance is developed, how the sensitivity to insulin is reset, and what factors contribute to its development and progression of type 2 diabetes remain unknown. Recently the publication of a number of studies using transgenic mice with global and/or tissue-specific knockout or knockin (overexpression) of G protein-coupled glucagon receptors (GCGRs) has reopened the debate on the potential roles of glucagon in the pathogenesis and therapeutic approaches of type 2 diabetes [8–18•]. The purpose of this article is to briefly review these recently published studies and provide some new insights and perspectives on the roles of hyperglucagonemia and/or hyperglycemia in the development of insulin resistance and target organ injury in type 2 diabetes.
An essential role of glucagon and GCGRs in glucose and metabolic homeostasis
Although it is well documented for more than five decades that body glucose and metabolic homeostasis is regulated by pancreatic bi-hormones, many diabetic researchers still hold the views that insulin alone contributes to the regulation of body glucose and metabolic homeostasis, and that insulin deficiency contributes to the development of type 1 and type 2 diabetes. Glucagon plays little or any role in diabetic development and treatment. These views, however, may be too simplistic, simply because pancreatic bi-hormones, insulin and glucagon play their respective YIN and YANG roles in the regulation of glucose metabolism and homeostasis [1–6••]. While insulin acts on insulin receptors to decrease blood glucose levels by stimulating glucose uptake in tissues and glycogen synthesis in the liver, glucagon counters these actions of insulin by stimulating hepatic glycogenolysis and gluconeogenesis, therefore raising blood glucose levels [1,2,4]. Glucagon plays an important role in overcoming hypoglycemic effects of insulin in isulin overdoses [3••,5••,6••]. The hyperglycemic role of glucagon has been supported by numerous studies in rodents and humans using specific GCGR antagonists [2,14,19–21]. Recent studies in mice with global GCGR knockout (Gcgr−/−) further affirm the important contribution of glucagon to normal glucose and metabolic homeostasis [10–12,15••–17•]. Gcgr−/− mice show lower basal blood glucose levels and increased glucose tolerance than wild-type Gcgr+/+ mice, suggesting that glucagon is essential for maintaining normal glycemia.
Glucagon, a 29-amino acid peptide hormone, is synthesized primarily in pancreatic α cells at the periphery of the islets of Langerhans, and released in response to hypoglycemic action of insulin [4,6••,22,23]. It may also be produced in extraislet tissues such as the gut or intestines in the absence of pancreases [24–26]. Glucagon and GCGRs have been molecularly characterized [2,27–29]. GCGRs in the rat and mouse have 485 amino acids, whereas the human GCGRs have 477 amino acids [2,28,29]. Both human and rodent GCGRs encode a common sequence motif, RLAR, in the third cytoplasmic domain, which is required for activation of Gs proteins and transduction of downstream signaling [2,28,29].
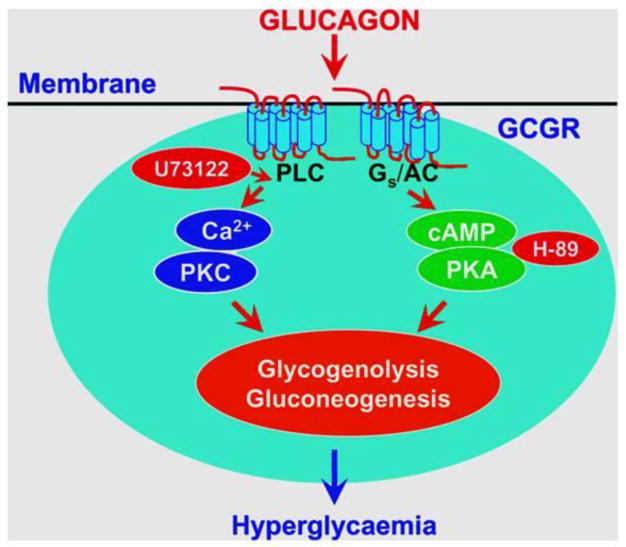
GCGRs are expressed predominantly in hepatocytes of the liver, skeletal muscles, and the kidney, but also to a lesser extent in cardiovascular, neural and gastrointestinal tissues [2,4,6••,30,31]. GCGRs mediate all known hyperglycemic effects of glucagon [1,2,4,5••,28,29]. Glucagon binds to its membrane-bound GCGRs through GTP-binding heterotrimeric G stimulatory (Gs) proteins and activates adenylate cyclase to increased intracellular cAMP levels [2,32,33]. cAMP via activation of protein kinase A (PKA) is the primary signaling molecule for the hyperglycemic action of glucagon [2,32,33]. However, the often overlooked signaling transduction pathway for glucagon-activted GCGRs is the phospholipase C (PLC)/inositol tri-phosphate (IP3)/Ca2+ pathway, which is responsible for increases in intracellular calcium ([Ca2+]i) and activation of protein kinase C [2,32–35]. PLC/IP3/Ca2+- and cAMP-dependent PKA signaling transduction likely mediate both hyperglycemic and/or growth-promoting effects of glucagon on pancreatic islets as well as non-pancreatic cells (Fig. 1).
Figure 1.
Two classic G protein-coupled glucagon receptor-mediated intracellular signaling pathways in glucagon-targeted cells. To induce an effect, glucagon binds to cell surface GTP-heterotrimeric Gs protein-coupled receptors and activates phospholipase C (PLC)/IP3/Ca2+ and cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) signaling. Both signaling pathways are closely involved in mediating glucagon-induced glycogenolysis and gluconeogenesis, leading to hyperglycemia. Pharmacological agents known to block these two signaling pathways also inhibit glucagon-induced hyperglycemic and growth effects in target cells. Abbreviations: AC, adenylyl cyclase; GCGP, the Gs protein-coupled glucagon receptor; H-89, a selective cAMP-dependent PKA inhibitor; PKC, protein kinase C; and U73122, a selective PLC inhibitor. Reprinted with permission from Li and Zhuo [38]. Targeting glucagon receptor signaling in treating metabolic syndrome and renal injury in type 2 diabetes: theory versus promise. Clinical Science 2007; 113, 183–193.
Evidence for or against a role of hyperglucagonemia in type 2 diabetes
The role of hyperglucagonemia in the pathogenesis of type 2 diabetes and its potentials as a therapeutic target for type 2 diabetes remain controversial at present. There are two schools of thoughts on whether hyperglucagonemia plays a role in the diabetic development and treatment [3••,5••,11••,12,14,15••,36••–38•]. The classic dogma argues that glucagon secretion from α islet cells is entirely dependent on insulin levels released from β islet cells; thus it is unlikely that circulating glucagon levels would be increased in type I diabetes, which has insulin deficiency, and in some type 2 diabetic patients, whose insulin levels may not be elevated [3••,6••]. Second, body glucose and metabolic homeostasis is reciprocally regulated by the hypoglycemic action of insulin and the hyperglycemic action of glucagon; a fall in the plasma glucose concentration due to insulin secretion would automatically triger glucagon secretion from α islet cells during hypoglycemia. Conversely, a rise in plasma glucose concentration due to glucagon secretion would stimulate insulin release from β islet cells [2–4,6••,39]. However, it is debatable whether this reciprocal interactions between insulin and glucagon may apply to established type 2 diabetes. Even in nondiabetic subjects, a recent study by Ramanathan et al. showed that partial inhibition of insulin secretion in nondiabetic subjects results in glucose intolerance but not hyperglucagonemia, suggesting that insulin is not the sole regulator of glucagon secretion in humans [17•]. An alternative hypothesis was proposed as early as in the 1970s, which suggests an important, but long debated, role of hyperglucagonemia in the pathophysiology of type 2 diabetes [5,15••,40–42]. Based on both human and animal studies, this hypothesis suggests that inappropriately elevated serum glucagon levels relative to insulin (also termed hyperglucagonemia) may at least in part contribute to the onset and progression of type 2 diabetes [5,15••,40–42].
The debate on whether hyperglucagonemia plays a role in type 2 diabetes in part appears to center on whether hyperglucagonemia is actually present in pre- and established diabetic patients [3••,5••,11••,12,14,15••,36••–38•]. At present, there are still no large scale, multi-center, randomized clinical trials to compare plasma glucagon levels in normal subjects and type 2 diabetic patients, therefore there is no conclusive evidence to indicate whether plasma glucagon levels are significantly elevated in most type 2 diabetic patients. However, Reaven et al. compared plasma glucose, insulin and glucagon levels over an 8-h period between normal subjects and patients with type 2 diabetes in an early study [43]. They demonstrated that plasma glucose and insulin concentrations were significantly higher than normal in patients with type 2 diabetes. The elevated plasma insulin levels were associated with higher, but not lower, plasma glucagon concentrations, as expected, in both nonobese and obese patients with type 2 diabetes [43]. These resuts suggest that despite the fact that plasma insulin levels are equal to or higher than normal, plasma glucagon and glucose levels remain significantly elevated in patients with type 2 diabetes [43]. Orskov et al. showed that proglucagon-like immunoreactivity was significantly elevated in type 2 diabetic patients compared with normal subjects [44]. Even after an oral glucose load, plasma insulin concentrations were lower, whereas proglucagon levels were higher in type 2 diabetic patients [44]. Indeed, in isolated mouse pancreatic islets and clonal hamster In-R1-G9 glucagon-releasing cells, hyperglycemia failed to suppress glucagon release [45•]. In the latter study, low glucose (~7 mmol/l) inhibited glucagon secretion while higher glucose concentrations stimulated glucagon release. In a recent study, Jamison et al. infused normal awake rats with glucose continuously for 10 days (2.5 to 3.3 g/kg/h) and measured plasma glucose and markers of islet and liver function throughout the infusion [46•]. The authors found that although rats adapted to glucose infusion and maintained euglycemia for first 4 days, hyperglycemia occurred in 69% of rats after 8 days and 89% after 10 days of glucose infusion. Interestingly, basal and stimulated plasma insulin and C-peptide concentrations were not different. By contrast, plasma glucagon levels increased fivefold. Infusion of anti-glucagon antibodies normalized plasma glucose to levels identical to those on day 4 [46•]. These paradoxical findings may explain why diabetic patients with pronounced hyperglycemia may still display hyperglucagonemia relative to the prevailing insulin levels [45].
Even if “absolute hyperglucagonemia” may not necessarily be present in most type 2 diabetic patients, there is still credible evidence that a “relative hyperglucagonemia” in the presence of “absolute” or “relative” insulin deficiency may contribute to hyperglycemia in type 1 and type 2 diabetes [1,3••,43,44,47]. Type 2 diabetes is characterized by defects in both α- and β cell function. In a normal subject, pancreatic α islet cells respond normally to glucose or food intake by decreasing glucagon secretion, but they are unable to decrease glucagon secretion appropriately in patients with type 2 diabetes in the presence of persistent hyperglycemia [1,43,44,47]. Shah et al. tested whether a lack of suppression of glucagon causes postprandial hyperglycemia in subjects with type 2 diabetes in two small studies involving 29 patients [47,48]. Somatostatin, insulin, or glucagon were infused to maintain portal glucagon concentrations constant or induce a transient decrease in glucagon. Their studies confirmed that the lack of suppression of glucagon indeed contributes to postprandial hyperglycemia in subjects with type 2 diabetes when insulin secretion is impaired [47,48]. Thus although clinical evidence is still limited, increased serum glucagon levels relative to insulin are likely responsible for persistent hyperglycemia and consequently increased insulin resistance in patients with type 2 diabetes [1,3••,5••,43,44,47,48]. Nevertheless, more lareg scale, multi-center, double-blinded clinical studies are required to confirm this hypothesis.
New insights for an important role of hyperglucagonemia from animal models
Although clinical studies have implicated absolute or relative hyperglucagonemia in the pathogenesis of type 2 diabetes, it has been very difficult to demonstrate a direct cause and effect relationaship between hyperglucagonemia and type 2 diabetes in humans [1,3••,5••,43,44,47,48]. For example, it is impossible to induce hyperglucagonemia in normal human subjects for weeks or months to determine whether long-term hyperglucagonnemia may cause type 2 diabetes. Animal models that either overproduce glucagon or overexpress GCGRs may be alternative approaches or models to determine the roles of glucagon. In keeping with this context, global or tissue-specific knockout or molecular knockdown of GCGRs would also be equally useful approaches. Gelling et al. were the first to examine the direct role of glucagon in glucose homeostasis by generating a null mutation of the glucagon receptor (Gcgr−/−) [10]. Gcgr−/− mice display lower basal blood glucose levels and improved glucose tolerance, but interestingly maintain normal insulin levels compared to wild-type animals. However, plasma glucagon levels were significantly elevated in Gcgr−/− mice [10]. Subsequent studies showed that Gcgr−/− mice are resistant to diet-induced obesity and streptozotocin-induced type 1 diabetic phenotypes [16•,18]. Lee et al. carefully compared pertinent clinical and metabolic phenotypes in Gcgr−/− and wild-type (Gcgr+/+) mice in response to equivalent destruction of pancreatic β cells by streptozotocin [16•]. Gcgr+/+ mice became hyperglycemic, hyperketonemic, polyuric, and cachectic, whereas Gcgr−/− mice failed to develop these diabetic phenotypes. In a further study, the same group of investigators expressed GCGRs in the livers of Gcgr−/− mice before and after β cell destruction by high-dose streptozotocin were performed [15••]. Wild type mice developed fatal diabetic ketoacidosis induced by streptozotocin, whereas Gcgr−/− mice with hepatic GCGR over expression or knockin remained clinically normal without hyperglycemia, impaired glucose tolerance, or hepatic glycogen depletion, despite of similar β cell destruction [15••]. These elegant studies provide evidence that glucagon plays a direct role in the development of type 1 diabetes in mice.
The important role of glucagon in the regulation of insulin secretion and homeostasis has been recently addressed using transgenic mice with GCGR overexpression [11••]. Gelling et al. generated mice with overexpression of GCGRs selectively in pancreatic β cells (RIP-Gcgr). Insulin secretion in response to glucagon was increased 1.7- to 3.9-fold in RIP-Gcgr mice compared with wild types, whereas the glucose excursion in response to a glucagon challenge and intraperitoneal glucose tolerance test (IPGTT) was significantly reduced in RIP-Gcgr mice compared with controls. Fasting hyperglycemia and impaired glucose tolerance (IGT) were reduced in RIP-Gcgr mice as well [11••]. However, this transgenic model has not been used to determine the role of glucagon in type 1 or type 2 diabetes.
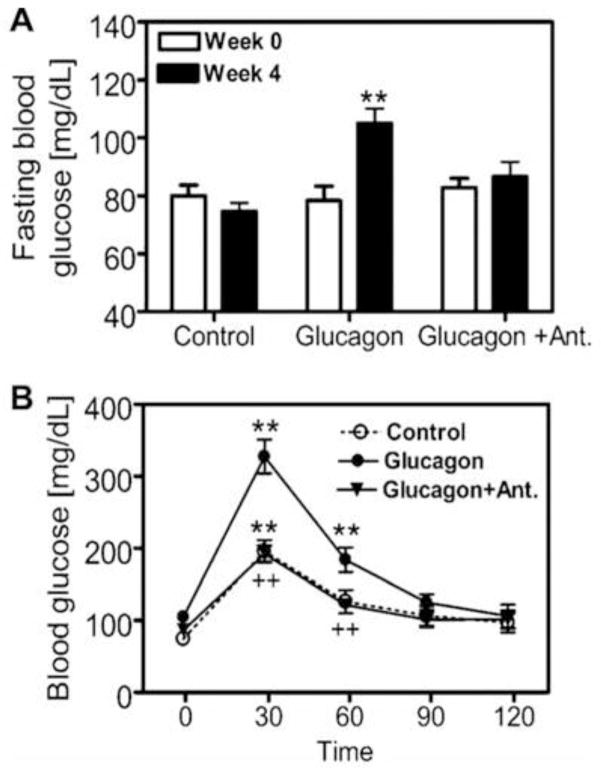
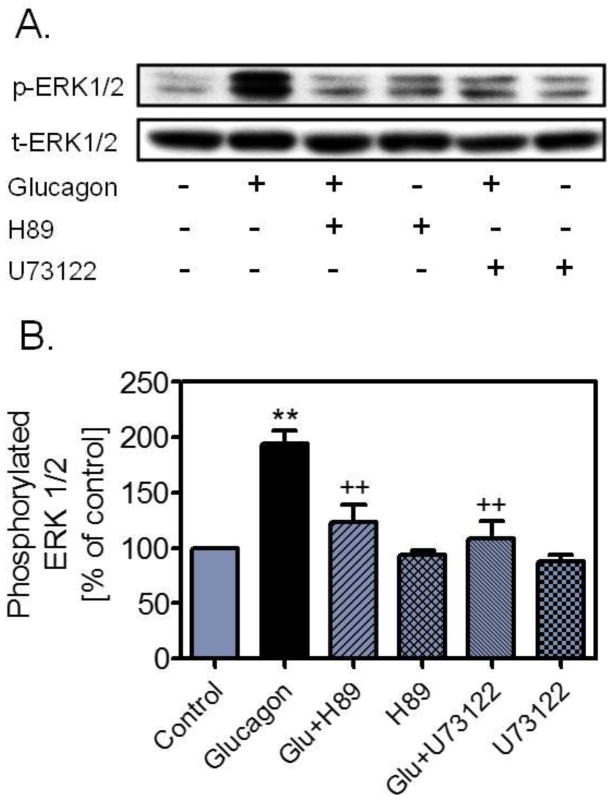
We have recently employed an alternative approach to study the role of hyperglucagonemia in the development of early phenotypes of type 2 diabetes [14]. Glucagon was chronically infused via an osmotic minipump for 4 weeks to induce hyperglucagonemia in male adult C57BL/6J mice, as described by Webb et al. [49]. We reasoned that imbalance of insulin and glucagon in favouring the latter may contribute to impaired glucose tolerance, persistent hyperglycaemia, microalbuminuria and glomerular injury. Compared with vehicle, infusion of glucagon alone at 1 μg/h for 4 weeks increased serum glucagon (p<0.01), elevated fasting blood glucose (p<0.01), impaired glucose tolerance (p<0.01), increased 24 h urinary albumin excretion (p<0.01), and induced glomerular mesangial expansion and extracellular matrix deposition (Fig. 2) [14]. We also found that serum insulin did not increase proportionally in response to glucagon infusion. All early type 2 diabetic phenotypes induced by long-term infusion of glucagon were blocked by concurrent administration of [Des-His1-Glu9]glucagon, a specific GCGR antagonist [14]. Interestingly, high glucose adminsitration alone for 4 weeks did not elevate fasting blood glucose levels, impair glucose tolerance or induce renal injury, as expected. These results demonstrate for the first time that long-term hyperglucagonaemia in mice may induce early metabolic and renal phenotypes of Type 2 diabetes by activating glucagon receptors. The renal phenotypes induced by long-term hyperglucagonemia are especially relevant to type 2 diabetic nephropathy. We have previously shown that glucagon induced growth and proliferation of rat mesangial cells associated with activation of MAP kinases ERK1/2 via protein kinase A and phospholipase C signaling transduction (Fig. 3) [34,35,38]. We and others have also demonstrated that glucagon induced significant glomerular hyperfiltration in anaesthetized rats [50,51]. Since glomerular hyperfiltration and subsequent development of albuminuria are two major hallmarks of glomerular injury in early type 2 diabetes [52,53], persistent glomerular hyperfiltration induced by hyperglucagonemia may lead to renal hypertrophy, glomerular injury and development of microalbuminuria in human type 2 diabetes [1,54–56].
Figure 2.
Effects of long-term hyperglucagonaemia induced by infusion of glucagon via osmotic minipump (1 μg/h, i.p., for 4 weeks) and concurrent blockade of GCGR by [des-His1-Glu9] glucagon (5 μg/h via osmotic minipump, i.p.) on fasting blood glucose (A) and glucose tolerance (B) in glucagon-infused mice. **p<0.01 compared with basal (week 0) for fasting blood glucose (in A) or compared with 0 min for the GTT within the same group (in B). ++p<0.01, compared with glucagon-infused mice at corresponding periods. Ant, [Des-His1-Glu9]glucagon. Reprinted with permission from Li et al. [14]. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of Type 2 diabetes in mice. Clin Sci (Lond) 2008; 114(9):591–601.
Figure 3.
Effects of cAMP-dependent protein kinase A- or phospholipase C-selective inhibitors on glucagon-induced MAP kinases ERK 1/2 phosphorylation in rat glomerular mesangial cells. Both H-89 and U73122 blocked the effects of glucagon on MAP kinases ERK 1/2 phosphorylation. (A) Western blot of phosphorylated and total ERK 1/2; (B) quantitative changes in phosphorylated ERK 1/2 from control. **p<0.01 vs control; ++p<0.01 vs glucagon. Reprinted with permission from Li et al. [35]. Glucagon receptor-mediated ERK 1/2 phosphorylation in rat mesangial cells: role of protein kinase A and phospholipase C. Hypertension 2006; 47, 1–6.
New perspectives for targeting glucagon receptors in type 2 diabetes
There has been tremendous progress being made in treating type 1 and type 2 diabetes during the last several decades as our understanding of the mechanisms underlying the development of diabetes is evolved [2–7]. Now type 1 diabetes can be treated with insulin replacement, cell transplantation, and/or embryonic stem cells, However, it is unlikely that the same approaches for type 1 diabetes are practical for patients with type 2 diabetes, since 95% of diabetic patients have type 2 rather than type 1 diabetes. More importantly, most of patients with type 2 diabetes do not have absolute insulin deficiency until the late stage of the disease when β cells can no longer produce insulin to maintain body glucose and metabolic homeostasis. The decrease in insulin sensitivity or increased insulin resistance plays a key role in the pathogenesis of type 2 diabetes [1–3••,5••,6••,38]. During last few decades, numerous therapeutic approaches have been proposed and developed to treat type 2 diabetes by improving insulin sensitivity and decreasing insulin resistance. Glucagon or its GCGRs is one of these approaches recently gaining renewed attention and debates [3••,5••,6••,38].
The idea to target glucagon or GCGRs in type 2 diabetes goes back to the 1970s, but the debate on its promises and limitations remains unabated [2–4,6••,40,41]. There are still major concerns on whether it is a good idea to block GCGRs in diabetes. For example, glucagon is widely used to treat hypoglycemia induced by insulin overdose in diabetic patients, blockade of GCGRs may cause severe hypoglycemic side effects [3••,6••]. Second, glucagon itself is the principle stimulator for insulin synthesis and secretion from β islet cells, blockade of GCGRs would further aggravate insulin deficiency in type 2 diabetes [3••,4,6••]. However, recent studies in global GCGR−/− mice appear to somewhat alleviate these concerns [10]. Gelling et al. showed that GCGR−/− mice have lower blood glucose levels, improved glucose tolerance, and supraphysiological glucagon and GLP-1 levels, but insulin levels are normal [10]. Nevertheless, subsequent studies in these mice suggest that GCGR deletion may increase fetal lethality and alter islet development and maturation [13]. Some of glucose, lipid and metabolic phenotypes observed in GCGR−/− mice may have negative implications on targeting GCGRs in the treatment of diabetes [36••]. The third concern is that there is little clinical evidence that orally active GCGR antagonists are effective in treating hyperglycemia and improving insulin resistance in type 2 diabetic patients [21]. A novel GCGR antagonist, Bay 27-9955, has previously been evaluated in a small double blind, placebo controlled, and crossover study [21]. Bay 27-9955 significantly blunted hyperglucagonemia-induced hyperglycemia, but long-term effects of this compound on type 2 diabetic patients have not been determined. Two recent unpublished studies also suggest that GCGR antagonists, MK-0893 [57••] and LY2409021 [58••], also show some promises in treating type 2 diabetic patients by reducing fast blood glucose and HbA1c. However, these are small studies involving only a small number of type 2 diabetic patients. More large-scale, multi-center, double blind, and placebo controlled studies are necessary to determine whether GCGRs are a novel target in the treatment of type 2 diabetes in humans.
However, there is accumulating evidence from animal experimentation that blockade of GCGRs with specific GCGR antagonists, antibodies, or antisense oligonucleotides may be beneficial in the treatment of type 2 diabetes in a number of proof of concept studies [8••,9,14,59]. For example, Gu et al. demonstrated that a 5-week treatment of diet-induced obese mice or db/db mice with GCGR mAb effectively normalized nonfasting blood glucose without inducing hypoglycemia or other undesirable metabolic perturbations [60]. No evidence of pancreatic α cell neoplastic transformation was found in mice treated with GCGR mAb for 18 weeks in this study. We demonstrated that the peptide GCGR antagonist, [Des-His1-Glu9]glucagon, effectively blocked the development of early type 2 diabetic phenotypes including hyperglycemia, impaired glucose tolerance, and microalbuminuria in C57BL/6J mice induced by long-term infusion of glucagon to produce hyperglucagonemia [14]. Sloop et al. targeted GCGRs in rodent models of type 2 diabetes using 2′-methoxyethyl-modified phosphorothioate-antisense oligonucleotide (ASO) inhibitors to determine whether blocking glucagon action would reverse hyperglycemia [8••]. These authors found that treatment with GCGR ASOs decreased GCGR expression, normalized blood glucose, improved glucose tolerance, and preserved insulin secretion. GCGR inhibition also increased serum GLP-1 concentrations and insulin levels in pancreatic islets [8••]. Similar beneficial results of GCGR inhibition by GCGR ASO were largely confirmed in a different study [9••]. In all above-mentioned studies, no significant hypoglycemic effects were observed [8••,9••,14,59,60•]. Taken together, these proof of concept studies provide new insights and perspectives on whether GCGRs may be targeted in the treatment of type 2 diabetes in the future.
Conclusions
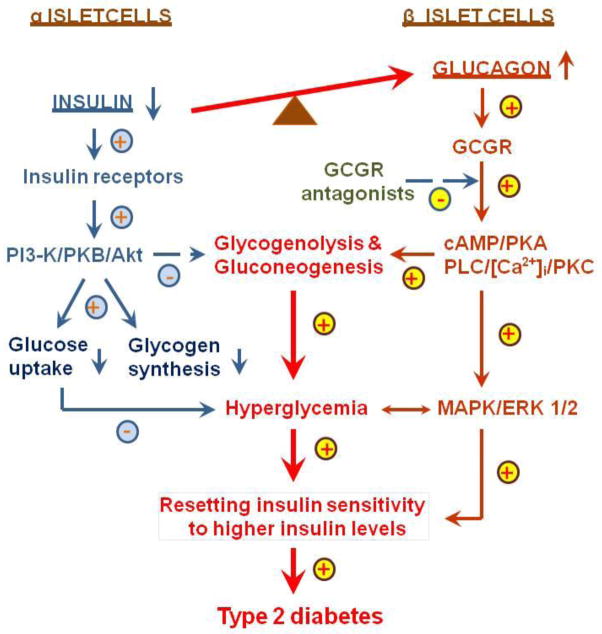
In summary, diabetes is a major risk factor for the development of cardiovascular, hypertensive, and renal target organ damage, leading to atherosclerosis, ischemic heart disease, stroke and chronic kidney diseases. Type 1 and type 2 diabetes affect ~26 million Americans of all ages, and ranks as the 7th leading cause of deaths in the United States. It is thus imperative to identify the factors contributing to the development and progression of type 2 diabetes, and to explore new therapeutic targets beyond insulin replacement. Clearly, imbalance in the actions of insulin and glucagon in favoring the latter contribute to increased insulin resistance, impaired glucose tolerance, persistent hyperglycemia, and target organ injury in type 2 diabetes (Fig. 4). Since glucagon is one of two key players along with insulin in the regulation of glucose metabolism and homeostasis in humans by promoting hyperglycemia and other growth and proliferation, glucagon and GCGRs should be considered as potential targets for treatment of type 2 diabetes (Fig. 4). It is expected that GCGR blockade alone, or in combination with angiotensin-converting enzyme inhibitors or AT1 receptor blockers, may provide clinical benefits by reducing hyperglycemia, improving or resetting insulin sensitivity and β cell function, and preventing type 2 diabetic cardiovascular and renal complications. However, at the same time the limitations or the side effects of GCGR blockade should also be carefully evaluated to ensure its safety and maximize its therapeutic promises in treating type 2 diabetes.
Figure 4.
A hypothesis that proposes a potential role of hyperglucagonemia in the development of hyperglycemic and metabolic phenotypes in type 2 diabetes. In human type 2 diabetes, there is an inappropriate increase in the hyperglycemic glucagon over the hypoglycemic insulin. Imbalance of the actions of insulin and glucagon in favoring the latter leads to increases in glycogenolysis and gluconeogenesis in the liver and other tissues, resulting in hyperglycemia, increased insulin resistance (resetting insulin sensitivity to higher levels of insulin) and impaired glucose tolerance. Moreover, glucagon, via direct activation of GCGR-mediated signaling pathways (cAMP-dependent protein kinase A, PI 3-K/PKB/Akt, PLC/[Ca2+]i/PKC, and MAPK/ERK 1/2), stimulates cellular growth and proliferation, leading to target organ injury. GCGR antagonists are expected to block these metabolic and growth effects of hyperglucagonemia in type 2 diabetes, and reset insulin sensitivity to lower insiulin levels. GCGR, G protein-coupled glucagon receptors; MAPK/ERK 1/2, mitogen-activated protein kinase/extracellular regulated kinases 1 and 2; PKA, protein kinase A; PI3-K, PI3-kinase; PKB/Akt, protein kinase B/AKT; and PLC/]Ca2+]i/PKC, phospholipase C/intracellular calcium/protein kinase C. Modified with permission from Li and Zhuo [38]. Targeting glucagon receptor signaling in treating metabolic syndrome and renal injury in type 2 diabetes: theory versus promise. Clinical Science 2007; 113, 183–193.
Acknowledgments
The authors’ work was supported in part by the National Institute of Diabetes, Digestive and Kidney Diseases Grants (5RO1DK067299, 2R56057299, and 2RO1DK067299.A2). The authors apologize to those investigators whose excellent work is not cited due to space and scope restrictions.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Xiao C. Li and Jia L. Zhuo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. All animal experiments as described in the work of the authors in this manuscript were approved by the Institutional Animal Care and Use Committees of Henry Ford Health System, Detroit, Michigan, and the University of Mississippi Medical Center, Jackson, Mississippi, USA.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Matthaei S, Stumvoll M, Kellerer M, Haring H-U. Pathophysiology and Pharmacological treatment of insulin resistance. Endocr Rev. 2000;21:585–618. doi: 10.1210/edrv.21.6.0413. [DOI] [PubMed] [Google Scholar]
- 2.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, Drucker DJ International Union of Pharmacology. XXXV. The glucagon receptor family [Review] [412 refs] Pharmacol Rev. 2003;55(1):167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- ••3.Cryer PE. Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3):1039–1048. doi: 10.1210/en.2011-1499. This is a comprehensive review on interactions between pancreatic islet alpha-cell glucagon secretion and islet beta-cell insulin secretion. The author argues that in absolute endogenous insulin deficiency (i.e. in type 1 diabetes and in advanced type 2 diabetes), beta-cell failure results in no decrease in beta-cell insulin secretion and thus no increase in alpha-cell glucagon secretion during hypoglycemia. However, the author also recognizes the increasing evidence that relative hyperglucagonemia, in the setting of deficient insulin secretion, plays a role in the pathogenesis of hyperglycemia in diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- ••5.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4–12. doi: 10.1172/JCI60016. The primary author is a well-recognized pioneer who advocated for the potential of targeting glucagon and/or GCGRs in the treatment of type 2 diabetes as early as in the 1970s. This is one of best overview articles summarizing the important role of the hormone glucagon in the pathogenesis of type 1 and type 2 diabetes. The authors provide the strong evidence and arguments for targeting glucagon and GCGRs in diabetes, and conclude that glucagon suppression or inactivation may provide therapeutic advantages over insulin monotherapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••6.Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab. 2009;296(3):E415–E421. doi: 10.1152/ajpendo.90887.2008. This is a constructive article to review glucagon actions in hepatic and extrahepatic tissues, and provide the arguments for and against the concept of targeting Gcgr signaling for the treatment of T2DM. [DOI] [PubMed] [Google Scholar]
- 7.Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA. 2008;299(10):1185–1187. doi: 10.1001/jama.299.10.1185. [DOI] [PubMed] [Google Scholar]
- 8.Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest. 2004;113(11):1571–1581. doi: 10.1172/JCI20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes. 2004;53:410–417. doi: 10.2337/diabetes.53.2.410. [DOI] [PubMed] [Google Scholar]
- 10.Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100(3):1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••11.Gelling RW, Vuguin PM, Du XQ, Cui L, Romer J, Pederson RA, Leiser M, Sorensen H, Holst JJ, Fledelius C, Johansen PB, Fleischer N, McIntosh CH, Nishimura E, Charron MJ. Pancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and mass. Am J Physiol Endocrinol Metab. 2009;297(3):E695–E707. doi: 10.1152/ajpendo.00082.2009. This study investigated the role of glucagon in the regulation of insulin secretion and whole body glucose homeostasis in vivo by generating mice overexpressing the Gcgr specifically on pancreatic beta-cells (RIP-Gcgr). The authors demonstrated that increased pancreatic beta-cell expression of the Gcgr increased insulin secretion, pancreatic insulin content, beta-cell mass, and, when mice were fed a HFD, partially protected against hyperglycemia and IGT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen H, Winzell MS, Brand CL, Fosgerau K, Gelling RW, Nishimura E, Ahren B. Glucagon receptor knockout mice display increased insulin sensitivity and impaired beta-cell function. Diabetes. 2006;55(12):3463–3469. doi: 10.2337/db06-0307. [DOI] [PubMed] [Google Scholar]
- 13.Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147(9):3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XC, Liao TD, Zhuo JL. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of Type 2 diabetes in mice. Clin Sci (Lond) 2008;114(9):591–601. doi: 10.1042/CS20070257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••15.Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A. 2012;109(37):14972–14976. doi: 10.1073/pnas.1205983109. This study tested whether suppression of glucagon action will eliminate manifestations of diabetes by expressing GCGRs in livers of glucagon receptor-null (GcgR(−/−)) mice before and after beta-cell destruction by high-dose streptozotocin. The authors conclude from their results that the metabolic manifestations of diabetes cannot occur without glucagon action and, once present, disappear promptly when glucagon action is abolished. The study supports the notion that glucagon suppression should be a major therapeutic goal in diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •16.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60(2):391–397. doi: 10.2337/db10-0426. In this article, the authors compared pertinent clinical and metabolic parameters in glucagon receptor-null (Gcgr(−/−)) mice and wild-type (Gcgr(+/+)) controls after equivalent destruction of beta-cells with streptozotocin. The study shows striking results that blocking glucagon action prevents the deadly metabolic and clinical derangements of type 1 diabetic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Ramanathan RP, Arbelaez AM, Cryer PE. Partial inhibition of insulin secretion results in glucose intolerance but not hyperglucagonemia. Diabetes. 2011;60(4):1324–1328. doi: 10.2337/db10-1586. This study tested the hypotheses that in nondiabetic individuals, partial inhibition of insulin secretion with the ATP-sensitive K(+) channel agonist (opener) diazoxide induces hyperglycemia and hyperglucagonemia after a mixed meal and after administration of the sulfonylurea glimepiride. The results show that partial inhibition of insulin secretion results in impairment of glucose tolerance after a mixed meal and after glimepiride administration in the absence of glucagon secretion. The study suggests that beta-cell secretion is not the only regulator of alpha-cell glucagon secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50(1):142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 19.Unson CG, Gurzenda EM, Merrifield RB. Biological activities of des-His1[Glu9]glucagon amide, a glucagon antagonist. Peptides. 1989;10:1171–1177. doi: 10.1016/0196-9781(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 20.Buggy JJ, Heurich RO, MacDougall M, Kelley KA, Livington JN, Yoo-Warren H, Rossomando AJ. Role of the glucagon receptor COOH-terminal domain in glucagon-mediated signaling and receptor internalization. Diabetes. 1997;46:1400–1405. doi: 10.2337/diab.46.9.1400. [DOI] [PubMed] [Google Scholar]
- 21.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44:2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 22.Bromer WW, Sinn LG, Staub A, Behrens OK. The amino acid sequence of glucagon. J Am Chem Soc. 1956;78:3858–3859. doi: 10.2337/diab.6.3.234. [DOI] [PubMed] [Google Scholar]
- 23.Baum J, Simon BF, Unger RH, Madison LL. Localization of glucagon in the alpha cells in the pancreatic islet by immunofluorescent techniques. Diabetes. 1962;11:371–374. [PubMed] [Google Scholar]
- 24.Villanueva ML, Hedo JA, Marco J. Plasma glucagon immunoreactivity in a totally pancreatectomized patient. Diabetologia. 1976;12(6):613–616. doi: 10.1007/BF01220639. [DOI] [PubMed] [Google Scholar]
- 25.Werner PL, Palmer JP. Immunoreactive glucagon responses to oral glucose, insulin infusion and deprivation, and somatostatin in pancreatectomized man. Diabetes. 1978;27(10):1005–1012. doi: 10.2337/diab.27.10.1005. [DOI] [PubMed] [Google Scholar]
- 26.Hatton TW, Yip CC, Vranic M. Biosynthesis of glucagon (IRG3500) in canine gastric mucosa. Diabetes. 1985;34(1):38–46. doi: 10.2337/diab.34.1.38. [DOI] [PubMed] [Google Scholar]
- 27.Lund PK, Goodman RH, Dee PC, Habener JF. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. PNAS. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jelinek LJ, Lok S, Rosenberg GB, Smith RA, Grant FJ, Biggs S, et al. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993;259:1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- 29.Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, et al. The human glucagon receptor encoding gene: structure, cDNA sequence and chromosomal localization. Gene. 1994;140:203–209. doi: 10.1016/0378-1119(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 30.Butlen D, Morel F. Glucagon receptors along the nephron: [125I]glucagon binding in rat tubules. Pflugers Arch. 1985;404:348–353. doi: 10.1007/BF00585347. [DOI] [PubMed] [Google Scholar]
- 31.Marks J, Debnam ES, Dashwood MR, Srai SK, Unwin RJ. Detection of glucagon receptor mRNA in the rat proximal tubule: potential role for glucagon in the control of renal glucose transport. ClinSci (Lond) 2003;104:253–258. doi: 10.1042/CS20020336. [DOI] [PubMed] [Google Scholar]
- 32.Rodbell M, Birnbaumer L, Pohl SL, Krans HM. The glucagon-sensitive adenylyl cyclase system in plasma membranes of rat liver. V. an obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971;246:1877–1882. [PubMed] [Google Scholar]
- 33.Wakelam MJ, Murphy GJ, Hruby VJ, Houslay MD. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature (Lond) 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- 34.Li XC, Carretero OA, Zhuo JL. Cross-talk between angiotensin II and glucagon receptor signaling mediates phosphorylation of mitogen-activated protein kinases ERK 1/2 in rat glomerular mesangial cells. Biochem Pharmacol. 2006;71(12):1711–1719. doi: 10.1016/j.bcp.2006.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XC, Carretero OA, Shao Y, Zhuo JL. Glucagon receptor-mediated ERK 1/2 phosphorylation in rat mesangial cells: role of protein kinase A and phospholipase C. Hypertension. 2006;47:1–6. doi: 10.1161/01.HYP.0000197946.81754.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36.Vuguin PM, Charron MJ. Novel insight into glucagon receptor action: lessons from knockout and transgenic mouse models. Diabetes Obes Metab. 2011;13 (Suppl 1):144–50. doi: 10.1111/j.1463-1326.2011.01447.x. This review article provides an overview on genetically modified animal models of glucagon signalling in metabolism. The authors summarize evidence that mice with a global deletion of the glucagon receptor gene (Gcgr) may be associated with poor foetal growth, altered cytoarchitecture of pancreatic islets and glucose, lipid and hormonal profiles; altered body composition and protection from diet-induced obesity; impaired hepatocyte survival; altered metabolic response to prolonged fasting and exercise, and prevention of development of diabetes in insulin-deficient mice. The authors caution that blockage of Gcgr activation may have negative implications in the treatment of diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •37.Maharaj A, Zhu L, Huang F, Qiu H, Li H, Zhang CY, Jin T, Wang Q. Ectopic expression of glucagon receptor in skeletal muscles improves glucose homeostasis in a mouse model of diabetes. Diabetologia. 2012;55(5):1458–1468. doi: 10.1007/s00125-012-2464-x. In this study, the investigators generated transgenic mice in which the expression of Gcgr is driven by the muscle specific creatine kinase (Mck) promoter, and assessed the effects of glucagon on the modulation of glucose homeostasis under conditions of extremes of glucose influx or efflux. The results suggest that mild and chronic hyperglucagonaemia provides beneficial effects on beta cell function and glucose homeostasis. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Zhuo JL. Targeting glucagon receptor signaling in treating metabolic syndrome and renal injury in type 2 diabetes: theory versus promise. Clinical Science. 2007;113:183–193. doi: 10.1042/CS20070040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab. 2008;295:E751–E761. doi: 10.1152/ajpendo.90295.2008. [DOI] [PubMed] [Google Scholar]
- 40.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;4:14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 41.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27(11):1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 42.Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28(8):574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia through the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 44.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55(8):2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- •46.Jamison RA, Stark R, Dong J, Yonemitsu S, Zhang D, Shulman GI, Kibbey RG. Hyperglucagonemia precedes a decline in insulin secretion and causes hyperglycemia in chronically glucose-infused rats. Am J Physiol Endocrinol Metab. 2011;301(6):E1174–E1183. doi: 10.1152/ajpendo.00175.2011. In this study, the investigators infusedglucaose in normal awake rats continuously for 10 days to induce hyperglycemia and determine its impact on markers of islet and liver function. Although rats adapted initially to hyperglycemia, and maintained euglycemia for approximately 4 days, continued infusion of glucose led to worsening hyperglycemia in 89% of rats after 10 days. Plasma insulin and C-peptide concentrations remained largely unchanged, whereas plasma glucagon concentrations increased fivefold. These data support the novel concept that hyperglycemia may paradoxically increase glucagon secretion in type 2 diabetic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 48.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277(2 Pt 1):E283–E290. doi: 10.1152/ajpendo.1999.277.2.E283. [DOI] [PubMed] [Google Scholar]
- 49.Webb GC, Akbar MS, Zhao C, Swift HH, Steiner DF. Glucagon replacement via micro-osmotic minipump corrects hypoglycemia and [alpha]-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes. 2002;51:398–405. doi: 10.2337/diabetes.51.2.398. [DOI] [PubMed] [Google Scholar]
- 50.Harris PJ, Skinner SL, Zhuo JL. The effects of atrial natriuretic peptide and glucagon on proximal glomerulotubular balance in anesthetized rats. J Physiol (Lond) 1988;402:29–42. doi: 10.1113/jphysiol.1988.sp017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahloulay M, Dechaux M, Hassler C, Bouby N, Bankir L. Cyclic AMP is a hepatorenal link influencing natriuresis and contributing to glucagon-induced hyperfiltration in rats. J Clin Invest. 1996;98:2251–2258. doi: 10.1172/JCI119035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hostetter TH. Hyperfiltration and glomerulosclerosis. Seminars in Nephrol. 2003;23:194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 53.Breyer MD, Bottinger E, Brosius FC, III, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 54.Gartner K. Glomerular hyperfiltration during the onset of diabetes mellitus in two strains of diabetic mice (c57bl/6j db/db and c57bl/ksj db/db) Diabetologia. 1978;15:59–63. doi: 10.1007/BF01219330. [DOI] [PubMed] [Google Scholar]
- 55.Levine DZ, Iacovitti M, Robertson SJ, Mokhtar GA. Modulation of single-nephron GFR in the db/db mouse model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R975–R981. doi: 10.1152/ajpregu.00693.2005. [DOI] [PubMed] [Google Scholar]
- 56.Ahloulay M, Dechaux M, Hassler C, Bouby N, Bankir L. Cyclic AMP is a hepatorenal link influencing natriuresis and contributing to glucagon-induced hyperfiltration in rats. J Clin Invest. 1996;98:2251–2258. doi: 10.1172/JCI119035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••57.Engel SS, Xu L, Andryuk PJ, Davies MJ, Kaufman K, Goldstein BJ. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist, in patients with type 2 diabetes. Diabetes. 2011;60:A85. (Abstract) This is a moderate phase II, randomized study invoving 342 type 2 diabetic patients treated with once-daily administration of the GCGR antagonistt MK-0893 in four different dosages, metformin, or placebo. 12 week-treatment with MK-0893 caused significant, dose-dependent reductions in fasting and postprandial plasma glucose, and HbA1c, compared with placebo. Low incidences of hypoglycemia were observed in all groups. This study provides clinical evidence that GCGRs ma be targeted for the treatment of type 2 diabetes in humans. [Google Scholar]
- ••58.Kelly RP, Garhyan P, Abu-Raddad EJ, Lim CN, Prince MJ, Pinaire JA, Loh MT, Deeg MA. Short-term treatment with glucagon receptor antagonist LY2409021 effectively reduces fasting blood glucose and HbA1c in patients with type 2 diabetes mellitus. Diabetes. 2011;60:A84. (Abstract). This is a small Phase 1, randomized, double-blind, placebo (PBO)-controlled study that evaluated the safety, tolerability, pharmacokinetics, and shortterm (4 weeks) efficacy of once-daily doses of GCGR antagonist LY2409021 (5, 30, 60, or 90 mg) in patients with T2DM treated with diet and exercise or metformin (N=47). LY2409021 significantly reduced fasting blood glucose by Day 14 by >30 mg/dL in 5 of 9 patients in the 90 mg dose group, with no clinical signs or significant elevations in bilirubin or alkaline phosphatase. Thus this study supports the concept that glucagon receptor antagonists may be developed for the treatment of T2DM. [Google Scholar]
- 59.Sorensen H, Brand CL, Neschen S, Holst JJ, Fosgerau K, Nishimura E, Shulman GI. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes. 2006;55(10):2843–2848. doi: 10.2337/db06-0222. [DOI] [PubMed] [Google Scholar]
- •60.Gu W, Yan H, Winters KA, Komorowski R, Vonderfecht S, Atangan L, Sivits G, Hill D, Yang J, Bi V, Shen Y, Hu S, Boone T, Lindberg RA, Veniant MM. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther. 2009;331(3):871–881. doi: 10.1124/jpet.109.157685. This study demonstrates that a 5-week treatment of diet-induced obese mice with mAb effectively normalized nonfasting blood glucose, reduced fasting blood glucose without inducing hypoglycemia or other undesirable metabolic perturbations. In addition, no hypoglycemia was found in db/db mice that were treated with a combination of insulin and mAb. [DOI] [PubMed] [Google Scholar]