Abstract
The flight muscles of many insects have a form of regulation enabling them to contract at high frequencies. The muscles are activated by periodic stretches at low Ca2+ levels. The same muscles also give isometric contractions in response to higher Ca2+. We show that the two activities are controlled by different isoforms of TnC (F1 and F2) within single myofibrils. F1 binds one Ca2+ with high affinity in the C-terminal domain and F2 binds one Ca2+ in the C-terminal domain and one exchangeable Ca2+ in the N-terminal domain. We have characterised the isoforms and determined their effect on the development of stretch-activated and Ca2+-activated tension by replacing endogenous TnC in Lethocerus flight muscle fibres with recombinant isoforms. Fibres with F1 gave stretch-activated tension and minimal isometric tension; those with F2 gave Ca2+-dependent isometric tension and minimal stretch-activated tension. Regulation by a TnC responding to stretch rather than Ca2+ is unprecedented and has resulted in the ability of insect flight muscle to perform oscillatory work at low Ca2+ concentrations, a property to which a large number of flying insects owe their evolutionary success.
Keywords: Ca2+ regulation, insect flight muscle, stretch activation, troponin C
Introduction
Striated muscles are regulated by changes in the concentration of Ca2+ in the fibres, which are activated when Ca2+ binds to the tropomyosin–troponin complex (Tm–Tn) on actin. Kinetic studies suggest that there are three states of the thin filament, referred to as blocked, closed and open (McKillop and Geeves, 1993; Maytum et al, 1999); these correspond to the B-state, C-state and M-state observed by electron microscopy (Lehman et al, 2000). According to the steric blocking model of regulation, in the absence of Ca2+, myosin-binding sites on the thin filament are blocked by Tm, which extends over seven actin monomers (the blocked state). Tn has three subunits, TnT, TnI and TnC (Greaser and Gergely, 1971); on activation, Ca2+ binds to TnC and changes in the structure of the whole complex result in movement of Tm, exposing myosin-binding sites. In the presence of Ca2+, there is an equilibrium between closed and open states of the thin filament. The closed state allows weak binding of crossbridges to actin; when a few crossbridges bind to the open state, there is a cooperative transition towards strong crossbridge binding, followed by the power stroke (Vibert et al, 1997; Maytum et al, 1999, 2003; Gordon et al, 2000; Lehman et al, 2000).
In an alternative model, regulation is explained by an effect of Ca2+ binding to Tn on the kinetics of the transition from weak to strong crossbridge binding (Brenner, 1988; Chalovich, 1992; Gordon et al, 2000). To understand muscle regulation, details of the structural changes in Tn that cause movement of Tm are needed. Recently, a change in the orientation of the N-terminal domain of cardiac TnC on the thin filament following activation has been reported (Ferguson et al, 2003), and a crystal structure of the tertiary troponin complex has been determined (Takeda et al, 2003), which may lead to a better understanding of how TnC acts in the complex to trigger contraction.
The flight muscles of many insects are not simply regulated by the Ca2+ switch proposed in the steric blocking model. The wing beat frequency of small insects is too high for individual contractions of the flight muscles to be activated by Ca2+, and in these muscles, oscillatory contractions are not synchronous with fluctuations in Ca2+ produced by nerve stimulation (Pringle, 1949). The wings of insects with asynchronous regulation are moved by resonant changes in the shape of the thorax produced by indirect flight muscle (IFM). The flight muscles contract in response to periodic stretches at Ca2+ concentrations too low to activate the fibres fully, and alternating stretch activation of opposing muscles produces oscillatory contractions (Pringle, 1978; Thorson and White, 1983; Tregear, 1983). This form of regulation is highly efficient and also occurs in larger insects with lower wing beat frequencies, such as the giant water bug, Lethocerus, which is commonly used for mechanical studies of IFM.
IFM can also be activated to give twitches synchronous with nerve impulses, and at high stimulation frequencies these produce a tetanus (Esch et al, 1991; Heinrich, 1996). This type of activation is used during the ‘warm-up' contractions that precede flight in large insects. Synchronous nerve impulses produce a tetanus simultaneously in opposing muscles, so that there is no resonant oscillation of the thorax such as occurs during flight (Esch et al, 1991; Heinrich, 1996). Isolated IFM fibres develop isometric tension at Ca2+ concentrations higher than that needed for stretch-activated contractions (Loxdale and Tregear, 1985; Kühn et al, 1985; Taylor et al, 1999); this type of activation (without stretch) is likely to be used in the Ca2+-activated twitches and tetani of IFM in vivo. Thus, IFM is activated in two different ways to produce different types of contraction.
The troponin components of IFM differ from those in other muscles. In vertebrate fast skeletal muscle, TnC has two sites in the C-terminal domain that bind Ca2+ with high affinity and also bind Mg2+ (sites III and IV), and two sites of lower affinity in the N-terminal domain that bind Ca2+ reversibly during activation (sites I and II) (Potter and Gergely, 1975). Cardiac TnC has sites III and IV, and a single regulatory Ca2+-binding site in the N-terminal domain (site II) (Johnson et al, 1980). We have shown that IFM has an unusual isoform of TnC with one Ca2+-binding site in the C-terminal domain (site IV) and no regulatory Ca2+ site in the N-terminal domain; an additional minor isoform has Ca2+ binding sites II and IV (Qiu et al, 2003). IFM TnT has a negatively charged extension at the C-terminus not present in vertebrate TnTs, and in Lethocerus the inhibitory component (TnH) is a fusion protein of TnI and a long C-terminal PA sequence rich in proline and alanine (Bullard et al, 1988; Qiu et al, unpublished data). Although it was likely that troponin regulated both types of contraction in IFM, it was not known how the complex could do this.
Here, we characterise the two isoforms of TnC and study their independent effects on regulation. The function of the isoforms is investigated by measuring the Ca2+ sensitivity of thin filaments reconstituted with one or the other isoform, and mutant proteins are used to show the importance of Ca2+ sites II and IV in regulation. Finally, the effect of the isoforms on the mechanics of IFM fibres is investigated. The results enable us to propose a model for dual regulation in IFM.
Results
Stoichiometry and affinity of Ca2+ binding to TnC isoforms
The formal names of Lethocerus TnCs, LiTnC4 and LiTnC1 (Qiu et al, 2003), have been replaced here by F1 and F2, respectively, indicating the number of Ca2+ ions bound to the IFM isoforms. Sequence analysis shows that F1 lacks key acidic residues at Ca2+-chelating positions (X, Y, Z, −Y, −X, −Z) of sites I, II and III, while F2 lacks these residues at sites I and III; therefore, F1 should bind one Ca2+ ion and F2 two. We previously found, using atomic absorption spectroscopy, that the mol bound Ca2+/mol protein was 1.01±0.37 for F1 and 1.86±0.58 (mean±s.d., n=6) for F2 (Qiu et al, 2003). To study Ca2+ binding further, the stoichiometry and the sites of bound Ca2+ were determined by mass spectrometry of recombinant F1, F2 and the mutants F1mIV and F2mII, with single residue substitutions (E to A) at the −Z position of the EF hand in Ca2+ -binding site IV and site II (Figure 1A–D). Comparison of the spectra of F1 in the presence of Ca2+ and apo F1 shows that there is an additional peak corresponding to F1 with one bound Ca2+. The spectrum of F2 with Ca2+ had two extra peaks compared to apo F2, corresponding to F2 with one or two bound Ca2+. The spectrum of F1mIV had no additional peak in the presence of Ca2+, while that of F2mII had a single additional peak; at saturating Ca2+, another peak appeared corresponding to F2mII with two Ca2+ (not shown). This confirmed predictions from the sequences that F1 binds one Ca2+ at site IV and F2 binds two at sites II and IV (Qiu et al, 2003.)
Figure 1.
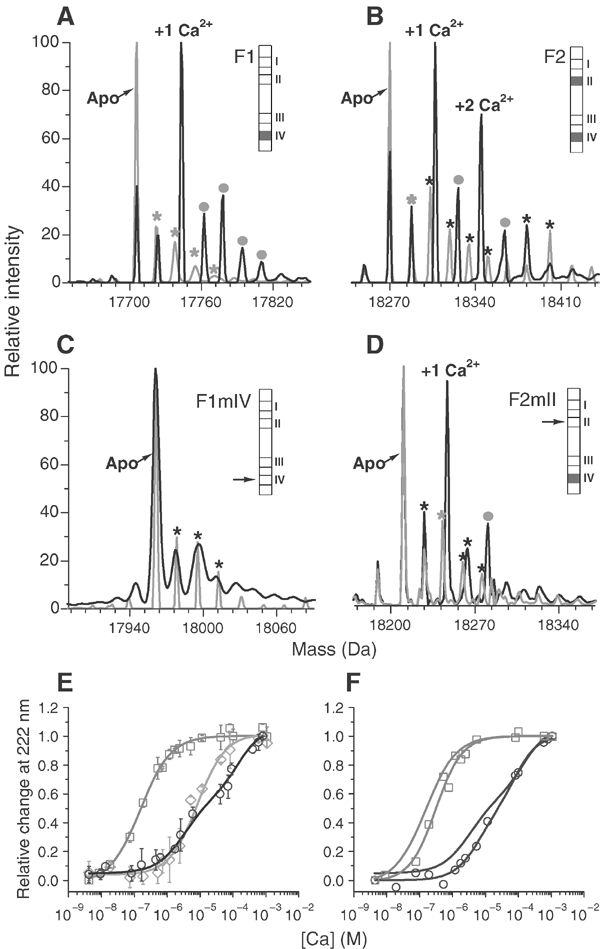
Stoichiometry and affinity of Ca2+ binding to TnC isoforms. (A–D) ESI-MS analysis of apo (grey) and Ca2+-loaded (black) TnC: (A) F1, (B) F2, (C) F1mIV, (D) F2mII. Minor apo-species within the apo-protein sample that are additively oxidised [+O16] (grey stars) and their corresponding oxidised-protein-Ca2+ adducts (grey dots) are marked; black stars are apoprotein adducts that do not bind Ca2+. Schematic bar diagrams of TnC sequences show Ca2+-binding sites I–IV. Filled sites are shown in grey, arrows indicate mutated sites; (E) Ca2+ titration of CD change in F1 (grey squares), F2 (black circles), F2mII (grey diamonds); values are mean±s.d. (n=5). (F) Effect of Mg2+ (1 mM) on Ca2+ titration of the CD change in F1 and F2 (symbols as in (E); values are the mean of two estimations. For comparison, titrations in the absence of Mg2+ are shown without symbols. See text for details.
The affinity of Ca2+-binding sites was estimated from changes in helical ellipticity measured by circular dichroism (CD) (Figure 1E). The CD change in F1 was fitted by a curve with a single transition of Kd 1.6(±0.1) × 10−7 M and Hill coefficient (nH) 0.98±0.05. The CD change in F2 had two transitions with Kd 2.6(±0.5) × 10−6 and 1.8(±0.4) × 10−4 M; nH was 0.9±0.1 and 1.25±0.20, respectively. The CD change in F2mII had a single transition with Kd 6.3(±0.6) × 10−6 M and nH 1.05±0.10. Values are mean±s.d. (n=5). There was no CD change for F1mIV because this mutant did not bind Ca2+. The absence of a second transition in F2mII shows that site II is the low-affinity site. Although both the single Ca2+ site in F1 and the higher affinity site in F2 are in position IV, the affinity of this site is more than 10 times lower in F2 and F2mII than in F1. The coordinating residues at site IV of F1 and F2 are identical (Qiu et al, 2003). Therefore, the observed difference in affinity is likely to be due to the effect of different noncoordinating residues within the Ca2+-binding loops of the two proteins, and/or the effect of sequence variation outside the EF hand. A similar effect has been observed in vertebrate TnCs (Wang et al, 1998; Tikunova et al, 2002).
Mg2+ binds with low affinity to both TnC isoforms and this had a small effect on the affinity of Ca2+ binding. The effect of Mg2+ on Ca2+ binding to site IV is shown in Figure 1F. Mg2+ increased the Kd for Ca2+ binding to the single binding site of F1 to 3.1 × 10−7 M, and increased the Kd for binding to the high-affinity site of F2 to 6.2 × 10−6 M. The Kd for the low-affinity site of F2 was not changed significantly by Mg2+, decreasing slightly to 1.3 × 10−4 M. Assuming competition between Ca2+ and Mg2+ for the high-affinity sites (Potter and Gergely, 1975), these numbers yield KdMg ∼1 × 10−3 M for F1 and ∼7 × 10−4 M for F2. Mg2+ binding by F1 and F2 has been confirmed by mass spectroscopy (not shown).
Regulation of actomyosin ATPase activity by TnC
The regulation of actomyosin ATPase by isoforms of TnC was investigated (Figure 2A). Thin filaments were reconstituted with a Lethocerus Tm–Tn complex lacking TnC (Figure 2B, first lane) to which recombinant TnC was added. F2 produced strong Ca2+-dependent activation of the ATPase, likely to be due to an exchangeable Ca2+ at site II. F2mII produced partial activation, probably due to an increase in the Ca2+ affinity of the mutated site II when TnC was in the troponin complex. In contrast, F1 produced little activation: F1mIV had an insignificant effect on the ATPase, showing that site IV is needed for the small Ca2+-dependent effect of F1. Binding of F1, F2 and mutant TnCs to the other Lethocerus troponin components was tested with immobilised recombinant TnC (Figure 2B). All the TnCs bound TnT and TnH; therefore, effects on the ATPase were not due to lack of association with other troponin components, and a functional site IV is not necessary for binding.
Figure 2.
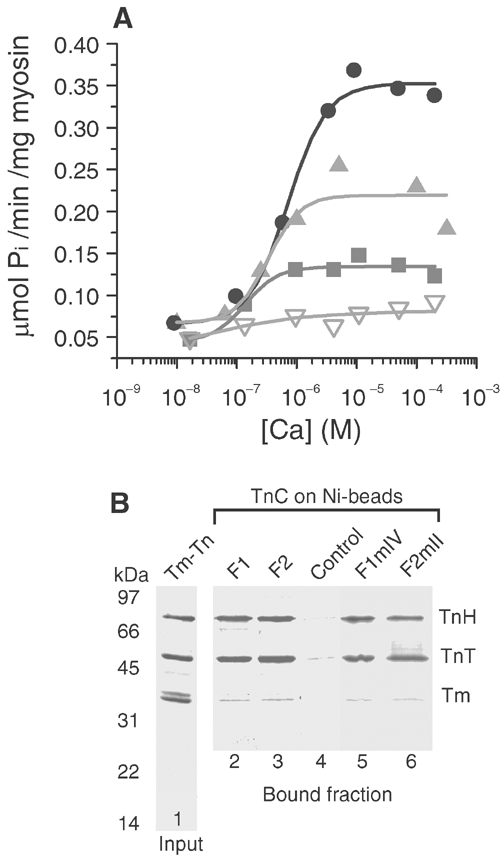
Effect of TnC isoforms on actomyosin ATPase. (A) Ca2+ dependence of ATPase with Tm–Tn reconstituted with F2 (black circles), F2mII (light grey triangles), F1 (dark grey squares) and F1mIV (inverted open triangles). K50 for F2 was 7.0 (±0.3) × 10−7 M; for F2mII it was 3.3 (±1.2) × 10−7 M and for F1 it was 1.4(±0.6) × 10−7 M (mean±s.d., n=3). (B) SDS–PAGE of Tm–Tn (lane 1) and TnC-binding assay. Lanes 2–6, fraction of Tm–Tn retained by TnCs on Ni-NTA-agarose beads; TnC isoforms on the beads are shown above the gel lanes (control, empty beads). TnT and TnH bind to all TnCs and the affinity is greater than that for Tm, which did not bind to the TnC-beads.
Effect of TnC on the mechanical response of flight muscle fibres
The function of the IFM TnC isoforms was studied by measuring the mechanical response of fibres before and after replacing the endogenous mixed TnC with single isoforms. Tension produced under isometric conditions and the transient response to a rapid stretch were recorded at different Ca2+ concentrations (see Materials and methods). TnC was extracted from fibre bundles with Na-orthovanadate, leaving TnT and TnH still bound to the fibres (Figure 3A). Added F1, F2 and the mutants F1mIV and F2mII bound to extracted fibres, but a third mutant, F1ΔC (lacking 18 residues from the C-terminus), did not, showing that the C-terminus is needed for association with thin filaments. F1ΔC also acts as a control demonstrating that association of the other TnCs is not due to nonspecific binding. Native fibres gave isometric contractions that decreased with decreasing Ca2+ concentration (Figure 3B). After extracting TnC, fibres produced no isometric or stretch-activated tension even at high Ca2+ concentration (Figure 3C). This was expected because TnT and the inhibitory component, TnH, remained bound to thin filaments. On adding F2, the fibres contracted isometrically in the presence of Ca2+ (Figure 3D); however, fibres with F2mII only produced significant tension at the highest Ca2+ concentration, probably due to some Ca2+ binding at site II (Figure 3E). In contrast to the effect of F2, fibres with added F1 developed little isometric tension and fibres with F1mIV did not respond at all (Figure 3F and G). Therefore, the exchangeable Ca2+ site II of F2 is essential for isometric contraction. This is in agreement with the effect of TnC isoforms on actomyosin ATPase.
Figure 3.
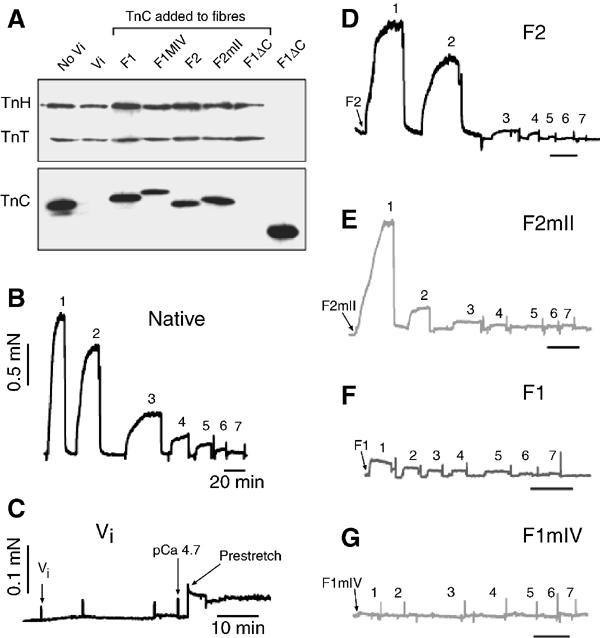
Effect of TnC isoforms on isometric tension in fibres. (A) Western blot of fibre bundles from which TnC was removed with vanadate (Vi) and replaced by different isoforms. First two lanes, fibres before and after removal of TnC; following lanes, replacement with TnC isoforms indicated; last lane, F1ΔC alone. Fibre bundles did not contain equal numbers of fibres. The blot was incubated in mixed anti-TnT, -TnH and -TnC. (B) Isometric tension in native fibres in solutions with decreasing free Ca2+ (1–7); fibres were put into relaxing solution between each contraction (see Methods). (C) Fibres after vanadate treatment, showing loss of tension response. (D–G) Isometric tension in vanadate-treated fibres with added TnC: (D) F2, (E) F2mII, (F) F1, (G) F1mIV; force bar in (B) also applies to traces (D–G) and time bars are 20 min. Spikes represent fibre stretching or buffer exchange. pCa from 1 to 7: 4.7, 5.5, 5.9, 6.1, 6.3, 6.6, 6.9.
The response of fibres to a step change in length was measured during the plateau of isometric tension. The time course of the response was analysed as the sum of exponentials, and the amplitude (A3) of the delayed tension phase was taken as a measure of stretch activation with rate constant r3 (Kawai and Brandt, 1980; Thorson and White, 1983) (see Figure 4F, inset). For native fibres, stretch-activated tension superimposed on isometric tension was Ca2+ dependent (Figure 4A). Fibres substituted with F1 produced greater stretch-activated tension than native fibres, but fibres with F1mIV were not stretch activated, showing that Ca2+ binding to site IV is necessary (Figure 4B and C). In contrast, fibres substituted with F2 responded very little to stretch (Figure 4D). Stretch-activated tension was only partially recovered in fibres with F2mII (Figure 4E); therefore, lack of bound Ca2+ at site II does not reproduce the effect of F1. The rate of rise of delayed tension (r3) was Ca2+ dependent for native fibres (Abbott, 1973) (Figure 4F). F1-substituted fibres had a much lower r3 at all Ca2+ concentrations than native fibres, while for F2-substituted fibres, r3 of the small response peaked at a higher level. Fibres with F2mII had a low r3 similar to that of fibres with F1, except at high Ca2+, when isometric tension was high and stretch-activated tension was low. Thus, the amplitude of stretch-activated tension is inversely related to the rate of tension development, as found previously for the effect of phosphate on Lethocerus fibres (Peckham et al, 1990).
Figure 4.
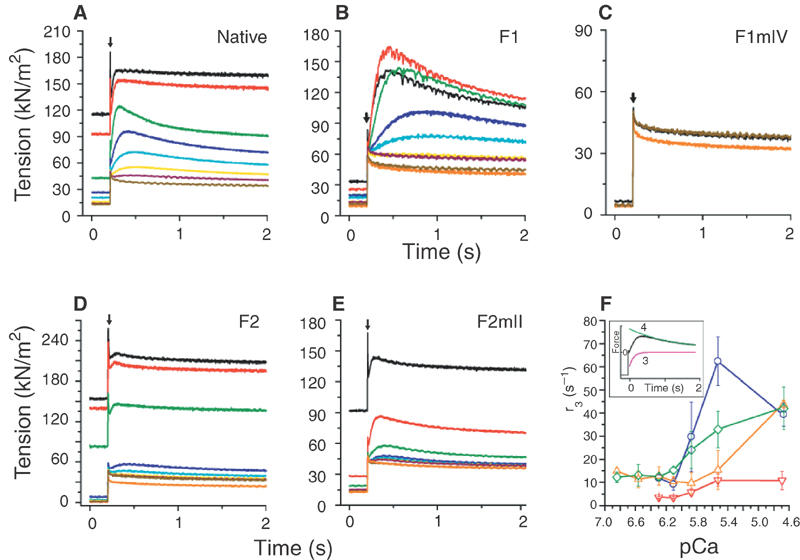
Effect of TnC isoforms on stretch-activated tension. Transient tension responses to a rapid 1% step change in length (arrow) were recorded at the plateau of isometric tension, in solutions of decreasing Ca2+. (A) Native fibres, (B) fibres in which TnC was substituted with F1, (C) F1mIV, (D) F2, (E) F2mII. Phases 3 and 4 of the tension response were fitted by the sum of two exponential processes (Thorson and White, 1983), using a least-squares algorithm. Stretch activation is characterised by the amplitude (A3) and rate constant (r3) of phase 3 (see (F) inset). Tension was normalised to fibre cross-sectional area (Dickinson et al, 1997). Ca2+ concentrations (pCa) were: 4.7 (black), 5.5 (red), 5.9 (green), 6.1 (dark blue), 6.3 (sky blue), 6.6 (yellow), 6.9 (wine), relaxing (<pCa 7.5) (yellow ochre). The orange curve is the response at pCa 4.7 after vanadate treatment. (F) Effect of Ca2+ on r3 for native fibres (green) and fibres substituted with F1 (red), F2 (blue) and F2mII (orange). Values are mean±s.d. (n=7) for native fibres, and mean and range (n=2) for substituted fibres. Inset: typical fit of phases 3 and 4 of a stretch-activated tension curve (black) by two exponentials (red and green).
The differences in the effect of F1, F2 and F2mII on isometric tension and stretch activation are shown in Figure 5. For native fibres, which gave both types of contraction, the stretch response reached a maximum at pCa 5.9; at higher Ca2+, stretch-activated tension fell as isometric tension increased (Figure 5A). For F1-substituted fibres in which isometric tension was low, stretch-activated tension was about 75% greater than in native fibres (Figure 5B). Conversely, for F2-substituted fibres, in which stretch activation was low, isometric tension increased to a greater maximum than in native fibres (Figure 5C). The Ca2+ dependence of isometric tension was also more cooperative in F2-substituted fibres than in native fibres (Figure 5A and C). Fibres with F2mII resembled fibres with F1 in the persistently low isometric tension at moderate Ca2+ levels, but stretch-activated tension was half that of F1-substituted fibres and isometric tension rose sharply at pCa 4.7 (Figure 5D). These results show that F1, which has no exchangeable Ca2+, is required for stretch activation, and F2 with one exchangeable Ca2+ is required for isometric tension. Lack of site II is not sufficient to produce the full stretch-activation response: a high-affinity site IV is also needed.
Figure 5.
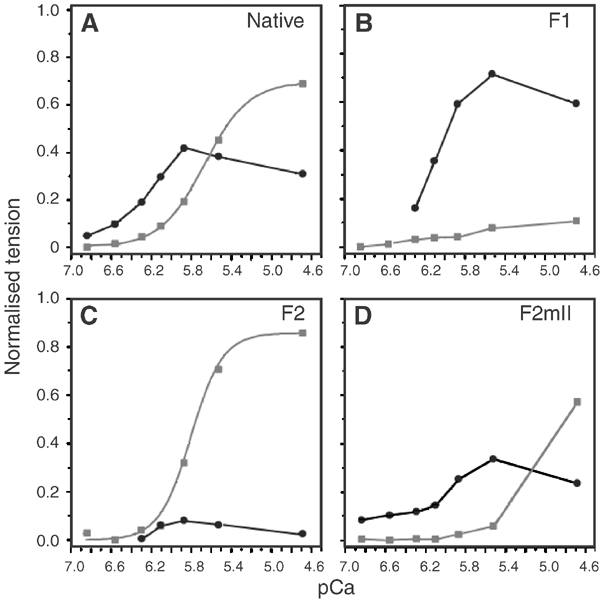
Ca2+ dependence of isometric tension and stretch-activated tension. (A) Native fibres, (B) fibres substituted with F1, (C) F2, (D) F2mII. Isometric tension (grey squares) and stretch-activated tension (black circles) are normalised to the total tension in native fibres at pCa 4.7. Stretch-activated tension is A3 (see Figure 4); total tension is isometric tension+stretch-activated tension. Isometric tension in native fibres at pCa 4.7 is 107±24 kN/m2 (mean±s.d., n=7). Values in (B) (C) and (D) are the mean of two estimations using the same protocol. pCa50 for isometric tension is 5.65±0.35 (n=7) for native fibres and 5.8 for F2-substituted fibres; Hill coefficients are 1.95±0.07 and 2.8, respectively.
Distribution of TnC isoforms in myofibrils
The distribution of F1 and F2 in IFM myofibrils was determined by immunofluorescence microscopy with monoclonal antibodies specific to each isoform. The specificity of the antibodies is shown in Figure 6A. Myofibrils were double labelled with anti-F1 and anti-F2. Both isoforms are in the same sarcomere and labelling is in the position of the thin filaments (Figure 6B). Immunoelectron microscopy with Protein A gold also showed that F1 and F2 are distributed across the sarcomere; the density of gold particles was greater in fibres labelled with anti-F1 than with anti-F2, as expected from the relative amounts of the two isoforms (Figure 7).
Figure 6.
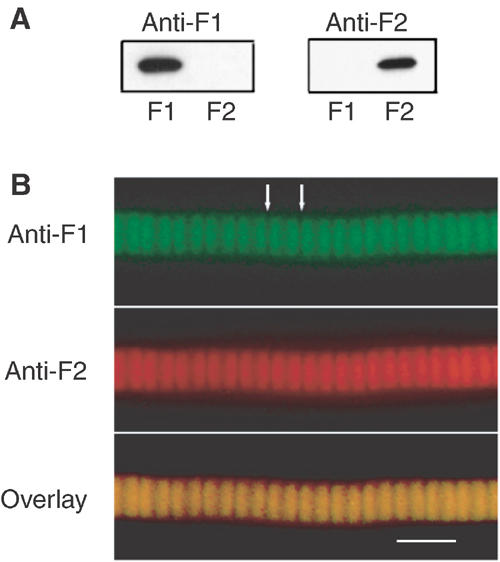
Position of F1 and F2 in myofibrils. (A) Western blot of F1 and F2 incubated with anti-F1 (left panel) and anti-F2 (right panel). (B) Lethocerus IFM myofibril double labelled with anti-F1 (rat antibody) and anti-F2 (mouse antibody), followed by FITC anti-rat and Texas red anti-mouse secondary antibodies. Overlay shows that both isoforms are in the same region of the sarcomere. White arrows mark the position of Z-discs. Scale bar 5 μm.
Figure 7.
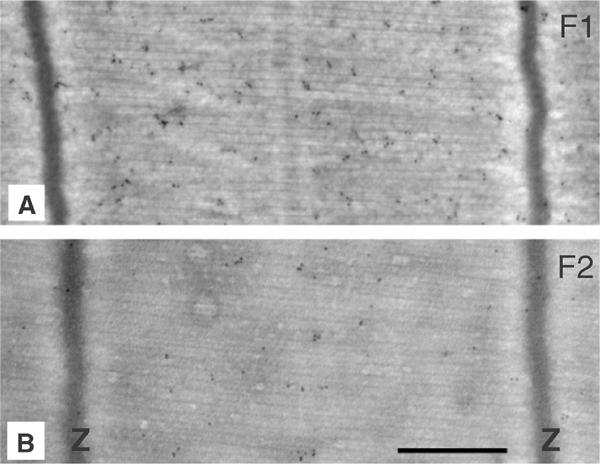
Distribution of F1 and F2 in Lethocerus IFM. Cryosections of the dorsal longitudinal muscle were labelled with anti-F1 (A) or anti-F2 (B) and Protein A gold (10 nm). Scale bar 0.5 μm.
Discussion
The important conclusion from these results is that the regulatory protein, TnC, determines whether IFM will give stretch-activated or isometric contraction. Current models explain stretch activation in IFM either by an effect of strain on the thick filament, which could affect the kinetics of the crossbridge cycle (Thorson and White, 1983; Lund et al, 1987, 1988; Tawada and Kawai, 1990), or by recruitment of crossbridges when fibres are stretched (Wray, 1979; Thorson and White, 1983). Certain properties of IFM myosin are necessary for maximum oscillatory power output by Drosophila flight muscle: myosin kinetics are affected by phosphorylation of a light chain (MLC2) (Tohtong et al, 1995), and a converter domain with the particular sequence in IFM myosin is needed for optimum performance (Swank et al, 2002). It has also been suggested that a link between thick and thin filaments, through the PA-rich extensions in TnH (Bullard et al, 1988; Reedy et al, 1994) or MLC2 (Tohtong et al, 1995), could transmit the effect of strain from thick to thin filaments. However, our results show that in IFM fibres with native thick filaments, it is the thin filament that determines the response to stretch or Ca2+. A possible explanation for the inability of F1-substituted fibres to produce isometric tension and F2-substituted fibres to produce stretch-activated tension is that crossbridges producing one type of tension are not available to produce the other. However, simple competition is unlikely to be the reason for the specificity of TnCs, because even at low levels of isometric tension F2-substituted fibres are incapable of producing appreciable stretch-activated tension.
Both TnC isoforms are found in the same myofibril and in the same sarcomere within the myofibril, and it is likely that each thin filament contains a mixture of isoforms. The ratio of F1 to F2 is approximately 5:1 (Qiu et al, 2003), but it is not known whether the distribution on the thin filament is random or periodic. The two isoforms probably regulate the same thin filament independently. F1 is expected to regulate at low Ca2+ levels, priming the filament for activation by stretch, and F2 at higher Ca2+, producing isometric tension. A possible model for the way in which F1 and F2 can act independently on the same filament is one where the two isoforms locally produce different states of the filament. The extent to which crossbridge target sites on actin were blocked by Tm would depend on whether the sites were under the local control of Tn containing F1 or F2. It is known that different states of the thin filament are produced by cardiac TnC, which has one exchangeable Ca2+, and skeletal TnC, which has two (Maytum et al, 2003). In the presence of Ca2+, both cardiac and skeletal Tn activate the thin filament to the same extent, but in the absence of Ca2+, the thin filament is less inhibited by cardiac Tn. Interpreted by the three-state model for regulation, in the absence of Ca2+, a greater proportion of the cardiac thin filament is in the closed or open state, compared with the skeletal thin filament (Maytum et al, 2003).
A similar, but more pronounced, effect may be produced in IFM thin filaments by Tn complexes containing F1, which has a single high-affinity Ca2+ site and no exchangeable Ca2+. At low Ca2+ concentrations, most of the thin filament would be in the less inhibited state, primed for myosin binding and the transition to the open state. In IFM, crossbridges on the thick filament and target sites on actin have the same lattice positions and, on stretching, the two could be brought into register (Wray, 1979). If regions of the thin filament were already in the closed or open state, the proximity of crossbridges and target sites might be sufficient to activate the muscle, even at low Ca2+ concentrations. A further parallel with cardiac Tn may be drawn from the observation that the equilibrium between closed and open states in the cardiac thin filament is insensitive to Ca2+ (Maytum et al, 2003). The model proposed here could be tested by comparing the affinity of myosin S1 for thin filaments regulated by F1- or F2-troponin, in the presence and absence of Ca2+. The model predicts that, in the absence of Ca2+, S1 would have a higher affinity for thin filaments with F1-troponin. Another test would be to compare the position of Tm in electron micrographs of thin filaments regulated by F1 or F2. In the absence of Ca2+, Tm in F1-regulated filaments might be nearer the C- and M-positions, which correspond to closed and open states. An alternative model is one in which stretch could trigger contraction by an effect of F1-troponin on strain-sensitive crossbridge kinetics. Stretch activation would be independent of reversible Ca2+ binding to TnC, although occupation of site IV in F1 by Ca2+ is evidently essential.
The interaction between TnC and TnI is an important element in regulation. In vertebrate skeletal muscle, the inhibitory region of TnI is bound to actin in the absence of Ca2+, keeping tropomyosin in the blocking position. When Ca2+ binds to the regulatory sites in TnC, the N-terminal domain opens up, exposing a hydrophobic patch. This binds the regulatory region of TnI (downstream of the inhibitory region), resulting in dissociation of TnI from actin (Farah et al, 1994; Gagné et al, 1995; Slupsky and Sykes, 1995). According to this model, the TnI part of TnH would either be unable to bind to F1 because the hydrophobic patch was not exposed, or it might be permanently bound to F1. However, in cardiac TnC, binding of the single Ca2+ does not expose this patch and TnI is needed to open the structure (Sia et al, 1997; Li et al, 1999). In IFM, there may be a similar interaction between TnH and F1, which is triggered by stretch instead of Ca2+, and the C-terminal PA extension in TnH may play a part in this. The failure of F1 to activate actomyosin ATPase fully in vitro at high Ca2+ concentrations shows that stretch is essential to F1 function. The relatively weak binding of the regulatory region of cardiac TnI to TnC is thought to allow more rapid association and dissociation than is the case with skeletal components (Li et al, 1999). This would clearly be an advantage in IFM. Determination of the structure of the complex of F1 with the regulatory peptide of TnH is needed for proper comparison with cardiac troponin.
In contrast to the action of F1, it is likely that a troponin complex containing F2 would regulate the thin filament through reversible Ca2+ binding to site II, according to the steric blocking model (Vibert et al, 1997; Gordon et al, 2000). However, as F2 is a minor component compared with F1, it would be necessary for F2 to act cooperatively over a number of tropomyosin repeats; a lower threshold for activation at F1-troponin sites might help the cooperative effect of F2. The difference in the Ca2+ dependence of stretch-activated and isometric tension in native fibres is crucial for IFM function. A low level of Ca2+ is needed for priming stretch activation; the high affinity of site IV in F1 compared to that of sites II and IV in F2 would ensure that this did not simultaneously fully activate F2.
In conclusion, a delicate balance between the distinct functions of the two Lethocerus TnC isoforms is necessary to achieve stretch-activated and isometric tension in the same muscle, in response to different Ca2+ concentrations in the fibre. As homologous TnC isoforms can be found in both the Drosophila and Anopheles genomes, it is likely that all asynchronous insect flight muscles are regulated in this way.
Materials and methods
Ca2+ binding
Recombinant F1, F2 and mutant proteins F1mIV (E148A in Ca2+-binding site IV), F1ΔC (F1 with 18 residues deleted from the C-terminus), F2mII (E69A in site II) were prepared according to Qiu et al (2003). A His6 tag was cleaved from purified proteins with TEV protease. Two extra residues remaining at the N-terminus after TEV cleavage may account for the difference in mobility between the endogenous and recombinant TnCs seen on SDS–PAGE gels (Figure 3). Proteins were decalcified using a Chelex-100 column and Ca2+ removal was confirmed by atomic absorption. For ESI-MS analysis, the mass ionisation spectra were recorded on a Q-Toff™2 mass spectrometer in positive mode using a μESI source. Mass spectra from apo-TnCs (50–100 μM) in 20 mM ammonium acetate, pH 6.8, were recorded and compared with spectra obtained from identical samples in Ca2+-titration experiments using Ca2+-acetate (incubated for 30 min at 22°C before injection). The specificity of Ca2+-adduct formation was verified by a rise in the intensity of the mass peaks with increasing free Ca2+. F1mIV was 199 Da heavier than F1, owing to extra QA residues at the N-terminus (from the expression vector). CD was measured with a Jasco J-710 spectropolarimeter. For Ca2+ titration of the CD signal, correct protein folding was assessed by far-UV CD scans, and then the ellipticity at 222 nm was followed after adding increasing amounts of standard solutions of CaCl2 (Orion Research) to proteins in 25 mM Mops, pH 7.2, 0.1 M KCl, 0.9 mM EGTA in a 2 ml cell, with continuous stirring. The pH at each titration point was maintained by adding KOH. Free Ca2+ concentration was determined using the program WinMaxc (Bers et al, 1994). Values were corrected for dilution and cell displacement and data were normalised to the maximum difference in ellipticity for each protein; values were plotted against free Ca2+, and fit by least squares to the Hill equation for one binding site (F1, F2mII) or two independent sites (F2). The procedure was repeated in the presence of 1 mM MgCl2.
Actomyosin regulation
A fraction containing tropomyosin and troponin components TnT and TnH was isolated from Lethocerus myofibrils and purified on a butyl-Sepharose column by a modification of the method of Wendt and Leonard (1999). For the ATPase assays, thin filament components (3.9 μg rabbit actin and 18 μg purified Tm–TnT–TnH) were titrated with recombinant TnCs (1.7–15 μg of F1, F2 or F2mII and 1.7–30 μg of F1mIV) in 20 mM Mops, pH 7.15, 0.5 M NaCl, 2 mM DTT on ice. Lethocerus myosin (11.4 μg) in the same buffer (Bullard et al, 1988) was added and the mixtures were placed in the wells of a microtitre plate with buffer and equilibrated at 22°C for 10 min. The reaction was started by adding ATP to a final concentration of 1 mM and followed for 7 min. The final assay medium (300 μl) was 10 mM Mops, pH 7.15, 40 mM NaCl, 1 mM MgCl2 and 0.1 mM CaCl2 or 1 mM EGTA. Phosphate was determined colorimetrically (Lanzetta et al, 1979). The Ca2+ dependence of actomyosin ATPase was determined with saturating amounts of TnCs (10 μg). Free Ca2+ concentration was varied with a Ca-EGTA buffer; the concentration was calculated as before and corrected for pH changes. Binding of TnT and TnH to recombinant TnCs was tested using His-tagged TnC bound to Ni-NTA-agarose beads (Qiagen). Tm–TnT–TnH (40 μg) was incubated with 50 μl TnC beads in binding buffer (0.2 M NaCl, 20 mM Na-phosphate, pH 8, 5 mM imidazole, 0.02% Triton X-100) in a total volume of 100 μl for 1 h at 22°C; beads were washed three times with binding buffer and protein was eluted with 50 μl SDS–PAGE sample buffer.
Replacement of TnC and fibre mechanics
Lethocerus indicus were obtained from Thailand. Bundles of fibres were dissected from the dorsal longitudinal muscle in half thoraces of Lethocerus stored in relaxing solution with 75% glycerol at −80°C (Reedy et al, 1988). The solutions used for mechanics were: relaxing solution with 20 mM Mops, pH 7.0, 5 mM NaN3, 11 mM MgCl2, 10 mM ATP, 5 mM EGTA, and activating solutions with the same components and varying amounts of CaCl2 up to 5 mM (Tregear et al, 1998; Taylor et al, 1999). Free Ca2+ concentration was determined as before. Mechanical measurements were made with a Güth workstation (Scientific Instruments for Muscle Research, Heidelberg) (Neagoe et al, 2002). Bundles containing 8–10 fibres were mounted on pins and the ends glued with cellulose nitrate glue. Fibres were prestretched by 1.5% (just taut) in relaxing solution. Static (isometric) force was recorded in activating solutions from high to low free Ca2+; fibres were placed in relaxing solution between each measurement. At the plateau of isometric tension, fibres were subjected to a step length change of 1% L0 in 5 ms and the stretch-activation response was followed for up to 40 s. The procedure was followed with native fibres before replacing TnC. TnC was extracted from fibres by a modification of the methods of Strauss et al (1992) and Allhouse et al (1999). Mounted fibres were incubated in 3 mM Na-orthovanadate (Sigma) in relaxing solution for 10–15 min at 22°C, and then washed twice in relaxing solution for 20 min. Fibre length was adjusted to bring tension to the level before vanadate treatment, and isometric tension and stretch-activation responses were tested in activating solution (pCa 4.7). Recombinant TnC (1 mg/ml) was then added to fibres in activating solution and the same procedure as for native fibres was followed. The results of two experiments using exactly the same procedure are shown; other results using variations of the protocol were consistent with these. Selective extraction of TnC with vanadate and replacement with recombinant TnCs was checked, after washing fibres in relaxing solution, by SDS–PAGE and Western blot with mixed antibodies to TnH (MAC 81), TnT (MAC 145) and TnC (MAC 352) (Bullard et al, 1988; Qiu et al, 2003).
Electrophoresis, immunoblotting and microscopy
Samples were run on SDS–PAGE gels with 15% acrylamide and Laemmli sample buffer. Western blotting was carried out by transferring proteins to nitrocellulose using a semi-dry blotting apparatus; transfer was for 1 h at 900 mA. After incubation with primary and secondary antibody (Sigma goat anti-rat), blots were developed using chemiluminescence (ECL, Amersham) (Kulke et al, 2001; Qiu et al, 2003).
Myofibrils were prepared from glycerinated dorsal longitudinal muscle of Lethocerus (Bullard et al, 1985). For immunofluorescence microscopy, myofibrils were incubated on a slide in relaxing solution with 1% BSA for 1 h and then with mixed hybridoma supernatents containing anti-F1 and anti-F2 diluted 1:10 in the same solution for 1 h. After washing, myofibrils were incubated with mixed secondary antibodies in relaxing solution with 1% BSA:FITC goat anti-rat (diluted 1:50) and Texas red goat anti-mouse (diluted 1:100) (Dianova) for 1 h. Monoclonal antibodies were anti-F1 (MAC 414) raised in a rat (Qiu et al, 2003) and anti-F2 (clone 7G6) raised in a mouse (Hybricore GmbH). Myofibrils were examined in a Zeiss Axioskop microscope and images were recorded with a colour CCD camera (Photonic Science). Sections of glycerinated fibres were prepared for immunoelectron microscopy and labelled with anti-F1 or anti-F2 and then anti-rat or anti-mouse bridging antibody, followed by Protein A linked to 10 nM gold particles, as described in Qiu et al (2003). Images were taken at 25 000 × magnification and 100 kV on a Philips Biotwin microscope and recorded on a Gatan CCD camera.
Acknowledgments
We are grateful to Dr T Franz (EMBL proteomics core facility) for mass spectroscopy measurements, to Mr A Sawyer (EMBL monoclonal antibody core facility and Hybricore GmbH) for producing monoclonal antibodies and to Sigrun Brendel for assistance with immunoelectron microscopy. We thank Dr MK Reedy for continual advice on fibre mechanics and Dr G Piazzesi and colleagues for communicating unpublished results. Mr T Poulsen kindly supplied Lethocerus indicus from Thailand. BA received a Marie Curie fellowship from the EU, and UK received an exchange fellowship from the DAAD (IASTE); WAL was supported by research grants and a Heisenberg fellowship from the Deutsche Forschungsgemeinschaft.
References
- Abbott RH (1973) The effects of fibre length and calcium ion concentration on the dynamic response of glycerol extracted insect fibrillar muscle. J Physiol 231: 195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allhouse LD, Potter JD, Ashley CC (1999) A novel method of extraction of TnC from skeletal muscle myofibrils. Pflügers Arch 437: 695–701 [DOI] [PubMed] [Google Scholar]
- Bers DM, Patton CW, Nuccitelli R (1994) A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol 40: 3–29 [DOI] [PubMed] [Google Scholar]
- Brenner B (1988) Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibres: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 88: 3265–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard B, Bell J, Craig R, Leonard K (1985) Arthrin: a new actin-like protein in insect flight muscle. J Mol Biol 182: 443–454 [DOI] [PubMed] [Google Scholar]
- Bullard B, Leonard K, Larkins A, Butcher G, Karlik C, Fyrberg E (1988) Troponin of asynchronous flight muscle. J Mol Biol 204: 621–637 [DOI] [PubMed] [Google Scholar]
- Chalovich JM (1992) Actin mediated regulation of muscle contraction. Pharmacol Ther 55: 95–148 [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Hyatt CJ, Lehmann FO, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW (1997) Phosphorylation-dependent power output of transgenic flies: an integrated study. Biophys J 73: 3122–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch H, Goller F, Heinrich B (1991) How do bees shiver? Naturwissenschaften 78: 325–328 [DOI] [PubMed] [Google Scholar]
- Farah CS, Miyamoto CA, Ramos CH, da Silva AC, Quaggio RB, Fujimori K, Smillie LB, Reinach FC (1994) Structural and regulatory functions of the NH2- and COOH-terminal regions of skeletal muscle troponin I. J Biol Chem 269: 5230–5240 [PubMed] [Google Scholar]
- Ferguson RE, Sun YB, Mercier P, Brack AS, Sykes BD, Corrie JE, Trentham DR, Irving M (2003) In situ orientations of protein domains: troponin C in skeletal muscle fibers. Mol Cell 11: 865–874 [DOI] [PubMed] [Google Scholar]
- Gagné SM, Tsuda S, Li MX, Smillie LB, Sykes BD (1995) Structures of the troponin C regulatory domains in the apo and calcium-saturated states. Nat Struct Biol 2: 784–789 [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80: 853–924 [DOI] [PubMed] [Google Scholar]
- Greaser ML, Gergely J (1971) Reconstitution of troponin activity from three protein components. J Biol Chem 246: 4226–4233 [PubMed] [Google Scholar]
- Heinrich B (1996) The Thermal Warriors: Strategies of Insect Survival. Cambridge, MA: Harvard University Press [Google Scholar]
- Johnson JD, Collins JH, Robertson SP, Potter JD (1980) A fluorescent probe study of Ca2+ binding to the Ca2+ specific sites of cardiac troponin and troponin C. J Biol Chem 225: 9635–9640 [PubMed] [Google Scholar]
- Kawai M, Brandt PW (1980) Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1: 279–303 [DOI] [PubMed] [Google Scholar]
- Kühn HJ, Bletz C, Güth K, Rüegg JC (1985) The effect of MgATP on forming and breaking actin-myosin linkages in contracted skinned insect flight muscle fibres. J Muscle Res Cell Motil 6: 5–27 [DOI] [PubMed] [Google Scholar]
- Kulke M, Neagoe C, Kolmerer B, Minajeva A, Hinssen H, Bullard B, Linke WA (2001) Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J Cell Biol 154: 1045–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem 100: 95–97 [DOI] [PubMed] [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R (2000) Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol 302: 593–606 [DOI] [PubMed] [Google Scholar]
- Li MX, Spyracopoulos L, Sykes BD (1999) Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry 38: 8289–8298 [DOI] [PubMed] [Google Scholar]
- Loxdale HD, Tregear RT (1985) Dissociation between mechanical performance and the cost of isometric tension maintenance in Lethocerus flight muscle. J Muscle Res Cell Motil 6: 163–175 [DOI] [PubMed] [Google Scholar]
- Lund J, Webb MR, White DC (1987) Changes in the ATPase activity of insect fibrillar flight muscle during calcium and strain activation probed by phosphate-water oxygen exchange. J Biol Chem 262: 8584–8590 [PubMed] [Google Scholar]
- Lund J, Webb MR, White DC (1988) Changes in the ATPase activity of insect fibrillar flight muscle during sinusoidal length oscillation probed by phosphate-water oxygen exchange. J Biol Chem 263: 5505–5511 [PubMed] [Google Scholar]
- Maytum R, Lehrer SS, Geeves MA (1999) Cooperativity and switching within the three-state model of muscle regulation. Biochemistry 38: 1102–1110 [DOI] [PubMed] [Google Scholar]
- Maytum R, Westerdorf B, Jaquet K, Geeves MA (2003) Differential regulation of the actomyosin interaction by skeletal and cardiac troponin isoforms. J Biol Chem 278: 6696–6701 [DOI] [PubMed] [Google Scholar]
- McKillop DF, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65: 693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA (2002) Titin isoform switch in ischemic human heart disease. Circulation 106: 1333–1341 [DOI] [PubMed] [Google Scholar]
- Peckham M, Molloy JE, Sparrow JC, White DC (1990) Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J Muscle Res Cell Motil 11: 203–215 [DOI] [PubMed] [Google Scholar]
- Potter J, Gergely J (1975) The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem 250: 4628–4633 [PubMed] [Google Scholar]
- Pringle JW (1978) The Croonian Lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc London B Biol Sci 201: 107–130 [DOI] [PubMed] [Google Scholar]
- Pringle JWS (1949) The excitation and contraction of the flight muscles of insects. J Physiol 108: 226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Lakey A, Agianian B, Hutchings A, Butcher GW, Labeit S, Leonard K, Bullard B (2003) Troponin C in different insect muscle types: identification of two isoforms in Lethocerus, Drosophila and Anopheles that are specific to asynchronous flight muscle in the adult insect. Biochem J 371: 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Leonard KR, Bullard B (1994) Gold/Fab immuno electron microscopy localization of troponin H and troponin T in Lethocerus flight muscle. J Mol Biol 239: 52–67 [DOI] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Tregear RT (1988) Two attached non-rigor crossbridge forms. Adv Exp Med Biol 226: 5–15 [PubMed] [Google Scholar]
- Sia SK, Li MX, Spyracopoulos L, Gagné SM, Liu W, Putkey JA, Sykes BD (1997) Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J Biol Chem 272: 18216–18221 [DOI] [PubMed] [Google Scholar]
- Slupsky CM, Sykes BD (1995) NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry 34: 15953–15964 [DOI] [PubMed] [Google Scholar]
- Strauss JD, Zeugner C, Van Eyk JE, Bletz C, Troschka M, Rüegg JC (1992) Troponin replacement in permeabilized cardiac muscle. Reversible extraction of troponin I by incubation with vanadate. FEBS Lett 310: 229–234 [DOI] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Suggs JA, Sarsoza F, Lee A, Maughan DW, Bernstein SI (2002) The myosin converter domain modulates muscle performance. Nat Cell Biol 4: 312–316 [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamashita A, Maeda K, Maeda Y (2003) Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature 424: 35–41 [DOI] [PubMed] [Google Scholar]
- Tawada K, Kawai M (1990) Covalent cross-linking of single fibers from rabbit psoas increases oscillatory power. Biophys J 57: 643–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Schmitz H, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Poole K, Lucaveche C, Edwards RJ, Chen LF, Winkler H, Reedy MK (1999) Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell 99: 421–431 [DOI] [PubMed] [Google Scholar]
- Thorson J, White DC (1983) Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J Physiol 343: 59–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikunova SB, Rall JA, Davis JP (2002) Effect of hydrophobic residue substitutions with glutamine on Ca2+ binding and exchange with the N-domain of troponin C. Biochemistry 41: 6687–6705 [DOI] [PubMed] [Google Scholar]
- Tohtong R, Yamashita H, Graham M, Haeberle J, Simcox A, Maughan D (1995) Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature 374: 650–653 [DOI] [PubMed] [Google Scholar]
- Tregear RT (1983) Physiology of insect flight muscle. In Peachey LD, Adrian RH, Geiger SR (eds) Skeletal Muscle. Bethesda, MD: Americal Physiological Society pp 487–506 [Google Scholar]
- Tregear RT, Edwards RJ, Irving TC, Poole KJ, Reedy MC, Schmitz H, Towns-Andrews E, Reedy MK (1998) X-ray diffraction indicates that active cross-bridges bind to actin target zones in insect flight muscle. Biophys J 74: 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W (1997) Steric-model for activation of muscle thin filaments. J Mol Biol 266: 8–14 [DOI] [PubMed] [Google Scholar]
- Wang S, George SE, Davis JP, Johnson JD (1998) Structural determinants of Ca2+ exchange and affinity in the C terminal of cardiac troponin C. Biochemistry 37: 14539–14544 [DOI] [PubMed] [Google Scholar]
- Wendt T, Leonard K (1999) Structure of the insect troponin complex. J Mol Biol 285: 1845–1856 [DOI] [PubMed] [Google Scholar]
- Wray JS (1979) Filament geometry and the activation of insect flight muscle. Nature 280: 325–326 [Google Scholar]


