Abstract
Lymphedema is a disorder of the lymphatic vascular system characterized by impaired lymphatic return and swelling of the extremities. Lymphedema is divided into primary and secondary forms based on the underlying etiology. Despite substantial advances in both surgical and conservative techniques, therapeutic options for the management of lymphedema are limited. Although rarely lethal, lymphedema is a disfiguring and disabling condition with an associated decrease in the quality of life. The recent impressive expansion of knowledge on the molecular mechanisms governing lymphangiogenesis provides new possibilities for the treatment of lymphedema. This review highlights the lymphatic biology, the pathophysiology of lymphedema, and the therapeutic lymphangiogenesis using hepatocyte growth factor.
1. Introduction
The lymphatic vascular system maintains tissue fluid homeostasis, plays a role in the afferent immune response, and carries proteins and large particulate matter away from the tissue spaces [1, 2]. Lymph stasis can accompany lymphatic anatomical or functional disorders as a result of both congenital and postnatal abnormalities. Because the lymphatic circulation provides the normal conduit for the return of interstitial fluid and protein to the blood circulation, abnormal lymph stasis creates an accumulation of protein and cellular metabolites in the extracellular space, resulting in an ensuing increase in tissue colloid osmotic pressure, water accumulation, and elevation of the interstitial hydraulic pressure. The lymphatic vascular system is a unidirectional transport system arising from blind ends. Fluid, cells, and macromolecules present in the interstitial space first enter blind-ended lymphatic capillaries. The lymphatic network permeates most organs in the body, as only the cornea, cartilage, epidermis, and central nervous system are devoid of lymphatic vessels. In addition to mammals, birds, fish, and amphibians also have a secondary lymphatic or lymphatic-like vascular system [3–6].
In addition to draining and transporting fluid, the lymphatic vascular system also plays an important role in the immune response by transporting leukocytes, antigens, and dendritic cells. Lymphatic vessels have many lymph nodes, which act as checkpoints for the immune response. The lymphatic system is also responsible for the absorption of dietary fats and fat-soluble vitamins from the digestive tract, in which specialized lymphatic capillaries (lacteals) in the intestinal villi absorb the lipid particles (chylomicrons) released by enterocytes.
Anatomic or functional obstruction of the lymphatic system can result in the progressive accumulation of protein-rich fluid in the interstitial spaces (lymphedema) [7, 8]. Lymphedema is divided into primary and secondary forms based on the underlying etiology. Primary (hereditary) lymphedema results from genetic damage, whereas secondary (acquired) lymphedema is a consequence of lymphatic failure resulting from trauma, surgery, radiotherapy, or parasitic infection. Primary lymphedema is thought to occur in approximately 1–3 out of every 10,000 live births [9], irrespective of race or geographic area, and the female-male ratio is 3.5 : 1 [10]. The vast majority of lymphedema worldwide is secondary lymphedema. The most common cause of secondary lymphedema is filariasis. According to a 2013 report from the World Health Organization (http://www.who.int/mediacentre/factsheets/fs102/en/), lymphatic filariasis afflicts more than 25 million men with genital disease and more than 15 million people with lymphedema in Southeast Asia and African regions. In industrialized countries, cancer therapy is the leading cause of secondary lymphedema. Advanced malignancies frequently require radical surgery, including lymph node removal with or without radiotherapy, resulting in the destruction of the lymphatic vessel network. Approximately 30% of patients who have undergone breast cancer surgery develop lymphedema of the upper limb [11]. Even among patients treated with sentinel navigation surgery, approximately 6% develop lymphedema [11]. Furthermore, 10%–30% of patients with gynecological cancer develop lymphedema [12–14], as do approximately 15% of other lymphedema-related malignant tumor patients (16% for melanoma, 10% for genitourinary, 4% for head/neck tumors, and 30% for sarcoma) [15].
Despite substantial advances in surgical and conservative techniques, therapeutic options for the management of lymphedema are limited [8, 16]. Although rarely lethal, lymphedema is a disfiguring and disabling condition which decreases the quality of life [17]. There is no cure for lymphedema at this time, but treatments to manage and reduce the swelling include physiotherapy, massage, and compression bandages, known as complex physical therapy [18].
2. Pathophysiology of Lymphedema
The pathophysiology of lymphedema is generally divided into two periods. During the first period, the pathological changes occur mainly only in the lymphatics and in the soft tissues lymphedema symptoms are not apparent (occult lymphedema = Stage 0). After this stage, the pathological changes occur in the soft tissue (fat, connective tissue, skin, etc.) of the limbs, resulting in the progressive swelling caused by systematic and combined pathologic factors. This clinical state is characterized not only by progressive swelling but also by fat and scar deposition, immunosuppression, a propensity for cellulitis, and microvascular proliferation (Figure 1).
Figure 1.
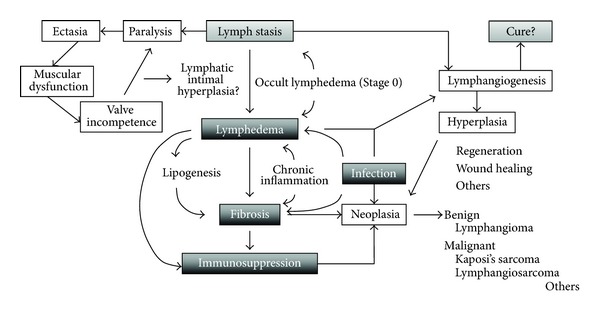
Schematic diagram of the pathophysiology of lymphedema.
2.1. Variable Period (Occult Lymphedema)
Congenitally deficient or obstructed lymphatics promote lymph stasis, which is accompanied by deranged truncal contractility, progressive valve incompetence, destruction of contractile elements (lymphangioparalysis), and gradual ectasia of lymphatic collectors. After a variable period (occult lymphedema), a series of events is initiated, culminating in chronic lymphedema. Because of difficulties in diagnosis, the pathophysiology of occult lymphedema is almost unknown for most patients.
2.2. Progress of Lymphedema and Exacerbation Factors
When lymphedema is apparent via the pathological changes in the lymphatic system, some findings may be confirmed by images [18]: (1) obstruction of the lymphatic main route; (2) dermal back flow; (3) lack of lymph nodes; (4) existence of collateral lymphatic flow. As lymphedema worsens (Table 1), a decrease of swelling after limb elevation of the limb (Stage 1) will be not seen (Stage 2), and subsequently, the edema changes from pitting edema into nonpitting edema (late Stage 2). In many cases, the symptoms of lymphedema may be resistant to most of the therapies during this late Stage 2. Furthermore, edema is irreversible, and sclerosis of the skin and subcutaneous tissue (elephantiasis) may be remarkable (Stage 3) [18].
Table 1.
Staging according to the “consensus document” of the International Society of Lymphology.
| Clinical stage | Evidence |
|---|---|
| 0 | Subclinical with possible clinical evolution |
| I | Edema regressing with treatments with positive pitting test |
| II | Edema partially regressing with treatments with negative pitting test |
| III | Elephantiasis with cutaneous complications and recurrent infections |
These pathological changes in soft tissues are induced by fibrosis and metabolic disorder. At this time, chronic inflammation is recognized as the important mechanism, involving lymphocytes, monocyte/macrophages, and dendritic cells. As previously reported, these inflammatory cells produce many inflammatory cytokines related to fibrosis, such as CTGF, TGF-β, and PDGF. Cellular proliferation and migration of fibroblasts are upregulated [19].
Infection and adipogenesis are exacerbation factors of lymphedema. The propensity for recurrent soft-tissue infection is one of the most troublesome aspects of long-standing lymphedema. Accumulated fluid and proteins provide a good substrate for bacterial growth. Lymphatic dysfunction impairs the local immune response, which plays a permissive role in the propagation of bacterial and fungal invasion. Furthermore, once established, soft tissue infection exacerbates the existing lymphatic dysfunction, sometimes irreversibly.
Although the connection between the lymphatic system and fat absorption/deposition has been recognized by clinicians for well over a hundred years, the subject received relatively little interest until recent publications raised theories regarding the mechanism of this association. It has long been recognized that a lymphedematous limb accumulates fat at an increased rate when compared to the rest of the body and that, conversely, when weight loss is undertaken, the lymphedematous limb loses fat at a slower rate than the rest of the body. The underlying cause of these observations is not well understood. A recent publication studying Prox1 haploinsufficient mice proposed that the lymph itself is stimulatory to fat cells [20]. According to recent reports, adipocytes recruit monocyte/macrophages via activation of NFκB and TNF-α [21]. Furthermore, it was reported that adipocytokines participate in fat absorption [22].
2.3. Primary Lymphedema Related Genes
Three genes were confirmed as the cause of lymphedema [23, 24]: (1) VEGFR-3 (familial Milroy lymphedema); (2) FOXC2 (lymphedema-distichiasis syndrome); (3) SOX18 (hypotrichosis-lymphedema-telangiectasia). In addition to these genes, the following genetic changes are associated with lymphedema: Aagenaes syndrome (chromosome 15), Noonan's syndrome (chromosome 12), trisomy disorders (chromosome 13, 18, 21, and 22), Klinefelter's syndrome (XXY), Turner's syndrome (XO), and chromosomal abnormalities of additions at 11p and deletions at 11q and 13q.Klippel-Trenaunay syndrome is recognized as a nonhereditary disease.
2.4. Malignancy in Patients with Lymphedema
Although lymphedema is recognized to generally not affect prognosis of mortality, in rare cases, chronic lymphedema may be complicated by the development of malignant tumors. One of these malignancies is lymphangiosarcoma (Stewart-Treves syndrome) [25]. The incidence rate of this syndrome in patients with lymphedema 5 years after breast cancer surgery is 0.07–0.45%, according to previous reports [26]. Other malignant tumors that appear with increased frequency in lymphedematous limbs include Kaposi's sarcoma, squamous cell carcinoma, malignant lymphoma, and melanoma. These malignancies frequently result in limb loss or even death. The mechanism of development of malignancy is unclear, but because these malignancies occur in the chronic lymphedema patients of any cause, the lymphedema state is thought to affect the development of these malignancies.
3. Therapeutic Lymphangiogenesis
3.1. Development on Lymphangiogenesis
Many reports regarding the molecular biology of lymphatics and lymphangiogenesis were published during the 1990s, in accordance with progress in the field of vascular biology. These developments were supported by the identification of lymphatic specific markers, such as LYVE-1 and Prox1, and subsequent improved ability to easily observe the lymphatics or lymphatic endothelial cells (LEC).
Recent studies suggest that lymphangiogenesis can be stimulated by various cytokines. For example, vascular endothelial growth factors (VEGF)-C and -D promote lymphangiogenesis by activating the VEGF receptor-3 (VEGFR-3), which is expressed on lymphatic endothelial cells (LEC) [27]. As further evidence, VEGF-C-deficient mice fail to develop a functional lymphatic system [28], transgenic expression of soluble VEGFR-3 results in pronounced lymphedema [29], and gene transfer of VEGF-C effectively reduces lymphedema in an animal model [30]. Another study reported that angiopoietin-1 also promotes lymphatic vessel formation through Tie2 [31] and that fibroblast growth factor 2 stimulates the growth of lymphatic vessels [32].
3.2. HGF Gene Therapy for Lymphedema
Hepatocyte growth factor (HGF) was originally identified as a potent mitogen for hepatocytes, and HGF is currently recognized as a mesenchyme-derived pleiotropic factor that regulates growth, motility, and morphogenesis of various types of cells [33–37]. Furthermore, HGF plasmid DNA is utilized for gene therapies targeting the heart [38], vascular system [39], brain [40], and lung [41]. HGF activates its tyrosine kinase receptor, c-Met [33, 42], and various c-Met-linked intracellular signaling pathways, such as the Ras-mitogen-activated protein kinase cascade (MAPK) or the phosphatidylinositol-3-OH kinase (PI3 K)-Akt cascade [43, 44].
We have previously investigated and reported the lymphangiogenic potency of HGF [45]. Canine lymphatic endothelial cells (cLEC) express c-Met as demonstrated by immunofluorescent staining, suggesting that cLEC are responsive to HGF. Indeed, the treatment of cLEC with HGF resulted in a dose-dependent increase in cellular proliferation and migration (Figure 2(a)). Furthermore, the extracellular signal-regulated protein kinase (ERK) or Akt was phosphorylated 5–15 minutes after the addition of HGF to cLEC, whereas total ERK or Akt protein levels were not altered by treatment with recombinant HGF (Figure 2(b)).
Figure 2.
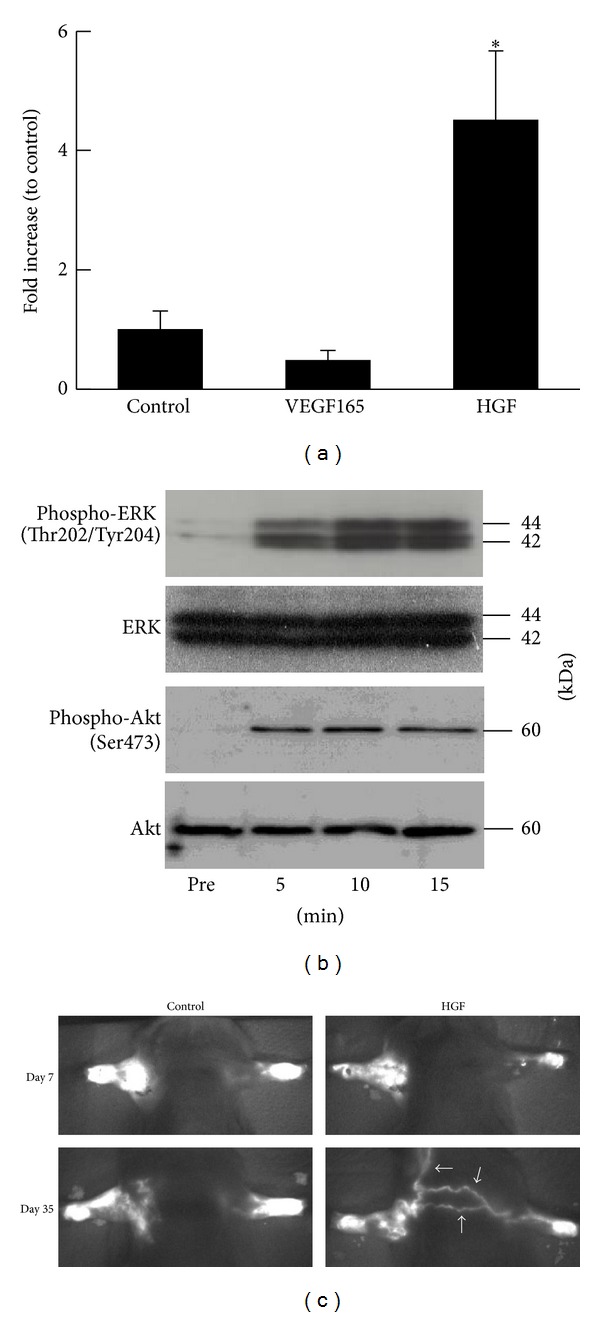
HGF lymphangiogenesis and gene therapy for lymphedema. (a) Effect of HGF plasmid on c-fos promoter activity in LEC. n = 4, *P < 0.05 versus control, VEGF165. (b) Typical western blot of ERK or Akt and phosphorylated ERK or Akt in cLEC before and 5, 10, and 15 minutes after treatment with human recombinant HGF (50 ng/mL). (c) Representative pictures of the fluorescent lymphography using PDE at day 7 and day 35 after surgery. “HGF” indicates human HGF plasmid (200 μg/0.1 mL) and “control” indicates GFP plasmid injection (200 μg/0.1 mL).
In accordance with in vitro data, we confirm the effect of HGF gene transfer for in vivo models. Using the mouse upper limb lymphedema model in a simulation of breast cancer related lymphedema, the operated arm volume began to increase 1 day after the operation and was stable at 7 days after operation in all animal groups. The arm volume of the HGF injected group was significantly decreased on postoperative day 7, compared with the arm volumes of the control group. This significant difference between the HGF injected group and the control group continued to postoperative day 35. Of note, new extra-anatomical lymphatic flow was observed only in the HGF injected group, as detected by the fluorescent lymphography system (PDE; Hamamatsu Photonics K.K. Hamamatsu, Japan, Figure 2(c)). We hypothesized that these lymphatic flows are induced by HGF lymphangiogenic potency.
In view of these results, we hypothesized that HGF gene therapy would stimulate the growth of the lymphatic vascular system and alter the course of lymphedema. In terms of HGF gene therapy, the safety of the use of HGF plasmid DNA in patients with critical limb ischemia has been investigated in a prospective open-labeled clinical trial [46]. There were no signs of systemic or local inflammatory reactions and no development of tumors or progression of diabetic retinopathy in this population. Of note, no edema was observed in this trial, in contrast to the transient lower-extremity edema that was reported after the use of clinical gene therapy using the VEGF-A gene [47]. We are currently preparing to start a clinical trial involving lymphedema patients and expect to observe successful therapeutic lymphangiogenesis management by HGF gene therapy.
4. Conclusion
The lymphatic vessels have three specific functions for maintenance of homeostasis: (1) drainage of tissue fluid; (2) immunosurveillance; and (3) the uptake of dietary fats. Furthermore, they play an important role in the pathogenesis of several diseases, including cancer, lymphedema, and various inflammatory conditions. Consequently, administration of lymphatic growth factors or related molecules provides the potential to target lymphatic vessels in human disease. In particular, therapeutic lymphangiogenesis is a promising gene therapy strategy for the treatment of lymphedema.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors are grateful to Professor N. Azuma and Professor T. Sasajima (Asahikawa Medical University) for kindly supporting their efforts.
References
- 1.Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microscopy Research and Technique. 2001;55(2):122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes and Development. 2002;16(7):773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 3.Jeltsch M, Tammela T, Alitalo K, Wilting J. Genesis and pathogenesis of lymphatic vessels. Cell and Tissue Research. 2003;314(1):69–84. doi: 10.1007/s00441-003-0777-2. [DOI] [PubMed] [Google Scholar]
- 4.Küchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires Vegfc signaling. Current Biology. 2006;16(12):1244–1248. doi: 10.1016/j.cub.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Ny A, Koch M, Schneider M, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nature Medicine. 2005;11(9):998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 6.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nature Medicine. 2006;12(6):711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 7.De Almeida AB, Freedman DO. Epidemiology and immunopathology of bancroftian filariasis. Microbes and Infection. 1999;1(12):1015–1022. doi: 10.1016/s1286-4579(99)80519-x. [DOI] [PubMed] [Google Scholar]
- 8.Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vascular Medicine. 1998;3(2):145–156. doi: 10.1177/1358836X9800300209. [DOI] [PubMed] [Google Scholar]
- 9.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 10.Smeltzer DM, Stickler GB, Schirger A. Primary lymphedema in children and adolescents: a follow-up study and review. Pediatrics. 1985;76(2):206–218. [PubMed] [Google Scholar]
- 11.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The Lancet Oncology. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 12.Ohba Y, Todo Y, Kobayashi N, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. International Journal of Clinical Oncology. 2011;16(3):238–243. doi: 10.1007/s10147-010-0171-5. [DOI] [PubMed] [Google Scholar]
- 13.Tada H, Teramukai S, Fukushima M, Sasaki H. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer. 2009;9, article 47 doi: 10.1186/1471-2407-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109(12):2607–2614. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 15.Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer. Cancer. 2010;116(22):5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- 16.Ko DSC, Lerner R, Klose G, Cosimi AB. Effective treatment of lymphedema of the extremities. Archives of Surgery. 1998;133(4):452–458. doi: 10.1001/archsurg.133.4.452. [DOI] [PubMed] [Google Scholar]
- 17.Girgis A, Stacey F, Lee T, Black D, Kilbreath S. Priorities for women with lymphoedema after treatment for breast cancer: population based cohort study. BMJ. 2011;342 doi: 10.1136/bmj.d3442.d3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Lymphology. 2009;42(2):51–60. [PubMed] [Google Scholar]
- 19.Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? European Respiratory Journal. 2007;30(5):835–839. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 20.Harvey NL, Srinivasan RS, Dillard ME, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature Genetics. 2005;37(10):1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 21.Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. Journal of Biological Chemistry. 2006;281(36):26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 22.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. Journal of Leukocyte Biology. 2010;88(1):33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- 23.Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. Journal of Investigative Dermatology. 2006;126(10):2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- 24.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annual Review of Pathology. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 25.Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer. 1948;1(1):64–81. doi: 10.1002/1097-0142(194805)1:1<64::aid-cncr2820010105>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Heitmann C, Ingianni G. Stewart-Treves syndrome: lymphangiosarcoma following mastectomy. Annals of Plastic Surgery. 2000;44(1):72–75. doi: 10.1097/00000637-200044010-00012. [DOI] [PubMed] [Google Scholar]
- 27.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiological Reviews. 2002;82(3):673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 28.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nature Immunology. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 29.Mäkinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Medicine. 2001;7(2):199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 30.Yoon Y-S, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. Journal of Clinical Investigation. 2003;111(5):717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105(12):4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 32.Chang LK, Garcia-Cardeña G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura Y, Morishita R, Higaki J, et al. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. Journal of Hypertension. 1996;14(9):1067–1072. doi: 10.1097/00004872-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Van Belle E, Witzenbichler B, Chen D, et al. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97(4):381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 35.Rappolee DA, Iyer A, Patel Y. Hepatocyte growth factor and its receptor are expressed in cardiac myocytes during early cardiogenesis. Circulation Research. 1996;78(6):1028–1036. doi: 10.1161/01.res.78.6.1028. [DOI] [PubMed] [Google Scholar]
- 36.Taniyama Y, Morishita R, Nakagami H, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102(2):246–252. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 37.Jung W, Castren E, Odenthal M, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. Journal of Cell Biology. 1994;126(2):485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniyama Y, Morishita R, Aoki M, et al. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40(1):47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 39.Aoki M, Morishita R, Taniyama Y, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. Journal of atherosclerosis and thrombosis. 2000;7(2):71–76. doi: 10.5551/jat1994.7.71. [DOI] [PubMed] [Google Scholar]
- 40.Koike H, Ishida A, Shimamura M, et al. Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model: a model of gene therapy for Parkinson’s disease. Gene Therapy. 2006;13(23):1639–1644. doi: 10.1038/sj.gt.3302810. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Ebina M, Orson FM, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Molecular Therapy. 2005;12(1):58–67. doi: 10.1016/j.ymthe.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. Journal of Cell Biology. 1992;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. Journal of Biological Chemistry. 1991;266(33):22087–22090. [PubMed] [Google Scholar]
- 44.Graziani A, Gramaglia D, Dalla Zonca P, Comoglio PM. Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. Journal of Biological Chemistry. 1993;268(13):9165–9168. [PubMed] [Google Scholar]
- 45.Saito Y, Nakagami H, Morishita R, et al. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114(11):1177–1184. doi: 10.1161/CIRCULATIONAHA.105.602953. [DOI] [PubMed] [Google Scholar]
- 46.Morishita R, Aoki M, Hashiya N, et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44(2):203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 47.Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. The Lancet. 1996;348(9024):370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]


