Abstract
Background
Young African-American women have the highest rates of Chlamydia trachomatis and Neisseria gonorrhoeae in the United States. The objective was to identify baseline predictors of repeat Chlamydia and/or gonorrhoea infections among African-American adolescent females.
Methods
Sociodemographic, psychosocial and behavioural data were collected at baseline and every 6 months for 2 years from 701 African-American females (14–20 years) enrolled in an HIV prevention trial. Vaginal swabs were self-collected at each visit and assayed for Chlamydia and gonorrhoea using DNA amplification. Among participants testing positive for Chlamydia and/or gonorrhoea at baseline, logistic regression analyses assessed baseline predictors of repeat infection.
Results
Of 618 (88%) participants with ≥1 follow-up assessment, 123 (20%) had a positive Chlamydia and/or gonorrhoea test result at baseline; 49 (40%) had a repeat infection during the study period. Of those with a repeat infection, 30 (61%) were positive at one follow-up visit, 18 (37%) at two and 1 (2%) at three follow-up visits. Controlling for age and intervention condition, impulsivity (AOR: 1.71, p=.018) was associated with an increased likelihood and having a boyfriend (AOR: 0.21, p=.006) a decreased likelihood of repeat infection.
Conclusions
Repeat Chlamydia and/or gonorrhoea infections are common among African-American adolescent females. Among young African-American women who test positive for Chlamydia and/or gonorrhoea, tailored interventions for more impulsive adolescents and those not in a relationship may reduce risk of repeat infections. Given high numbers of repeat infections after receipt of an evidence-based intervention, enhanced screening and treatment services for young men may be warranted.
Keywords: Adolescent, Chlamydia trachomatis, Gonorrhoea
Introduction
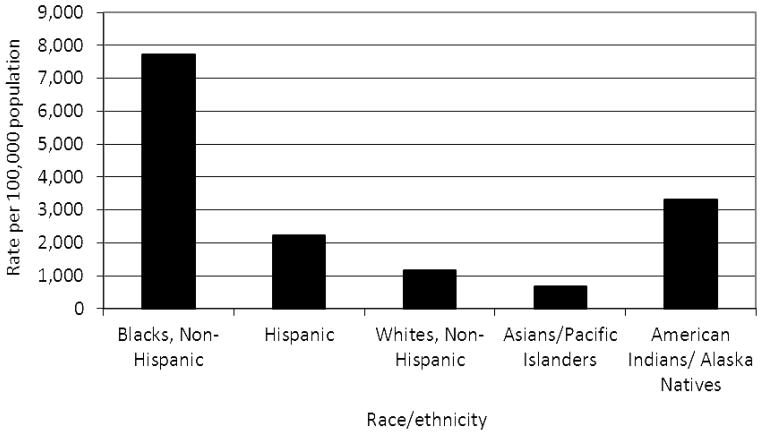
In the United States, Black females 15–19 years of age have the highest rates of both Chlamydia trachomatis and Neisseria gonorrhoeae among all age, race/ethnicity and gender categories.1 Figures 1 and 2 present the rates of Chlamydia and gonorrhoea, respectively, among females 15–19 years in the United States in 2010, by race/ ethnicity.1 Studies have estimated that up to 53% of all Chlamydia diagnoses are repeated infections and persons with repeat infections account for up to 48% of gonorrhoea diagnoses.2,3
Figure 1.
Rate of Chlamydia per 100,000 population among females 15–19 years, by race/ethnicity, United States, 20101
Figure 2.
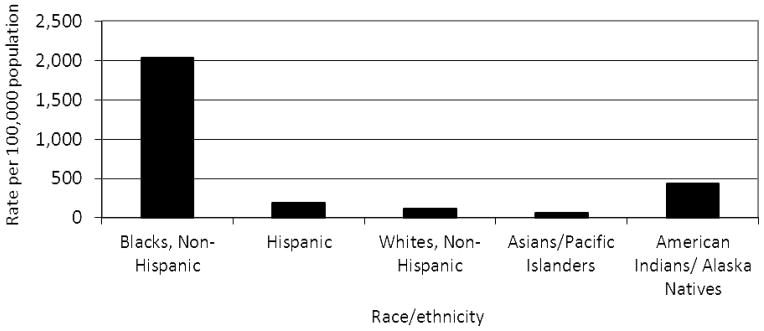
Rate of gonorrhea per 100,000 population among females 15–19 years, by race/ethnicity, United States, 20101
Both Chlamydia and gonorrhoea are associated with adverse reproductive health outcomes such as pelvic inflammatory disease, infertility, ectopic pregnancy and perinatal infections.4 Reinfection is associated with increased risk of adverse reproductive health outcomes.4 Both Chlamydia and gonorrhoea also facilitate human immunodeficiency virus (HIV) transmission.5 The annual direct medical costs of treating sexually transmitted infections (STIs), excluding HIV, and their sequelae is $8 billion.6 The time of diagnosis may be an important opportunity to prevent subsequent infections.2 Examining factors that predict repeat infection may help researchers and programmers develop and target interventions to reduce infection rates and their public health burden.
Individual and partner behaviour and relationship and psychosocial factors influence STI risk. Individuals who have been adequately treated for Chlamydia and/or gonorrhoea may become reinfected through a new infected partner or an existing partner who has not been adequately treated or has a newly acquired infection. Partner-level factors associated with STIs among adolescent females include older partner age, a partner with concurrent sexual partners, sex with a high or drunk partner and a partner recently released from incarceration.7–10 Psychosocial factors, such as stress and depression, are associated with increased sexual risk behaviour among adolescents.11 Among African-American adolescent females, heavy alcohol use has been shown to predict STI acquisition and sexual risk behaviour, including inconsistent condom use, multiple sexual partners and sex while high or drunk.12 Abuse and dating violence also impact STI risk. Evidence shows that African-American young women who experienced sexual violence as youth have increased risk for STIs, earlier sexual debut and a greater number of lifetime sex partners.13,14 Among adolescents, dating violence is associated with increased sexual risk behaviour and substance use.15 Adolescents who experience dating violence may be at increased risk of STIs through forced sex or reduced capacity negotiate to safer sex practices.13,15,16 Among African-American adolescent females, a history of dating violence is associated with increased fear about the consequences of condom negotiation and fear of talking to a partner about pregnancy prevention.16
Despite marked disparities in the rates of Chlamydia and gonorrhoea, we are not aware of studies that have explored predictors of repeat infection exclusively among young African-American females. The objective of this study was to identify baseline predictors of repeat Chlamydia and/or gonorrhoea infections among African-American adolescent females enrolled in an HIV prevention trial who tested positive for Chlamydia and/or gonorrhoea at baseline.
Methods
Study sample
From July, 2005, to June, 2007, African-American adolescent females were recruited from three reproductive health clinics in Atlanta, GA, to participate in an HIV prevention trial. Potential participants were approached in clinic waiting areas by a female African-American recruiter who assessed study eligibility. Eligibility criteria included age 14–20 years and at least one episode vaginal sex without a condom in the past 6 months. Individuals who were married, pregnant or attempting to become pregnant were excluded from participating. On a return visit to the clinic, written informed consent was obtained with parental consent waived for individuals younger than 18 years. Participants then completed baseline assessments and were randomized to trial conditions. Of 1,684 individuals screened, 745 met eligibility criteria. Of eligible adolescents, 94% (N=701) enrolled and were randomized (intervention condition n=342; comparison condition n=359). Participants were compensated $75 for travel and childcare to attend intervention sessions and complete assessments. This analysis reports on 618 (88%) participants who, in addition to the baseline assessment, completed ≥1 assessment during two years of follow-up post-intervention. The Emory University Institutional Review Board approved all study protocols.
Study procedures
The parent study was a randomized controlled supplemental treatment trial. As such, all participants received the same “primary” treatment, and, depending on study condition, received one of two supplemental treatments. The purpose of the trial was to assess whether supplemental treatment could enhance maintenance of an efficacious HIV risk reduction intervention.17 All participants received a single 5-hour, group-delivered HIV risk reduction intervention based on HORIZONS, a two-session intervention shown to be efficacious among African-American females.18 Participants assigned to the intervention condition then received 12 brief phone calls during which a health educator conducted an HIV risk assessment and provided individualized HIV prevention counseling. Participants assigned to the control condition also received 12 brief phone calls, but the health educator conducted a nutritional risk assessment and provided nutrition counseling. Participants received calls approximately every 8 weeks for 24 months following the primary treatment.
Data collection occurred at baseline and 6-, 12-, 18- and 24-months post-intervention and consisted of an audio computer-assisted self-interview (ACASI) and a self-collected vaginal swab. The ACASI assessed sociodemographics, sexual history and psychosocial constructs associated with sexual risk behaviours. After completing the ACASI, participants provided a self-collected vaginal swab that was assayed for Chlamydia and gonorrhoea, using the BDProbeTec ET Chlamydia trachomatis and Neisseria gonorrhoeae Amplified DNA Assays (Becton Dickinson and Company, Sparks, MD).19 Participants with a positive STI test result received directly observed single-dose antimicrobial treatment and risk-reduction counseling per Centers for Disease Control and Prevention recommendations and were encouraged to refer sex partners for treatment. The County Health Department was notified of reportable STIs.
Summary of main trial findings
There were no differences between study conditions in terms of sociodemographic characteristics, STIs or behavioural outcomes at the time of randomization. Retention was similar with 89.2% of individuals in the intervention condition and 87.2% in the comparison condition completing ≥1 follow-up assessments (p=0.414). There were statistically significant differences in the primary and secondary outcomes between the two arms through 18 months. Participants in the intervention arm had a lower proportion of incident Chlamydia infections (7.0% vs. 13.6%, p=0.009), lower mean frequency of sex while high on drugs or alcohol (1.42 vs. 2.42; p=0.009) and higher mean percent condom-protected sex acts (58% vs. 52%; p=0.049).17
Measures
Repeat infection was defined as a positive Chlamydia and/or gonorrhoea test following a negative result or documented treatment, among individuals positive for Chlamydia and/or gonorrhoea at baseline.
Sociodemographic factors considered as potential baseline predictors included age (years) and having a paid job (yes/no). Family aid was assessed with a 4-item index. Participants respond yes/no to “In the past 12 months, did you or anyone you live with receive any money or services from”: a) “Welfare (including TANF (Temporary Assistance to Needy Families) or SSI)?”; b) “food stamps?”; c) “WIC (Women, Infants and Children?” and d) “Section 8 housing (housing subsidies)?”.
Cigarette smoking and substance use
Smoking was assessed with the question “Do you smoke cigarettes?” (yes/no). Alcohol use in the 90 days prior to baseline assessment was assessed with “In the past 90 days, how many days have you used alcohol?” and “How many alcoholic drinks do you usually have at one time?” Participants who indicated they used alcohol on ≥1 days were categorized as users. Participants who indicated they usually had ≥3 drinks on one occasion were categorized as heavy alcohol users. Marijuana use prior to baseline assessment was assessed with “In the past 90 days, how many days have you used marijuana?” Participants were dichotomized as having or not having used marijuana.
Sexual risk behaviours assessed at baseline included: age at first sex (years), lifetime number of vaginal sex partners (above or equal to the median number vs. less than median) and self-reported STI history (yes/no). Sexual behaviours in the 90 days prior to baseline assessment included: a) number of vaginal sex partners; b) percent condom use during vaginal sex, calculated by dividing the number of times the respondent had vaginal sex by the number of times condoms were used; c) consistent condom use, defined as 100% use during vaginal sex (yes/no). We also assessed the participant’s report of being drunk or high during sex (yes/no) as a potential baseline predictor. Participants’ responses were dichotomized based on the question “In the past 90 days, how many times did you have sex while high on alcohol or drugs?”; reports of ≥1 times were categorized as engaging in the behaviour. In addition, we explored whether hormonal contraceptive use (“pills/ patch/ depo”) at last sex (yes/no) predicted repeat infection.
Partner-level factors included: a) currently having a boyfriend (yes/no); b) participant’s perception of whether her current boyfriend had sex with another woman during the relationship (yes/no); c) currently having a casual partner (yes/no); d) general age of partners (≥2 years older vs. same age or younger) and e) sex in the past 6 months with a male partner recently released from jail, prison or detention (yes/no) and f) sex with a partner who was high or drunk during sex in the past 90 days (yes/no). Participants were asked “In the past 90 days, how many times did you have sex while your partner was high on alcohol or drugs?”; reports of ≥1 times were categorized as engaging in the behaviour.
Psychosocial Constructs Associated with Sexual Risk Behaviour included perceived interpersonal stress, depressive symptomology, fear of condom negotiation, partner communication self-efficacy, impulsivity and sexual sensation-seeking. Perceived stress was assessed with a 14-item scale, in which respondents indicated their level of stress around various interpersonal situations and their overall stress level from 1= no stress to 5=extreme stress; a sample item was, “relationships with family members” (α=0.88). Depression was measured with an 8-item scale (α=0.90);20 participants were asked how frequently during the past week they experienced depressive symptoms from 1=less than one day to 4= 5–7 days. A sample item was “I felt sad”. Participants with a score of 16 or greater were classified as having elevated depressive symptoms. Fear of consequences of condom negotiation was measured using a 7-item scale (α=0.88); a sample item was “I have been worried that if I talked about using condoms with my boyfriend or sex partner he would threaten to leave me”. Participants responded from 1=never to 5=always. Partner communication self-efficacy was assessed with a 6-item index (α=0.82); a sample item was, “How hard is it for you to demand that he use a condom?” Participants responded from 1=very hard to 4=very easy. Impulsivity was measured with a 15-item scale (α=0.82);21 a sample item was “I just react without thinking.” Participants responded from 1=never to 5=always. A 10-item scale measured sexual sensation-seeking (α=0.74). The original 9-item scale was modified to include “Videotaping or photographing myself and my partner having sex is exciting”.22 Participants responded from 1=strongly disagree to 4=strongly agree. With the exception of depressive symptomology, all psychosocial measures used in regression models were standardized to mean zero and unit variance.
History of abuse
A history of emotional abuse was assessed with “Have you ever been emotionally abused? (threatened, called names, etc.)” (yes/no). Physical abuse was assessed with “Have you ever been physically abused? (hit, punched, kicked, slapped, etc.)” (yes/no). Sexual abuse was assessed with “Has anyone ever forced you to have vaginal sex when you didn’t want to?” (yes/no).
Data analysis
T-tests and chi-square statistics compared selected baseline characteristics among study participants according to completion of at least one post-intervention assessment. Descriptive statistics assessed the frequencies of repeat infections. Simple and multivariable logistic regression analyses identified baseline factors associated with repeat infection during the 2-year follow-up period among participants positive for Chlamydia and/or gonorrhoea at baseline. Thus, the comparator groups are individuals positive for 1 or both STIs at baseline and at a subsequent visit and individuals positive for 1 or both STIs at baseline only. First, simple logistic models assessed associations between potential baseline predictors and repeat infection. Factors significant at p<0.1 were entered together into a multivariable model, controlling for age and treatment assignment.
Results
Sample characteristics
Table 1 compares selected baseline characteristics among study participants according to their completion of at least one post-intervention assessment. Participants excluded from analysis because they only completed the baseline assessment were more likely to smoke (27.7% vs. 14.9%, p=0.003), use alcohol heavily (21.7% vs. 9.9%, p=0.001), report being high or drunk during sex in the past 90 day (39.8% vs. 26.5%, p=0.012) and experience sexual abuse (36.1% vs. 22.3%, p=0.006). They also reported a greater proportion of protected sex acts in the past 90 days (38.2% vs. 36.9%, p=0.023). Of participants who completed ≥1 post-intervention assessment (n=618), 19.9% (n=123) had a laboratory-confirmed diagnosis of Chlamydia and/or gonorrhoea at baseline; 16.5% were positive for Chlamydia, 6.5% were positive for gonorrhoea and 3.1% were positive for both Chlamydia and gonorrhoea.
Table 1.
Selected baseline characteristics among African-American females 14–20 years of age participating in an HIV prevention trial according to their completion of at least one post-intervention assessment
| Variable | At least one post-intervention assessment (n=618) | Baseline assessment only (n=83) | p-value |
|---|---|---|---|
| n (%) | |||
| Sociodemographic characteristics | |||
| Age (years), mean (SD) | 17.6 (1.7) | 17.8 (1.5) | 0.296 |
| Paid job | 231 (37.4) | 24 (28.9) | 0.132 |
| Family aid index, mean (SD) | 0.9 (1.0) | 0.7 (1.0) | 0.216 |
| Current cigarette smoking and substance use in past 90 days | |||
| Smoking | 92 (14.9) | 23 (27.7) | 0.003 |
| Alcohol | 318 (51.1) | 46 (55.4) | 0.463 |
| Heavy alcohol | 61 (9.9) | 18 (21.7) | 0.001 |
| Marijuana | 238 (38.5) | 36 (43.4) | 0.394 |
| STIs diagnosed at baseline | |||
| Chlamydia | 102 (16.5) | 18 (21.7) | 0.239 |
| Gonorrhea | 40 (6.5) | 4 (4.8) | 0.560 |
| Chlamydia and gonorrhea | 19 (3.1) | 1 (1.2) | 0.337 |
| Chlamydia and/or gonorrhea | 123 (19.9) | 21 (25.3) | 0.253 |
| Lifetime sexual history | |||
| Age at first sex (years), mean (SD) | 14.8 (1.6) | 15.1 (1.4) | 0.092 |
| Lifetime number of vaginal sex partners, mean (SD) | 8.1 (12.3) | 8.7 (12.8) | 0.652 |
| Self-reported STI history | 349 (56.5) | 48 (57.8) | 0.815 |
| Hormonal contraceptive use at last sex | 221 (35.8) | 21 (25.3) | 0.060 |
| Sexual behaviour in past 90 days | |||
| Number of vaginal sex partners, mean (SD) | 1.7 (1.8) | 1.7 (1.3) | 0.997 |
| Percent condom use | 49.2 (36.9)∫ | 39.1 (38.2)∞ | 0.023 |
| Consistently used condoms | 102 (17.4)∫ | 11 (14.1)∞ | 0.466 |
| Drunk or high during sex | 164 (26.5) | 33 (39.8) | 0.012 |
| Psychosocial constructs related to sexual risk behaviour | |||
| Stress, mean (SD) | 28.2 (13.1) | 29.8 (13.6) | 0.303 |
| Elevated depressive symptoms | 215 (35.0) | 37 (44.6) | 0.081 |
| Fear of condom negotiation, mean (SD) | 8.3 (3.3) | 8.2 (2.6) | 0.797 |
| Partner communication self-efficacy, mean (SD) | 20.5 (3.5) | 20.7 (3.8) | 0.763 |
| Impulsivity, mean (SD) | 38.8 (7.3) | 38.0 (9.5) | 0.342 |
| Sexual sensation-seeking, mean (SD) | 19.2 (4.3) | 19.3 (4.5) | 0.850 |
| Partner-level factors | |||
| Current boyfriend | 488 (79.0) | 69 (83.1) | 0.377 |
| Boyfriend had sex with someone else during relationship | 124 (27.5)‡ | 15 (25.0)§ | 0.683 |
| Current casual partner | 214 (34.6) | 28 (33.7) | 0.872 |
| Partners generally 2 or more years older | 391 (63.3) | 55 (66.3) | 0.594 |
| Partner recently released from incarceration | 96 (15.5) | 12 (14.5) | 0.799 |
| Partner drunk or high during sex in past 90 days | 274 (44.3) | 41 (49.4) | 0.384 |
| History of abuse | |||
| Emotional | 346 (56.0) | 46 (55.4) | 0.922 |
| Physical | 243 (39.3) | 33 (39.8) | 0.939 |
| Sexual | 138 (22.3) | 30 (36.1) | 0.006 |
n=586 (missing 32 responses)
n=451 (missing 37 responses)
n=78 (missing 5 responses)
n=60 (missing 9 responses)
Frequency of repeat infections
Forty-nine participants (intervention condition n=30; comparison condition n=19) who were positive for Chlamydia and/or gonorrhoea at baseline had a repeat infection during the study period (40.0%). Of those with a repeat infection, 30 (61.2%) were positive at a single follow-up visit, 18 (36.7%) at two visits and one (2.0%) at three total follow-up visits.
Baseline predictors of repeat infection
Table 2 presents unadjusted and adjusted associations between potential baseline predictors and repeat Chlamydia and/or gonorrhoea infection among 123 participants positive for at least one of these infections at baseline. In both unadjusted and adjusted models, greater impulsivity (AOR: 1.69, 95% CI: 1.08, 2.65, p=0.021) predicted an increased likelihood of repeat infection and having a boyfriend (AOR: 0.21, 95% CI: 0.07, 0.62, p=0.005) predicted a decreased likelihood of repeat infection.
Table 2.
Associations between potential baseline predictors and repeat Chlamydia trachomatis and/or Neisseria gonorrhoeae infection among 123 young African-American women positive for at least one of these infections at baseline
| Variable | OR | CI | p-value | Final model | ||
|---|---|---|---|---|---|---|
| AOR | CI | p-value | ||||
| Sociodemographic factors | ||||||
| Age | 0.92 | 0.73, 1.16 | 0.482 | 0.85 | 0.65, 1.11 | 0.219 |
| Paid job | 1.11 | 0.51, 2.45 | 0.790 | -- | -- | -- |
| Family aid index | 1.22 | 0.85, 1.74 | 0.278 | -- | -- | -- |
| Current cigarette smoking and substance use in past 90 days | ||||||
| Smoking | 1.68 | 0.68, 4.11 | 0.260 | -- | -- | -- |
| Alcohol | 0.91 | 0.44, 1.87 | 0.797 | -- | -- | -- |
| Heavy alcohol | 0.20 | 0.02, 1.67 | 0.137 | -- | -- | -- |
| Marijuana | 0.84 | 0.41, 1.73 | 0.632 | -- | -- | -- |
| Lifetime sexual history | ||||||
| Age at first sex | 1.02 | 0.81, 1.29 | 0.838 | -- | -- | -- |
| Number of sexual partners, median number (5) or greater | 0.71 | 0.33, 1.52 | 0.379 | -- | -- | -- |
| STI history | 0.82 | 0.39, 1.74 | 0.610 | -- | -- | -- |
| Hormonal contraceptive use at last sex | 0.80 | 0.36, 1.79 | 0.587 | -- | -- | -- |
| Sexual behaviour in past 90 days | ||||||
| Number of sexual partners, median number (1) or greater | 0.32 | 0.03, 3.65 | 0.360 | -- | -- | -- |
| Percent condom use | 1.21 | 0.43, 3.35 | 0.718 | -- | -- | -- |
| Consistent condom use∫ | 0.78 | 0.28, 2.26 | 0.649 | -- | -- | -- |
| Drunk or high during sex | 1.29 | 0.60, 2.76 | 0.515 | -- | -- | -- |
| Psychosocial constructs related to sexual risk behaviour | ||||||
| Stress∞ | 0.89 | 0.64, 1.25 | 0.509 | -- | -- | -- |
| Elevated depressive symptoms | 1.47 | 0.70, 3.09 | 0.310 | -- | -- | -- |
| Fear of condom negotiation∞ | 1.06 | 0.67, 1.69 | 0.802 | -- | -- | -- |
| Partner communication self-efficacy∞ | 1.08 | 0.77, 1.52 | 0.661 | -- | -- | -- |
| Impulsivity∞ | 1.55 | 1.03, 2.34 | 0.035 | 1.69 | 1.08, 2.65 | 0.021 |
| Sexual sensation-seeking∞ | 0.91 | 0.64, 1.30 | 0.616 | -- | -- | -- |
| Partner-level factors | ||||||
| Current boyfriend | 0.39 | 0.16, 0.97 | 0.043 | 0.21 | 0.07, 0.62 | 0.005 |
| Boyfriend ever had sex with someone else during relationship‡ | 0.91 | 0.35, 2.35 | 0.846 | -- | -- | -- |
| Current casual partner | 1.04 | 0.50, 2.15 | 0.914 | -- | -- | -- |
| General age of partners, 2 or more years older | 0.78 | 0.36, 1.66 | 0.515 | -- | -- | -- |
| Partner recently released from incarceration | 1.22 | 0.51, 3.02 | 0.634 | -- | -- | -- |
| Partner drunk or high during sex in past 90 days | 1.33 | 0.64, 2.76 | 0.438 | -- | -- | -- |
| Lifetime abuse | ||||||
| Emotional abuse | 0.88 | 0.43, 1.83 | 0.742 | -- | -- | -- |
| Physical abuse | 0.81 | 0.38, 1.70 | 0.568 | -- | -- | -- |
| Sexual abuse | 1.39 | 0.58, 3.33 | 0.460 | -- | -- | -- |
Abbreviations: OR: odds ratio; CI: 95% confidence interval; AOR: adjusted odds ratio. Adjusted for age, treatment assignment and other covariates in the model
n=116 (missing 5 responses)
Standardized to zero mean and unit standard deviation prior to estimation. The reported coefficients give the expected change in the expected odds of repeat infection per 1 SD increase in the predictor variable
n=93 (missing 5 responses)
Conclusions
Repeat Chlamydia and/or gonorrhoea infections were common among African-American adolescent females in this study; 40% who were positive at baseline experienced a repeat infection within two years of the intervention. Other studies among adolescents in the United States with a follow-up period of at least 20 months have reported Chlamydia reinfection estimates from 18.0% to 23.3%.4 Gonorrhoea reinfection estimates of up to 40% within 20 months of follow-up have also been reported.4 Fortenberry et al found 41.1% of adolescent girls positive for gonorrhoea, Chlamydia and/or trichomonas experienced a subsequent infection within 12 months.23 The high proportion of repeat infection in this study may suggest a reservoir of infection among asymptomatic males. Given that all participants received an evidence-based intervention as the primary treatment, enhanced screening and treatment efforts for young men may be warranted.
Greater impulsivity predicted repeat infection. Research suggests that individuals with poor impulse control may be more likely to engage in risky sexual behaviours or have sex with risky partners;24,25 however, to our knowledge, impulsivity has not previously been identified as a factor related to repeat Chlamydia or gonorrhoea infections. Interestingly, sexual sensation-seeking, often related to impulsivity and sexual risk behaviours,22,24,25 was unrelated to repeat infection. Although impulsivity and sensation seeking are related, and usually moderately correlated, they represent unique traits. The ability to differentiate between the two and understand how they independently relate to repeat infection is important when considering strategies to reduce repeat infections.
Having a boyfriend predicted a decreased likelihood of repeat infection. Increased risk of repeat Chlamydia and/or gonorrhoea infection among individuals without a boyfriend could have been due to an increased likelihood to obtain a new partner or of continued sex with an untreated partner. Among a sample of women at least 15 years of age, Niccolai et al found the population attributable risk of recurrent Chlamydia infections was similar for sex with a new sex partner and sex with a partner not known to be treated.26
Strengths and Limitations
This study is subject to a number of limitations. Some of the factors assessed were subject to change over time, and some were targeted by the intervention. In addition, the intervention was effective in reducing incident STIs. Despite these limitations, many types of potential factors were assessed, and two significant baseline predictors of repeat Chlamydia and/or gonorrhoea infection were identified. It is also possible that participants may have been diagnosed and treated between study visits or after the study ended. These findings are relevant only to the two years of follow-up post-intervention and could be subject to error to the degrees that participants were diagnosed and treated between study visits and their baseline characteristics varied from those who were not. The study did not achieve 100% retention, therefore the proportion of participants who were categorized as experiencing a repeat infection may be over- or underestimated. Participants excluded from this analysis because they did not return for follow-up assessment were more likely to report heavy alcohol use, being high or drunk during sex and sexual abuse, which increase the likelihood of STI acquisition, and they were only slightly more likely to use condoms; therefore, our findings might underestimate repeat infections. Nevertheless, particular strengths of this study are its duration of assessment and high retention rate. The number of cases available limited the ability to assess baseline predictors of repeated Chlamydia and gonorrhoea infections separately. However, assessing these infections together is consistent with a public health approach in that both infections are prevalent in this population, are transmitted by unprotected sex with an infected partner and cause adverse reproductive health outcomes. Lastly, the possibility exists that treatment failures were misclassified as repeat infections; therefore, the findings may overestimate repeat infections and the strength of the relationship between the baseline predictors and repeat infection may be underestimated.
Implications
These results confirm the need to rescreen young women diagnosed with Chlamydia or gonorrhoea. The findings suggest that individual baseline factors influence repeat infection despite receipt of an efficacious intervention. Among young African-American females who test positive for Chlamydia and/or gonorrhoea, assessing impulsivity and relationship status may allow for tailored interventions to help prevent repeat infections. For example, more impulsive adolescents may benefit by learning about the role of impulsivity and its potential impact on sexual decision-making as well as how to identify and utilize strategies to avoid situations where they are likely to behave impulsively. In addition, enhanced STI screening and treatment efforts among young men may be warranted, given the limitations of current interventions for female adolescents to adequately reduce the number of repeat infections.
Key Messages.
Among African-American adolescent females, repeat Chlamydia trachomatis and/or Neisseria gonorrhoeae infections were common.
Greater impulsivity and not having a boyfriend at baseline predicted repeat infection during two years of follow-up.
Tailored interventions for more impulsive adolescents and those not in a steady relationship may reduce risk of repeat infections.
Given high numbers of repeat infections after receipt of an evidence-based intervention, enhanced screening and treatment services for young men may also be warranted.
Acknowledgments
Funding statement: This work was supported by the National Institute of Mental Health grant number 5R01 MH070537-08 and Emory Center for AIDS Research grant number P30-A150409. Jennifer L. Brown was supported by National Institute of General Medical Sciences grant number K12 GM000680. Jessica M. Sales was supported by the National Institute of Mental Health grant number K01 MH085506.
Footnotes
Conflicts of interest and financial disclosures: None reported
Clinical Trials Registration: www.clinicaltrials.gov (NCT00279799)
License for Publication: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in Sexually Transmitted Infections and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence http://group.bmj.com/products/journals/instructions-for-authors/licence-forms.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 2.Niccolai LM, Hochberg AL, Ethier KA, Lewis JB, et al. Burden of recurrent Chlamydia trachomatis infections in young women: Further uncovering the “hidden epidemic”. Arch Pediatr Adolesc Med. 2007;161(3):246–251. doi: 10.1001/archpedi.161.3.246. [DOI] [PubMed] [Google Scholar]
- 3.Noble RC, Kirk NM, Slagel WA, et al. Recidivism among patients with gonococcal infection presenting to a venereal disease clinic. Sex Transm Dis. 1977;4(2):39–43. doi: 10.1097/00007435-197704000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: A systematic review of the literature. Sex Transm Dis. 2009;36(8):478–489. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. The role of STD detection and treatment in HIV prevention. [Accessed August 20, 2012.];CDC Fact Sheet. http://www.cdc.gov/std/hiv/stdfact-std-hiv.htm.
- 6.Chesson H, Gift T, Owusu-Edusei K, et al. A brief review of the estimated economic burden of sexually transmitted diseases in the United States: Inflation-adjusted updates of previously published cost studies. Sex Transm Dis. 2011;38(10):889–91. doi: 10.1097/OLQ.0b013e318223be77. [DOI] [PubMed] [Google Scholar]
- 7.Crosby RA, Diclemente RJ, Wingood GM, et al. Co-occurrence of intoxication during sex and sexually transmissible infections among young African American women: Does partner intoxication matter? Sex Health. 2008;5(3):285–289. doi: 10.1071/sh07098. [DOI] [PubMed] [Google Scholar]
- 8.Begley E, Crosby RA, DiClemente RJ, et al. Older partners and STD prevalence among pregnant African American teens. Sex Transm Dis. 2003;30(3):211–213. doi: 10.1097/00007435-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Drumright LN, Gorbach PM, Holmes KK. Do people really know their sex partners? Concurrency, knowledge of partner behavior, and sexually transmitted infections within partnerships. Sex Transm Dis. 2004;31(7):437–442. doi: 10.1097/01.olq.0000129949.30114.37. [DOI] [PubMed] [Google Scholar]
- 10.Swartzendruber A, Brown JL, Sales JM, et al. Sexually transmitted infections, sexual risk behavior and intimate partner violence among African-American adolescent females with a male sex partner recently released from incarceration. J Adolesc Health. 2012;51(2):156–63. doi: 10.1016/j.jadohealth.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzaferro KE, Murray PJ, Ness RB, et al. Depression, stress, and social support as predictors of high-risk sexual behaviors and STIs in young women. J Adolesc Health. 2006;39(4):601–603. doi: 10.1016/j.jadohealth.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Seth P, Sales JM, DiClemente RJ, et al. Longitudinal examination of alcohol use: A predictor of risky sexual behavior and Trichomonas vaginalis among African-American female adolescents. Sex Transm Dis. 2011;38(2):96–101. doi: 10.1097/OLQ.0b013e3181f07abe. [DOI] [PubMed] [Google Scholar]
- 13.Wingood GM, DiClemente RJ. Child sexual abuse, HIV sexual risk, and gender relations of African-American women. Am J Prev Med. 1997;13(5):380–384. [PubMed] [Google Scholar]
- 14.Wyatt GE. The relationship between child sexual abuse and adolescent sexual functioning in Afro-American and white American women. Ann N Y Acad Sci. 1988;528:111–122. doi: 10.1111/j.1749-6632.1988.tb50854.x. [DOI] [PubMed] [Google Scholar]
- 15.Silverman JG, Raj A, Mucci LA, et al. Dating violence against adolescent girls and associated substance use, unhealthy weight control, sexual risk behavior, pregnancy, and suicidality. JAMA. 2001;286(5):572–579. doi: 10.1001/jama.286.5.572. [DOI] [PubMed] [Google Scholar]
- 16.Wingood GM, DiClemente RJ, McCree DH, et al. Dating violence and the sexual health of black adolescent females. Pediatrics. 2001;107(5):E72. doi: 10.1542/peds.107.5.e72. [DOI] [PubMed] [Google Scholar]
- 17.DiClemente RJ, Wingood G, Rose E, et al. Brief cell-phone delivered counseling as a novel strategy to enhance maintenance of HIV behavioral intervention efficacy: Results from a supplemental treatment effectiveness trial. International AIDS Conference; Vienna, Austria. 2010. [Google Scholar]
- 18.DiClemente RJ, Wingood GM, Rose ES, et al. Efficacy of sexually transmitted disease/human immunodeficiency virus sexual risk-reduction intervention for African American adolescent females seeking sexual health services: A randomized controlled trial. Arch Pediatr Adolesc Med. 2009;163(12):1112–1121. doi: 10.1001/archpediatrics.2009.205. [DOI] [PubMed] [Google Scholar]
- 19.Van Der Pol B, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39(3):1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiClemente RJ, Wingood GM, Crosby RA, et al. A prospective study of psychological distress and sexual risk behavior among black adolescent females. Pediatrics. 2001;108(5):E85. doi: 10.1542/peds.108.5.e85. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman RS, Donohew L. Sensation seeking, impulsive decision-making, and adolescent sexual behaviors. American Public Health Association; New York: 1996. [Google Scholar]
- 22.DiClemente R, Milhausen RR, Salazar LF, et al. Development of the sexual sensation-seeking scale for African American adolescent women. Int JSex Health. 2010;22(4):248–261. [Google Scholar]
- 23.Fortenberry JD, Brizendine EJ, Katz BP, et al. Subsequent sexually transmitted infections among adolescent women with genital infection due to Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis. Sex Transm Dis. 1999;26(1):26–32. doi: 10.1097/00007435-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, Abell R. Sensation seeking, impulsive decision-making, and risky sex: Implications for risk-taking and design of interventions. Pers Individ Dif. 2000;28(6):1079–1091. [Google Scholar]
- 25.Cooper ML, Agocha VB, Sheldon MS. A motivational perspective on risky behaviors: The role of personality and affect regulatory processes. J Pers. 2000;68(6):1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- 26.Niccolai LM, Livingston KA, Laufer AS, et al. Behavioural sources of repeat Chlamydia trachomatis infections: Importance of different sex partners. Sex Transm Infect. 2011;87(3):248–253. doi: 10.1136/sti.2010.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]