Abstract
In CKD, the risk of kidney failure and death depends on the severity of proteinuria, which correlates with the extent of podocyte loss and glomerular scarring. We investigated whether proteinuria contributes directly to progressive glomerulosclerosis through the suppression of podocyte regeneration and found that individual components of proteinuria exert distinct effects on renal progenitor survival and differentiation toward a podocyte lineage. In particular, albumin prevented podocyte differentiation from human renal progenitors in vitro by sequestering retinoic acid, thus impairing retinoic acid response element (RARE)-mediated transcription of podocyte-specific genes. In mice with Adriamycin nephropathy, a model of human FSGS, blocking endogenous retinoic acid synthesis increased proteinuria and exacerbated glomerulosclerosis. This effect was related to a reduction in podocyte number, as validated through genetic podocyte labeling in NPHS2.Cre;mT/mG transgenic mice. In RARE-lacZ transgenic mice, albuminuria reduced retinoic acid bioavailability and impaired RARE activation in renal progenitors, inhibiting their differentiation into podocytes. Treatment with retinoic acid restored RARE activity and induced the expression of podocyte markers in renal progenitors, decreasing proteinuria and increasing podocyte number, as demonstrated in serial biopsy specimens. These results suggest that albumin loss through the damaged filtration barrier impairs podocyte regeneration by sequestering retinoic acid and promotes the generation of FSGS lesions. Our findings may explain why reducing proteinuria delays CKD progression and provide a biologic rationale for the clinical use of pharmacologic modulators to induce regression of glomerular diseases.
CKD affects approximately 600 million people throughout the world.1 These individuals are at increased risk of death from cardiovascular causes and progression to ESRD, which requires dialysis or kidney transplantation.2 Glomerulosclerosis accounts for 90% of ESRD cases, and, independently of the initial insult, it shares common pathogenic mechanisms leading to glomerular scarring and progressive loss of renal function.3 Indeed, depletion of podocytes, which are critical components of the glomerular filtration barrier, is key for progression to glomerulosclerosis.3–5 Different degrees of podocyte loss correlate with the degrees of proteinuria and, as an increasing proportion of glomeruli become involved, a measurable reduction of the clearance function of the kidney ensues.3–5 Proteinuria independently predicts progression and renal outcome in diabetic and nondiabetic disease.6 Lowering proteinuria retards renal disease progression5,6 and can induce regression of glomerulosclerosis, supported by experimental6–10 and clinical6,11,12 studies. For example, in animal models, reduction of proteinuria induced by treatment with angiotensin-converting enzyme (ACE) inhibitors is associated with increased number of podocytes and regression of glomerulosclerosis.13 Indeed, although podocytes have limited capacity to divide, they can potentially get replaced by a population of renal progenitor cells (RPCs) localized within the Bowman capsule.14–17 In this study, we hypothesized that proteinuria may interfere with the generation of novel podocytes via direct or indirect impairment of regenerative mechanisms.
Results
Transferrin and IgG Overload Affects Human RPC Survival, while Albumin Overload Impairs RPC Differentiation into Podocytes
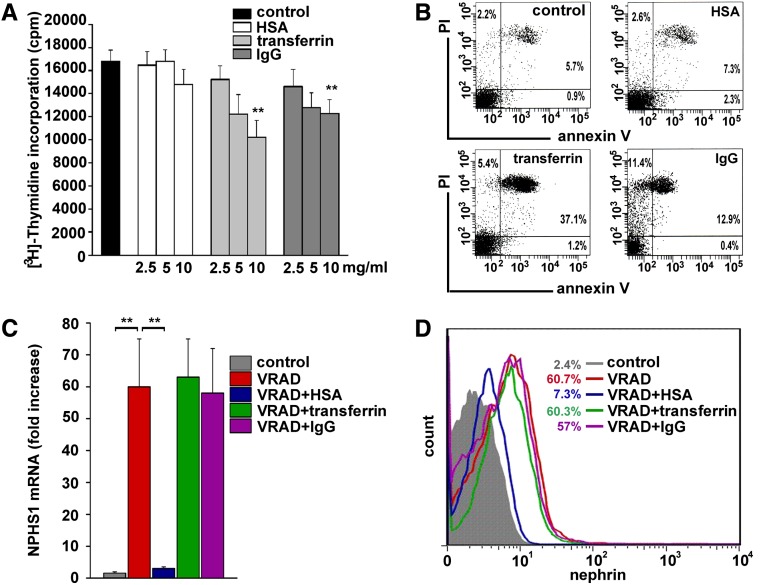
To investigate the effects of protein overload, primary cultures of human RPCs were exposed to different concentrations of human serum albumin (HSA), transferrin, or IgG, which are the circulating proteins that are mostly lost when proteinuria occurs. Treatment with transferrin and IgG induced a reduction of [3H]-thymidine incorporation (Figure 1A), which was not observed with HSA treatment (Figure 1A). However, viability of HSA-treated and -untreated RPCs was similar but was reduced after treatment with transferrin and IgG, as shown with a combined flow cytometry analysis for propidium iodide and annexin V staining (Figure 1B). The effect of exposure to HSA, transferrin, or IgG on RPC differentiation into podocytes was then evaluated. In agreement with a previous study,15 culturing of human RPCs in the differentiative medium VRAD (Vitamin D3, retinoic-acid-supplemented DMEM-F12) resulted in novel expression of nephrin (NPHS1) mRNA and protein (Figure 1, C and D). This effect was abolished in the presence of HSA (Figure 1, C and D), but not in the presence of transferrin and IgG (Figure 1, C and D). HSA also blocked VRAD-induced acquisition of other podocyte-specific markers, such as podocin, podocalyxin (PODXL), and KLF1518 (Supplemental Figure 1A) and upregulation of other molecules necessary for podocyte function, such as p21, p27, and cyclin I (Supplemental Figure 1B). These results demonstrate that transferrin and IgG overload affects human RPC survival, while albumin overload impairs RPC differentiation into podocytes.
Figure 1.
Transferrin and IgG overload affects human RPC survival, while albumin overload impairs RPC differentiation into podocytes. (A) Effect of HSA, transferrin, or IgG treatment on the proliferative capacity of RPC, as assessed by [3H]-thymidine incorporation (n=4). (B) Flow cytometry analysis of RPC viability after treatment with HSA, transferrin, or IgG, as assessed by annexin-V and propidium iodide costaining. One representative of four experiments is shown (control: 90.82%±0.69% live cells; HSA: 90.35%±1.44%, transferrin: 51.68%±4.83%, IgG: 72.48%±5.28%; P=0.75 control versus HSA, P=0.01 control versus transferrin-treated and control versus IgG-treated cells by Mann-Whitney test, n=4). (C) NPHS1 mRNA levels in RPCs cultured in control medium or VRAD medium alone, or supplemented with HSA, transferrin, or IgG (n=6). (D) Nephrin protein expression assessed by FACS analysis in RPCs cultured in control medium or VRAD medium alone or supplemented with HSA, transferrin, or IgG. Representative histograms obtained in one of three independent experiments are shown. Percentages of nephrin-positive cells are calculated over their respective isotype control. All data are means ± SEM, **P<0.01 by ANOVA with Bonferroni post hoc analysis (A) and by Mann-Whitney test (C).
Albumin Overload Impairs Podocyte Differentiation by Sequestering Retinoic Acid
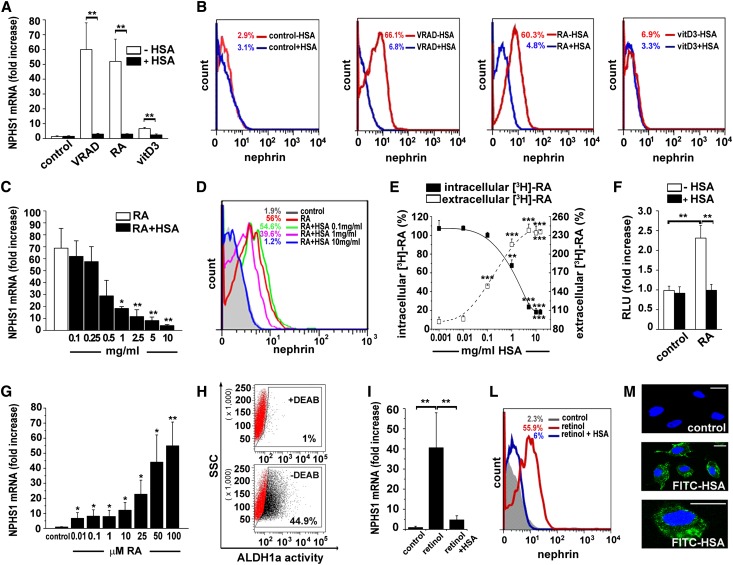
We then analyzed the effects of distinct VRAD components on nephrin mRNA levels and demonstrated that retinoic acid (RA) exerted the strongest differentiative effect, while the effect of vitamin D3 was mild (Figure 2, A and B). HSA abolished RA-induced RPC differentiation into podocytes starting at 0.1 mg/ml (Figure 2, C and D). HSA can generate retinoid-HSA complexes through interaction with specific binding sites. We thus investigated the capacity of HSA to impair RA entry within RPCs by exposing them to [3H]-RA in the presence or absence of HSA and measuring radioactivity in supernatant and cells. In the absence of HSA, [3H]-RA could enter RPCs, whereas in the presence of HSA it remained in the supernatant (Figure 2E). Because RA can upregulate NPHS1 and other podocyte genes through activation of RA response elements (RARE) on their promoters, we infected RPCs with a RARE-reporter luciferase vector. As expected, HSA abolished the RA-induced activation of the RARE–reporter (Figure 2F). Taken together, these results demonstrate that HSA blocks the differentiation activity of 100 μM RA, which is contained in the VRAD medium. However, RA could induce RPC differentiation into podocytes starting already from a 10 nM RA dose (Figure 2G). In addition, RPCs displayed retinaldehyde dehydrogenases (Aldh1a1,a2,a3) mRNA expression (Supplemental Figure 2A) and Aldh1a enzymatic activity, as demonstrated by Aldefluor staining and sensitivity to diethylaminobenzaldehyde (DEAB) treatment (Figure 2H), thus showing that RPCs can synthesize RA from retinol. Consistently, retinol administration induced differentiation of RPCs into podocytes, an effect that was abolished by HSA (Figure 2I-L and Supplemental Figure 2B). This suggests that albumin can enter RPC and sequester endogenously synthesized RA. Consistently, after 1 hour exposure of RPCs to 1 mg/ml FITC-HSA, green fluorescence could be easily revealed within living cells (Figure 2M). In addition, pretreatment of RPCs with an inhibitor of protein kinase C, which is a mediator of albumin endocytosis, partially reverted the inhibitory effect of HSA on retinol-induced RPC differentiation into podocytes (54.5%±5.3%; P<0.05).19 Taken together these results demonstrate that HSA blocks RPC differentiation into podocytes induced by RA by preventing transcription of podocyte-specific genes.
Figure 2.
Albumin overload impairs RPC differentiation into podocyte by sequestering RA. (A) Effect of HSA (10 mg/ml) on NPHS1 mRNA levels in human cells cultured in control medium, VRAD medium, or RA- or vitamin D3 (vitD3)–containing medium (n=5). (B) Nephrin protein expression assessed by FACS analysis in RPCs cultured in control medium, VRAD medium, or RA- or vitamin D3–containing medium in presence or absence of HSA (10 mg/ml). Representative histograms obtained in one of three independent experiments are shown. Percentages of nephrin-positive cells are calculated over their respective isotype control. (C) Dose-response effect of HSA on NPHS1 mRNA levels in human RPCs cultured in RA-containing medium (n=4); P=0.0006 by ANOVA. (D) Nephrin protein expression assessed by FACS analysis in human RPCs cultured in control medium or in RA-containing medium, in presence of different doses of HSA (0.1, 1, or 10 mg/ml). Representative histograms obtained in one of three independent experiments are shown. Percentages of nephrin-positive cells are calculated over their respective isotype control. (E) Effect of increasing concentrations of HSA on the uptake of radiolabeled-RA by human RPCs (n=6); P<0.0001 by ANOVA for both curves. (F) Effect of HSA (10 mg/ml) on the transcriptional activity of RARE-reporter plasmid in infected human RPCs in control or RA-containing medium (n=6). (G) NPHS1 mRNA levels in human RPCs in response to increasing concentrations of RA (n=6). (H) Flow cytometry analysis of ALDH1a activity of human RPCs in presence (top) or absence (bottom) of the ALDH1a inhibitor DEAB. The flow cytometry gatings used are marked by boxes (ALDH1apositive fraction+DEAB: 0.67%±0.17% and–DEAB: 40.1%±1.83; n=4; P=0.02). (I) Effect of HSA (10 mg/ml) on NPHS1 mRNA induced by retinol treatment in human RPCs (n=6). (L) Nephrin protein expression assessed by FACS analysis in human RPCs cultured in control medium, retinol-containing medium, or retinol+HSA (10 mg/ml)–containing medium. Representative histograms obtained in one of three independent experiments are shown. Percentages of nephrin-positive cells are calculated over their respective isotype control. (M) Confocal microscopy analysis of FITC-HSA uptake by human RPC (green HSA signal, blue Topro-3 nuclear staining). One representative experiment of four is shown. Bars = 20 μm. All data are means ± SEM. *P<0.05, **P<0.01, ***P<0.001 by ANOVA with Bonferroni post hoc analysis for C, E, and G and by Mann-Whitney test for A, F, H, and I.
In Vivo Models of FSGS to Evaluate the Effect of Albuminuria and RA on Podocyte Regeneration
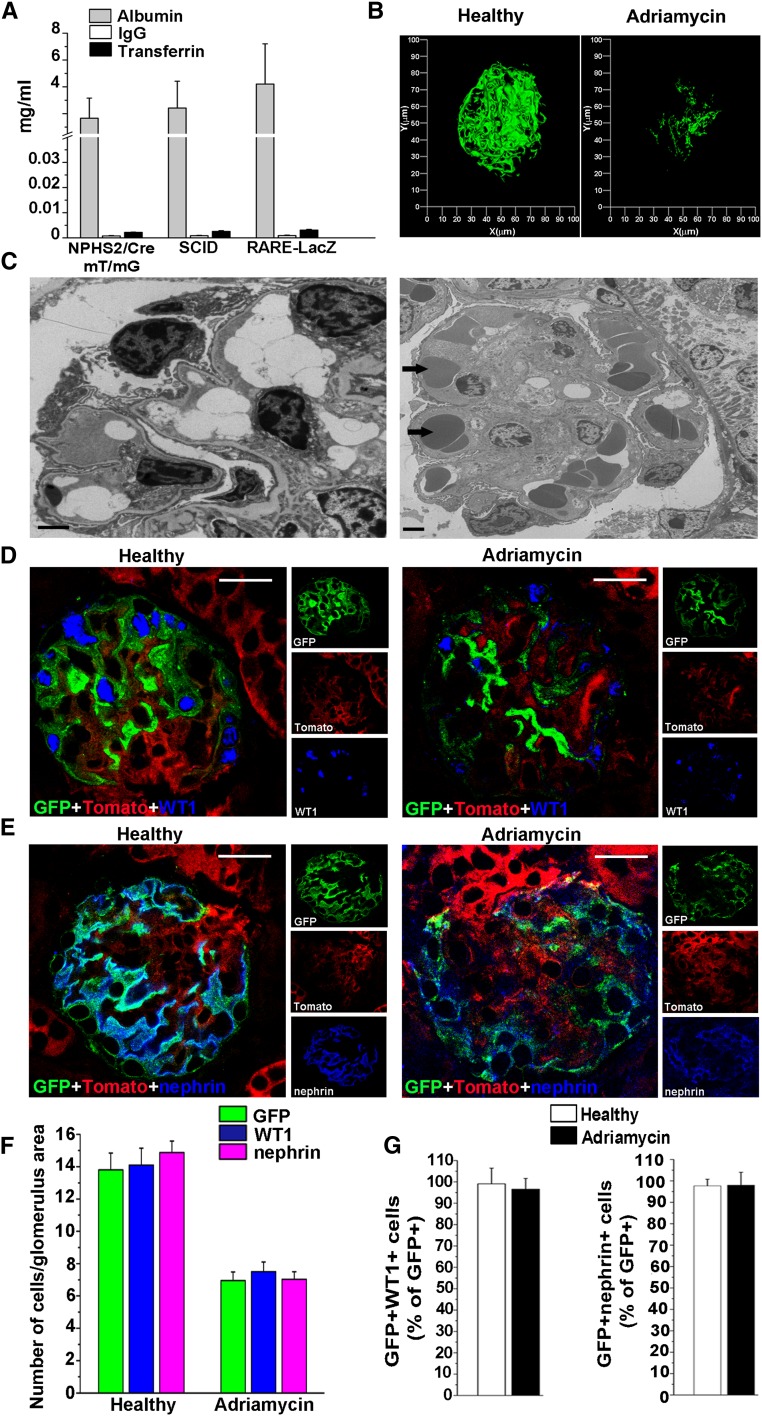
To evaluate the effect of albuminuria and RA on podocyte regeneration in vivo, we used the Adriamycin nephropathy (AN) model, which mimics human FSGS and can be induced in different mice strains. We selected the three following backgrounds: (1) NPHS2.Cre;mT/mG transgenic mice, to validate markers that could be used in the other strains for analysis of podocyte loss and regeneration through podocyte genetic labeling. (2) Balb/c severe combined immunodeficiency (SCID) mice, to analyze the effects of endogenously synthesized RA in podocyte injury without confounding effects related to its immunomodulatory activity; (3) RARE-lacZ transgenic mice, to visualize which cells are responding to RA. Evaluation of albumin, transferrin, and IgG levels in the urine through specific ELISAs after induction of AN demonstrated that in the experimental conditions established, all three mouse strains displayed similar levels of a nearly selective albuminuria (Figure 3A and Supplemental Figure 3). Evaluation of glomeruli in NPHS2.Cre;mT/mG transgenic mice demonstrated that proteinuria was related to a severe podocyte loss (Figure 3, B–G). Indeed, in NPHS2.Cre;mT/mG transgenic mice, green fluorescent protein (GFP) is specifically and permanently expressed by podocytes independent of their functional status or phenotypic appearance, making GFP loss a direct quantitation of podocyte loss. Three-dimensional reconstruction of glomeruli of NPHS2.Cre;mT/mG transgenic mice demonstrated severe podocyte depletion following induction of AN (Figure 3B). As a further confirmation, electron microscopy demonstrated podocytopenia in AN (Figure 3C). Interestingly, GFP expression coincided with that of Wilms tumor 1 (WT1) or nephrin in both healthy and AN mice (Figure 3, D and E); counting podocyte numbers as GFP-, or as WT1-, or as nephrin-expressing cells gave identical results (Figure 3F); GFP-expressing cells that had lost WT1 or nephrin were not observed (Figure 3G). Taken together, these results demonstrate that AN is characterized by a nearly selective albuminuria and that WT1 and nephrin provide an exact quantitation of podocyte numbers in this model.
Figure 3.
Evaluation of urinary protein levels and podocyte markers in mice with genetically tagged podocytes validate AN as a model to study podocyte loss and regeneration. (A) Evaluation of albumin, transferrin, and IgG levels in the urine of three different mice strains (n=15 each) after induction of AN (day 11). (B) Three-dimensional reconstruction of glomerulus of healthy and Adriamycin-treated NPHS2.Cre;mT/mG transgenic mouse (green = GFP). (C) Representative electron micrographs of glomerular podocytes in AN show podocyte loss with extensive denudation of the capillary loops (arrows). Bars = 2 μm. (D) WT1 staining (blue) in renal sections of healthy (left) and of Adriamycin-treated (right) NPHS2.Cre;mT/mG transgenic mice (green = GFP; red = Tomato). Bars = 20 μm. (E) Nephrin staining (blue) in renal sections of healthy (left) and of Adriamycin-treated (right) NPHS2.Cre;mT/mG transgenic mice (green = GFP; red = Tomato). Bars = 20 μm. (F) Number of GFP, WT1, or nephrin-positive cells/glomerulus area in healthy (n=6) and in Adriamycin-treated (n=10) NPHS2.Cre;mT/mG transgenic mice. (G) Percentage of GFP cells coexpressing WT1 (left) or nephrin (right) in healthy (n=6) and in Adriamycin-treated (n=10) NPHS2.Cre;mT/mG transgenic mice. Data are means ± SEM; P=NS by Mann-Whitney test.
Blocking Endogenous RA Synthesis Decreases Podocyte Number, Increases Proteinuria, and Enhances Mortality in SCID Mice with FSGS
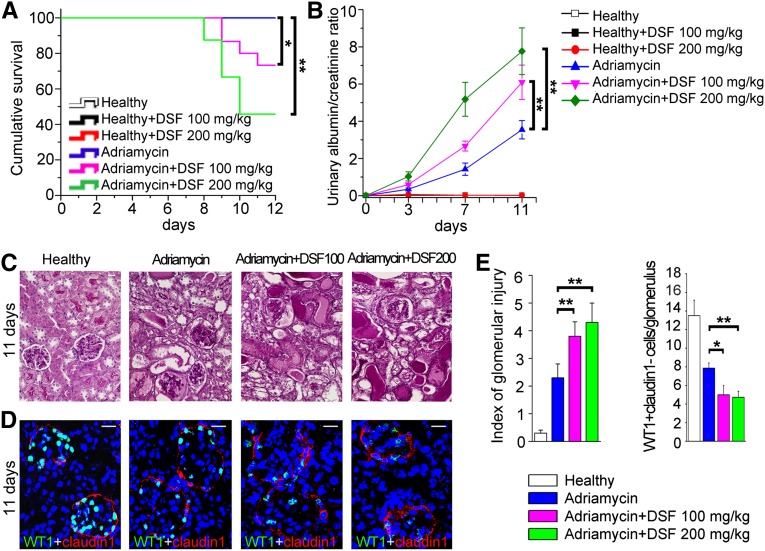
To evaluate the in vivo effects of retinoids, we treated SCID mice affected by AN with different doses of Aldh1a inhibitor disulfiram (DSF) or its vehicle. Treatment with 100 mg/kg and 200 mg/kg of DSF reduced survival in AN mice, while similar doses of DSF had no effect on healthy mice (Figure 4A). AN mice treated with DSF displayed a significantly higher albuminuria in comparison with vehicle-treated mice (Figure 4B). Indeed, at day 11, these mice displayed mean ± SD albuminuria values of 272.8±54.5 mg/dl versus 432.9±71.2 mg/dl in mice treated with DSF 100 mg/kg and 642.3±147.6 mg/dl in mice treated with DSF 200 mg/kg (P=0.05 and P=0.05). Albuminuria was absent in DSF-treated healthy mice, suggesting that blocking endogenous RA synthesis is not causing albuminuria per se (Figure 4B). Healthy mice consistently showed normal podocyte numbers, even after treatment with 100 mg/kg and 200 mg/kg of DSF (Supplemental Figure 4, A and B). In contrast, treatment with DSF in AN mice increased glomerulosclerosis (Figure 4C) by reducing podocyte number counted as WT1+claudin1− cells, representing differentiated podocytes that lost the progenitor marker claudin1 (Figure 4, D and E), or as nephrin+ cells (Supplemental Figure 4, A and B). These results demonstrate that endogenous synthesis of RA following podocyte injury limits albuminuria and glomerulosclerosis by increasing podocyte numbers.
Figure 4.
Blocking endogenous retinoic acid synthesis decreases podocyte number, increases proteinuria and enhances mortality in SCID mice with FSGS. (A) Survival curve of Adriamycin-treated SCID mice exposed to DSF 100 mg/kg (purple line) (n=15) or 200 mg/kg (green line) (n=24). Survival curve of healthy mice (n=6), mice treated with DSF 100 mg/kg (n=6) and 200 mg/kg (n=6), and mice with Adriamycin alone (n=14) was 100%. (B) Time course assessment of albumin-to-creatinine ratio in mice with AN (blue triangle) (n=14) in comparison with mice with AN treated with DSF 100 mg/kg (pink inverted triangle) (n=11) or 200 mg/kg (green diamond) (n=11). Albumin-to-creatinine ratio in healthy mice and healthy mice treated with DSF 100 and 200 mg/kg (n=6 for each group) was all around 0. P=0.0001 by ANOVA test. (C) Representative periodic acid-Schiff staining of renal sections of healthy mice and of mice with AN, untreated or treated with DSF 100 and 200 mg/kg. (D) Representative WT1 (green) and claudin1 (red) costaining of renal sections of healthy mice and of mice with AN, untreated or treated with DSF 100 and 200 mg/kg. (E) Left: index of glomerular injury assessed as described in the Concise Methods section. Right: quantitation of number of podocytes (WT1+claudin1− cells)/glomerulus is shown (healthy, n=6; Adriamycin, n=14; Adriamycin plus DSF 100 mg/kg, n=11; Adriamycin plus DSF 200 mg/kg, n=11). Topro-3 counterstains nuclei (blue). Bars = 20 µm. All data are means ± SEM. *P<0.05, **P<0.01 by Kaplan-Meier survival analysis and log-rank test for A, by ANOVA with Bonferroni post hoc analysis for B, and by Mann-Whitney test for E.
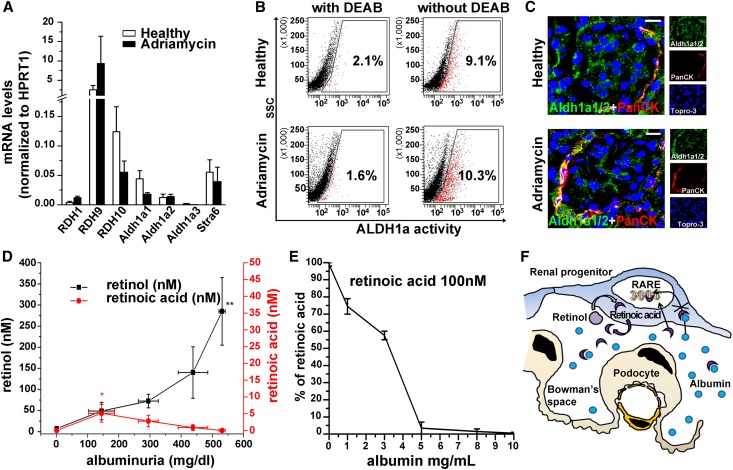
RA Is Neutralized by Albuminuria after Podocyte Injury
To clarify the mechanisms of the RA-mediated effect, the RA synthetic pathway was assessed in RARE-lacZ transgenic AN mice. mRNA levels of key components of the RA biosynthetic pathway (RDH1,9,10, Aldh1a1,2,3) and the membrane receptor for retinol-binding protein (STRA6) (Figure 5A), Aldh1a activity (Figure 5B), as well as expression of the Aldh1a enzymes (Figure 5C) were similar in glomeruli of AN mice and in normal glomeruli (Figure 5, A–C), suggesting similar levels of RA synthesis before and after injury. We then assessed RA and retinol levels in relation to levels of albuminuria in the urine of mice by using liquid chromatography tandem mass spectrometry (LC-MS/MS). In healthy mice, a small amount of retinol could be detected in the urine, while RA could not (Figure 5D). However, following podocyte injury, retinol levels increased proportionally to the severity of albuminuria. In contrast, RA became detectable in the urine when albuminuria was <150 mg/dl, decreased between 150 and 300 mg/dl, and disappeared when albuminuria increased to >300 mg/dl (Figure 5D and Supplemental Figure 5). Consistently, exposure of 100 nM RA in a standard urine solution to increasing doses of albumin led to gradual disappearance of RA (Figure 5E). These results demonstrate that following injury, passage of retinol through the glomerular filtration barrier increases proportionally to the severity of damage and allows RA synthesis to occur (Figure 5F). However, urinary levels of RA do not increase in parallel with retinol levels but rather decrease when albuminuria levels increase, suggesting that RA is progressively sequestered by albumin (Figure 5F).
Figure 5.
Following podocyte injury, RA is neutralized by albuminuria. (A) mRNA levels of RDH1, RDH9, RDH10, Aldh1a1, Aldh1a2, Aldh1a3, and Stra6 in glomeruli from healthy or Adriamycin-treated mice (n=4). (B) Flow cytometry analysis of ALDH1a activity of murine glomerular cells isolated from healthy or Adriamycin-treated mice, and stained with Aldefluor in presence (left) or absence (right) of the ALDH1a inhibitor DEAB. The flow cytometry gatings used are marked by boxes (in healthy animals: ALDH1apositive fraction+DEAB 1.025%±0.4%, and –DEAB 8.875%±1.07%, n=4, P=0.02; in Adriamycin-treated animals: ALDH1apositive fraction+DEAB 1.325%±0.24%, and –DEAB 7.975%±1.18%, n=4, P=0.02 by Mann-Whitney test). (C) Representative immunofluorescence staining of glomeruli from healthy or Adriamycin-treated mice showing Aldh1a1/2 (green) and PanCK (red). (D) Graph representing retinol (black) or RA (red) concentration (nM) in urine of mice with various levels of proteinuria. n=3–6, P=0.003 for retinol measurement by ANOVA test. (E) Percentage of RA (100 nM) assessed by LC-MS/MS in a urine standard solution in presence of increasing doses of albumin. (F) Hypothetic scheme summarizing the blocking effect of albumin on RARE activation induced by retinoids in renal progenitors. *P<0.05, **P<0.01 by Mann-Whitney test for D. Topro-3 counterstains nuclei (blue). Bars = 20 µm.
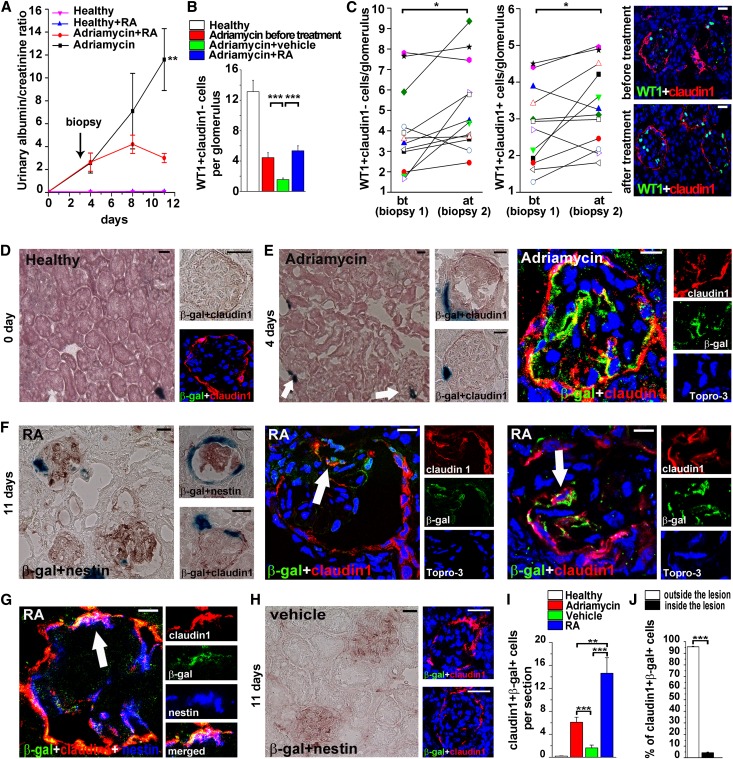
RA Treatment Increases Podocyte Number by Reversing Albuminuria-Impaired RARE Activation in RPC
We then aimed to counteract the harmful effect of albuminuria by administrating RA and evaluating which cells were responding to RA treatment in RARE-lacZ transgenic mice. Thus, 4 days after Adriamycin injection, mice were divided in two groups with identical proteinuria levels: one group was treated with RA and the other with vehicle. Mice treated with RA exhibited urinary doses of RA of 197±85.7 nM measured by LC-MS/MS. RA treatment (20 mg/kg) diminished proteinuria in RARE-lacZ transgenic mice, an effect that was significant after 1 week of treatment (Figure 6A). At day 11 after injury, vehicle-treated mice displayed high proteinuria, while proteinuria in RA-treated mice was significantly lower (774.4±214.7 mg/dl versus 170±50.2 mg/dl in RA-treated mice; P=0.04). There was no effect in healthy mice treated with RA or vehicle (Figure 6A).
Figure 6.
RA treatment increases podocyte number by reversing proteinuria-impaired RARE activation in RPC. (A) Albumin-to-creatinine ratio in RARE-lacZ healthy transgenic mice, in healthy mice treated with RA, and in mice with AN with or without RA treatment. n=32 at days 0 and 4, n=11 for each group at the following time points. P=0.0001 by ANOVA. (B) Quantitation of podocyte number (WT1+claudin1− cells)/glomerulus in healthy (n=10), Adriamycin before treatment (n=10), Adriamycin plus vehicle (n=11), Adriamycin plus RA (n=11). (C) Left: quantitation of podocyte number (WT1+claudin1− cells)/glomerulus in renal biopsy samples (n=12) before (bt) and after (at) RA treatment (3.98±0.6 versus 5.16±0.62). Center: quantitation of number of RPCs that are differentiating toward podocytes (WT1+claudin1+ cells)/glomerulus in renal biopsy specimens (n=12) before (bt) and after (at) RA treatment (2.80±0.31 versus 3.33±0.31). Each symbol represents one single mouse. Right: representative staining of WT1 (green) and claudin1 (red) in renal biopsy specimens of mice with AN before (top) and after (bottom) RA treatment. (D) Left: RARE activity (blue) in healthy mouse kidney. Right: higher magnification of colocalization of β-gal staining (blue) and claudin1 (red) assessed by immunohistochemistry (top) and immunofluorescence (bottom, β-gal green and claudin1 red). (E) Low and high magnification images showing RARE activity in the kidney of mice with AN at day 4 as seen by colocalization of X-gal staining (blue) and claudin1 (red) by immunohistochemistry (left) and by immunofluorescence (right) for β-gal (green) and claudin1 (red). Arrows point to positive glomeruli. (F) Low and high magnification images demonstrating RARE activity in the kidney of mice with AN treated with RA (day 11) as seen by colocalization of X-gal staining (blue) and nestin (red) by immunohistochemistry (left) and by immunofluorescence (center and right) for β-gal (green) and claudin1 (red). White arrows pointed to β-gal+claudin1+ cells entering in the glomerular tuft. (G) Immunofluorescence for β-gal (green), claudin1 (red) and nestin (blue) in the kidney of mice with AN treated with RA (day 11). White arrow points to β-gal+claudin1+nestin+ cells demonstrating the acquisition of podocyte markers by β-gal+ cells. (H) Absence of RARE activity in the glomeruli of mice with AN treated with vehicle (day 11) as seen by colocalization of β-gal staining (blue) and nestin (red) by immunohistochemistry (left) and by immunofluorescence (right) for β-gal (green) and claudin1 (red). (I) Quantitation of number of claudin1+β-gal+ cells/section in healthy (n=10), Adriamycin before treatment (n=10), Adriamycin plus vehicle (n=11), and Adriamycin plus RA mice (n=11). (J) Percentage of claudin+β-gal+ cells outside and inside the lesions in Adriamycin plus RA treated mice (n=11). Topro-3 counterstains nuclei (blue). Bars = 20 µm. All data are means ± SEM. *P<0.05, **P<0.01, ***P<0.001 by ANOVA with Bonferroni post hoc analysis for A; by Mann-Whitney test for B, I, and J; and by Wilcoxon test for C.
Counting WT1+claudin1− or nephrin+ cells, we demonstrated that in RA-treated mice podocyte number is restored to a level slightly higher than the one observed at day 4 after Adriamycin treatment, although the difference was not statistically significant (Figure 6B and Supplemental Figure 6A). Thus, to establish whether RA was inducing generation of novel podocytes, avoiding the interindividual variability in podocyte numbers and in response to treatment, we performed serial renal biopsies in an additional group of 12 mice before and after RA treatment. Counting podocytes, we indeed demonstrated that RA increased WT1+claudin1− cells in 10 of 12 mice (Figure 6C). Similar results were obtained once podocytes were counted as nephrin+ cells (Supplemental Figure 6B). Interestingly, RA-treated mice also displayed more WT1+claudin1+ (RPCs that are differentiating into podocytes) compared with vehicle-treated mice, suggesting that RA was promoting RPC differentiation into podocytes.
While in healthy kidneys β-gal activity was not detected within glomeruli, but exclusively in scattered rare tubules (Figure 6D), 4 days after induction of podocyte injury, and just before starting RA or vehicle treatment, β-gal activity appeared in some RPCs lining the Bowman capsule or that were entering the glomerular tuft (Figure 6E). However, 7 days after RA treatment (i.e., 11 days after Adriamycin administration), β-gal activity was very different in RA-treated animals than in vehicle-treated mice (Figure 6, F and G). Indeed, at day 11, β-gal activity persisted and was further enhanced in RPCs of RA-treated mice (Figure 6F), which also started to acquire podocyte markers (Figure 6G), while β-gal activity was almost absent in vehicle-treated kidneys (Figure 6H). The results suggest that in mice treated with vehicle, endogenously produced RA was not available to induce RARE activation in RPCs. Indeed, RA-treated mice displayed more claudin1+β-gal+ RPCs compared with not only vehicle-treated mice (Figure 6I) but also mice analyzed immediately before initiation of treatment (Figure 6I). Among claudin1+β-gal+cells, 52.3%±6.4% also coexpressed the podocyte marker nestin, and thus represented RPCs that were differentiating into podocytes. Interestingly, 16.02%±2.9% of RA-treated glomeruli showed at least one claudin1+β-gal+ cells, compared with only 1.7%±0.5% of glomeruli in vehicle-treated mice (P<0.0001) and 6.1%±0.77% of glomeruli in mice analyzed immediately before initiation of treatment (P=0.003). Although numerous claudin1+ RPCs also localized within cellular FSGS lesions, almost all claudin1+ RPCs that exhibited β-gal activity localized outside these lesions, suggesting that RPCs within FSGS lesions cannot respond to RA and differentiate into podocytes (Figure 6J). Finally, 1 month after induction of injury, mice treated with RA exhibited improved renal function compared with vehicle-treated mice (Supplemental Figure 6C). Taken together, these results suggest that RA induces podocyte regeneration and that RA sequestration by proteinuria favors the generation of FSGS lesions.
Discussion
Far from being a simple prognostic clinical measure, proteinuria has been proposed as a pivotal causative factor that perpetuates kidney damage and precedes the relentless deterioration of renal function in progressive nephropathies.6 Previous studies showed that proteinuria has an intrinsic renal toxicity because of excessive reabsorption by proximal tubular cells, which causes activation of interstitial inflammation and fibrosis.6 However, why and how reduction of proteinuria is renoprotective for glomerular structures and preserves GFR is still unknown.
This study demonstrated that exposure to albumin can impair RPC differentiation into podocytes by sequestering RA, which is a critical driver of progenitor differentiation in multiorgan systems.20 Interestingly, RA was previously found to provide protection in multiple experimental models of kidney disease, including mesangioproliferative GN,21 puromycin-induced nephrosis,22 lupus nephritis,23 diabetic nephropathy,24 and HIV nephropathy.25 A recent study suggested that RA upregulates podocyte protein expression on parietal epithelial cells.26 In addition, treatment with RA reduced proteinuria in two patients with lupus nephritis.27 However, how RA improves kidney disease remains mostly unclear. Our data suggest that RA is essential for appropriate differentiation of RPCs into podocytes and that this process is altered by albumin in a dose-dependent manner. Albumin represents the specific carrier of RA in the blood28 and binds it with high affinity,29 which explains why even a small amount of albumin within the Bowman space can be detrimental for an appropriate regenerative process to proceed. In agreement with results of in vitro human RPC experiments, blocking endogenous RA synthesis in experimental FSGS induced in SCID mice markedly increased albuminuria and mortality, thus suggesting that endogenous RA synthesis is essential for podocyte number maintenance independent of its immunomodulatory effect.
Of note, by using genetic labeling of podocytes, we were able to demonstrate that in AN proteinuria was related to podocyte loss and not to dedifferentiation, indicating that RA modulates podocyte numbers. More important, using LC-MS/MS and confocal microscopy in RARE-lacZ transgenic mice, we provided evidence that following podocyte injury, retinol lost through the injured glomerular filtration barrier is transformed by Aldh1a into RA within the Bowman space. This activates RPC response to RA, thus initiating their differentiation into podocytes. However, once podocyte damage leads to a progressive increase of proteinuria, RPC response to RA is impaired and differentiation into podocytes is abolished.
Taken together, these results provide a novel explanation of why albuminuria is a predictive factor of progression to glomerulosclerosis in patients with diabetic and nondiabetic nephropaties.6 Indeed, although podocytes can potentially be replaced by RPCs localized along the Bowman capsule, the regenerative capacity observed during glomerular disorders seems limited.17 Instead, aberrant RPC proliferation following podocyte injury may drive generation of hyperplastic glomerular lesions in patients with FSGS or collapsing glomerulopathy.17,29,30 Our results suggest that this occurs when RPC proliferate to replace injured podocytes, but they cannot fully differentiate into podocytes because of the presence of albuminuria. This leads to the amplification of an immature podocyte population, which is not able to interconnect with other podocytes within the glomerular tuft, thus generating glomerular hyperplastic cellular lesions. However, the regenerative process can possibly be rescued once the differentiative capacity of RPCs into podocytes is adequately restored, bypassing the neutralizing activity of albumin through exogenous administration of RA.
This is possible also because at the doses analyzed in this study, albuminuria does not appear to have a death-promoting effect in RPCs, in contrast to what was previously reported for podocytes.31 Indeed, progenitors usually exhibit an increased resistance to death compared with differentiated cells, which allows preservation of the possibility of starting a regenerative response following injury.32 Consistently, previous evidence in animals suggested that glomerulosclerosis can be reversed and the podocyte number increased once proteinuria is reduced.6,10 Similar results have also been reported in human studies.6,11,12,33 Indeed, findings from several clinical studies suggest that if proteinuria is reduced upon treatment with ACE inhibitors, recovery of kidney function due to regeneration is possible.6,11,12,33 Notably, a previous study reported that mouse treatment with ACE inhibitors induced regression of FSGS lesions and increased expression of podocyte markers by RPCs and podocyte number, suggesting that remodeling of RPCs is a key feature of ACE inhibitor renoprotection.34,35 Finally, our results also suggest that when both albuminuria and larger proteins, such as IgG and transferrin, are lost, a combined toxic effect not only blocks RPC differentiation into podocytes but also promotes their death. This potentially explains the observation that when nonselective proteinuria occurs, renal function declines faster.36
Taken together, these results support the concept that proteinuria represents not only a marker of progression but also a cause for glomerulosclerosis; they also explain why reducing proteinuria through proteinuria-lowering drugs delays progression of CKD.37 More important, the results of this study provide a biologic rationale for the clinical use of pharmacologic modulators to promote regression of glomerular disease.
Concise Methods
Human RPC Cultures
Human RPCs were obtained and cultured as previously described,32 in agreement with the Ethics Committee on human experimentation of the Azienda Ospedaliero-Universitaria Careggi, Florence, Italy. Cell proliferation was assessed by [3H]-thymidine incorporation. HSA, fatty acid–free HSA, transferrin, and IgG were obtained from Sigma (St. Louis, MO). All experiments were also performed by using fatty acid–free HSA with identical results.
For podocyte differentiation, cells were treated for 2 days with VRAD medium composed of DMEM-F12 (Sigma) supplemented with 10% FBS (Hyclone Laboratories, South Logan, UT), 100 nM vitamin D3, and 100 μM RA (all-trans retinoic acid) (all from Sigma). RA-induced differentiation was obtained by culturing the cells for 2 days in endothelial basal medium plus 5% FBS and either 25 µM retinol (Sigma) or 100 µM RA. In some experiments, differentiation with retinol was performed in the presence of 100 nM calphostin C (Tocris, Bristol, United Kingdom) to inhibit endocytosis.
Propidium Iodide/Annexin V Staining
Apoptosis and/or necrosis were evaluated using propidium iodide (Invitrogen, Carlsbad, CA) and the Annexin V kit (BD Biosciences, San Diego, CA) following manufacturer’s instructions, and the FACSDiva software.
Real-Time Quantitative RT-PCR
TaqMan RT-PCR was performed as described15 using commercially available Assay on Demand kits (Applied Biosystems, Warrington, United Kingdom). For luciferase quantification, the following primers and probes were used (Applied Biosystems): probe 5′-VIC-ACGCCGGTGAACTTCCCGCC-TAMRA-3′; forward 5′-CGCAGGTCTTCCCGACG-3′; reverse 5′-TTCCGTGCTCCAAAACAACA-3′.
Immunofluorescence and Confocal Microscopy
Confocal microscopy was performed by using an LSM510 META confocal microscope (Carl Zeiss, Jena, Germany) as described.15
Primary antibodies (pAbs) were antinephrin pAb, antiretinaldehyde dehydrogenase 1/2 pAb (Aldh1a1/2, clone H-85) (all from Santa Cruz Biotechnology, Santa Cruz, CA), anti-Pan Cytokeratin mAb (PanCK, clone C-2562, Sigma), anti–β-galactosidase pAb (Abcam, Cambridge, United Kingdom), anticlaudin1 pAb (Invitrogen), anti-WT1 mAb (clone F-6, Santa Cruz Biotechnology), antipodocin pAb (Alpha Diagnostics, MD), antinestin mAb (Santa Cruz Biotechnology). Secondary antibodies were AlexaFluor 488/647-labeled rabbit antigoat IgG, AlexaFluor 488/546-labeled goat antirabbit IgG, AlexaFluor 488/546/633-labeled goat antimouse IgG1, AlexaFluor 633-labeled goat antirat (all from Molecular Probes, Invitrogen), AlexaFluor 488-labeled donkey antichicken (Jackson ImmunoResearch, West Grove, PA). Staining with FITC-conjugated HSA (Abcam) was performed following manufacturer’s instructions. Topro-3 (Molecular Probes) was used for nuclear staining.
Flow Cytometry
Intracytoplasmic staining for nephrin was performed as described elsewhere.15 A Goat IgG (Calbiochem, San Diego, CA, USA) was used as isotype control.
RA Uptake Assay
Cells were incubated in endothelial basal medium plus 5% FBS in the presence of 100 µM radiolabeled RA (all-trans-[11,12–3H(N)] RA, NEN Life Science Products, Boston, MA) and increasing concentrations of HSA (from 0 to 10 mg/ml). After 24 hours, supernatant and cells were collected and frozen, and radioactivity was determined using a β-counter (1205 Betaplate; Wallac/PerkinElmer, Waltham, MA).
Human RPC Infection
pGF1 (pGreenFire1)-RARE, pGF1-cytomegalovirus (CMV), and pGF1-mCMV (minimal CMV) plasmids were obtained from System Biosciences (Mountain View, CA). Lentiviral particles were produced by cotransfection of the lentiviral plasmid and the packaging vectors into Lenti-X 293T cells (Clontech, Mountain View, CA, USA). Cells were infected at a multiplicity of infection of 30, in the presence of 8 μg/ml of polybrene (Sigma). The pGF1-CMV plasmid was used as a positive control to estimate the infection efficiency by flow cytometry. Infection levels were further verified by quantitative PCR of luciferase DNA.
For luciferase assay a commercially available kit (Promega, Milan, Italy) was used. The reporter activity of the pGF1-RARE–infected cells was measured on a GloMax 96 Luminometer (Promega) and normalized to the cells infected with pGF1-mCMV.
Evaluation of ALDH1a Activity
ALDH1a activity was assessed on human RPCs and mouse glomeruli using an Aldefluor staining kit (Stem Cell Technology, Vancouver, British Columbia, Canada) following the manufacturer’s recommendations.
Animals
Animal experiments were approved by the institutional review board; were performed in accordance with institutional, regional, and state guidelines; and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
In Vivo Models of FSGS
AN was induced in three background strains by using doxorubicin (trade name Adriamycin). Because different mouse strains display variable sensitivity to Adriamycin treatment, different doses of the drug were administered to obtain damage of similar severity, as detailed below.38
NPHS2.Cre;mT/mG Transgenic Mice
The NPHS2.Cre;mT/mG mice were developed to stably and irreversibly tag podocytes. Indeed, by crossing the transgenic NPHS2.Cre. mice with the mT/mG transgenic reporter strain, which carries a loxP-flanked Tomato cassette, Cre-mediated excision of membrane-targeted tandem dimer Tomato (mT) occurs, and an alternate reporter protein, membrane-targeted GFP, is selectively expressed in podocytes. Once recombination has occurred, GFP expression by the podocyte is not under control of the podocin promoter anymore but is continuously transcribed under control of enhancer elements from the chick β-actin gene and from CMV, thus allowing podocyte genetic labeling. B6.Cg-Tg(NPHS2-cre)295Lbh/J (here abbreviated NPHS2.Cre) mice and B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (here abbreviated mT/mG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). NPHS2.Cre;mT/mG reporter mice were generated by crossing mT/mG mice with NPHS2.Cre mice. To establish a model of Adriamycin-induced nephropathy in these mice, 6- to 10-week-old male mice were treated with Adriamycin by two successive retro-orbital injections on day −7 (22 mg/kg in PBS) and on day 0 (18 mg/kg in PBS) (n=10). Urinary albumin-to-creatinine ratio was evaluated on days 3, 5, 8, and 11. On day 11, mice were euthanized and kidneys collected. Left kidneys were fixed for 2 hours in ice-cold 4% paraformaldehyde, then washed 30 minutes in ice-cold PBS and finally frozen; right kidneys were frozen immediately after collection. Six additional healthy mice were euthanized as controls. On day 11 after Adriamycin treatment, a 11.3%±0.45% decrease in body weight was observed.
SCID Mice
AN was induced in 6-week-old female SCID mice (Harlan, Udine, Italy) by a retro-orbital injection of Adriamycin (6 mg/kg in PBS; Sigma) on day 0 in a total of three independent experiments. Control mice received PBS. DSF (Sigma), 100 or 200 mg/kg in corn oil, or vehicle alone (corn oil) was given by oral gavage twice a week starting from 1 day before Adriamycin or PBS injection. Groups were defined as follows: (1) PBS plus vehicle (n=6); (2) PBS plus DSF 100 mg/kg (n=6); (3) PBS plus DSF 200 mg/kg (n=6); (4) Adriamycin plus vehicle (n=14); (5) Adriamycin plus DSF 100 mg/kg (n=15); (6) Adriamycin plus DSF 200 mg/kg (n=24). BUN levels were measured by Reflotron System (Roche Diagnostics, Rotkreuz, Switzerland). Kidneys were collected at the end of the experiment for analysis of renal morphology. Urinary albumin-to-creatinine ratio in 24-hour urine samples was evaluated on surviving mice on days 0, 3, 7, and 11 using Albuwell M kit (Exocell, Philadelphia, PA), and a creatinine assay kit (Cayman Chemical, Ann Arbor, MI). For the determination of urinary transferrin and IgG, a mouse transferrin ELISA kit (Bethyl Laboratories Inc., Montgomery, TX) and mouse IgG ELISA kit (Cusabio, Wuhan, Hubei, P.R. China) were used, respectively. On day 11 after Adriamycin treatment, a 10.1%±0.5% decrease in body weight was observed. DSF treatment did not significantly affect body weight.
RARE-lacZ Transgenic Mice
RARE-lacZ transgenic mice (CD1 background) were purchased from the Jackson Laboratory. AN was induced in 6- to 10-week-old female mice by two successive retro-orbital injections of Adriamycin (14 mg/kg in PBS) on day −14 and on day 0 (n=32). Urinary albumin and creatinine in 24-hour urine specimens was evaluated on day 0, and on day 4, when mice were divided into two groups of 16 mice with similar proteinuria and 5 mice for each group were euthanized before starting treatment and kidneys collected for immunohistochemical analysis. Then, one group was subcutaneous injected with 20 mg/kg RA (stock 40 mg/ml in DMSO, diluted 1:4 in corn oil, n=11) and one with vehicle (DMSO, diluted 1:4 in corn oil, n=11) for 5 consecutive days. Proteinuria was evaluated again at days 8 and 11. BUN levels were measured by Reflotron System. Finally, on day 11 mice were euthanized and kidneys collected. Ten additional healthy mice were euthanized to evaluate control kidneys.
In an additional experiment AN was induced in 12 mice as described above. Animals were monitored for proteinuria and underwent biopsy on day 3 as follow: after anesthetization by intraperitoneal injection of 250 mg/kg 2,2,2-tribromoethanol (Avertin, Sigma), the kidney was exteriorized and a small piece of tissue was cut off using small scissors. A sterile gauze saturated with tranexamic acid (Tranex, Lusofarmaco, Peschiera Borromeo, Italy) was applied to the wound, which was then cauterized. The remaining kidney was pushed back in the body cavity and the animal sutured. From the day after the surgery, the animals were given daily subcutaneous injections of RA 20 mg/kg (n=12) for 5 consecutive days. On day 11, the animals were euthanized and kidneys were collected. On day 11 after Adriamycin treatment, a 13.4%±0.7% decrease in body weight was observed. RA treatment did not significantly affect body weight.
Transmission Electron Microscopy
Kidneys were immersed in cold modified Karnovsky fixative containing 3% glutaraldehyde and 1% paraformaldehyde in PBS, postfixed in phosphate-buffered 2% osmium tetroxide for 1 hour, dehydrated in graded ethanol with a final dehydration in propylene oxide, and embedded in Embed-812 (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections (approximately 90 nm thick) were stained with uranyl acetate and Venable lead citrate and viewed with a JEOL model 1200EX electron.
Analysis of Glomerular Injury and Podocyte Count
Kidney sections of 5 μm were fixed in ethanol and stained with periodic acid-Schiff reagent (Carlo Erba, Milan, Italy). Fifty randomly selected glomeruli were assessed for glomerular damage (well developed exudative, mesangial proliferation, and glomeruli hypertrophy) and graded as follows: 0, normal; 1, slight glomerular damage of the mesangial matrix and/or hyalinosis with focal adhesion involving <10% of the glomerulus; 2, sclerosis of 10%–20%; 3, sclerosis of 20%–30%; 4, sclerosis of 30%–40%; and 5, sclerosis >40% of the glomerulus. All scoring was performed in a blinded manner. For quantitation of podocytes, the number of nephrin-positive cells or of WT1+claudin1− cells or GFP-positive cells per glomerulus area was evaluated in 15 glomeruli of at least four sections for each mouse by two independent observers.
LC-MS/MS
One hundred microliters of mouse urine was treated by adding 1 µl of formic acid and then extracted twice with 0.5 ml of hexane. After phase separation, the hexane phase was dried under N2 stream and the residue dissolved in 100 µl of acetonitrile plus 0.1% of formic acid. Calibration curve was prepared by adding RA (stock solution 3 g/l in ethyl acetate) to urine to obtain concentrations of 0, 3, 30, and 300 µg/L and then treated as described above. The samples were immediately analyzed on an AB-Sciex QTRAP 5500 LC/MS/MS System (Applied Biosystems, Toronto, Ontario, Canada) equipped with an atmospheric pressure chemical ionization (APCI)source and coupled to a Series 1290 Infinity LC System (Agilent Technologies, Waldbronn, Germany) UHPLC Capillary Pump and the analytical column Kinetex C18 2.6 µm, 3mm × 75mm (Phenomenex, Torrance, CA). APCI source operated in positive ion mode, using the following setting: curtain gas 23, collision gas flow 9, temperature 400°C, nebulizer current 3. The following transitions were monitored for the multiple reaction monitoring experiment: m/z 301.1>205.3 (quantifier) and m/z 301.1>283.2 (qualifier). Optimal CE (collision energy), CXP (collision exit potential), and DP (declustering potential) were found at 17 and 50 V for both transitions.
Chromatographic separation was performed at room temperature at 0.4 ml/min flow rate using water (A) and acetonitrile (B), both containing 0.1% formic acid. The fast gradient started from a 100% of solution A. One hundred percent of B was reached in 2 minutes and maintained for 5 minutes; initial conditions were restored in 30 seconds and the column equilibrated for 2.5 minutes. The total running time was 10 minutes, and the RA retention time was fixed to 3.39 minutes. The eluent from the column was directed into the APCI source without split. Five microliters of the extracted sample was injected for the ultra-high-performance liquid chromatography-MS/MS experiments.
System control and data acquisition were performed with Analyst 1.5.2 software. Calibration curves were constructed with the Analyst Quantitation program using a linear least-square regression, nonweighted.
β-Gal Assays
Kidneys were incubated for 30 minutes in an ice-cold fixative (3% paraformaldehyde, 0.2% glutaraldehyde, 2.5 mM EGTA, and 4 mM MgCl2 in 0.5× PBS [pH, 7.6]) and then for 30 minutes in PBS. Staining was performed on 8-μm cryosections by overnight incubation at 37°C in a humidified atmosphere in staining solution (1 mg/ml X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS [pH, 7.8]). Sections were counterstained with eosin, washed in tap water, and mounted. The quantitation of β-gal–positive cells was performed by two independent observers by counting blue cells in an entire section/mouse in a total of 10 healthy mice, 10 mice with AN before treatment, and 11 mice for the two groups of treatment (Adriamycin plus vehicle, Adriamycin plus RA).
When stated, X-gal staining was combined with immunohistochemistry for the expression of claudin1 and nestin (mAb; Santa Cruz Laboratories). As secondary antibodies we used biotinylated goat antirabbit and goat antirat, respectively (Vector Laboratories, United Kingdom). Detection was carried out with Vectastain ABC kit (Vector Laboratories) and 3-amino-9-ethylcarbazole (Vector Laboratories) as peroxidase substrate.
Statistical Analyses
The results were expressed as mean ± SEM. Comparison between groups was performed by the Mann-Whitney test, the Wilcoxon test, or ANOVA for multiple comparisons with Bonferroni post hoc analysis. A P value <0.05 was considered to represent a statistically significant difference. Statistical analysis was performed using the OriginPro 9.0.0 software (OriginLab Corporation, Northampton, MA).
Disclosures
None.
Supplementary Material
Acknowledgments
This study was funded by the European Community under the European Community’s Seventh Framework Programme (FP7/2012-2016), grant number 305436; the Tuscany Ministry of Health; the Italian Ministry of Health; and the Associazione Italiana per la Ricerca sul Cancro. M.L.A. is a recipient of the FIRC fellowship. A.P. is the recipient of a two-year AXA Research Fund Post-Doctoral Fellowship. We thank Rosa Mancina for her help with the RA uptake assay.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A New Mechanism for Albuminuria-Induced Podocyte Injury,” on pages 1709–1711.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012090950/-/DCSupplemental.
References
- 1.Couser WG, Remuzzi G, Mendis S, Tonelli M: The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80: 1258–1270, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Perico N, Benigni A, Remuzzi G: Present and future drug treatments for chronic kidney diseases: Evolving targets in renoprotection. Nat Rev Drug Discov 7: 936–953, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, Remuzzi G, Benigni A: Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol 297: F1448–F1456, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Marinides GN, Groggel GC, Cohen AH, Border WA: Enalapril and low protein reverse chronic puromycin aminonucleoside nephropathy. Kidney Int 37: 749–757, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Ikoma M, Kawamura T, Kakinuma Y, Fogo A, Ichikawa I: Cause of variable therapeutic efficiency of angiotensin converting enzyme inhibitor on glomerular lesions. Kidney Int 40: 195–202, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Adamczak M, Gross ML, Krtil J, Koch A, Tyralla K, Amann K, Ritz E: Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol 14: 2833–2842, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Perna A, Benini R, Bertani T, Zoccali C, Maggiore Q, Salvadori M, Remuzzi G: In chronic nephropathies prolonged ACE inhibition can induce remission: Dynamics of time-dependent changes in GFR. Investigators of the GISEN Group. Gruppo Italiano Studi Epidemiologici in Nefrologia. J Am Soc Nephrol 10: 997–1006, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Wilmer WA, Hebert LA, Lewis EJ, Rohde RD, Whittier F, Cattran D, Levey AS, Lewis JB, Spitalewitz S, Blumenthal S, Bain RP: Remission of nephrotic syndrome in type 1 diabetes: Long-term follow-up of patients in the Captopril Study. Am J Kidney Dis 34: 308–314, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Remuzzi A, Gagliardini E, Sangalli F, Bonomelli M, Piccinelli M, Benigni A, Remuzzi G: ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int 69: 1124–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Romagnani P: Toward the identification of a “renopoietic system”? Stem Cells 27: 2247–2253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagni L, Romagnani P: Glomerular epithelial stem cells: The good, the bad, and the ugly. J Am Soc Nephrol 21: 1612–1619, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P, Chuang PY, He JC: Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287: 19122–19135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hryciw DH, Pollock CA, Poronnik P: PKC-alpha-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol 288: F1227–F1235, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gudas LJ, Wagner JA: Retinoids regulate stem cell differentiation. J Cell Physiol 226: 322–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shima Y, Iwano M, Yoshizaki K, Tanaka T, Kawase I, Nishimoto N: All-trans-retinoic acid inhibits the development of mesangial proliferative glomerulonephritis in interleukin-6 transgenic mice. Nephron, Exp Nephrol 100: e54–e62, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ: ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int 68: 133–144, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Wagner J, Dechow C, Morath C, Lehrke I, Amann K, Waldherr R, Floege J, Ritz E: Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol 11: 1479–1487, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, Kang SW, Han DS, Han JY, Cha DR: Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol 82: 568–576, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Ratnam KK, Feng X, Chuang PY, Verma V, Lu TC, Wang J, Jin Y, Farias EF, Napoli JL, Chen N, Kaufman L, Takano T, D’Agati VD, Klotman PE, He JC: Role of the retinoic acid receptor-α in HIV-associated nephropathy. Kidney Int 79: 624–634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ: Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol 121: e23–e37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinoshita K, Kishimoto K, Shimazu H, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M: Successful treatment with retinoids in patients with lupus nephritis. Am J Kidney Dis 55: 344–347, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Niederreither K, Dollé P: Retinoic acid in development: Towards an integrated view. Nat Rev Genet 9: 541–553, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P: Renal progenitor cells contribute to hyperplastic glomerular lesions of different types of podocytopathies and in crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura K, Dummer P, Kopp J, Qiu L, Levi M, Faubel S, Blaine J: Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS ONE 8: e54817, 2013. 10.1371/journal.pone.0054817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P: Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Gadegbeku C, Lipkowitz MS, Rostand S, Lewis J, Wright JT, Appel LJ, Greene T, Gassman J, Astor BC, African-American Study of Kidney Disease and Hypertension Group : Kidney function can improve in patients with hypertensive CKD. J Am Soc Nephrol 23: 706–713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting ACE promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deegens JK, Wetzels JF: Fractional excretion of high- and low-molecular weight proteins and outcome in primary focal segmental glomerulosclerosis. Clin Nephrol 68: 201–208, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Remuzzi G, Bertani T: Is glomerulosclerosis a consequence of altered glomerular permeability to macromolecules? Kidney Int 38: 384–394, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Pavlidis P, Chua S, D’Agati VD, Gharavi AG: An ancestral haplotype defines susceptibility to doxorubicin nephropathy in the laboratory mouse. J Am Soc Nephrol 17: 1796–1800, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.