Abstract
Glucose-containing peritoneal dialysis solutions may exacerbate metabolic abnormalities and increase cardiovascular risk in diabetic patients. Here, we examined whether a low-glucose regimen improves metabolic control in diabetic patients undergoing peritoneal dialysis. Eligible patients were randomly assigned in a 1:1 manner to the control group (dextrose solutions only) or to the low-glucose intervention group (IMPENDIA trial: combination of dextrose-based solution, icodextrin and amino acids; EDEN trial: a different dextrose-based solution, icodextrin and amino acids) and followed for 6 months. Combining both studies, 251 patients were allocated to control (n=127) or intervention (n=124) across 11 countries. The primary endpoint was change in glycated hemoglobin from baseline. Mean glycated hemoglobin at baseline was similar in both groups. In the intention-to-treat population, the mean glycated hemoglobin profile improved in the intervention group but remained unchanged in the control group (0.5% difference between groups; 95% confidence interval, 0.1% to 0.8%; P=0.006). Serum triglyceride, very-low-density lipoprotein, and apolipoprotein B levels also improved in the intervention group. Deaths and serious adverse events, including several related to extracellular fluid volume expansion, increased in the intervention group, however. These data suggest that a low-glucose dialysis regimen improves metabolic indices in diabetic patients receiving peritoneal dialysis but may be associated with an increased risk of extracellular fluid volume expansion. Thus, use of glucose-sparing regimens in peritoneal dialysis patients should be accompanied by close monitoring of fluid volume status.
Peritoneal dialysis (PD) is a well established treatment for renal replacement therapy that provides clinical outcomes similar to those with hemodialysis (HD)1 and similar and possibly better quality of life.2–4 However, registry data from Australia/New Zealand, Europe, and the United States show that dialysis patients continue to have a high risk of cardiovascular (CV) mortality compared with the general population of the same age.5 Although overall survival appears no different between patients treated with PD or HD,1,6,7 features unique to both modalities may contribute to differences in the underlying mechanisms leading to morbidity and death. PD populations have multiple modifiable CV risk factors, including dyslipidemia, hypertension, smoking, obesity, and factors that are associated with uremia (such as vascular calcification, inflammation, endothelial dysfunction, and oxidative stress).8 Fluid overload and glucose exposure are postulated to contribute significantly to CV mortality in PD patients.8,9
Unique to PD therapy is the exposure of the patient to large amounts of glucose contained in the dialysis fluids. Glucose serves as the osmotic agent for the PD-based solutions, and patients can absorb up to 200 g per day, depending on the glucose concentration in the PD fluid used and the patient’s peritoneal membrane transport status.10,11 Patients treated with PD experience a heightened exposure to metabolic risk factors, including dyslipidemia (elevated triglycerides and very-low-density lipoprotein [VLDL]) and hyperglycemia. This group of risk factors (including hypertension) composes the metabolic syndrome, a disorder estimated to occur in about 50% of patients undergoing PD.12,13 For patients with diabetes, the glucose loading can aggravate glycemic control and potentially require increased doses of insulin or other hypoglycemic agents.
Poor glycemic control is associated with increased mortality in PD patients.14 Adjusted all-cause mortality hazard ratio for time-averaged hemoglobin A1c (HbA1c) values of 7.0%–7.9% and 8.0%–8.9% were 1.10 and 1.28, respectively, compared with 6.0%–6.9% as reference.14 In addition, higher HbA1c is associated with increased CV mortality in nondiabetic patients undergoing PD.15
A recent randomized, controlled trial performed in Mexico demonstrated improved control of multiple metabolic variables with an icodextrin-based intervention in diabetic PD patients with high average peritoneal transport status.16 In addition, amino acid–containing solutions, such as Nutrineal, have been shown to improve glucose and lipid metabolism.17
The Improved Metabolic Control of Physioneal, Extraneal, Nutrineal (P-E-N) versus Dianeal Only in DIAbetic continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD) Patients (IMPENDIA) trial was designed with these considerations in mind. IMPENDIA was a prospective, randomized, controlled, open-label, parallel-group, multicenter, multinational trial designed to investigate whether a glucose-sparing PD prescription (P-E-N) improves metabolic control in diabetic PD patients compared with a glucose-only prescription (Dianeal only) regimen over 6 months. The Evaluation of Dianeal, Extraneal and Nutrineal (D-E-N) versus Dianeal only in Diabetic CAPD Patients (EDEN) study was a separate study of patients from Colombia in which Dianeal was substituted for Physioneal as part of an otherwise identical trial design. The EDEN trial was added as a result of insufficient enrollment into the IMPENDIA study. However, because of the unavailability of the Physioneal solutions in Colombia, Dianeal was used instead. Given that both Dianeal and Physioneal have a similar glucose concentration, the analysis of the combined results of both studies provides a legitimate evaluation of the safety and efficacy of glucose-sparing PD regimens compared with conventional PD regimens in diabetic PD patients. It was decided a priori, before the statistical plan was completed and the databases were locked, to pool the data from both studies into a combined analysis.
Results
Patient Characteristics at Baseline
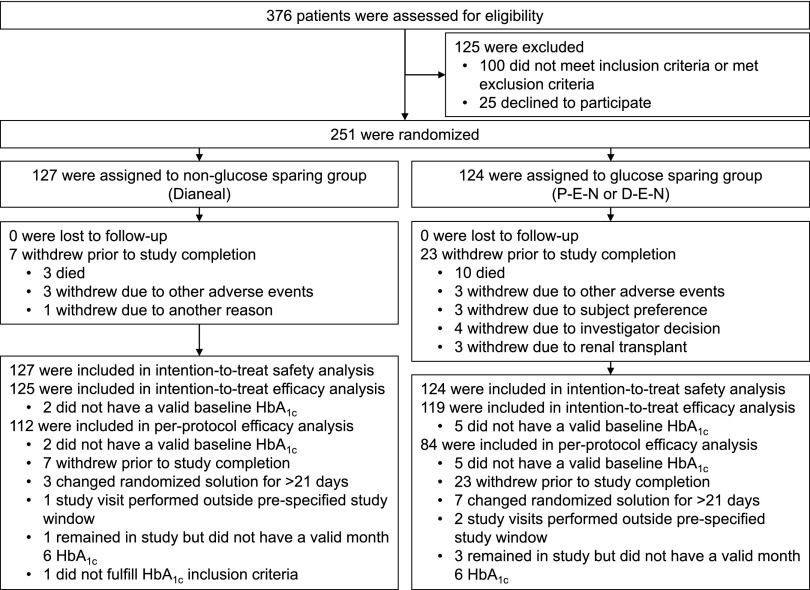
Between February 2008 and January 2011, 251 patients (180 in IMPENDIA and 71 in EDEN) were enrolled and randomly assigned to the intervention (n=124) or the control (n=127) group. Complete follow-up was achieved for all participants (Figure 1). Significantly more patients withdrew from the intervention group than the control group before study completion. The difference between groups in the withdrawal rate was driven by an increased number of deaths, renal transplants, and voluntary withdrawals by the investigators and the patients, as shown in Figure 1.
Figure 1.
Enrollment, randomization, and follow-up of study participants. D-E-N, Dianeal, Extraneal and Nutrineal; HbA1c, hemoglobin A1c; P-E-N, Physioneal, Extraneal, Nutrineal.
Patient baseline characteristics are shown in Table 1. The two groups were well balanced with respect to other baseline characteristics.
Table 1.
Baseline characteristics (IMPENDIA and EDEN studies combined)
| Variable | Control Group (Dianeal Only) (n=127) | Intervention Group (P-E-N or D-E-N) (n=124) |
|---|---|---|
| Age (yr) | 58±13 | 57±12 |
| Women, n (%) | 59 (46) | 64 (52) |
| Men, n (%) | 68 (54) | 60 (48) |
| Race, n (%) | ||
| Asian | 41 (32) | 42 (34) |
| Caucasian | 41 (32) | 41 (33) |
| Hispanic | 32 (25) | 31 (25) |
| Other | 13 (10) | 10 (8) |
| Country, n (%) | ||
| Australia | 8 (6) | 9 (7) |
| Canada | 6 (5) | 3 (2) |
| Colombia | 36 (28) | 35 (28) |
| France | 1 (1) | 0 (0) |
| Hong Kong | 21 (17) | 19 (15) |
| Korea | 13 (10) | 12 (10) |
| New Zealand | 9 (7) | 8 (6) |
| Portugal | 1 (1) | 0 (0) |
| Russia | 29 (23) | 30 (24) |
| Singapore | 1 (1) | 2 (2) |
| Taiwan | 2 (2) | 6 (5) |
| BMI (kg/m2) | 27±5 | 27±4 |
| CAPD, n (%) | 118 (93) | 122 (98) |
| Diabetes, n (%) | ||
| Type 1 | 21 (17) | 27 (22) |
| Type 2 | 106 (83) | 97 (78) |
| Dialysis vintage (yr) | 1.7±2.0 | 1.5±1.8 |
| SGA classification, n (%) | ||
| Well nourished | 96 (80) | 84 (81) |
| Mild to moderate malnutrition | 24 (20) | 20 (19) |
| Severe malnutrition | 0 (0) | 0 (0) |
| BP (mmHg) | ||
| Systolic | 138±19 | 142±18 |
| Diastolic | 77±12 | 79±11 |
| HbA1c (%) | 7.6±1.1 | 7.7±1.3 |
| Hemoglobin (g/L) | 108±14 | 110±13 |
| BUN (mmol/L) | 21±6 | 21±7 |
Data are presented as n (%) or mean ± SD. IMPENDIA, Improved Metabolic Control of Physioneal, Extraneal, Nutrineal versus Dianeal Only in DIAbetic continuous ambulatory peritoneal dialysis and automated peritoneal dialysis Patients; EDEN, Evaluation of Dianeal, Extraneal and Nutrineal versus Dianeal only in Diabetic CAPD Patients; P-E-N, Physioneal, Extraneal, Nutrineal; D-E-N, Dianeal, Extraneal and Nutrineal; (BMI, body mass index; CAPD, continuous ambulatory peritoneal dialysis; SGA, Subjective Global Assessment.
Primary Outcome: Change in HbA1c
Ninety-seven percent (n=244) of patients were included in the intention-to-treat (ITT) primary efficacy analysis. During the 6 months of therapy, in the ITT population the mean HbA1c profile improved in the intervention group but remained unchanged in the control group (0.5% difference between groups; 95% confidence interval [CI], 0.1% to 0.8%; P=0.006) (Figure 2). In the per-protocol analysis, a similar difference of 0.5% (95% CI, 0.1% to 0.8%; P=0.009) in the mean change in HbA1c profile between groups was observed. In both the ITT and per-protocol populations, the separation between treatment groups was observed as early as 3 months and continued until the end of study assessment. In a post hoc analysis, no relationship between these treatment differences for HbA1c and the use of insulin or oral hypoglycemic agents was found.
Figure 2.
Mean hemoglobin A1c (±SEM) at baseline, month 3, and end of study by treatment group in the intention-to-treat population. HbA1c, hemoglobin A1c.
Secondary Outcomes
Significant treatment differences between the two groups were observed for several lipids and lipoproteins (Table 2). Notably, treatment differences between groups were detected for serum triglyceride, VLDL cholesterol, and apolipoprotein B levels. In a post hoc analysis, no relationship between these treatment differences and the use of lipid-lowering agents was found.
Table 2.
Secondary outcomes (IMPENDIA and EDEN studies combined)
| Endpointa | Control Group (Dianeal Only) | Intervention Group (P-E-N or D-E-N) | Treatment Difference between Groupsb | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n=127) | Month 3 (n=110) | Month 6 (n=120) | Baseline (n=124) | Month 3 (n=91) | Month 6 (n=107) | Control–Intervention (95% CI) | P Value | |
| Metabolic control | ||||||||
| Total cholesterol (mmol/L) | 5.1±1.5 | 5.2±1.6 | 5.1±1.6 | 5.2±1.4 | 4.8±1.3 | 4.8±1.3 | 0.3 (0.0 to 0.7) | 0.07 |
| LDL cholesterol (mmol/L) | 2.8±1.1 | 2.9±1.3 | 2.9±1.3 | 3.0±1.2 | 2.7±1.1 | 2.8±1.1 | 0.1 (−0.2 to 0.4) | 0.59 |
| HDL cholesterol (mmol/L) | 1.1±0.4 | 1.1±0.3 | 1.0±0.4 | 1.1±0.3 | 1.1±0.4 | 1.1±0.3 | 0.0 (−0.1 to 0.1) | 0.30 |
| VLDL cholesterol (mmol/L) | 1.0 (0.2–12.6) | 0.9 (0.3–6.0) | 0.9 (0.1–7.7) | 0.9 (0.3–6.8) | 0.8 (0.2–2.7) | 0.8 (0.2–3.3) | 0.3 (0.1 to 0.5) | 0.003 |
| Serum TG (mmol/L) | 2.1 (0.4–27.7) | 2.1 (0.6–13.2) | 2.2 (0.5–16.9) | 1.9 (0.6–15.0) | 1.8 (0.4–6.0) | 1.7 (0.7–7.3) | 0.7 (0.3 to 1.1) | 0.002 |
| Apolipoprotein A1 (mg/dl) | 134±26 | 133±26 | 130±25 | 135±25 | 127±26 | 125±26 | 5.0 (−1.5 to 11.5) | 0.13 |
| Apolipoprotein B (mg/dl) | 94±28 | 99±34 | 99±33 | 96±27 | 88±25 | 90±28 | 8.4 (0.8 to 15.9) | 0.03 |
| Lipoprotein(a) (mg/dl) | 14 (2–125) | 14 (2–150) | 17 (2–171) | 13 (2–102) | 18 (2–113) | 18 (2–140) | −1.9 (−8.2 to 4.4) | 0.56 |
| Proinsulin (pmol/L) | 17 (3–1187) | 17 (3–193) | 15 (3–1475) | 16 (3–240) | 18 (3–752) | 19 (3–285) | 13.5 (−9.4 to 36.4) | 0.25 |
| Insulin (pg/ml) | 485 (70–3674) | 462 (137–3873) | 471 (137–6190) | 416 (87–7354) | 462 (131–4592) | 478 (137–8155) | −29.2 (−269 to 211) | 0.81 |
| C-peptide (pg/ml) | 3745 (69–20058) | 3756 (69–20743) | 3725 (69–19581) | 2744 (69–17287) | 3635 (69–22325) | 3903 (69–23126) | 121 (−928 to 1171) | 0.82 |
| Glycemic control medications | ||||||||
| Daily insulin use (units) | 20 (0–156) | 22 (0–244) | 25 (0–286) | 24 (0−168) | 22 (0–168) | 24 (0–258) | 4.7 (−5 to 14.4) | 0.34 |
| Severe hypoglycemia | ||||||||
| Number of events | 0 | 0 | 0 | 4 | NA | NA | ||
| Nutritional statusc | ||||||||
| Serum albumin (g/L) | 34.8±4.4 | 34.5±5.1 | 35.4±4.7 | 34.7±4.2 | 34.3±4.6 | 34.2±4.5 | 1.3 (0.1 to 2.5) | 0.03 |
| Total protein (g/L) | 64.2±6.7 | 63.7±7.1 | 64.9±6.3 | 64.1±6.3 | 64.3±5.8 | 63.7±5.8 | 1.4 (−0.2 to 2.9) | 0.09 |
| Body mass index (kg/m2) | 26.6±4.8 | 26.5±4.8 | 26.7±4.8 | 26.7±4.2 | 26.7±4.5 | 26.7±4.5 | 0.3 (−0.8 to 1.5) | 0.58 |
| Drained body weight (kg) | 70.6±15.4 | 70.3±15.0 | 70.8±15.2 | 70.8±13.6 | 71.1±14.7 | 70.9±14.9 | 0.7 (−3.0 to 4.3) | 0.64 |
| Quality of life | ||||||||
| EuroQOL-5D Health Status | 63.6±25.5 | 60.8±25.6 | 61.2±26.5 | 61.5±26.7 | 59.8±26.9 | 61.4±27.6 | 0.02 (−6.79 to 6.83) | 0.99 |
| EuroQOL-5D Index | 0.81±0.16 | 0.80±0.18 | 0.77±0.19 | 0.82±0.17 | 0.78±0.22 | 0.80±0.21 | −0.02 )−0.07 to 0.03) | 0.49 |
| Abdominal fat compositiond | ||||||||
| Visceral fat volume (ml) | 234 (71–885) | 197 (42–906) | 227 (77–858) | 150 (33–510) | 55 (−7 to 118) | 0.08 | ||
| Subcutaneous fat volume (ml) | 307 (107–693) | 326 (115–664) | 245 (99–828) | 270 (85–664) | 25 (−47 to 96) | 0.50 | ||
| LV mass and functiond | ||||||||
| LV mass (g) | 128±37 | 132±60 | 128±45 | 124±39 | 2.3 (−18.9 to 23.4) | 0.83 | ||
| Ejection fraction (%) | 59±14 | 58±17 | 57±14 | 58±14 | −0.2 (−5.3 to 7.7) | 0.72 | ||
IMPENDIA, Improved Metabolic Control of Physioneal, Extraneal, Nutrineal versus Dianeal Only in DIAbetic continuous ambulatory peritoneal dialysis and automated peritoneal dialysis Patients; EDEN, Evaluation of Dianeal, Extraneal and Nutrineal versus Dianeal only in Diabetic CAPD Patients; D-E-N, Dianeal, Extraneal and Nutrineal; P-E-N, Physioneal, Extraneal, Nutrineal; CI, confidence interval; LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein; TG, triglycerides; EuroQOL-5D, European Quality of Life 5 Dimensions; LV, left ventricular.
Data are presented as mean ± SD or median (range).
Difference between groups calculated using ANOVA with repeated measures and represents the comparison of change from baseline between groups.
Subjective Global Assessment was also a secondary endpoint within the nutritional status category. Results are not shown in this table because of the ordered ordinal nature of the data. Using a proportional odds logistic regression model with a working independence correlation structure, no difference was observed between treatment groups for Subjective Global Assessment.
Abdominal fat composition and left ventricular mass and function were measured at baseline and end of study in a subset of IMPENDIA patients (n=82).
No differences between treatment groups were observed for most of the remaining secondary endpoints, with the exception of change in serum albumin. In the control group, mean serum albumin increased by 0.6 g/L, while in the intervention group mean serum albumin decreased by 0.5 g/L. Through use of ANOVA with repeated measures, the difference between groups for the change from the baseline profile of serum albumin was 1.3 g/L (95% CI, 0.1 to 2.5 g/L; P=0.03).
Results for the primary and secondary endpoint analyses for the individual IMPENDIA and EDEN trials are provided in the Supplemental Material.
Safety
Adverse events were actively solicited during the conduct of both studies, and the relatedness to the PD solutions was judged by the clinical trial site investigator. Seventy-nine percent (n=199) of the participants experienced at least one adverse event. The number of patients who experienced an adverse event and the overall number of adverse events was similar between the two groups. However, the total number of adverse events judged to be related to the PD solutions was higher in the intervention group (34 related adverse events) compared with the control group (14 related adverse events). As shown in Table 3, more patients in the control group experienced related CV adverse events, while more patients in the intervention group experienced related adverse events that were gastrointestinal, endocrine, or neurologic/musculoskeletal in origin.
Table 3.
Adverse events and serious adverse events by treatment group (IMPENDIA and EDEN studies combined)
| Variable | All Adverse Events, n (Related Adverse Events, n)a | Serious Adverse Events, n (Related Serious Adverse Events, n)a | ||
|---|---|---|---|---|
| Control Group (n=127) | Intervention Group (n=124) | Control Group (n=127) | Intervention Group (n=124) | |
| Cardiovascular | ||||
| Ischemic heart disease | 9 (1) | 2 | 5 | 2 |
| Edema/fluid overload | 38 (7) | 19 (3) | 7 (2) | 8 (1) |
| Hypertension | 14 (1) | 8 | 1 (1) | 1 |
| Hypotension/dehydration | 8 | 8 (3) | 3 | 1 (1) |
| Peripheral vascular | 8 | 5 | 5 | 3 |
| Hypertensive crisis/urgency/encephalopathy | 1 | 7 | 1 | 7 |
| Heart failure | 1 | 6 | 1 | 6 |
| Cardiac arrest/sudden death | 1 | 3 | 1 | 3 |
| Other | 5 (2) | 5 | 0 | 0 |
| Subtotal | 85 (11) | 63 (6) | 24 (3) | 31 (2) |
| Infectious | ||||
| Peritonitis | 29 | 27 | 11 | 17 |
| Respiratory | 10 | 18 | 4 | 6 |
| Abscess/cellulitis | 4 | 10 | 3 | 8 |
| Catheter site/exit site infection | 6 | 7 | 0 | 0 |
| Other | 22 | 20 | 10 | 7 |
| Subtotal | 71 (0) | 82 (0) | 28 (0) | 38 (0) |
| Gastrointestinal and hepatobiliary | ||||
| Pain and discomfort | 13 | 26 (3) | 3 | 4 |
| Nausea, vomiting, or decreased appetite | 14 | 20 (3) | 0 | 0 |
| Malnutrition | 5 | 7 | 0 | 0 |
| Other | 11 | 11 | 0 | 2 |
| Subtotal | 43 (0) | 64 (6) | 3 (0) | 6 (0) |
| Endocrine | ||||
| Hypoglycemia | 4 (1) | 15 (5) | 0 | 4 (2) |
| Hyperglycemia | 4 (1) | 5 (2) | 2 (1) | 1 |
| Hyperparathyroidism or increased PTH | 5 | 1 | 0 | 0 |
| Other | 9 (1) | 5 | 2 | 1 |
| Subtotal | 22 (3) | 26 (7) | 4 (1) | 6 (2) |
| Neuromuscular/musculoskeletal | ||||
| Pain | 12 | 12 (3) | 2 | 1 |
| Peripheral neuropathy | 7 | 7 | 1 | 1 |
| Cerebrovascular | 4 | 1 | 4 | 1 |
| Dizziness | 3 | 2 | 1 | 0 |
| Seizures | 0 | 3 | 0 | 3 |
| Headache | 0 | 3 (1) | 0 | 0 |
| Other | 7 | 8 | 1 | 2 |
| Subtotal | 33 (0) | 36 (4) | 9 (0) | 8 (0) |
| Respiratory | ||||
| Cough | 8 | 4 | 0 | 0 |
| Other | 5 | 6 | 0 | 1 |
| Subtotal | 13 (0) | 10 (0) | 0 (0) | 1 (0) |
| Nonspecific skin rash and other skin disorders | 6 | 12 (3) | 0 | 2 |
| Allergic/immune system disorders | 0 | 5 (1) | 0 | 2 (1) |
| Abnormal blood test results, not otherwise specified | 19 | 20 | 2 | 1 |
| Noninfectious catheter or exit site complications | 10 | 10 | 2 | 3 |
| Eye, ear, and throat disorders | 12 | 9 | 1 | 2 |
| Blood and lymphatic system disorders | 8 | 9 | 2 | 2 |
| Other | 23 | 27 (7) | 3 | 3 |
| Total | 345b (14) | 373b (34) | 78c (4) | 105c (5) |
| Number of patients with any adverse event or serious adverse event (% of total group sample) | 101b (80) | 98b (79) | 41c (32) | 58c (47) |
IMPENDIA, Improved Metabolic Control of Physioneal, Extraneal, Nutrineal versus Dianeal Only in DIAbetic continuous ambulatory peritoneal dialysis and automated peritoneal dialysis Patients; EDEN, Evaluation of Dianeal, Extraneal and Nutrineal versus Dianeal only in Diabetic CAPD Patients; PTH, parathyroid hormone.
Relatedness was judged by the clinical trial site investigator. The numbers of related events are shown in brackets.
P=0.15 for difference between groups in adverse event rate; P=0.92 for difference between groups in the number of patients with an adverse event.
P=0.06 for difference between groups in serious adverse event rate; P=0.02 for difference between groups in number of patients with a serious adverse event.
Of all the adverse events, 78 events (in 41 patients) in the control group and 105 events (in 58 patients) in the intervention group were considered serious (life-threatening and/or resulted in hospitalization or death). More patients in the intervention group also experienced at least one serious adverse event (47% versus 32%; P=0.02). Most of these serious adverse events were CV or infectious in origin.
Five patients in the control group and 11 in the intervention group died. None of these deaths were judged by the investigators to be related to the study solutions. The causes of these deaths are outlined in Table 4.
Table 4.
Deaths by treatment group (IMPENDIA and EDEN studies combined)
| Cause of Death | Treatment Group | Days on Randomized PD Solutions before Death | Age (yr) | Sex | Country | Association with Study PD Solutionsa |
|---|---|---|---|---|---|---|
| Cardiorespiratory arrest | D-E-N | 165 | 70 | Female | Colombia | No |
| Pneumonia | D-E-N | 29 | 64 | Male | Colombia | No |
| Hypertensive encephalopathy | D-E-N | 98 | 38 | Male | Colombia | No |
| Hypertensive encephalopathy | D-E-N | 83 | 54 | Female | Colombia | No |
| Sepsis/peritonitis | D-E-N | 16 | 54 | Female | Colombia | No |
| Coronary artery disease | P-E-N | 126 | 84 | Male | New Zealand | No |
| Cardiac arrest | P-E-N | 147 | 58 | Female | Hong Kong | No |
| Gastrointestinal bleeding | P-E-N | 72 | 30 | Male | Russia | No |
| Acute heart failure | P-E-N | 72 | 43 | Male | Russia | No |
| Acute heart failure | P-E-N | 33 | 43 | Male | Russia | No |
| Bacterial peritonitisb | P-E-N | 179 | 50 | Female | Russia | No |
| Intracerebral hemorrhage | Dianeal only | 75 | 61 | Female | New Zealand | No |
| Multisystem organ failure | Dianeal only | 46 | 55 | Male | Russia | No |
| Sudden death | Dianeal only | 42 | 54 | Male | Colombia | No |
| Cerebrovascular accidentc | Dianeal only | 173 | 74 | Female | Canada | No |
| Acute heart failured | Dianeal only | 200 | 76 | Male | Russia | No |
IMPENDIA, Improved Metabolic Control of Physioneal, Extraneal, Nutrineal versus Dianeal Only in DIAbetic continuous ambulatory peritoneal dialysis and automated peritoneal dialysis Patients; EDEN, Evaluation of Dianeal, Extraneal and Nutrineal versus Dianeal only in Diabetic CAPD Patients; D-E-N, Dianeal, Extraneal and Nutrineal; P-E-N, Physioneal, Extraneal, Nutrineal.
Relatedness to the study PD solutions was judged by the clinical trial site investigator.
Patient died 30 days after completing all clinical trial visits.
Patient died 12 days after completing all clinical trial visits.
Patient died 12 days after completing all clinical trial visits.
Results of the safety analyses for the individual IMPENDIA and EDEN trials are provided in the Supplemental Material.
Discussion
This is the largest multinational, multicenter, randomized, controlled study investigating the effect of low-glucose PD solutions on metabolic control in diabetic patients.
Studies performed to date support the hypothesis that glucose-sparing regimens can improve glycemic control in patients with diabetes undergoing PD. In a study involving eight diabetic PD patients, replacement of a glucose-based regimen with a P-E-N regimen was associated with a reduction in the 24-hour variability of glucose concentrations as measured by a subcutaneous probe in the interstitial fluid of the abdominal wall.18 In another small study, HbA1c decreased significantly among PD patients with diabetes (n=12) when icodextrin was introduced into the dialysis regimen.19 Several other small, nonrandomized studies have reported that nondiabetic PD patients treated with icodextrin exhibited significantly lower serum insulin levels and significantly higher insulin sensitivity than those treated with glucose-based solutions.20–23 Although these preliminary studies suggest that use of biocompatible and glucose-sparing solutions may improve metabolic control in PD patients with diabetes, most of these studies involved only small numbers of patients or lacked adequate controls.16,17,24
The present study has now demonstrated that, in diabetic PD patients, a glucose-sparing prescription using icodextrin and amino acid–based dialysate for two of the daily PD exchanges improves HbA1c compared with a prescription of all glucose-based solutions. HbA1c is the most widely used measure of long-term glycemic control in diabetic patients.25 Laboratory assays for measuring HbA1c levels are well established, and screening has been standardized by international consensus.25 Although some studies have indicated that HbA1c only loosely correlates with glycemic control, especially in patients with CKD,26–28 many other studies do support the hypothesis that HbA1c is a reliable indicator of glycemic control and morbidity outcomes in dialysis patients.
Wu et al. studied 137 HD patients with type 2 diabetes and reported that the cumulative survival was lower in the group with poor glycemic control.29 Similarly, it has been shown that higher HbA1c is associated with increased death risk in patients treated with HD.30 Recently, Williams et al. reported a higher risk for death only in patients with type 2 diabetes undergoing HD with HbA1c levels >11%.31 Adoption of HbA1c as the primary efficacy variable in IMPENDIA/EDEN is further supported by a recent study that found patients receiving HD with HbA1c levels >8% had a greater than two-fold increase in the risk of sudden death compared with patients with HbA1c levels ≤6%.32
A previous study on the relationship between glycemic control and outcome in 101 PD patients found poor glycemic control to be associated with a higher death risk.33 Poor glycemic control, as measured by the change in HbA1c level, was also associated with higher mortality in PD patients with diabetes in another recent study.14 The researchers demonstrated that in a cohort of 2798 diabetic PD patients, the adjusted all-cause death hazard ratio for time-averaged HbA1c increments of 7.0%–7.9%, 8.0%–8.9%, 9.0%–9.9%, and >10%, compared with 6.0%–6.9% as reference, were 1.10, 1.28, 1.34, and 1.81, respectively.14 However, HbA1c has not always been associated with outcomes, as shown in a recent retrospective analysis of 91 diabetic patients undergoing PD.34 Time-averaged follow-up HbA1c in increments of <6.5%, ≥6.5%–8%, and >8% showed no significant survival difference among groups in this smaller PD cohort.34
The present study also demonstrated modest but significant improvements in several lipid measures, including triglycerides, VLDL, and apolipoprotein B, in the glucose-sparing group. The prominent features of uremic dyslipidemia are an increase in serum triglyceride levels (due to elevated VLDL remnants and intermediate-density lipoprotein) and low HDL cholesterol.35 Hyperapobetalipoproteinemia appears to be a prevalent dyslipoproteinemia in PD patients and, as such, might be another factor that places patients undergoing CAPD at particularly increased risk of atherosclerosis.36 In a large, prospective study in the general population of >7500 patients in the United States, apolipoprotein B measurements significantly predicted coronary heart disease death, independent of conventional lipids and other CV risk factors.37 Impaired clearance of TG-rich lipoproteins and accumulation of their oxidation-prone, atherogenic remnants in patients with advanced CKD is associated with major adverse consequences. Accumulation of oxidation-prone intermediate-density lipoprotein, chylomicron remnants, and TG-containing small dense LDL promotes accelerated atherosclerosis.38 Also, increased triglyceride levels are associated with chronic low-grade inflammation,39,40 which may lead to the accelerated mortality observed in these patients.
The improvements in the lipid profiles of the glucose-sparing group shown in our study could conceivably reduce atherosclerotic risk in the longer term. However, this potential benefit requires an outcome study. Certainly it is clear from studies to date with conventional lipid-lowering strategies in dialysis patients that the effect is absent or more difficult to demonstrate.
Subjective Global Assessment, quality of life, and abdominal magnetic resonance imaging (MRI) results did not significantly differ between the two groups. A glucose-sparing regimen may not lead to measureable differences in these particular outcomes, or this finding may be related to the short duration of the study.
An unexpected finding was the imbalance between groups on serum albumin. This imbalance remains unexplained, but several hypotheses are possible. First, it is unlikely that this is a dilutional effect due to icodextrin and its metabolites. Although icodextrin and its metabolites can theoretically expand plasma volume through a colloidal effect, peritoneal ultrafiltration from icodextrin typically outweighs any colloid-related plasma volume increase. Prior randomized controlled trials have also not demonstrated any effect of icodextrin on serum albumin.41,42 Second, we have also been unable to find any reports of icodextrin or amino acids interfering with the methods used for serum albumin determination. Third, a different cause of dilution, independent of icodextrin, could lead to an imbalance between groups for serum albumin. This could occur if the vigorous pursuit of lowering exposure to glucose was done at the expense of extracellular fluid volume control. In other words, if higher-dextrose solutions needed for adequate ultrafiltration were purposely avoided to optimize metabolic endpoints, progressively volume overload could occur. In the combined analyses, no difference between groups was observed for weight gain, physician assessments of edema or euvolemia, or end-diastolic volume as measured by CV MRI in a subgroup of IMPENDIA participants. We also did not observe strong correlations between change in serum albumin and change in other markers of dilution, including serum sodium and hemoglobin. Furthermore, adverse events that could be related to volume expansion (Table 3) were actually more common in the control group. A fourth explanation for the imbalance in serum albumin between groups is related to the difference between groups in serious adverse events (hospitalizations, for example). Serum albumin is an acute phase reactant and is known to decrease abruptly with acute medical illnesses, such as infection-related events. Insofar as more patients in the intervention group experienced more serious adverse events, the lower serum albumin may reflect this imbalance of events. Finally, the use of nonconventional PD solutions has been associated with the development of a more rapid transmembrane transport status.43,44 The change in membrane transport could lead to increased flux of albumin into the peritoneal cavity with consequent reduction in serum albumin.
Another unexpected finding was the greater number of adverse events, related adverse events, serious adverse events and deaths in the treatment group. Given that the intervention consisted of three “drugs” versus one “drug” in the control group, some of this imbalance in number and relatedness of adverse events could be explained by known drug-specific adverse effects. For example, skin rash and other skin disorders, adverse effects known to occur with icodextrin, were more frequently observed in the intervention group. Nausea, vomiting, and decreased appetite, reported adverse effects with intraperitoneal amino acids, were also more likely to occur in the intervention group. However, a surprising number of hypertensive crises and episodes of heart failure occurred in the intervention group. We found no association between these events and use of erythropoietin or increase in hemoglobin. Given that the intervention group received icodextrin as part of the glucose-sparing regimen, this result is even more surprising. Although the purpose of using icodextrin in this trial was to spare glucose exposure, it usually produces good ultrafiltration and is commonly used to maintain normal volume status when there is insufficient ultrafiltration with dextrose-based solutions. In addition, as a result of the continuous ultrafiltration with PD, compared with intermittent HD and perhaps other reasons, BP tends to be well controlled in patients receiving this modality, and episodes of malignant-range hypertension are uncommon. Again, a possible explanation is that in the pursuit of better metabolic values, investigators used lower concentrations of dextrose-based dialysis fluid, when more hypertonic dialysate was indicated to optimize ultrafiltration. If this were the case, the investigators may not have recognized the association between reduced ultrafiltration and these adverse events. It follows, then, that a preference for less hypertonic dialysate could have contributed to the improved metabolic results seen in the intervention group. If this were the case, it points out that fluid balance control should not be compromised in pursuit of better metabolic endpoints.
As with any regimen that strives to improve glycemic control, there may be an increased risk of hypoglycemic events. Four serious hypoglycemic events (in three patients) developed in the treatment group, compared with none in the control group.
The IMPENDIA and EDEN trials were multicenter, randomized, and controlled, and therefore free from the biases and limitations inherent in observational studies and small, single-centered randomized trials. However, the statistical blending together of two different treatment regimens into a single analysis presents challenges. Given that the two solutions have the same glucose concentration, it was decided before the inception of the EDEN trial that the results of the two trials could be combined. In this way, the patient numbers afforded sufficient statistical power to detect a clinically meaningful difference in the primary outcome measure of HbA1c. HbA1c is an unvalidated surrogate outcome measure, such that the improvement in HbA1c demonstrated in this study may not necessarily translate into improved clinical outcomes. Another limitation of the trial was the lack of collection of data on peritoneal ultrafiltration and peritoneal glucose exposure, which made it difficult to assess the effect of glucose-sparing regimens on overall fluid management in PD patients. Likewise, methods to accurately measure residual kidney function or volume overload were not used, thus limiting the assessment of adverse events associated with hypertension or heart failure. The trial was open label which also potentially introduced observer and performance biases. The assessment by investigators of related adverse events was subjective and open to potential bias. There was also a significant difference in withdrawal of patients from the intervention and control groups before study completion (19% versus 6%), raising the possibility of informative censoring bias.
In conclusion, in diabetic PD patients, a glucose-sparing prescription improves metabolic control, as evidenced by reductions in HbA1c, serum VLDL cholesterol, serum triglycerides, and apolipoprotein B. This benefit was counterbalanced by a significant reduction in serum albumin and increases in related adverse events. The numbers of serious adverse events and deaths were also higher in the group given glucose-sparing solutions. Use of glucose-sparing regimens in PD patients should be accompanied by close monitoring of fluid volume status.
Concise Methods
Trial Design
The low glucose clinical study program consisted of the IMPENDIA (Improved Metabolic control of Physioneal, Extraneal, and Nutrineal [P-E-N] versus Dianeal-only treatment in DIAbetic peritoneal dialysis patients) and EDEN (Evaluation of Dianeal, Extraneal, and Nutrineal [D-E-N] in diabetic PD patients) clinical trials. IMPENDIA was a phase III protocol in Canada, Australia, and New Zealand (Clinicaltrials.gov registration NCT00567398) and a phase IV protocol in Europe and Asia (NCT00567489). The EDEN trial was a phase III protocol performed in Colombia (NCT01219959). The EDEN trial was added as a result of insufficient enrollment into the IMPENDIA study. However, because of the unavailability of the Physioneal solution in Colombia, Dianeal was used instead. The phase III nature of the trials in Canada, Australia, New Zealand, and Colombia was due to country-specific regulatory requirements for the use of Nutrineal. Both IMPENDIA and EDEN were randomized, controlled, open-label, parallel-group, multicenter trials that compared the effects of a P-E-N or D-E-N PD regimen to a Dianeal-only regimen in diabetic CAPD and APD patients during a 6-month study period.
Patients were randomly assigned to the intervention group (P-E-N or D-E-N) or the control group (Dianeal only) using a centralized randomization scheme implemented locally using a web-based automated randomization system. Patients were assigned the next available patient number at the time of randomization. A 1:1 stratified randomization scheme was carried out in which randomization was stratified by informed consent status (i.e., informed consent to participate in the MRI subgroup evaluation, yes or no). Blocks of size 4 were used, so that in each block two patients were randomly assigned to the intervention group and two to the control group.
The primary efficacy endpoint of the glucose-sparing clinical trial program was change in HbA1c from baseline to 6 months. Secondary efficacy endpoints included the following:
Change from baseline value in metabolic control measures, including total cholesterol, LDL cholesterol, HDL cholesterol, VLDL, serum triglycerides, lipoprotein(a), apolipoprotein A1, apolipoprotein B, pro-insulin, insulin and C-peptide.
Change in glycemic control medication use, as defined by change in medication dose and use.
Change in the number of severe hypoglycemic events requiring medical intervention.
Change from baseline value in nutritional status, as measured by the Subjective Global Assessment test, total protein, serum albumin, body mass index, and drained body weight.
Quality of life as measured by the Diabetes Symptom Checklist and the European Quality of Life 5 Dimensions (EuroQol-5D) score.
Change in composition and distribution of abdominal fat and in left ventricular structure and function, as measured by abdominal and cardiac MRI, respectively. This secondary endpoint was assessed only in a prespecified subgroup of IMPENDIA study participants.
Primary and secondary outcome measures were assessed at screening and baseline visits, a 3-month visit (for most patients), and an end-of-study visit 6 months after the start of study solutions. HbA1c and other biochemical secondary endpoint measures were collected with patients fasting (nil per os and no dwelling PD solution) for 10 hours and were measured at a central laboratory (Baxter’s Clinical Laboratory Services, Round Lake, IL) using validated techniques. For the HbA1c measurement, a Tina-quant immunologic assay (Roche Diagnostic) suitable for samples from dialysis patients, including patients with icodextrin metabolites, was used.
The sample size for the glucose-sparing clinical study program was based on the goal to detect a 10% difference in the mean change from the baseline value of HbA1c between patients randomly assigned to the intervention and control groups. To calculate the sample size required to detect this 10% difference, the following assumptions were made: (1) the mean HbA1c would be between 7.1% and 7.9%, with an SD of 2.0, and a correlation between the baseline value and 6-month HbA1c of 0.50 (unpublished data from Baxter Novum, Sweden); (2) the control group would have a mean HbA1c at baseline of 7.5%, with no change anticipated during the 6 months of follow-up; (3) the intervention group would have a mean HbA1c of 7.5% at the baseline measurement and a 10% reduction (i.e., an average absolute decline of 0.75 in HbA1c) in the average HbA1c during the 6 months of follow-up. On the basis of a two-group repeated-measures ANOVA F-test carried out at the 5% level of significance, a sample of 100 evaluable patients per group would provide 90% power to detect a 10%, in aggregate, average difference in the mean change from the baseline value for HbA1c. It was anticipated that an annual dropout rate of approximately 30% would occur during the study. Therefore, to achieve a target of 100 evaluable patients per group, randomization of 118 patients per group would be necessary, for a desired total sample of 236 patients.
The eligible study population included patients with incident and prevalent type 1 and type 2 diabetes, aged 18 years or older, who had been receiving CAPD or APD for at least 30 days. Patients using only Dianeal and/or Physioneal solutions were included. To prevent volume depletion upon randomization to the intervention group that included icodextrin, eligible patients were also required to have at least one exchange per day of 2.5% or 4.25% dextrose (2.27% or 3.86% glucose) during screening with no prescribed dry (dialysate-free) time. Eligibility criteria also included an HbA1c level >6.0% but ≤12.0%, a blood hemoglobin concentration of ≥8.0 g/dl but ≤13.0 g/dl, and a total Kt/V urea ≥1.7. Patients entering into the study were expected to continue PD for at least 6 months, the duration of the study period. A full list of exclusion criteria, as well as other details on the study methods, is available in the Supplemental Material.
Patients randomly assigned to the intervention received a 24-hour combination of Physioneal (one to three exchanges daily for CAPD patients, up to 16L for APD patients), Nutrineal (1 exchange daily), and Extraneal (the long dwell exchange once daily). In the EDEN trial, patients assigned to the intervention group received Dianeal instead of Physioneal. Both Dianeal and Physioneal have similar glucose concentrations, thereby assuring that the glucose-sparing hypotheses was tested in a similar manner for IMPENDIA and EDEN patients. Patients randomly assigned to the control group continued on Dianeal for all exchanges in a 24-hour period, with three to five daily exchanges permitted for CAPD patients and up to 20 L daily for APD patients. Dry periods were not allowed in the 24-hour prescriptions of either group. PD prescriptions in both treatment groups were tailored to reach a minimum target total Kt/V urea of 1.7 throughout the study as per local standards of care.
The IMPENDIA trial commenced enrollment of eligible CAPD patients in February 2008. To address lower than anticipated recruitment, the study protocol was amended in November 2008 (see Supplemental Material for details) and the EDEN trial initiated with commencement of patient enrollment in October 2010. Enrollment was complete in January 2011 for both the IMPENDIA and EDEN trials, and the last patient finished all study-related procedures in July 2011. The IMPENDIA trial was conducted at 37 sites in Russia (7), Hong Kong (4), Korea (6), Australia (8), New Zealand (2), Canada (4), Taiwan (2), Singapore (2), France (1), and Portugal (1). The EDEN trial was conducted at 16 sites in Colombia.
All patients were required to provide informed consent after the nature of the study had been explained but before the initiation of any trial-related activities. Ethics approval for the IMPENDIA and EDEN trials was obtained from the local research ethics boards in all participating centers prior to study initiation and patient enrollment. The studies were conducted in accordance with the Declaration of Helsinki and applicable International Conference on Harmonization guidelines, and all study coordinators and investigators followed international good clinical practice guidelines.
Statistical Analyses
Before completion of either clinical trial or database lock, the statistical plan was developed to combine both clinical trials in order to achieve the desired sample size for the primary endpoint.
Two populations were identified for the primary endpoint efficacy analysis: (1) the ITT population included all patients who were randomly assigned and for whom, at a minimum, the baseline value of HbA1c measured at screening was determined and one PD exchange using a study solution was performed, and (2) the per-protocol patient population, which included all ITT patients who completed the study and had, at a minimum, their 6-month HbA1c value measured. A safety ITT analysis was also performed that included all patients who were randomly assigned regardless of subsequent measurement of HbA1c or exposure to study solutions.
The primary efficacy analysis was carried out for both the ITT and per-protocol patient populations. A repeated-measures ANOVA compared the HbA1c mean change from the baseline profile between the two groups using a generalized estimating equations approach. No confounding baseline variables were found, so no additional covariates were added to the model. Mean change from baseline value at mid-study and 6 months was summarized for each treatment group, and comparisons between the two groups were based on the repeated-measures ANOVA model. To safeguard against model misspecification with respect to assumptions about the SD and common correlation (compound symmetry) over time, all analyses (e.g., CIs, tests of hypotheses) were done using robust SEM estimates. A P value <0.05 for the treatment effect or the treatment-by-time interaction was evidence that the treatment groups were different.
For the secondary efficacy endpoints, a repeated-measures ANOVA was also carried out that is consistent with the generalized linear model and link function appropriate to the particular endpoint analyzed. In each case, the repeated-measures ANOVA incorporated time (corresponding to those visits when the endpoint of interest was measured), treatment group (Dianeal only versus P-E-N/D-E-N), and their interaction (time-by-treatment group) as the primary independent class variables.
Disclosures
A.A. is a contractor for RTS Ltd Colombia, an affiliate of Baxter Healthcare. J.M.B. has received consultancy fees and speaker’s honoraria from Amgen, Baxter Healthcare Corporation, DaVita Healthcare, Otsuka Pharmaceuticals and Roche. B.C. is an employee of Baxter Healthcare. J.-Y.D. has received speaker’s honoraria and research funding from Baxter Healthcare and Fresenius Medical Care. D.J. has received consultancy fees, speaker’s honoraria, travel sponsorships and research funding from Baxter Healthcare Pty Ltd. He was previously a member of several Baxter medical advisory boards. He has also received consultancy fees, speaker’s honoraria, travel sponsorships, and research funding from Fresenius Medical Care and Roche, as well as consultancy fees and speaker’s honoraria from Boehringer-Ingelheim, Shire, Genzyme, Amgen, Janssen-Cilag, Roche, Sanofi-Aventis, and Sigma. He is a current recipient of a Queensland Government Health Research Fellowship. P.K.T.L. received speaker honoraria from Astellas, Baxter Healthcare, Fresenius Medical Care, and Roche and is a member of Baxter Trial Advisory Board. M.S. is an employee of Baxter Healthcare. T.R.S. is an employee of Baxter Healthcare. K.S. is an employee of Baxter Healthcare. M.V. has previously received speaker’s honoraria, travel sponsorships, and research funding from Baxter Healthcare Corporation. A.Y. was an employee of Baxter Healthcare but during the execution of all study activities he was affiliated with the Alice Ho Miu Ling Nethersole Hospital, Hong Kong.
Supplementary Material
Acknowledgments
IMPENDIA and EDEN were funded by Baxter Healthcare Corporation. Baxter was involved in the design, implementation, and conduct of the study; provided logistic support and study products during the trial; and performed the statistical analyses. The manuscript was prepared by Drs. Li, Bargman, Culleton and other authors. Editorial support by Catherine Lee of Gardiner-Caldwell Communications was funded by Baxter Healthcare. Cathy Hoff (Baxter Healthcare) contributed to the outline of the manuscript. Baxter was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the authors. P.K.T.L. and J.M.B. had full access to all data and take responsibility for its integrity and the accuracy of all analyses.
Results from this study were presented in abstract form at the 2012 ERA-EDTA Meeting (Li PKT, Ariza A, Culleton B, Do J-Y, Johnson D, Shockley T, Vatazin A, Verrelli M, Yu A, Bargman J. The impact of glucose-sparing peritoneal dialysis solutions on metabolic control in diabetic patients in a randomized, controlled, clinical trial program: The IMPENDIA and EDEN trials, 2012, late breaking clinical trials platform presentation) and the 2012 ASN Annual Meeting (Bargman JM, Culleton BF, Sniderman A, Do J-Y, Gomez RA, Yu AW, Prichard SS, Story K, Li PKT. The impact of a low glucose peritoneal dialysis solution regimen on serum lipids and lipoproteins in diabetic patients: The IMPENDIA and EDEN randomized controlled clinical trials).
Members of the IMPENDIA/EDEN Study Group are as follows: Australia: David Johnson, Princess Alexandra Hospital, Wolloongabba, QLD; Fiona Brown, Monash Medical Centre, Clayton, Victoria; Dwarakanathan Ranganathan, Royal Brisbane Hospital, Herston QLD; Gopi Rangan, Blacktown Hospital & Westmead Hospital, Sydney, NSW; Rajiv Juneja, Flinders Medical Centre, Adelaide, South Australia; Maureen Lonergan, Wollongong Hospital, Wollongong, NSW; Michael Suranyi, Liverpool Hospital, Sydney, NSW; Paul Snelling, Royal Prince Alfred Hospital, Camperdown, NSW; Canada: Joanne Bargman, University Health Network–Toronto General Hospital, Toronto, Ontario; Murray Vasilevsky, Montreal General Hospital, Montreal, Quebec; Mauro Verrelli, Saint Boniface General Hospital, Winnipeg, Manitoba; Marc Dorval, Beausejour Hospital Corporation–Dr George Dumont Hospital Site, Moncton, New Brunswick; Colombia: All investigators are employees of RTS Ltd Colombia, an affiliate of Baxter Healthcare, except where stated. Amaury Ariza (contractor), Cartagena; David Camargo, Bogota; Rafael Alberto Gomez A., Cali; Mauricio Guerrero, Barranquila; Yolanda Guevara, Giradot; Richard Jacome, Sogamoso; Mauricio Lopera, Medellin; Pedro Lopera, Cali; Gustavo Marin, Medellin; Ivan Nieto (contractor), Villavicencio; Alvaro Ordonez, Bucaramanga; Maria Pas Dazzarola, Cali; Edgar Vargas (contractor), Ibague; David Varon, Cucuta; Edward Martinez, Armenia; Jairo González, Valle. France: Françoise Heibel, CHU Nouvel Hôpital Civil Service de Dialyze Peritoneale, Strasbourg; Hong Kong: Alex Yu, Alice Ho Miu Ling Nethersole Hospital, Tai Po, NT; Philip Li, Kai Ming Chow, Prince of Wales Hospital, Sha Tin, NT; Siu Ka Mak, Kwong Wah Hospital, Kowloon; Tze-Hoi Kwan, Tuen Mun Hospital, Tuen Mun; Korea: Sung Ho Kim, Daegu Fatima Hospital, Daegu; Shin-Wook Kang, Severance Hospital, Yonsei University College of Medicine, Seoul; Yong-Lim Kim, Kyungpook National University Hospital, Daegu; Kook Hwan Oh, Seoul National University Hospital; Seoul; Jun-Young Do, Yeungnam University Hospital, Daegu; Joong Kyung Kim, Bongseng Memorial Hospital, Busan; New Zealand: Maha Yehia, Auckland City Hospital, Grafton, Auckland; Peter Sizeland, Waikato Hospital, Hamilton, Waikato; Portugal: Manuel Amoedo, Hosp.do Espirito Santo, Nephrology Department, Evora; Russia: Evgeny V. Shutov, Moscow State Healthcare Institution Municipal Clinical Hospital n.a. S.P. Botkin; Anton M. Andrusev, Moscow State Healthcare Institution Municipal Clinical #52; Andrey V. Vatazin, State Institution Moscow Regional Clinical Research Institute n.a. M.F. Vladimirsky; Alexander Yu. Zemchenkov, St. Petersburg State Healthcare Institution Municipal Mariinskaya Hospital; Albert S. Navasardian, State Healthcare Institution Samara Regional Clinical Hospital n.a. M.I. Kalinin; Anastasia B. Sabodash, St. Petersburg State Medical Institution: “St. Elizabeth Municipal Hospital”; Igor V. Nesterenko, Moscow State Medical Institution: “Municipal Clinical Hospital #7 ” under Moscow Committee for Healthcare; Singapore: Evan Lee, National University Hospital, Singapore; Adrian Liew Seng Teck, Tan Tock Seng Hospital, Singapore; Taiwan: Chiu-Ching Huang, China Medical University Hospital, Taichung; Kwan-Dun Wu, National Taiwan University Hospital, Taipei.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Should Glucose-Sparing Prescriptions Be Expected to Reduce the Cardiovascular Risk of Peritoneal Dialysis Patients?,” on pages 1713–1716.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012100987/-/DCSupplemental.
References
- 1.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Li PK, Szeto CC: Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant 23: 1475–1478, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Boateng EA, East L: The impact of dialysis modality on quality of life: A systematic review. J Ren Care 37: 190–200, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Zhang AH, Cheng LT, Zhu N, Sun LH, Wang T: Comparison of quality of life and causes of hospitalization between hemodialysis and peritoneal dialysis patients in China. Health Qual Life Outcomes 5: 49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL: Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis 58: 64–72, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ: Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrotra R: Comparing outcomes of hemodialysis and peritoneal dialysis patients: Consider the pitfalls. Contrib Nephrol 178: 30–34, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Krediet RT, Balafa O: Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol 6: 451–460, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Li PK, Chow KM: The clinical and epidemiological aspects of vascular mortality in chronic peritoneal dialysis patients. Perit Dial Int 25[Suppl 3]: S80–S83, 2005 [PubMed] [Google Scholar]
- 10.Holmes CJ: Reducing cardiometabolic risk in peritoneal dialysis patients: Role of the dialysis solution. J Diabetes Sci Tech 3: 1472–1480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkart J: Metabolic consequences of peritoneal dialysis. Semin Dial 17: 498–504, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, Prins JB, Isbel NM: Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 12: 391–398, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Li PK, Kwan BC, Ko GT, Chow KM, Leung CB, Szeto CC: Treatment of metabolic syndrome in peritoneal dialysis patients. Perit Dial Int 29[Suppl 2]: S149–S152, 2009 [PubMed] [Google Scholar]
- 14.Duong U, Mehrotra R, Molnar MZ, Noori N, Kovesdy CP, Nissenson AR, Kalantar-Zadeh K: Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin J Am Soc Nephrol 6: 1041–1048, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dheir H, Ozkahya M, Kircelli F, Sezis Demirci M, Asci G, Toz H, Ertilav M, Kose T, Ok E: Glycosylated hemoglobin levels are associated with cardiovascular events in nondiabetic peritoneal dialysis patients. J Nephrol 25: 107–112, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Paniagua R, Ventura MD, Avila-Díaz M, Cisneros A, Vicenté-Martínez M, Furlong MD, García-González Z, Villanueva D, Orihuela O, Prado-Uribe MD, Alcántara G, Amato D: Icodextrin improves metabolic and fluid management in high and high-average transport diabetic patients. Perit Dial Int 29: 422–432, 2009 [PubMed] [Google Scholar]
- 17.Martikainen T, Teppo AM, Gronhagen-Riska C, Ekstrand A: Benefit of glucose-free dialysis solutions on glucose and lipid metabolism in peritoneal dialysis patients. Blood Purif 23: 303–310, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Marshall J, Jennings P, Scott A, Fluck RJ, McIntyre CW: Glycemic control in diabetic CAPD patients assessed by continuous glucose monitoring system (CGMS). Kidney Int 64: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Johnson DW, Arndt M, O’Shea A, Watt R, Hamilton J, Vincent K: Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrol 2: 2, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amici G, Orrasch M, Da Rin G, Bocci C: Hyperinsulinism reduction associated with icodextrin treatment in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 17: 80–83, 2001 [PubMed] [Google Scholar]
- 21.Furuya R, Odamaki M, Kumagai H, Hishida A: Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant 21: 494–498, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gürsu EM, Ozdemir A, Yalinbas B, Gürsu RU, Canbakan M, Güven B, Atasoyu EM, Keskin AT, Elçi A, Baru Y: The effect of icodextrin and glucose-containing solutions on insulin resistance in CAPD patients. Clin Nephrol 66: 263–268, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Canbakan M, Sahin GM: Icodextrine and insulin resistance in continuous ambulatory peritoneal dialysis patients. Ren Fail 29: 289–293, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H, Nakao K, Wada J, Makino H: Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: A randomized controlled trial. Clin J Am Soc Nephrol 6: 1337–1344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanas R, John G, International HbA Consensus Committee : 2010 consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Diabet Med 27: 737–738, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y, Osaka CKD Expert Research Group : Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol 18: 896–903, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, Calles-Escandon J, Russell GB, Freedman BI: Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int 73: 1062–1068, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Shurraw S, Majumdar SR, Thadhani R, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Glycemic control and the risk of death in 1,484 patients receiving maintenance hemodialysis. Am J Kidney Dis 55: 875–884, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Wu MS, Yu CC, Yang CW, Wu CH, Haung JY, Hong JJ, Fan Chiang CY, Huang CC, Leu ML: Poor pre-dialysis glycaemic control is a predictor of mortality in type II diabetic patients on maintenance haemodialysis. Nephrol Dial Transplant 12: 2105–2110, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kopple JD, Regidor DL, Jing J, Shinaberger CS, Aronovitz J, McAllister CJ, Whellan D, Sharma K: A1C and survival in maintenance hemodialysis patients. Diabetes Care 30: 1049–1055, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Williams ME, Lacson E, Jr, Wang W, Lazarus JM, Hakim R: Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: Comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol 5: 1595–1601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drechsler C, Krane V, Ritz E, März W, Wanner C: Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 120: 2421–2428, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Wu MS, Yu CC, Wu CH, Haung JY, Leu ML, Huang CC: Pre-dialysis glycemic control is an independent predictor of mortality in type II diabetic patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 19[Suppl 2]: S179–S183, 1999 [PubMed] [Google Scholar]
- 34.Sekercioglu N, Dimitriadis C, Pipili C, Elias RM, Kim J, Oreopoulos DG, Bargman JM: Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Int Urol Nephrol 44: 1861–1869, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Quaschning T, Krane V, Metzger T, Wanner C: Abnormalities in uremic lipoprotein metabolism and its impact on cardiovascular disease. Am J Kidney Dis 38[Suppl 1]: S14–S19, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Sniderman A, Cianflone K, Kwiterovich PO, Jr, Hutchinson T, Barre P, Prichard S: Hyperapobetalipoproteinemia: The major dyslipoproteinemia in patients with chronic renal failure treated with chronic ambulatory peritoneal dialysis. Atherosclerosis 65: 257–264, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J, Walldius G, Hellenius ML, Hamsten A: Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: Findings from a multi-ethnic US population. Eur Heart J 30: 710–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaziri ND, Norris K: Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif 31: 189–196, 2011 [DOI] [PubMed] [Google Scholar]
- 39.März W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, Winkelmann BR: Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: The Ludwigshafen Risk and Cardiovascular Health study. Circulation 110: 3068–3074, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Rivera JJ, Nasir K, Campbell C, Carvalho JA, Blumenthal RS, Santos RD: Relation of plasma lipoprotein levels with low-grade inflammation in white men without clinical evidence of myocardial ischemia. Am J Cardiol 100: 450–454, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, Bosselmann HP, Heimbürger O, Simonsen O, Davenport A, Tranaeus A, Divino Filho JC: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Wolfson M, Piraino B, Hamburger RJ, Morton AR, Icodextrin Study Group : A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis 40: 1055–1065, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J, Euro Balance Trial Group : The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 66: 408–418, 2004 [DOI] [PubMed] [Google Scholar]
- 44.le Poole CY, Welten AG, ter Wee PM, Paauw NJ, Djorai AN, Valentijn RM, Beelen RH, van den Born J, van Ittersum FJ: A peritoneal dialysis regimen low in glucose and glucose degradation products results in increased cancer antigen 125 and peritoneal activation. Perit Dial Int 32: 305–315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.