Abstract
Whether novel biomarkers improve the assessment of incident kidney disease and related adverse outcomes remains to be tested in longitudinal observational studies. We tested 14 urinary biomarkers for association with incident kidney, cardiovascular, and mortality outcomes in 2948 Framingham Heart Study participants. Baseline examinations were performed between 1995 and 1998; mean follow-up was 10.1 years for renal outcomes and 11.2 years for survival analyses. Primary outcomes were incident CKD, incident albuminuria, incident cardiovascular disease, and all-cause mortality. Secondary analyses assessed incident congestive heart failure (CHF) and mortality with coexistent kidney disease. Biomarkers were tested for association with renal end points using logistic regression and incident cardiovascular and mortality outcomes in proportional hazards models; α1-microglobulin, Kim-1, and TFF-3 predicted all-cause mortality (hazard ratio per SD increase in log-transformed biomarker [HR] range, 1.15 to 1.21; 95% confidence interval [CI] range, 1.04 to 1.34; P values=0.007 to <0.001), whereas α1-microglobulin, β2-microglobulin, KIM-1, and TFF-3 associated with death with coexistent kidney disease (HR range, 1.72–2.25; 95% CI, 1.17 to 3.24; P values<0.01). KIM-1 also associated with the risk of incident CHF (HR, 1.32; 95% CI, 1.07 to 1.63; P=0.008). CTGF associated nominally with CKD (HR, 0.83; 95% CI, 0.71 to 0.98; P=0.03), but no other biomarkers associated with incident CKD or albuminuria. Addition of α1-microglobulin and TFF-3 resulted in a nonsignificant net reclassification index (NRI) of 3% for all-cause mortality beyond clinical risk factors. In conclusion, components of a panel of 14 subclinical biomarkers of kidney injury were associated with important clinical outcomes and merit additional investigation.
During the past 50 years, cardiac injury biomarkers, such as creatine-kinase MB isoenzyme and troponin, have transformed the assessment and management of patients with heart disease.1 During this time, clinically validated biomarkers available for the assessment of kidney disease have remained unchanged. The current benchmarks, serum creatinine and albuminuria, lack sensitivity in early disease and provide limited insight into the nature of the underlying renal injury.2,3
There is a need for novel biomarkers that identify at-risk individuals before the development of overt kidney disease. Kidney injury biomarkers can provide mechanistic insights by precisely localizing the affected kidney compartment and permit monitoring and assessment of distinct renal pathophysiological pathways, which may have prognostic implications for patients. Furthermore, they can provide insight into disease biology and help to better classify at-risk individuals in the absence of overt clinical disease.
Several novel renal biomarkers, detectable as proteins in urine, have been proposed for these purposes in experimental and clinical settings, but they require validation in prospective human cohorts.2,4,5 We used a panel of 14 biomarkers identified in animal studies of AKI as well as cross-sectional human studies.2,4,5 The panel includes all five biomarkers recently proposed by the Food and Drug Administration and the European Medicines Evaluation Agency for use as indicators of drug-induced AKI through the Critical Path Initiative: kidney injury molecule 1 (KIM-1), β2-microglobulin, cystatin C, clusterin, and trefoil factor-3 (TFF-3).6 We hypothesized that these biomarkers would be associated with an increased risk of kidney disease and related adverse outcomes. Our specific aim was to determine if the urinary biomarker panel is associated with incident CKD (estimated GFR<60 ml/min per 1.73 m2), incident albuminuria, cardiovascular disease (CVD), and mortality. To address this question, we performed prospective analyses in unselected participants from the Framingham Heart Study (FHS).
Results
Baseline Characteristics of the Cohort
Baseline characteristics of the cohort and median indexed biomarker levels are presented in Table 1. The mean age was 59 years, 53% were women, and mean follow-up was 10.1 years for renal outcomes and 11.2 years for survival analyses.
Table 1.
Baseline characteristics of the study sample
| Characteristic | Overall |
|---|---|
| N | 2948 |
| Mean age, yr | 59 (10) |
| Women | 53 (1561) |
| Mean BMI, kg/m2 | 28.0 (5.2) |
| Mean systolic BP, mmHg | 128 (19) |
| Hypertension | 40.7 (1199) |
| Diabetes | 9.4 (278) |
| HDL cholesterol, mg/dl | 51 (16) |
| Triglycerides, mg/dla | 117 (82, 171) |
| CKD | 8.9 (261) |
| Mean eGFR, ml/min per 1.73 m2 | 87 (25) |
| Albuminuria | 17.5 (517) |
| Median biomarker levels indexed to urinary creatinine (25th, 75th percentiles) | |
| α1-microglobulin, ng/ml | 5.0 (2.0, 20.0) |
| β2-microglobulin, ng/ml | 0.3 (0.05, 0.8) |
| Calbindin, ng/ml | 0.4 (0.08, 1.0) |
| Clusterin (Apolipoprotein J), ng/ml | 0.1 (0.01, 0.5) |
| CTGF, pg/ml | 5.0 (2.0,10.0) |
| Cystatin-C, ng/ml | 0.08 (0.01, 0.2) |
| GST-α, pg/ml | 2.0 (1.0, 6.0) |
| Kim-1, pg/mL | 4.0 (2.0, 7.0) |
| NGAL, ng/ml | 0.1 (0.04, 0.4) |
| Osteopontin, ng/ml | 9.2 (5.2, 13.5) |
| TFF-3, ng/ml | 4.0 (2.0, 7.0) |
| Tamm–Horsfall Protein (uromodulin), μg/ml | 0.09 (0.04, 0.2) |
| Tissue inhibitor of matrix metalloproteinase-1, pg/ml | 9.0 (3.0, 20.0) |
| Vascular endothelial growth factor, pg/ml | 2.2 (1.01, 3.5) |
Data are shown as mean (SD) for continuous variables and percent (n) for categorical variables. Albuminuria was defined as a spot UACR of ≥17 mg/g in men and ≥25 mg/g in women.
Median (25th, 75th percentiles).
Correlation Coefficients between Biomarkers
Pearson correlations of log-transformed urinary biomarkers are presented in Supplemental Table 1. In general, biomarkers showed moderate to high correlations with each other, with the majority of r values between 0.25 and 0.75.
Correlations of Urinary Biomarkers with CKD Risk Factors
Pearson correlations between indexed urinary biomarkers and CKD risk factors are presented in Table 2. Moderate to weak positive correlations were observed between the urinary biomarkers and urinary albumin-to-creatinine ratio. In general, correlations with other CKD covariates were weak.
Table 2.
Correlations between biomarkers indexed to urinary creatinine and clinical traits
| Biomarker | Age | BMI | SBP | Glucose | HDL Cholesterol | Triglycerides | eGFR | Cystatin C | UACRa |
|---|---|---|---|---|---|---|---|---|---|
| α1-microglobulin | 0.14b | −0.04c | 0.12b | 0.10b | −0.05d | 0.06b | −0.09b | 0.21b | 0.22b |
| β2-microglobulin | 0.06b | −0.002 | 0.05c | 0.04c | −0.04c | 0.03 | −0.09b | 0.21b | 0.16b |
| Calbindin | 0.06b | 0.007 | 0.08b | 0.01 | −0.008 | −0.005 | −0.008 | −0.01 | 0.12b |
| Clusterin | 0.03 | −0.05c | 0.002 | −0.06b | 0.07b | −0.04c | −0.001 | −0.04c | 0.10b |
| CTGF | 0.07b | −0.05d | 0.02 | −0.03 | 0.06d | −0.04 | 0.04 | 0.03 | 0.13b |
| Cystatin-C | 0.04c | 0.004 | 0.04c | 0.01 | −0.02 | 0.03 | −0.09b | 0.16b | 0.13b |
| GST-α | 0.03 | −0.01 | 0.02 | −0.01 | 0.02 | −0.03 | −0.02 | 0.01 | 0.07b |
| Kim-1 | 0.19b | 0.02 | 0.13b | 0.08b | 0.003 | 0.05d | −0.03 | 0.17b | 0.19b |
| NGAL | 0.06b | −0.02 | 0.02 | 0.01 | 0.04c | 0.03 | −0.06d | 0.04 | 0.15b |
| Osteopontin | 0.01 | 0.06d | 0.006 | 0.02 | −0.05d | 0.001 | 0.04c | −0.03 | −0.17 |
| TFF-3 | 0.02 | −0.08b | 0.02 | 0.008 | 0.10b | 0.03 | −0.06d | 0.07b | 0.001 |
| Tamm–Horsfall Protein | 0.02 | −0.12b | −0.02 | −0.13b | 0.16b | −0.08b | 0.04 | −0.09b | 0.02 |
| TIMP-1 | 0.04c | −0.001 | 0.01 | 0.02 | −0.03 | −0.02 | −0.03 | 0.02 | 0.06b |
| VEGF | 0.07b | −0.04c | 0.03 | −0.004 | −0.04c | −0.02 | −0.05c | 0.05d | 0.10b |
SBP, systolic BP; TIMP-1, tissue inhibitor of matrix metalloproteinase-1; VEGF, vascular endothelial growth factor.
To avoid spurious correlation caused by creatinine being the denominator in both cases, the correlation with biomarker unindexed to urinary creatinine is presented for UACR.
P<0.001.
P<0.05.
P<0.01.
Primary End Points
Associations of Urinary Biomarkers with Incident CKD, Albuminuria, and CVD
Of 2141 participants free of baseline CKD, 194 (9.1%) participants developed CKD at 10 years. Each 1 SD increase in log-connective tissue growth factor (CTGF) was associated with a decreased risk of CKD (odds ratio, 0.83; 95% confidence interval, 0.71 to 0.98; P=0.03) (Table 3), consistent with a prior report in a subset of these participants.7 Results were similar for CTGF after additional adjustment for serum cystatin C concentration at exam 7 (CTGF odds ratio, 0.80; P=0.01). No other urinary biomarker was associated with risk of incident CKD. Similarly, no biomarker was significantly associated with continuous rapid decline in eGFR, which was defined as the loss of >3 ml/min per 1.73 m2 eGFR per year (Supplemental Table 2).
Table 3.
Multivariable-adjusted Cox proportional hazard and logistic regression of log-transformed urinary biomarker levels indexed to urinary creatinine and the primary outcomes of incident CKD, incident albuminuria, incident CVD, and all-cause mortality
| Biomarker | Incident CKD | Incident Albuminuria | Incident CVD | All-Cause Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| OR [95% CI] | P Value | OR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | |
| N cases/total n | 194/2141 | 175/1812 | 225/2795 | 404/2948 | ||||
| α1-microglobulin | 1.03 [0.88 to 1.21] | 0.7 | 1.09 [0.92 to 1.28] | 0.3 | 1.08 [0.94 to 1.23] | 0.3 | 1.26 [1.13 to 1.40] | <0.001 |
| β2-microglobulin | 0.97 [0.83 to 1.13] | 0.7 | 0.95 [0.81 to 1.11] | 0.5 | 1.10 [0.97 to 1.26] | 0.1 | 1.15 [1.05 to 1.27] | 0.004 |
| Calbindin | 0.90 [0.77 to 1.05] | 0.2 | 0.98 [0.84 to 1.15] | 0.8 | 1.04 [0.91 to 1.19] | 0.6 | 1.14 [1.02 to 1.26] | 0.02 |
| Clusterin | 0.94 [0.80 to 1.11] | 0.5 | 0.95 [0.81 to 1.12] | 0.5 | 1.00 [0.87 to 1.14] | 0.9 | 1.05 [0.95 to 1.16] | 0.4 |
| CTGF | 0.83 [0.71 to 0.98] | 0.03 | 1.06 [0.90 to 1.24] | 0.5 | 1.08 [0.95 to 1.24] | 0.2 | 1.09 [0.98 to 1.20] | 0.1 |
| Cystatin-C | 0.85 [0.73 to 1.00] | 0.05 | 0.93 [0.79 to 1.10] | 0.4 | 1.12 [0.98 to 1.28] | 0.09 | 1.06 [0.96 to 1.17] | 0.2 |
| GST-α | 0.96 [0.81 to 1.12] | 0.6 | 1.10 [0.94 to 1.28] | 0.2 | 1.07 [0.94 to 1.21] | 0.3 | 1.10 [1.00 to 1.20] | 0.04 |
| Kim-1 | 1.16 [0.97 to 1.38] | 0.1 | 1.19 [0.99 to 1.41] | 0.06 | 1.09 [0.94 to 1.26] | 0.2 | 1.17 [1.04 to 1.31] | 0.007 |
| NGAL | 1.00 [0.85 to 1.17] | 1.0 | 1.11 [0.95 to 1.31] | 0.2 | 1.04 [0.91 to 1.18] | 0.6 | 1.06 [0.97 to 1.17] | 0.2 |
| Osteopontin | 0.92 [0.79 to 1.06] | 0.3 | 0.96 [0.82 to 1.11] | 0.6 | 1.00 [0.88 to 1.14] | 1.0 | 1.00 [0.91 to 1.10] | 1.0 |
| TFF-3 | 1.00 [0.85 to 1.16] | 0.9 | 0.95 [0.80 to 1.11] | 0.5 | 1.04 [0.92 to 1.19] | 0.5 | 1.21 [1.10 to 1.34] | 0.001 |
| Tamm–Horsfall Protein | 1.00 [0.85 to 1.20] | 0.9 | 1.09 [0.91 to 1.29] | 0.4 | 1.02 [0.89 to 1.18] | 0.8 | 1.06 [0.95 to 1.19] | 0.3 |
| TIMP-1 | 0.90 [0.76 to 1.06] | 0.2 | 0.97 [0.82 to 1.14] | 0.7 | 1.09 [0.96 to 1.25] | 0.2 | 1.11 [1.01 to 1.23] | 0.03 |
| VEGF | 0.99 [0.84 to 1.18] | 0.9 | 1.02 [0.86 to 1.20] | 0.8 | 1.03 [0.89 to 1.18] | 0.7 | 1.07 [0.96 to 1.19] | 0.2 |
Risks associated with a 1 SD increment in the natural logarithm of the biomarker. CKD is defined as eGFR<60 ml/min per 1.73 m2. Incident CKD analysis adjusted for age, sex, baseline GFR, hypertension, diabetes, and dipstick proteinuria. Albuminuria defined as UACR≥17 mg/g in men and ≥25 mg/g in women. Incident albuminuria analysis adjusted for age, sex, hypertension and diabetes, and dipstick proteinuria. Incident CVD was defined as one or more of fatal or nonfatal myocardial infarction, stroke, or transient ischemic attacks. Incident CVD analyses were adjusted for age, sex, BMI, smoking, hypertension, diabetes, total to HDL cholesterol ratio, eGFR, and UACR after exclusion of prevalent CVD cases. Incident CVD and all-cause mortality results are presented per SD increment of the biomarker using Cox proportional hazards models. All-cause mortality analyses were adjusted for age, sex, BMI, smoking, hypertension, diabetes, total to HDL cholesterol ratio, eGFR, UACR, and baseline CVD. OR, odds ratio; 95% CI, 95% confidence interval; TIMP-1, tissue inhibitor of matrix metalloproteinase-1; VEGF, vascular endothelial growth factor.
Among 1812 participants with data available, 175 (9.7%) participants developed albuminuria during follow-up. No biomarker was significantly associated with incident albuminuria in the overall analysis (Table 3). However, when stratified by sex, KIM-1 was associated with an increased risk of albuminuria in women (hazard ratio [HR], 1.65; 95% confidence interval, 1.21 to 2.25; P=0.001). Results were otherwise unchanged from the primary analysis (data not shown).
Of 2795 participants free of CVD at baseline, 225 individuals developed CVD. No biomarker was significantly associated with risk of incident CVD (Table 3).
Associations of Urinary Biomarkers with All-Cause Mortality
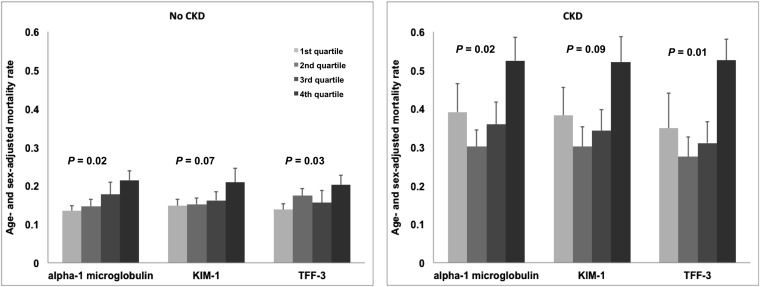
There was a total of 404 deaths among 2948 participants during the follow-up period. Three biomarkers were associated with all-cause mortality: α1-microglobulin, KIM-1, and TFF-3. The increased mortality risk ranged from 1.17 (KIM-1) to 1.26 (α1-microglobulin; P range=0.007 to <0.001) (Table 3). Age- and sex-adjusted mortality rates increased by quartile of urinary biomarker, even among participants without CKD (Figure 1).
Figure 1.
Mortality rates increase by quartile of α1-microglobulin, KIM-1, and TFF-3 in participants with and without CKD. Bars represent age- and sex-adjusted mortality rates, and error bars represent standard errors quartiles of α1-microglobulin, KIM-1, and TFF-3.
In participants free of baseline CKD, mortality HRs for α1-microglobulin and TFF-3 showed a significant upward trend by quartile (α1-microglobulin: Q4 versus Q1 HR, 1.44 [0.64 to 3.24]; Ptrend=0.02; TFF-3: Q4 versus Q1 HR, 1.51 [1.09 to 2.10]; Ptrend=0.03). Results were similar in participants with baseline CKD (α1-microglobulin: Q4 versus Q1 HR, 1.44 [0.64 to 3.24]; Ptrend=0.02; TFF-3: Q4 versus Q1 HR, 1.75 [0.91 to 3.36]; Ptrend=0.03).
Secondary End Points
Exploration of Association between Urinary Biomarkers and Cause of Death
Of 404 total deaths, 86 deaths were attributed to CVD. We observed no associations between biomarkers and CVD mortality (HR, 0.94 to 1.15) (Table 4). Conversely, six of seven significant biomarkers were associated with non-CVD mortality (α1-microglobulin, β2-microglobulin, calbindin, glutathione S-transferase-α (GST-α), KIM-1, and TFF-3; P value range=0.02 to <0.001) (Table 4). Additional adjustment for C-reactive protein (CRP) and brain natriuretic peptide (BNP) did not materially affect these results (P for non-CVD mortality=0.03 to <0.001; data not shown).
Table 4.
Multivariable-adjusted Cox proportional hazard and logistic regression of log-transformed urinary biomarker level, indexed to urinary creatinine and the secondary outcomes of incident CHF, cardiovascular mortality, noncardiovascular mortality, and mortality with kidney disease
| Biomarker | Incident CHF | Cardiovascular Mortality | Noncardiovascular Mortality | Mortality with Kidney Disease | ||||
|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | HR [95% CI] | P Value | |
| N cases/total n | 131/2915 | 86/2948 | 318/2948 | 34/318 | ||||
| α1-microglobulin | 1.17 [0.97 to 1.40] | 0.1 | 1.06 [0.85 to 1.32] | 0.6 | 1.31 [1.17 to 1.49] | <0.001 | 2.20 [1.49 to 3.24] | <0.001 |
| β2-microglobulin | 1.09 [0.92 to 1.29] | 0.3 | 1.03 [0.85 to 1.26] | 0.7 | 1.19 [1.06 to 1.32] | 0.002 | 1.72 [1.24 to 2.39] | 0.001 |
| Calbindin | 1.04 [0.87 to 1.24] | 0.7 | 1.08 [0.86 to 1.36] | 0.5 | 1.15 [1.02 to 1.30] | 0.02 | 1.22 [0.84 to 1.77] | 0.3 |
| Clusterin | 1.13 [0.95 to 1.35] | 0.2 | 1.04 [0.83 to 1.31] | 0.7 | 1.05 [0.93 to 1.18] | 0.4 | 1.13 [0.79 to 1.63] | 0.5 |
| CTGF | 1.03 [0.87 to 1.22] | 0.7 | 1.01 [0.81 to 1.25] | 1.0 | 1.11 [0.99 to 1.24] | 0.07 | 1.21 [0.86 to 1.70] | 0.3 |
| Cystatin-C | 1.06 [0.89 to 1.25] | 0.5 | 1.00 [0.81 to 1.23] | 1.0 | 1.07 [0.96 to 1.20] | 0.2 | 1.15 [0.84 to 1.59] | 0.4 |
| GST-α | 1.02 [0.87 to 1.21] | 0.8 | 0.96 [0.78 to 1.18] | 0.7 | 1.14 [1.03 to 1.26] | 0.01 | 1.22 [0.91 to 1.63] | 0.2 |
| Kim-1 | 1.32 [1.07 to 1.63] | 0.008 | 1.15 [0.90 to 1.47] | 0.3 | 1.17 [1.03 to 1.33] | 0.02 | 1.86 [1.17 to 2.95] | 0.009 |
| NGAL | 1.02 [0.87 to 1.21] | 0.8 | 1.01 [0.84 to 1.23] | 0.9 | 1.07 [0.96 to 1.20] | 0.2 | 1.30 [0.98 to 1.73] | 0.07 |
| Osteopontin | 1.04 [0.86 to 1.24] | 0.7 | 1.03 [0.83 to 1.29] | 0.8 | 0.99 [0.89 to 1.10] | 0.8 | 0.86 [0.66 to 1.11] | 0.2 |
| TFF-3 | 1.02 [0.86 to 1.21] | 0.9 | 0.94 [0.76 to 1.15] | 0.5 | 1.30 [1.17 to 1.46] | <0.001 | 2.25 [1.62 to 3.12] | <0.001 |
| Tamm–Horsfall Protein | 1.09 [0.89 to 1.33] | 0.4 | 1.10 [0.85 to 1.44] | 0.5 | 1.05 [0.92 to 1.19] | 0.5 | 0.93 [0.65 to 1.33] | 0.7 |
| TIMP-1 | 1.13 [0.96 to 1.34] | 0.1 | 1.11 [0.91 to 1.36] | 0.3 | 1.11 [0.99 to 1.24] | 0.07 | 1.28 [0.94 to 1.74] | 0.1 |
| VEGF | 1.08 [0.89 to 1.30] | 0.4 | 1.07 [0.85 to 1.35] | 0.6 | 1.07 [0.95 to 1.21] | 0.3 | 0.98 [0.68 to 1.40] | 0.9 |
Results are presented per SD increment of the biomarker using Cox proportional hazards models. Incident CHF analyses excluded prevalent CHF and were adjusted for age, sex, BMI, smoking, systolic BP, hypertension treatment, diabetes, total to HDL cholesterol ratio, baseline CVD, and history of valvular heart disease. Cardiovascular and noncardiovascular mortality models were adjusted for age, sex, BMI, smoking, hypertension, diabetes, total to HDL cholesterol ratio, eGFR, UACR, and baseline CVD. Mortality with kidney disease was defined as kidney disease contributing to death based on assessment of the death adjudication forms by a trained nephrologist. 95% CI, 95% confidence interval; TIMP-1, tissue inhibitor of matrix metalloproteinase-1; VEGF, vascular endothelial growth factor.
Of 318 deaths from noncardiovascular causes, 34 participants died with coexisting kidney disease. Of six markers associated with non-CVD mortality, four biomarkers were associated with death with coexistent kidney disease: α1-microglobulin (HR, 2.20 [1.49 to 3.24]; P<0.001), β2-microglobulin (HR, 1.72 [1.24 to 2.39]; P=0.001), KIM-1 (HR, 1.86 [1.17 to 2.95]; P=0.009), and TFF-3 (HR, 2.25 [1.62 to 3.12]; P<0.001) (Table 4). These four biomarkers remained significant after additional adjustment for CRP and BNP (P=0.02 to <0.001; data not shown). Finally, after excluding cases of baseline CKD (n=67), only TFF-3 remained significant (HR, 1.99 [1.14 to 3.48]; P=0.02).
Associations of Urinary Biomarkers with Incident Cancer
Of the entire cohort of 2948 participants, 175 participants developed cancer during follow-up. Two biomarkers, α1-microglobulin and TFF-3, were associated with this outcome after adjustment for multiple testing (α1-microglobulin: HR, 1.28 [1.09 to 1.51]; P=0.003; TFF-3: HR, 1.32 [1.14 to 1.54]; P<0.001; data not shown).
Associations of Urinary Biomarkers with Incident Congestive Heart Failure
Of 2915 participants free of congestive heart failure (CHF) at baseline, 131 participants developed CHF during follow-up. Overall, 13 of 14 biomarkers tested were not associated with incident CHF, with the exception of KIM-1, which was associated with a 32% increased hazard of CHF per 1 SD (HR, 1.32 [1.07 to 1.63]; P=0.008) (Table 4). The association with KIM-1 and incident CHF was not attenuated after additional adjustment for plasma BNP (HR, 1.48 [1.18 to 1.86]; P<0.001).
Reclassification Analyses
When three significant biomarkers from the all-cause mortality analysis (α1-microglobulin, KIM-1, and TFF-3) were included in a clinical model for all-cause mortality, α1-microglobulin (P=0.002) and TFF-3 (P=0.03) remained significant. In multivariable-adjusted analyses, a multimarker model comprising α1-microglobulin and TFF-3 resulted in a nonsignificant net reclassification improvement beyond clinical predictors of 3.0% [0.0 to 7.8].
Discussion
Principle Findings
We have systematically examined biomarkers of subclinical kidney injury and related them to clinically meaningful longitudinal end points. Overall, five key findings emerge. First and most strikingly, a panel of markers of kidney injury was most strongly associated with mortality rather than incident kidney disease. Second, despite the well established connection between established CKD and CVD, the majority of this association seemed to be driven by deaths from noncardiovascular causes. Third, there was an excess of coexistent kidney disease at death for four specific biomarkers, namely α1-microglobulin, β2-microglobulin, KIM-1, and TFF-3. Fourth, KIM-1 was associated with a range of related adverse outcomes, including incident albuminuria in women, incident CHF, all-cause mortality, and death with coexistent kidney disease. Fifth, the biomarkers failed to meaningfully improve reclassification of all-cause mortality risk beyond the improvement achievable by clinical risk factors alone.
In the Context of the Current Literature
Several population-based studies have evaluated a multiple biomarker approach for the prediction of renal, cardiovascular, and mortality events. In general, the incremental risk described by these markers is modest, and little improvement is seen in traditional measures of discrimination.8,9 We have previously reported a small improvement in reclassification of risk for incident CKD and albuminuria beyond traditional risk factors by homocysteine, aldosterone, and BNP.10 UACR, BNP, CRP, homocysteine, and renin were also shown to predict all-cause mortality in FHS.11 A more recent study in a unique cohort of elderly men showed that cystatin C, troponin I, BNP, and CRP improved risk stratification for death from cardiovascular causes.12
Our findings advance the literature in this area in several ways. We present data on an extensive panel of urinary biomarkers specific for kidney injury and disease, and many of these biomarkers have not previously been examined in a community-based setting. Furthermore, our findings were robust after adjustment for many previously identified mortality biomarkers, including eGFR, UACR, cystatin C, BNP, and CRP. Finally, the inclusion of the end point of death with coexisting kidney disease sheds light on the cause of death in at least some of those participants at increased risk, suggesting that the excess mortality may have been mediated, at least in part, by kidney damage.
Of the biomarkers that we tested, KIM-1 in particular was associated with a range of important clinical outcomes, including all-cause mortality, incident albuminuria in women, death with coexistent kidney, and incident CHF. KIM-1 is a transmembrane tubular protein solely expressed in response to kidney injury,13 which has been proposed as an early marker of AKI as well as a player in the transition from AKI to CKD.14,15 Furthermore, KIM-1 is elevated in symptomatic heart failure and predicts adverse outcomes in that setting.16,17 We extend these findings by showing that KIM-1 also predicts new cases of CHF in members of the general population. Taken together, these findings suggest that KIM-1 predicts adverse outcomes across the clinical spectrum, highlighting its potential as a biomarker of promise.
TFF-3 was strongly associated with all-cause mortality and mortality with kidney disease in this analysis, extending a previously identified association with incident CKD identified in the Atherosclerosis Risk in Communities Study.18 TFF-3 is a member of the trefoil factor peptide family secreted by mucin-producing epithelial cells of the human urinary tract, and it is the predominant trefoil subclass in the proximal and distal tubules and cortical collecting duct.19 TFF-3 seems to play an important role in mucosal repair through restitution early postinjury20–22 and later by effects on epithelial differentiation.23–25 We speculate that TFF-3 plays a role in repair of tubular epithelial damage, with higher urinary levels indicative of previous or persistent injury. Interestingly, we also observed an increased risk of cancer mortality with higher urinary TFF-3. Pathologic expression of TFF-3 has been observed in several cancers, including endometrial and prostate adenocarcinoma, gastric cancer, and several lung cancer histologic subtypes.26–28
Although there has been considerable focus on the cardiovascular consequences of kidney disease, the importance of non-CVD outcomes as a cause of death in CKD is increasingly recognized. People with CKD experience premature morbidity and death from a wide range of causes compared with age-matched members of the general population. For example, in the elderly, there is a graded and independent increase in all-cause mortality risk as CKD advances,29,30 and kidney function predicts death from a range of causes, including pulmonary disease, infection, and cancer.31 In younger adults, the risk of hospitalization, death from pneumonia,32 and non-CVD mortality all increase as GFR declines.33 Albuminuria similarly predicts non-CVD mortality in the general population.34 Our findings extend the literature in this regard by showing that the excess mortality associated with biomarkers of subclinical kidney injury is linked to non-CVD outcomes.
Although results for the incident kidney disease analyses were generally negative, it is notable that reduced levels of CTGF were associated with incident CKD, which is in a direction consistent with a prior small case-control study in FHS.7 This counterintuitive finding may perhaps be explained by the homeostatic challenge model described for the closely related cytokine TGF-β.35 This hypothesis proposes a physiologic custodial function for these cytokines under basal conditions, such that a deficiency in signaling results in tubular cell injury, impaired repair, and fibrosis.
Implications
There are several important implications to this work. First, elevated urinary α1-microglobulin, KIM-1, and TFF-3 levels are associated with a range of important clinical outcomes. Indeed, because one half of the 14 biomarkers tested were at least nominally associated with an increased risk of death, urinary biomarker leak may be a novel presentation of kidney disease in the general population. Second, the panel captures different prognostic information to creatinine, because there was no association between any biomarker and baseline eGFR; also, the findings were independent of adjustment for baseline eGFR, albuminuria, and cystatin C. Future research should focus on validation of our findings in independent studies as well as examination of whether it is possible to intervene to improve outcomes of individuals identified as being at risk. Examining whether these biomarkers predict propensity of disease progression in a sample enriched for CKD would also be of interest.
Strengths and Limitations
Our results may potentially have been attenuated by urinary biomarker degradation during prolonged frozen storage. However, although long-term stability measurements for these biomarkers are not available, medium-term measures for those biomarkers that have been tested seem acceptable. For example, storage for 2 years at −80°C, including several freeze–thaw cycles, did not have a significant effect on the stability of neutrophil gelatinase-associated lipocalin (NGAL) or KIM-1.36 Equally, serum β2-microglobulin measurements were robust to a single freeze–thaw cycle in long-term stored samples from the National Health and Nutrition Examination Survey.37 More importantly, biomarker degradation would not explain our findings, because degradation would not be expected to occur differentially with regard to outcomes. If significant degradation did occur, it would be expected to bias our results to the null and potentially explain attenuated results for individual biomarkers. This result may have been a particular issue for GST-α, for which the addition of a buffer solution to fresh urine samples is recommended to prevent degradation until assay. No buffer was added at the time of urine collection in FHS, and samples were instead frozen and stored for later use. However, although long-term stability measures for GST-α are not available, it remained detectable at the time of assay in our sample. If partial degradation of GST-α occurred, it may partly explain the negative findings for that biomarker. Lastly, suboptimal (i.e., >10%) individual intra-assay coefficient of variations (CVs) for clusterin, GST-α, NGAL, and tissue inhibitor of matrix metalloproteinase-1 may have blunted their predictive performance and biased the results for these biomarkers to the null.
Loss of participants to follow-up is a limitation in longstanding cohort studies such as FHS. Nonreturning participants in our study tended to be in poorer health, which was evidenced by a higher prevalence of chronic disease, including kidney disease (data not shown). This finding may have attenuated the observed associations by excluding sicker participants. Although confirmatory testing of serum creatinine or UACR to confirm the presence of CKD would be desirable, it is not feasible in a large epidemiologic study such as FHS, where participants only return every 4–8 years. Again, any imprecision introduced would be expected to yield attenuated associations. Lastly, because our sample was composed of white individuals of European ancestry, the generalizability of our findings is uncertain.
Strengths of our study include the prospective design and large, well defined cohort as well as the inclusion of biomarkers that are both biologically plausible and supported by the literature. The ability to assess performance of the biomarker panel across a broad range of adjudicated outcomes is also a strength.
Components of a panel of 14 subclinical biomarkers of kidney injury are associated with important outcomes among members of the general population, and KIM-1 in particular was associated with several cardiovascular and mortality outcomes. Elevations in α1-microglobulin and TFF-3 were associated with an excess mortality risk that may be missed by traditional markers of kidney disease. Future studies are needed to establish the role of these biomarkers in reducing risks for these outcomes.
Concise Methods
Study Participants
Participants were from the Framingham Offspring cohort, and they have been examined approximately every 4 years since its inception in 1971.38 Participants who attended the sixth examination cycle (1995–1998) were included, with follow-up at the eighth exam cycle (2005–2008) for longitudinal analyses and December 31, 2009 for survival analyses. Of 3532 participants who attended the baseline examination, 2948 participants had urine samples taken and stored, permitting biomarkers to be assayed, and they were included in the mortality analyses. Participants had to attend follow-up to be assessed for the development of CKD and albuminuria. Of 2948 participants, 261 participants had baseline CKD, 2 participants were missing exposures or covariates, and 544 participants did not return for follow-up, leaving 2141 participants in the incident CKD analysis. For the incident albuminuria analysis, 373 participants had baseline albuminuria, 1 participant was missing covariates, 186 participants did not have follow-up albuminuria measured, and 576 participants did not return for follow-up, leaving 1812 participants. Participants provided written informed consent, and the institutional review board of the Boston Medical Center (Boston, Ma) approved the study.
Urinary Biomarker Determination
Morning urine samples were collected at baseline and initially stored in a large aliquot at −20°C before transfer to −80°C. In January of 2010, each large aliquot was thawed and stored in smaller aliquots until assaying in May of 2010. Urinary biomarker concentrations were measured using a multiplex immunoassay (Human KidneyMAP v1.0 panel; Rules Based Medicine, Austin, TX). There was good stability of biomarker measurements and no freezer decay. Intra-assay coefficients of variation were α1-microglobulin (8.8%), β2-microglobulin (11.8%), calbindin (7.5%), clusterin (20.7%), CTGF (28.1%), cystatin-C (6.3%), GST-α (19.9%), KIM-1 (7.4%), NGAL (12.5%), osteopontin (5.7%), TFF-3 (9.0%), Tamm–Horsfall Protein (7.6%), tissue inhibitor of matrix metalloproteinase-1 (18.3%), and vascular endothelial growth factor (7.4%). Undetectable values were set at the limit of detection.
Outcome Definitions
CKD
The National Kidney Foundation practice guidelines definition of CKD was used (eGFR<60 ml/min per 1.73 m2 by Modification of Diet in Renal Disease equation).39 Continuous rapid decline in eGFR was defined as the loss of >3 ml/min per year during follow-up. Serum creatinine (Jaffé method) was calibrated as follows: a correction factor of 0.23 mg/dl was applied to NHANES III creatinine values to align them with the Cleveland Clinic Laboratory values, and FHS serum creatinine values were then calibrated to the age- and sex-specific mean values from NHANES III.40
Albuminuria
Albuminuria was assessed by UACR. For baseline UACR assessment, spot urine samples were kept at −20°C until quantification in October of 1998. Urinary albumin was measured using immunoturbidimetry (Tina-quant albumin assay; Roche; http://www.roche-diagnostics.us/). Urinary creatinine was assessed using a modified Jaffé method. Albuminuria was defined as UACR≥17 (men) or ≥25 mg/g (women).41
Incident CVD, CHF, Cancer, and Mortality Definitions
CVD was defined as one or more of fatal or nonfatal myocardial infarction, stroke, or transient ischemic attack. Incident CVD, CHF, and mortality events were confirmed by a panel of three physicians after review of outpatient, hospital, and death records using established protocols and criteria.42
Death adjudication forms were abstracted for evidence of kidney disease at the time of death by a trained nephrologist. Death with coexisting kidney disease was adjudicated if renal failure or CKD were recorded as an immediate or contributing cause of death or if they coexisted at the time of death.
Covariate Assessment
The average of two BP measurements, recorded using a mercury sphygmomanometer after 5 minutes seated rest, was used. Hypertension was defined as systolic BP≥140mmHg, diastolic BP≥90mmHg, or use of antihypertensive medication. BMI was defined as weight (kg) divided by the square of height (m). Persons that smoked cigarettes during the previous year were classified as current smokers. Fasting levels of HDL cholesterol and plasma glucose were measured using standardized assays. Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dl or medication. Plasma BNP was measured with high-sensitivity immunoradiometric assays (intra-assay CV=12.2%; Shionogi, Japan). CRP was quantified using a high-sensitivity assay (intra-assay CV=3.2%; Dade Behring BN100 nephelometer; Dade Behring Diagnostic, Marburg, Germany). Serum cystatin C was measured using particle-enhanced immunonephelometry (intra-assay CV=2.4%; Dade Behring BN 100).
Statistical Analyses
Urinary biomarkers were log-transformed for all analyses. Urinary biomarkers were indexed to urinary creatinine for all analyses to adjust for variability in urine concentration, with the exception of assessment of the interbiomarker Pearson correlations to avoid detecting spurious urinary creatinine-to-creatinine correlation. Correlations of biomarkers with CKD risk factors as well as interbiomarker correlations were assessed using multivariable-adjusted Pearson partial correlation coefficients.
We tested four primary outcomes: incident CKD, incident microalbuminuria, incident CVD, and all-cause mortality. Associations of individual biomarkers with incident CKD and albuminuria were determined using multivariable logistic regression adjusted for standard risk factors: age, sex, baseline eGFR, hypertension, diabetes, and dipstick proteinuria (in lieu of UACR to avoid collinearity with urinary creatinine).43 Associations of individual biomarkers with incident albuminuria were also assessed separately in men and women. Multivariable proportional hazards regression models were constructed to test the association of individual urinary biomarkers with incident cardiovascular and mortality events.44 Mortality models were adjusted for age, sex, BMI, smoking, hypertension, diabetes, total HDL-to-cholesterol ratio, eGFR, UACR, and baseline CVD. Incident CVD analyses excluded baseline CVD and were adjusted for the same covariates (except baseline CVD). Proportional hazards assumptions were met for these models.
We also tested four secondary outcomes: incident CHF, cardiovascular and noncardiovascular mortality, and death with coexistent kidney disease. Incident CHF analyses excluded prevalent CHF and were adjusted for age, sex, BMI, smoking, systolic BP, hypertension treatment, diabetes, total to HDL cholesterol ratio, baseline CVD, and valvular heart disease. Cardiovascular and noncardiovascular mortality models were adjusted for the same covariates as all-cause mortality.
We performed four sensitivity analyses. First, all mortality analyses were additionally adjusted for baseline BNP and CRP, because these biomarkers are associated with mortality beyond standard risk factors.11 Second, analyses for death with coexistent kidney disease were also assessed after exclusion of baseline CKD. Third, we determined age- and sex-adjusted mortality rates in participants stratified by CKD status. Finally, incident CKD and albuminuria analyses were additionally adjusted for serum cystatin C.
We estimated the net reclassification improvement for the significant biomarkers from the all-cause mortality analysis in a multimarker model containing established risk factors.45,46 Categories for the reclassification analysis were derived from tertiles of the number of deaths.
We adjusted for multiple testing in analyses of the exploratory biomarkers to reduce the likelihood of false positives. KIM-1, NGAL, and CTGF were not considered exploratory based on the extensive literature on these biomarkers and our own prior work. Because biomarkers were highly intercorrelated, we constructed a correlation matrix to determine the number of independent markers. There were four eigenvalues>1, indicating four independent sets of markers, which explained 50.9%, 9.1%, 7.3%, and 7.2% of the total variance, respectively. The P value for declaring significance for the primary end points was, thus, corrected by a factor of 16 (four independent tests × four independent outcomes), yielding a threshold P value=0.003 (0.05/16). A P value<0.05 was considered statistically significant for the secondary end points. All statistical analyses were performed using SAS version 9.2.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Framingham Heart Study for their important contributions.
The Framingham Heart Study is supported by National Heart, Lung, and Blood Institute Grant N01-HC-25195.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010019/-/DCSupplemental.
References
- 1.Alpert JS, Thygesen K, Jaffe A, White HD: The universal definition of myocardial infarction: A consensus document: Ischaemic heart disease. Heart 94: 1335–1341, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F: Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu I, Parikh CR: Screening for kidney diseases: Older measures versus novel biomarkers. Clin J Am Soc Nephrol 3: 1895–1901, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Parikh CR, Devarajan P: New biomarkers of acute kidney injury. Crit Care Med 36[Suppl]: S159–S165, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Endre ZH, Westhuyzen J: Early detection of acute kidney injury: Emerging new biomarkers. Nephrology (Carlton) 13: 91–98, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Coons SJ: The FDA’s critical path initiative: A brief introduction. Clin Ther 31: 2572–2573, 2009 [DOI] [PubMed] [Google Scholar]
- 7.O’Seaghdha CM, Hwang SJ, Bhavsar NA, Köttgen A, Coresh J, Astor BC, Fox CS: Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis 57: 841–849, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, Ibsen H, Torp-Pedersen C, Hildebrandt PR: N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population. Eur Heart J 28: 1374–1381, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Buring JE, Rifai N, Cook NR: Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA 297: 611–619, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, Meigs JB, Levy D, Wang TJ, Jacques PF, Benjamin EJ, Vasan RS: A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol 21: 2143–2149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D’Agostino RB, Vasan RS: Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 355: 2631–2639, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlöv J: Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 358: 2107–2116, 2008 [DOI] [PubMed] [Google Scholar]
- 13.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H: Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol 298: F1472–F1483, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL: Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart 96: 1297–1302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, Endemann D, Banas B, Mack M, Böger CA, Riegger G, Luchner A: Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: Possible biomarkers of cardiorenal syndrome. Eur J Heart Fail 13: 1104–1110, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Köttgen A, Hwang SJ, Bhavsar N, Fox CS, Coresh J: Trefoil factor 3 predicts incident chronic kidney disease: A case-control study nested within the Atherosclerosis Risk in Communities (ARIC) study. Am J Nephrol 34: 291–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinnert M, Hinz M, Buhtz P, Reiher F, Lessel W, Hoffmann W: Synthesis and localization of trefoil factor family (TFF) peptides in the human urinary tract and TFF2 excretion into the urine. Cell Tissue Res 339: 639–647, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann W: Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 62: 2932–2938, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawar S, Kartha S, Toback FG: Differential gene expression in migrating renal epithelial cells after wounding. J Cell Physiol 165: 556–565, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Romih R, Koprivec D, Martincic DS, Jezernik K: Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int 25: 531–537, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Veranic P, Romih R, Jezernik K: What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 83: 27–34, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann W: Regeneration of the gastric mucosa and its glands from stem cells. Curr Med Chem 15: 3133–3144, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mhawech-Fauceglia P, Wang D, Samrao D, Liu S, Dupont NC, Pejovic T: Trefoil factor family 3 (TFF3) expression and its interaction with estrogen receptor (ER) in endometrial adenocarcinoma. Gynecol Oncol 130: 174–180, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Im S, Yoo C, Jung JH, Choi HJ, Yoo J, Kang CS: Reduced expression of TFF1 and increased expression of TFF3 in gastric cancer: Correlation with clinicopathological parameters and prognosis. Int J Med Sci 10: 133–140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu Y, Yang Y, Ma D, Xiao W: Increased trefoil factor 3 levels in the serum of patients with three major histological subtypes of lung cancer. Oncol Rep 27: 1277–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DD, Hubbard RB, Bulpitt CJ, Fletcher AE: CKD and mortality risk in older people: A community-based population study in the United Kingdom. Am J Kidney Dis 53: 950–960, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Emberson JR, Haynes R, Dasgupta T, Mafham M, Landray MJ, Baigent C, Clarke R: Cystatin C and risk of vascular and nonvascular mortality: A prospective cohort study of older men. J Intern Med 268: 145–154, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 32.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, Hemmelgarn BR, Alberta Kidney Disease Network : CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 54: 24–32, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V: Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: Prospective population based cohort study. BMJ 341: c4986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wakefield LM, Stuelten C: Keeping order in the neighborhood: New roles for TGFbeta in maintaining epithelial homeostasis. Cancer Cell 12: 293–295, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juraschek SP, Coresh J, Inker LA, Rynders GP, Eckfeldt JH, Selvin E: The effects of freeze-thaw on β-trace protein and β2-microglobulin assays after long-term sample storage. Clin Biochem 45: 694–696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP: An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 110: 281–290, 1979 [DOI] [PubMed] [Google Scholar]
- 39.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 40.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 42.McKee PA, Castelli WP, McNamara PM, Kannel WB: The natural history of congestive heart failure: The Framingham study. N Engl J Med 285: 1441–1446, 1971 [DOI] [PubMed] [Google Scholar]
- 43.O’Seaghdha CM, Lyass A, Massaro JM, Meigs JB, Coresh J, D’Agostino RB, Sr, Astor BC, Fox CS: A risk score for chronic kidney disease in the general population. Am J Med 125: 270–277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox DR: Regression models and life-tables. J R Stat Soc Ser A 34: 187–220, 1972 [Google Scholar]
- 45.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.