Abstract
Dense deposit disease (DDD) and C3 glomerulonephritis (C3GN) are widely recognized subtypes of C3 glomerulopathy. These ultra-rare renal diseases are characterized by fluid-phase dysregulation of the alternative complement pathway that leads to deposition of complement proteins in the renal glomerulus. Disease triggers are unknown and because targeted treatments are lacking, progress to end stage renal failure is a common final outcome. We studied soluble CR1, a potent regulator of complement activity, to test whether it restores complement regulation in C3 glomerulopathy. In vitro studies using sera from patients with DDD showed that soluble CR1 prevents dysregulation of the alternative pathway C3 convertase, even in the presence of C3 nephritic factors. In mice deficient in complement factor H and transgenic for human CR1, soluble CR1 therapy stopped alternative pathway activation, resulting in normalization of serum C3 levels and clearance of iC3b from glomerular basement membranes. Short-term use of soluble CR1 in a pediatric patient with end stage renal failure demonstrated its safety and ability to normalize activity of the terminal complement pathway. Overall, these data indicate that soluble CR1 re-establishes regulation of the alternative complement pathway and provide support for a limited trial to evaluate soluble CR1 as a treatment for DDD and C3GN.
Dense deposit disease (DDD) and C3 glomerulonephritis (C3GN) are two widely recognized subtypes of C3 glomerulopathy (C3G).1,2 These ultra-rare renal diseases are caused by fluid-phase dysregulation of the C3 convertase of the alternative pathway (AP) of complement, with variable concomitant dysregulation of the C5 convertase. Consistent with complement-mediated disease acting through the AP, C3G is strongly positive for C3 and notably negative for Igs by immunofluorescence microscopy.2 Electron microscopy distinguishes DDD from C3GN, with the former characterized by pathognomonic electron-dense transformation of the lamina densa of the glomerular basement membrane (GBM).3 In C3GN, the electron microscopy deposits are lighter in color, and are more often mesangial and/or subendothelial, intramembranous, and subepithelial in location.4 In both diseases, mass spectroscopy of laser dissected glomeruli is highly enriched for proteins of the AP and terminal complement cascade.4,5 Although long-term outcome data are not available for C3GN, nearly half of all DDD patients progress to end stage renal failure (ESRF) within 10 years of diagnosis.6,7 In virtually all cases of DDD, transplantation is associated with histologic recurrence, explaining the 5-year graft failure rate of 50%.7,8 There are no target-specific treatments for C3G; however, its pathophysiology suggests that therapeutic approaches to restore C3 convertase control, impair C3 convertase activity, or remove C3 breakdown products from the circulation warrant consideration.1,9
Similar to human DDD, the complement factor H (Cfh)–deficient mouse, reclassified recently as a murine model of C3GN, accumulates C3 fragments within glomeruli as the first pathologic abnormality to develop.10 Systemic administration of either murine or human complement factor H (fH) to the Cfh−/− mouse rapidly reverses both the renal deposition of C3 and its plasma depletion, suggesting that fH replacement therapy may be an effective therapy to restore the underlying protein deficiency and correct the disease process in C3G patients with CFH mutations.11,12 However, it is unlikely that fH administration would be therapeutically successful in the absence of CFH-inactivating mutations. For example, fH would be ineffective in C3G patients carrying C3 mutations that render C3 convertase fH resistant or in the presence of autoantibodies to C3 convertase that block regulation by fH.13,14
Among the non-fH proteins that regulate C3 convertase is soluble CR1. CR1 is a cell-surface glycoprotein expressed on erythrocytes, monocytes, neutrophils, B cells, some T cells, follicular dendritic cells, and podocytes, and modulates the complement cascade at multiple levels (Supplemental Figures 1 and 2).15–18 It is composed of 30 short consensus repeats (SCRs) linked to transmembrane and cytosolic domains.19 On the basis of SCR sequence homology, the 30 extracellular SCRs can be grouped into four long homologous repeats, which are sequentially lettered A through D. These long homologous repeats give CR1 C3 and C5 convertase-controlling activity. CR1 is also the only cofactor of factor I (fI) to promote cleavage of inactive C3b and inactive C4b into their smaller protein fragments, C3dg and C4d.20–22
The soluble form of CR1 (sCR1) is present in the circulation at extremely low concentrations precluding any recognized physiologic significance. However, super-physiologic single-dose treatment with sCR1 has been used in >500 patients (infants and adults) undergoing a variety of cardiac surgeries or after myocardial infarctions to arrest complement activation.23–26 These studies showed that sCR1 was well tolerated with no adverse events clearly or consistently associated with its use. Importantly, the possible development of anti-sCR1 antibodies was monitored but never observed in >350 patients.23,26
Results
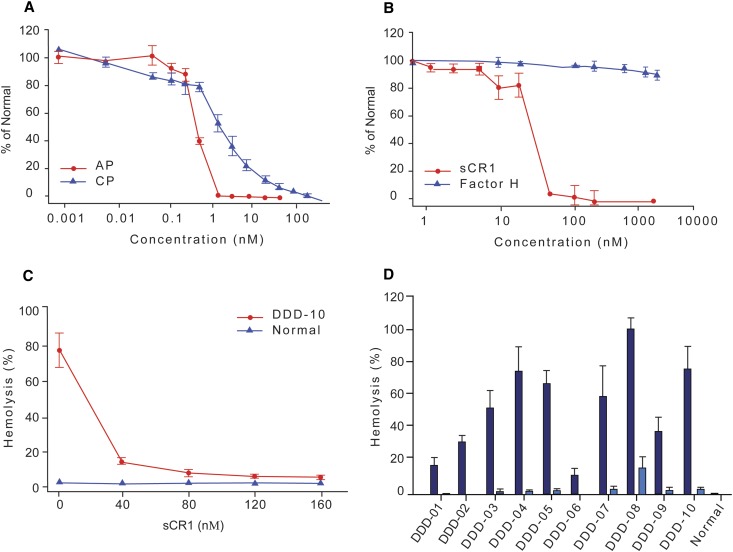
We assessed the efficacy of sCR1 as a complement regulator in vitro in normal and DDD sera. In normal pooled human sera, it prevented classic pathway (CP) and AP complement activation (IC50 values of 2.55±0.55 nM and 0.71±0.08 nM, respectively; Figure 1A). Confirmatory hemolytic assays were performed with rabbit and sheep erythrocytes. Rabbit erythrocytes are a complement-activating surface in human sera; however, lysis could be prevented by the addition of sCR1 (IC50=29.46±4.64 nM). In comparison, fH did not prevent hemolysis even at high concentrations (Figure 1B). Sheep erythrocytes do not activate complement in normal sera; however, hemolysis occurred when tested against DDD sera. The addition of sCR1 restored AP control in a dose-dependent manner (Figure 1C) and prevented hemolysis even when DDD sera contained C3 convertase-stabilizing autoantibodies called C3Nefs (Figure 1D).
Figure 1.
sCR1 prevents complement activation. (A) The ability of sCR1 to prevent activation of the alternative pathway (AP, red) and classical pathway (CP, blue) was measured by Wieslab complement assay using pooled normal human serum (PNHS). Results are expressed as percent activation by PNHS in presence of increasing concentrations of sCR1. Half-maximal inhibitory concentration (IC50) of sCR1 for the CP and AP are 2.55 ± 0.55 nM and 0.71 ± 0.08 nM, respectively. (B) Rabbit erythrocytes are normally an activating surface for the AP. sCR1 (red) protects rabbit erythrocytes from AP-mediated complement lysis (IC50 = 29.46 ± 4.64 nM) while FH (blue) does not. (C) Sheep erythrocytes normally do not activate the human complement system, but DDD patient serum (20% v/v) causes hemolysis due to the presence of C3 convertase-stabilizing C3 nephritic factors. sCR1 suppresses C3Nef activity in a dose-dependent manner (serum from patient DDD-10). (D) In all DDD sera (20% v/v) tested, sheep erythrocyte hemolysis was reduced by sCR1. All patients except DDD-07 and 08 were positive for C3Nefs. Data represent mean ± SD of triplicates (dark blue, 0nM sCR1; light blue, 160nM sCR1).
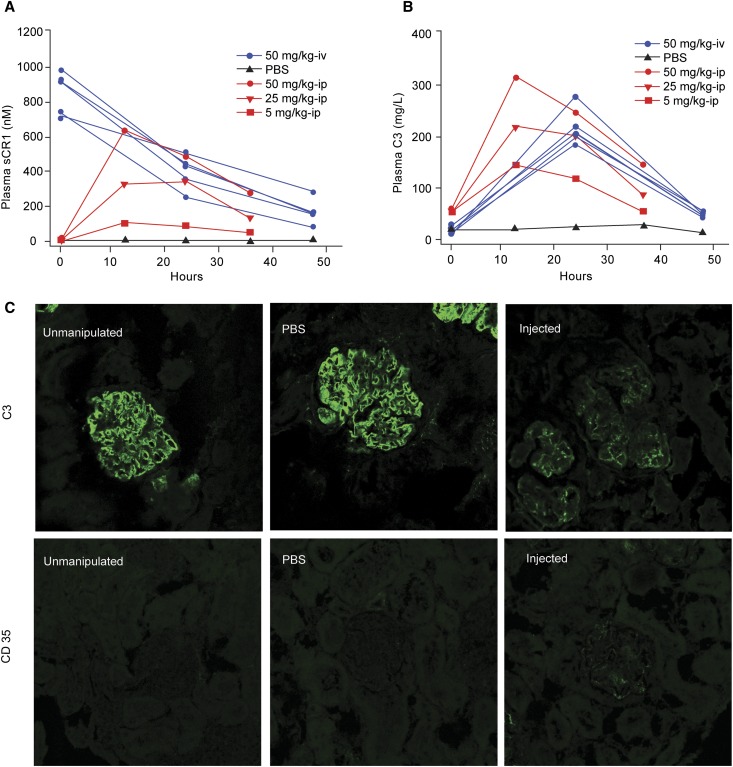
We next measured the systemic effect of sCR1 in vivo in both Cfh−/− and Cfh−/−/huCR1-Tg mice (the latter express CR1 on erythrocytes only) by introducing sCR1 by the tail vein or intraperitoneally, respectively, after first verifying that it prevented hemolysis of rabbit erythrocytes in mouse sera (IC50=6.15±0.18 nM; Figure 2 and Supplemental Figure 3). After a single tail vein injection of sCR1 in Cfh−/− mice (50 mg/kg, n=5; Figure 2A, blue), serum C3 levels rose dramatically within 24 hours from 12.03±12.29 mg/L to 219.77±42.32 mg/L but fell to near preinjection levels by 48 hours (41.84±24.29 mg/L; Figure 2B, blue). Single-dose intraperitoneal injections in Cfh−/−/huCR1-Tg mice at concentrations of 5 mg/kg, 25 mg/kg, or 50 mg/kg showed that changes in serum C3 levels were sCR1 dose dependent (Figure 2, A and B, red). In all sCR1-treated animals, glomerular C3 deposition was markedly reduced (Figure 2C, top panel) with only trace glomerular staining for sCR1 (Figure 2C, bottom panel). In PBS-treated and unmanipulated animals, glomerular C3 deposition was clearly evident (Figure 2C, top panel) and sCR1 was undetectable (Figure 2C, bottom panel).
Figure 2.
Human sCR1 restores serum C3 levels and reduces C3 glomerular staining in Cfh−/− and Cfh−/−/huCR1-Tg mice. (A) Plasma levels of sCR1 dropped rapidly following a single tail-vein injection in 5 Cfh−/− mice (blue lines; t1/2 approximately 18 hours). After a single IP injection of sCR1 (5 mg/kg, 25 mg/kg or 50 mg/kg) in Cfh−/−/huCR1-Tg mice, plasma levels rose and then fell slowly (red lines). Each line represents a single mouse. (B) Serum C3 levels in Cfh−/−mice (blue lines) increased 24 hours after a single tail-vein injection of sCR1 (50 mg/kg) but returned to baseline 48 hours later. Serum C3 levels in Cfh−/−/huCR1-Tg mice (red lines) also increased 12 hours after a single IP injection (5 mg/kg, 25 mg/kg, or 50 mg/kg) and closely followed the sCR1 concentration curves in A. All data (A and B) represent the mean of triplicate assays. (C) C3 glomerular deposition decreased after 48 hours in sCR1-injected Cfh−/− mice (50 mg/kg by tail-vein injection) but not in PBS-injected or non-injected controls (top panel). Trace glomerular staining for human CR1 (CD35) was seen in sCR1-injected mice 48 hours after treatment (bottom).
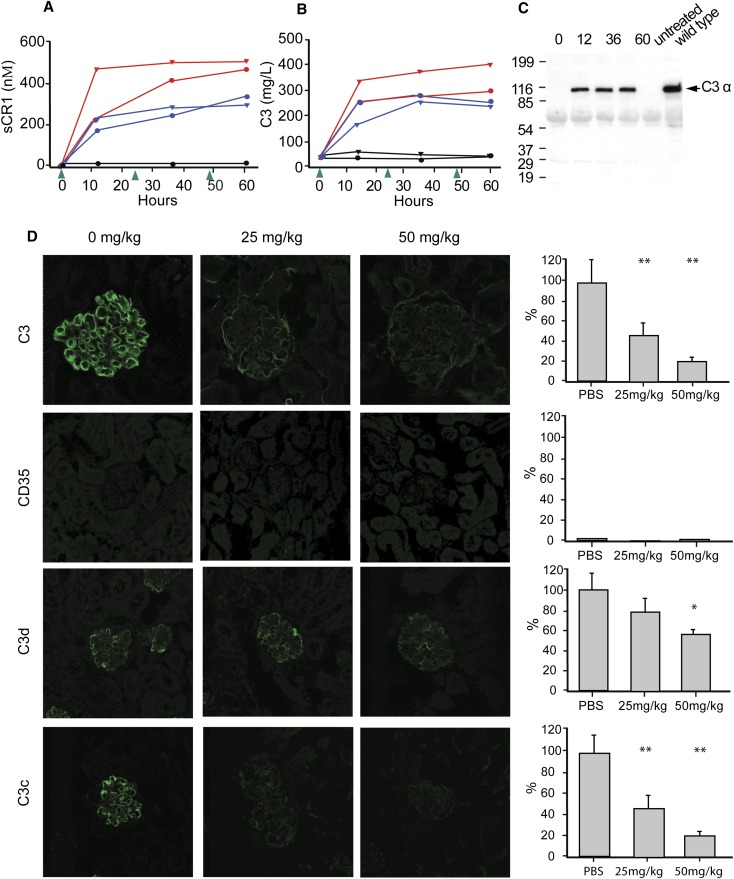
We calculated the serum t1/2 of sCR1 in mice to be approximately 18 hours. On the basis of the dose-response curves to single intraperitoneal injections, we treated four Cfh−/−/huCR1-Tg mice with three intraperitoneal injections (25 mg/kg, n=2 mice; 50 mg/kg, n=2 mice) at 24-hour intervals (Figure 3A). In all sCR1-treated animals, serum C3 levels rose to near normal by 12 hours and remained high throughout the experiment (Figure 3B). Glomerular immunofluorescence studies showed that there was a significant decrease in new C3 deposition, with clearance of old C3 demonstrated by the marked reduction in C3c staining. C3d staining also decreased and sCR1 staining was absent (Figure 3D). To verify that control of the C3 convertase had been restored and that it was an increase in serum C3 and not its degradation products that we were measuring, we performed Western blotting under reducing conditions with a C3α-chain–specific antibody to document the appearance of the 106-kD C3α chain. In PBS-treated and untreated animals, serum C3 levels remained nearly undetectable and the 106-kDa C3α chain was not seen (Figure 3C). Thus, sCR1 treatment restored fluid-phase AP regulation in these murine models of C3G.
Figure 3.
Multi-dose sCR1 treatment clears iC3b glomerular deposition in mouse. (A) Daily dosing (arrows) maintained sCR1 serum levels (blue, 25mg/kg; red, 50mg/kg, black, PBS). (B) Serum C3 levels in Cfh−/−/huCR1-Tg mice increased and remained high with daily IP dosing (0, 24, and 48 hours) of 25 (blue) or 50 (red) mg/kg sCR1 (black, PBS; normal range: 300∼1500 mg/L). Data represent the mean of triplicate assays. (C) Intact C3α (992 amino acids) was detected by Western blotting in reducing conditions using a murine C3α-specific antibody. The 106-kDa band (equivalent to the C3α chain in wild-type mice) appeared 12 hours after sCR1 treatment but was absent before treatment and in untreated controls. (D) IF of glomeruli harvested 60 hours after injection with relative fluorescence quantitation (averaged control value set to 100% with the exception of CD35 where averaged C3 control was set to 100%; scale bar: average of 10 glomeruli ± SD). From top to bottom: C3 was dramatically decreased; CD35 (CR1) was absent; C3d decreased significantly with the higher dose of sCR1; C3c was greatly reduced. *P<0.05, **P<0.001; controls versus experiments.
On the basis of these data, we sought permission from the US Food and Drug Administration (FDA) for a limited multidose safety trial with sCR1 when presented with a C3G patient in ESRF. The patient was an 8-year-old girl with biopsy-proven DDD (Figure 4A) who had presented 3 months earlier with lower extremity swelling, nephritic urine sediment, nephrotic-range proteinuria, positive C3Nefs, and a low serum C3 level (21.6 mg/dl; normal range, 90–180 mg/dl). She transitioned to rapidly progressive GN (eGFR <10 ml/min per 1.73 m2) necessitating dialysis. Approval for a seven-dose trial of sCR1 was obtained from the FDA and from the University of Iowa Institutional Review Board.
Figure 4.
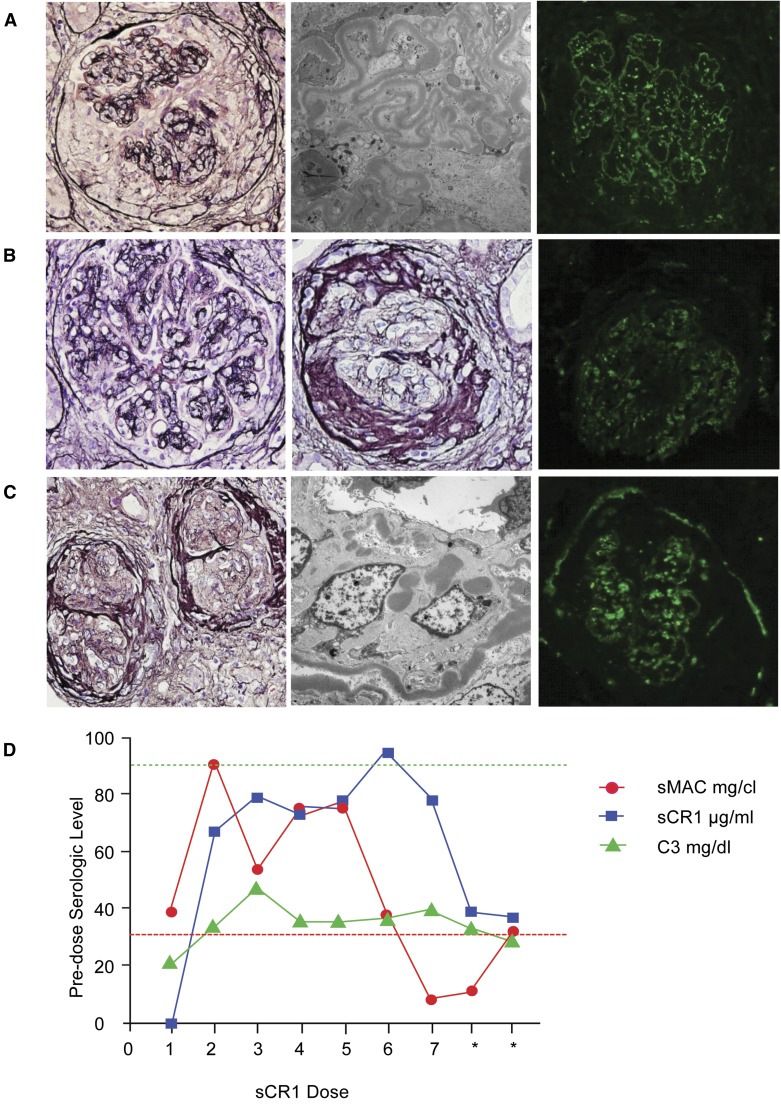
sCR1 restores soluble MAC levels in a DDD patient. (A) Biopsy at presentation showed a proliferative-appearing glomerulus with cellular crescents, endocapillary and mesangial hypercellularity and capillary loop “double contours” (light microscopy [LM] ×400). No global glomerulosclerosis was noted, and interstitial fibrosis and tubular atrophy were minimal. Glomerular “ribbon-like” electron-dense deposits were present on electron microscopy (EM, ×8200) with discrete, coarsely granular C3 immunofluorescence of the mesangium and capillary loops (IF, ×400). (B) The pre-sCR1 biopsy, 6 weeks after the initial biopsy, showed persistent glomerular hypercellularity and capillary loop “double contours” (first LM, ×400), and a glomerular fibrocellular crescent (second LM, ×400) consistent with disease progression to chronicity; moderate interstitial fibrosis and tubular atrophy were present. IF showed “chunky” C3 staining in the glomerular mesangium and capillary loops (×400). (C) Post-sCR1 biopsy, 6 days after the final dose, showed glomerular fibrocellular and fibrous crescents with periglomerular fibrosis (LM, ×200). The ribbon-like capillary loop and mesangial electron dense deposits remained unchanged (EM, ×8200). IF (×400) continued to show bright C3 staining because the biopsy was done 6 days after sCR1 was stopped. (D) Immediately before receiving sCR1 serum C3 was low and sMAC was high. Although the C3 level initially doubled during treatment it trended back to the pre-treatment level. Soluble MAC normalized after the sixth dose (<30 mg/cL) but within 96 hours after termination of therapy was abnormal (asterisks, levels measured at 48 and 96 hours after the last dose of sCR1; red dotted line, normal level of sMAC; green dotted line low normal of plasma C3; sMAC in mg/cl, C3 in mg/dl, sCR1 in μg/ml to allow representation on same graph; sCR1, 1 μg/ml = 4nM).
The patient received a 10 mg/kg loading dose of sCR1 followed by six maintenance doses at 5 mg/kg every 48 hours. During treatment, serum C3 rose to a peak of 48 mg/dl but dropped to pretreatment levels by the end of the trial. Soluble C5b-9 levels were elevated before treatment, normalized after the sixth dose of sCR1, and became abnormal after terminating therapy (Figure 4D). Biopsies taken before and after treatment were similar (Figure 4, B and C, and Table 1). The patient continues on dialysis at this time.
Table 1.
Patient biopsies at presentation and before and after treatment with sCR1
| Histology | Presentation | Before Treatment | After Treatment |
|---|---|---|---|
| Crescents (n) | 21 of 25 | 6 of 18 | 3 of 13 |
| Glomerular sclerosis (n) | 0 of 25 | 9 of 18 | 3 of 13 |
| Interstitial fibrosis | Minimal | Moderate | Moderate |
| C3 immunofluorescence | 2–3+ | 2–3+ | 2–3+ |
Although the trial was very short, there were no adverse effects associated with the administration of sCR1 and immunogenicity was not detected. Assays for anti-sCR1 antibodies on plasma obtained before dosing and at days 2, 4, 6, 8, 10, 12, and 14 and 1 month after dosing remained consistently negative (A450 values ≤0.076 at dilutions of 1:50 at all time points; positive control, A450 1.10, 0.68, and 0.40 at 1:100, 1:200, and 1:400, respectively; negative control, 0.05 at 1:50). Pharmacokinetic data from measured sCR1 serum concentrations in this patient were in good agreement with dose-based simulations using pharmacokinetic data from adult patients undergoing high-risk cardiac surgery (Supplemental Figure 4).23
Discussion
The in vitro activity of sCR1 in complement inhibition is dependent on the specific conditions of the assay and varies with the concentration and hemolytic potential of the serum complement source and the efficiency of the complement-activating mechanisms. In the assays we used, sCR1 prevented C3 convertase activity in normal (rabbit erythrocyte hemolytic assay) and pathologic conditions (sheep erythrocyte hemolytic assay with DDD sera). In vivo experiments in two mouse models of C3G confirmed the in vitro data—sCR1 stopped AP dysregulation and restored plasma C3 levels to normal. These changes were accompanied by reduced deposition of new iC3b and clearance of old iC3b in the GBM.
Our results are consistent with the known role that CR1 plays as a central complement regulator of both the C3 and C5 convertases. In addition to regulating these convertases, CR1 is the only cofactor of fI that can cleave iC3b into smaller fragments (C3c and the thioester-containing fragment C3dg), thus explaining the rapid clearance of iC3b from the GBM. The slower clearance of C3d is consistent with its surface-binding properties (Figure 3D). Thus, by bringing complement dysregulation under control, the deposition of new iC3b is arrested (Figure 3).
These observations provide strong evidence that iC3b is deposited in the GBM and is an important component of the glomerular dense deposits, a conclusion consistent with work done by Pickering and colleagues demonstrating that the presence of fI is an absolute requirement in Cfh−/− mice for the development of a C3GN renal phenotype. In mice deficient in both fH and fI (Cfh−/−.Cfi−/− mice), although excess C3b is present in the circulation, no iC3b forms and GBM dense deposits are not seen.27 The Cfh−/−/huCR1-Tg mouse also provides an excellent animal model in which to study long-term gene therapy to assess the effect of constitutive hepatic expression of sCR1 on the renal phenotype.
The patient with DDD who we treated received only seven doses of sCR1; however, we demonstrated that multidose treatment was safe and nonimmunogenic in this limited study. We did not expect to, nor did we, see a histologic improvement in the renal biopsy results. sCR1 administration was associated with a transient improvement in serum C3 and soluble C5b-9 normalized. These biomarker changes were expected based on in vitro and murine data and support a longer trial using sCR1 in a carefully selected patient population to evaluate the potential of sCR1 as a specific therapy for C3G. Therapeutic entities that incorporate select domains of sCR1 also warrant investigation.
Concise Methods
Patient
An 8-year-old patient with rapidly progressive DDD was enrolled under an FDA-approved compassionate-use investigational new drug study designed to administer seven doses of sCR1 under IRB-approved guidelines. DDD was diagnosed by renal biopsy.
Patient and Normal Sera
Ten patients with biopsy-proven DDD consented to provide sera under IRB-approved guidelines. All patients were positive for C3Nefs except for patients DDD-07 and DDD-08. Patient DDD-07 carries a genetic rearrangement between CFHR2 and CFHR5, and patient DDD-06 carries a genetic variant in CFHR5 (c.1541 T>G; p.Met514Arg). No other genetic mutations were identified in CFH, CFI, CFB, MCP, or C3. Pooled normal human sera were obtained from Innovative Research (Novo, MI); pooled murine sera were collected from five 2-month-old C57BL/6 animals by cardiac puncture.
sCR1
sCR1 (also called TP10 or CDX-1135) was produced by recombinant DNA technology using Chinese hamster ovary cells and purified using standard filtration and chromatography methods as a single-chain polypeptide of 1931 amino acids with a protein molecular mass of 212 kD (Celldex Therapeutics, Needham, MA). Due to N-linked glycosylation, the final total molecular mass was 247 kD.28
Complement Activity Assays
AP and CP activity were evaluated using the appropriate Wieslab complement assay (Wieslab AB, Lund, Sweden) following the manufacturer’s protocols (serum dilution 1:34 for AP and 1:100 for CP). To measure complement inhibitory effects, increasing amounts of sCR1 were added to diluted sera before conducting these assays.
Hemolytic Assays
AP hemolytic activity was measured using rabbit or sheep erythrocytes. Rabbit erythrocytes activate AP-mediated lysis in human and mouse sera; sheep erythrocytes do not. For the rabbit erythrocyte hemolytic assay, increasing concentrations of sCR1 (0.2 nM to 200 nM) or fH (0.2 nM to 2000 M) were mixed with normal human or mouse sera (20% v/v) and incubated for 30 minutes at 37°C with 1×108 rabbit erythrocytes in the presence of 10 mM EGTA/0.15 mM Mg2+/gelation veronal buffer (GVB) or EDTA-GVB as a nonhemolytic control. The reaction was stopped by adding 150 μl of 20 mM EDTA-GVB. After centrifugation, the supernatant was transferred to a clean 96-well plate and absorbance was recorded at 415 nm. Percent lysis was calculated as a fraction of the maximum A415 absorbance in the absence of sCR1 [(A415sCR1-EGTA- A415EDTA)/(A415EGTA) × 100].
To test whether sCR1 prevents C3Nef stabilization of C3 convertase, 10 μl of patient serum was added to 10 μl of sheep erythrocytes (1×109/ml) coated with preformed C3 convertase in a total 50 μl (EGTA-Mg-GVB) reaction as described.29 The mixture was allowed to decay at 30°C (water bath) for 20 minutes. Hemolysis was assayed by adding 50 μl of rat serum (1:9 diluted in GVB-EDTA buffer) as a source of C5-9. Sheep red blood cells were lysed in the presence of nondecayed C3 convertase stabilized by C3Nefs. The reaction was stopped and the percentage of hemolysis was measured as described above. In parallel tests, varying concentrations of sCR1 (0 nM, 40 nM, 120 nM, 160 nM) were added to patient serum before mixing with sheep erythrocytes and incubating on ice for 15 minutes. Repetition of this experiment was done with sera from 10 patients with DDD.
Mice
The Cfh−/− mutant mouse, obtained from Dr. Matthew Pickering, was generated as described.10 On this background, human CR1 was introduced to create the Cfh−/−/huCR1-Tg mutant.30 The latter mouse line expresses CR1 only on mouse erythrocytes. The line was used to avoid any murine immunoresponse. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
sCR1 and C3 Concentrations
sCR1 concentrations were measured by sandwich ELISA in a microtiter plate format using two mouse mAbs specific for CR1. Microtiter plates were coated with mAb 6B1.H12; the detection antibody was horseradish peroxidase–conjugated mAb 4D6.1.31 Murine total C3 serum levels were measured using a two-site ELISA (Kamiya Biomedical, Seattle, WA); human total C3 was measured by radial immunodiffusion (The Binding Site, San Diego, CA). Data were collected in three independent assays and expressed as the mean for each group.
Immunohistochemical Analyses
Kidneys were harvested at the time of euthanasia and imbedded in Tissue-Tek OCT medium for routine processing (Sakura Finetek, Torrance, CA). For glomerular C3 deposition, sections were stained with FITC-conjugated mouse C3 antibody (MP Biomedicals, Solon, OH) at a dilution of 1:800 for 1 hour. For glomerular sCR1, C3c, and C3d staining, sections were treated with goat anti-human CR1 antibody (1:400; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human C3c (1:8000; Abcam, Cambridge, MA), or goat anti-mouse C3d (1:400; R&D Systems, Minneapolis, MN) for 1 hour, respectively, followed by staining with the corresponding Alexa Fluor 488 (1:500; Molecular Probes, Eugene, OR) for 1 hour at room temperature. Fluorescence images were acquired on a Leica model TCS SP5 laser system (Leica Microsystems, Buffalo Grove, IL). Relative fluorescence intensities (average of 10 glomeruli; ± SD) were quantified by ImageJ software (http://rsbweb.nih.gov/ij/; National Institutes of Health, Bethesda, MD).
Immunoblot Analyses
Mouse sera were diluted 1:40 in Laemmli buffer, separated by 7.5% SDS-PAGE, and transferred to nitrocellulose membranes (Whatman Schleicher and Schuell Inc., Dassel, Germany). Membranes were blocked with 5% skim milk in PBS at room temperature for 30 minutes and incubated with anti-mouse C3 antibody (1:500; in-house antibody targeting the fI cleavage site on C3). Each membrane was washed three times with 0.05% Tris-buffered saline Tween-20 followed by incubation with horseradish peroxidase–conjugated anti-mouse secondary antibody (Jackson ImmunoResearch, Inc., West Grove, PA). Specific protein bands were visualized with an Enhanced Chemiluminescent System (GE Healthcare, Piscataway, NJ).
sCR1 Dose Simulations
Pharmacokinetic data from adult cardiac surgery patients treated with sCR1 were used to estimate sCR1 serum concentrations over time using empirical calculations that assumed concentrations from multiple doses to be additive because sCR1 pharmacokinetics has been shown to be dose proportional (dose versus area under the curve are linear).23 Averaged data from 26 cardiac surgery patients receiving a single 10 mg/kg bolus of sCR1 were used to simulate the dosing regimen used in the single DDD patient. The dose regimen was designed to keep trough levels of sCR1 above 20 µg/ml throughout the dosing period.
Immunogenicity Assays
Patient plasma samples were screened for anti-sCR1 antibodies using a microtiter plate ELISA format. Microtiter plates were coated with sCR1 and blocked with BSA in PBS. Patient samples were diluted 1:50 in PBS 10 mM EDTA 0.5% Tween and incubated in coated plates. Antibodies were detected using horseradish peroxidase-conjugated F(ab)2 goat anti-human IgG antibody (KPL Inc., Gaithersburg, MD). Normal human serum was used as a negative control. Knops-McCoy blood group antiserum, which is specific for CR1, served as a positive control.32 Positive patient samples were confirmed specific for sCR1 by preincubating with excess sCR1 and rerunning the assay.
Statistical Analyses
IC50 values were calculated by GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA). Statistical significance was calculated with the t test.
Disclosures
H.C.M., R.A.H., and L.J.T. own stock and/or options and are employees of Celldex Therapeutics.
Supplementary Material
Acknowledgments
We are grateful to those patients with DDD whose participation made this research possible. The Cfh−/− and Cfh−/−/huCR1-Tg mutant mice were kindly provided by Drs. Matthew Pickering and Rick Quigg.
This work was supported in part by National Institutes of Health Grant DK074409 to R.J.H.S. and S.S.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010045/-/DCSupplemental.
References
- 1.Smith RJ, Harris CL, Pickering MC: Dense deposit disease. Mol Immunol 48: 1604–1610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S, Nester CM, Smith RJ: Membranoproliferative glomerulonephritis and C3 glomerulopathy: Resolving the confusion. Kidney Int 81: 434–441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Córdoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Würzner R, Zipfel PF, Dense Deposit Disease Focus Group : New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447–2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJH: C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, 3rd, Zipfel PF, Dogan A, Smith RJ: Glomeruli of dense deposit disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu DF, McCarthy AM, Lanning LD, Delaney C, Porter C: A descriptive study of individuals with membranoproliferative glomerulonephritis. Nephrol Nurs J 34: 295–302, quiz 303, 2007 [PubMed] [Google Scholar]
- 7.Lu DF, Moon M, Lanning LD, McCarthy AM, Smith RJ: Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol 27: 773–781, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF: Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol 16: 2225–2233, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Nester CM, Smith RJH: Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens 22: 231–237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M: Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31: 424–428, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Fakhouri F, de Jorge EG, Brune F, Azam P, Cook HT, Pickering MC: Treatment with human complement factor H rapidly reverses renal complement deposition in factor H-deficient mice. Kidney Int 78: 279–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paixão-Cavalcante D, Hanson S, Botto M, Cook HT, Pickering MC: Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol 46: 1942–1950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP, Llorca O, Harris CL, Rodríguez de Córdoba S: Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 120: 3702–3712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Meyer NC, Wang K, Nishimura C, Frees K, Jones M, Katz LM, Sethi S, Smith RJ: Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol 7: 265–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, Xu C, Fu YX, Holers VM, Molina H: Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol 160: 5273–5279, 1998 [PubMed] [Google Scholar]
- 16.Kazatchkine MD, Fearon DT: Deficiencies of human C3 complement receptors type 1 (CR1, CD35) and type 2 (CR2, CD21). Immunodefic Rev 2: 17–41, 1990 [PubMed] [Google Scholar]
- 17.Rødgaard A, Christensen LD, Thomsen BS, Wiik A, Bendixen G: Complement receptor type 1 (CR1, CD35) expression on peripheral T lymphocytes: Both CD4- and CD8-positive cells express CR1. Complement Inflamm 8: 303–309, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Weiss L, Fischer E, Haeffner-Cavaillon N, Jouvin MH, Appay MD, Bariety J, Kazatchkine M: The human C3b receptor (CR1). Adv Nephrol Necker Hosp 18: 249–269, 1989 [PubMed] [Google Scholar]
- 19.Klickstein LB, Wong WW, Smith JA, Weis JH, Wilson JG, Fearon DT: Human C3b/C4b receptor (CR1). Demonstration of long homologous repeating domains that are composed of the short consensus repeats characteristics of C3/C4 binding proteins. J Exp Med 165: 1095–1112, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HD, Svergun DI, Johansson CM, Uhrín D, Barlow PN, Hannan JP: Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol 18: 463–470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medof ME, Nussenzweig V: Control of the function of substrate-bound C4b-C3b by the complement receptor Cr1. J Exp Med 159: 1669–1685, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masaki T, Matsumoto M, Nakanishi I, Yasuda R, Seya T: Factor I-dependent inactivation of human complement C4b of the classical pathway by C3b/C4b receptor (CR1, CD35) and membrane cofactor protein (MCP, CD46). J Biochem 111: 573–578, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Lazar HL, Bokesch PM, van Lenta F, Fitzgerald C, Emmett C, Marsh HC, Jr, Ryan U, OBE and the TP10 Cardiac Surgery Study Group : Soluble human complement receptor 1 limits ischemic damage in cardiac surgery patients at high risk requiring cardiopulmonary bypass. Circulation 110[Suppl 1]: II274–II279, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lazar HL, Keilani T, Fitzgerald CA, Shapira OM, Hunter CT, Shemin RJ, Marsh HC, Jr, Ryan US, TP10 Cardiac Surgery Study Group : Beneficial effects of complement inhibition with soluble complement receptor 1 (TP10) during cardiac surgery: Is there a gender difference? Circulation 116[Suppl]: I83–I88, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Li JS, Jaggers J, Anderson PA: The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev Cardiovasc Ther 4: 649–654, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Li JS, Sanders SP, Perry AE, Stinnett SS, Jaggers J, Bokesch P, Reynolds L, Nassar R, Anderson PA: Pharmacokinetics and safety of TP10, soluble complement receptor 1, in infants undergoing cardiopulmonary bypass. Am Heart J 147: 173–180, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC: Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest 118: 608–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT: Soluble human complement receptor type 1: In vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249: 146–151, 1990 [DOI] [PubMed] [Google Scholar]
- 29.West CD: A hemolytic method for the measurement of nephritic factor. J Immunol Methods 335: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Alexander JJ, Hack BK, Jacob A, Chang A, Haas M, Finberg RW, Quigg RJ: Abnormal immune complex processing and spontaneous glomerulonephritis in complement factor H-deficient mice with human complement receptor 1 on erythrocytes. J Immunol 185: 3759–3767, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Nickells M, Hauhart R, Krych M, Subramanian VB, Geoghegan-Barek K, Marsh HC, Jr, Atkinson JP: Mapping epitopes for 20 monoclonal antibodies to CR1. Clin Exp Immunol 112: 27–33, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moulds JM, Nickells MW, Moulds JJ, Brown MC, Atkinson JP: The C3b/C4b receptor is recognized by the Knops, McCoy, Swain-langley, and York blood group antisera. J Exp Med 173: 1159–1163, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.