Abstract
Cell division autoantigen 1 (CDA1) enhances TGF-β signaling in renal and vascular cells, and renal expression of CDA1 is elevated in animal models of diabetes. In this study, we investigated the genetic deletion of Tspyl2, the gene encoding CDA1, in C57BL6 and ApoE knockout mice. The increased renal expression of TGF-β1, TGF-β type I and II receptors, and phosphorylated Smad3 associated with diabetes in wild-type mice was attenuated in diabetic CDA1 knockout mice. Notably, CDA1 deletion significantly reduced diabetes-associated renal matrix accumulation and immunohistochemical staining for collagens III and IV and attenuated glomerular and tubulointerstitial injury indices, despite the presence of persistent hyperglycemia, polyuria, renal hypertrophy, and hyperfiltration. Furthermore, CDA1 deletion reduced gene expression of TGF-β1 receptors in the kidney, resulting in a functionally attenuated response to exogenous TGF-β, including reduced levels of phosphorylated Smad3 and ERK1/2, in primary kidney cells from CDA1 knockout animals. Taken together, these data suggest that CDA1 deletion reduces but does not block renal TGF-β signaling. Because direct antagonism of TGF-β or its receptors has unwanted effects, CDA1 may be a potential therapeutic target for retarding DN and perhaps, other kidney diseases associated with TGF-β–mediated fibrogenesis.
Diabetic nephropathy (DN) is the most common cause of ESRD.1,2 The histologic hallmarks of DN are basement membrane thickening and accumulation of extracellular matrix (ECM) in the mesangium and tubulointerstitium.3–6 TGF-β is a key player in this process.3,5,7–10 Although renal fibrogenesis in DN has been clearly shown to be TGF-β–dependent and may be attenuated in experimental models by anti–TGF-β strategies,11,12 TGF-β is not an ideal target for treating this condition because of its other biologic functions, including regulation of cell proliferation, apoptosis, differentiation, and immune tolerance. TGF-β1 knockout mice die before birth or within several weeks after birth from massive inflammation.13–17 A similar lethal effect has been described for the deletion of the TGF-β type 1 receptor gene.18
Our group has previously identified that the pathologic profibrotic effects of TGF-β in DN are, at least in part, mediated by enhanced TGF-β signaling modulated by cell division autoantigen 1 (CDA1).19 CDA1 knockdown by siRNA not only attenuates TGF-β–stimulated Smad3 (mothers against decapentaplegic homolog 3) phosphorylation and transcriptional activities but also ultimately blocks the stimulated expression of TGF-β target genes in association with fibrogenesis, such as connective tissue growth factor (CTGF), collagens, and fibronectin.19,20 These findings support the hypothesis that CDA1 is a potential molecular target for retarding the pathologic activities of TGF-β and renal scarring in diabetes.
To validate CDA1 as an effective target in DN, a CDA1 gene knockout (KO) mouse was generated. In addition, this mouse was crossed with a mouse on an Apolipoprotein E (ApoE) KO background, because previous studies have identified that the induction of streptozotocin (STZ) diabetes in ApoE KO mice is associated with accelerated renal injury and prominent ECM accumulation.21 Here, we explore the renal effects of deletion of the gene encoding CDA1, Testis-specific Y-encoded-like protein 2 (Tspyl2), with a particular focus on TGF-β signaling as well as diabetes-associated renal injury and ECM accumulation.
Results
CDA1 KO Mice Are Viable and Fertile with No Abnormal Phenotypes
To further characterize the role of CDA1, we used our newly generated CDA1 KO mouse to validate CDA1 as a critical molecular target for retarding TGF-β signaling and renal fibrosis. The CDA1 encoding gene, Tspyl2, was located on the X chromosome. Therefore, a colony of breeder mice with the CDA1 gene targeted was maintained with various genotypes, including CDA1 wild-type (WT) and hemizygous CDA1 KO males as well as heterozygous and homozygous CDA1 KO females. There were no obvious abnormal phenotypes observed in these mice during the breeding process. The homozygous female and hemizygous male CDA1 KO breeders produced litters with an average size of 6.3±0.3 (n=39) pups per litter, which was similar to the WT breeders with 5.9±0.6 pups per litter (n=20; P=0.43). The sexes of the pups produced by the KO breeders were equal (female/male ratio=0.99; 87 females and 88 males from 30 litters).
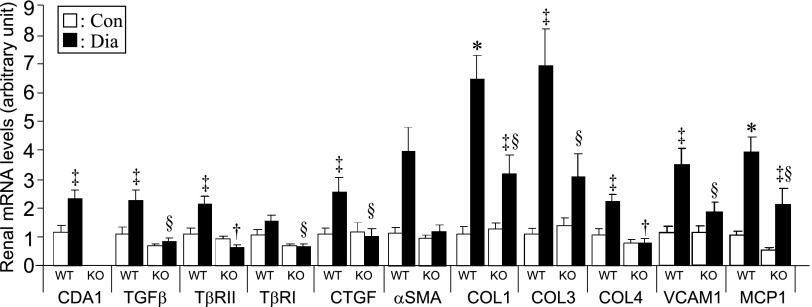
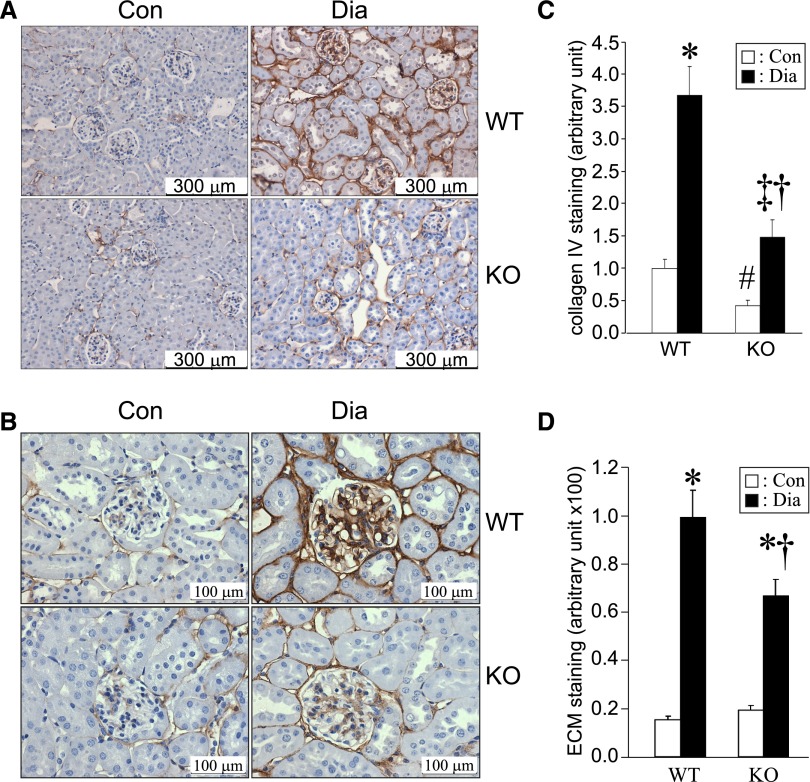
Deletion of CDA1 had no significant effect on animal health. There was no difference for the body weight, growth rate, and water or food consumption between the CDA1 WT and KO genotypes or between ApoE KO and CDA1/ApoE double KO (dKO) mice (Supplemental Table 1). In particular, there was no effect on renal function, because creatinine clearance, renal weight, and urinary albumin excretion in KO mice were not significantly different from their respective WT controls (Table 1). However, deletion of CDA1 resulted in lower mRNA levels for TGF-β and TGF-β type I receptor (TβRI) in the renal cortex (Figure 1). Furthermore, basal levels of collagen IV staining in the nondiabetic CDA1 KO mice were lower than the levels in WT mice (P=0.003) (Figure 2).
Table 1.
Metabolic data for CDA1 WT and KO mice with and without diabetes for 20 weeks
| Genotype | CDA1 WT | CDA1 KO | ||
|---|---|---|---|---|
| Nondiabetic (n=15) | Diabetic (n=12) | Nondiabetic (n=13) | Diabetic (n=9) | |
| Plasma glucose (mmol/L) | 13.5±0.9 | 28.6±1.1a | 11.2±0.6 | 27.4±1.7a |
| Glycated Hb (%) | 4.3±0.1 | 11.7±0.5a | 4.8±0.3 | 12.3±0.8a |
| Body weight (g) | 33.3±0.9 | 25.7±0.5a | 31.2±0.5b | 26.8±0.6a |
| Kidney/body weight (%) | 0.55±0.02 | 0.83±0.04a | 0.55±0.02 | 0.77±0.04a |
| Urine volume (ml/d) | 0.8±0.1 | 19.7±1.8a | 0.7±0.2 | 19.0±3.6a |
| Albuminuria (μg/d) | 119±20 | 302±37a | 153±24 | 312±38c |
| Creatinine clearance (ml/min per m2) | 14.8±1.6 | 42.7±2.0a | 14.3±1.7 | 33.3±4.0c |
Data are shown as mean ± SEM.
P<0.001 versus nondiabetic.
P<0.05 versus nondiabetic CDA1 WT.
P<0.05 versus nondiabetic.
Figure 1.
CDA1 deletion attenuates diabetes-associated increase in renal mRNA levels of prosclerotic and proinflammatory genes in mice. Male WT and CDA1 KO mice were rendered diabetic by injections of STZ (Dia; black columns) or buffer only to serve as nondiabetic controls (Con; white columns) for 20 weeks. Renal mRNA levels (arbitrary unit) are shown as mean ± SE (n=8–10/group). *P<0.001 and ‡P<0.05 versus Con; †P<0.001 and §P<0.05 versus WT Dia. COL, collagen; MCP1, monocyte chemoattractant protein-1; VCAM1, vascular cell adhesion molecule 1.
Figure 2.
Diabetes-associated increase in collagen IV and ECM accumulation in the kidney is attenuated in CDA1 KO mice. (A and B) Representative immunohistochemical staining of collagen IV (brown) is shown for kidney paraffin sections from Con and 20-week Dia WT and CDA1 KO mice. Original magnification, ×200 in A and ×400 in B. (C) The staining of collagen IV (quantified by Image-Pro Plus program) is shown as mean ± SE (n=7–8/group). *P<0.001 and ‡P<0.05 versus Con; †P<0.001 versus WT Dia; #P<0.05 versus WT control group. (D) Quantification of ECM accumulation in kidney, which was determined by Masson’s Trichrome staining, is shown as mean ± SE (n=8 for nondiabetic, n=17 for WT diabetic, and n=15 for KO diabetic groups). *P<0.001 versus Con; †P<0.001 versus WT Dia.
General Parameters of the Diabetic CDA1 WT and KO Mice
The induction of diabetes by five daily injections of STZ was associated with increased plasma glucose and glycated hemoglobin (Hb) levels in both CDA1 WT and KO mice, with no effect of CDA1 deletion on glucose homeostasis (Table 1). Similarly, polyuria, polydipsia, renal hypertrophy, hyperfiltration, and increased urinary albumin excretion were observed after the induction of diabetes in both CDA1 WT and KO mice (Table 1 and Supplemental Table 1).
CDA1 Deficiency Retards Diabetes-Associated Renal Fibrosis in C57BL6 Mice
Twenty weeks of diabetes in WT mice resulted in an approximately 2.5-fold increase in gene expression of CDA1 as well as similar increase in TGF-β1, TβRI, and TGF-β type II receptor (TβRII) mRNA levels. This result was associated with increased expression of key target genes, including CTGF, alpha-smooth muscle actin, and collagens I, III, and IV as well as monocyte chemoattratant protein-1 and vascular cell adhesion molecule 1 (Figure 1). Renal ECM accumulation was also increased by approximately sevenfold in diabetic WT mice, which was determined by Masson’s Trichrome staining. In addition, the expression of collagen IV was increased by at least threefold in the diabetic WT group as determined by immunohistochemical staining (Figure 2, A–C).
By contrast, the induction of diabetes in CDA1 KO mice was not associated with increased gene expression of CTGF, alpha smooth muscle actin, and collagen IV, and the expression of collagens I and III as well as monocyte chemoattractant protein-1 and vascular cell adhesion molecule 1 were attenuated by ∼50% in diabetic CDA1 KO mice (Figure 1). Moreover, diabetes-associated increases in renal ECM accumulation and collagen IV immunostaining were also attenuated in CDA1 KO mice (P<0.05) (Figure 2, C and D). The staining pattern of collagen IV showed a diabetes-associated increase in expression of collagen IV in the tubulointerstitial region in diabetic WT mouse (Figure 2A) as well as the glomerular region (Figure 2B). CDA1 deficiency significantly attenuated the collagen IV accumulation in both glomerular and tubulointerstitial compartments in diabetic CDA1 KO mice (Figure 2, A–C).
CDA1 Deficiency Retards Diabetes-Associated Renal Injury in ApoE KO Mice
To show the possible effects of CDA1 deletion in reducing renal fibrosis in a diabetic model with accelerated and more severe renal fibrosis,21,22 we generated CDA1/ApoE dKO mice. Diabetes was induced after five injections of STZ, and mice were then followed for 20 weeks. The metabolic data again showed similar plasma glucose and glycated Hb levels in both diabetic ApoE KO and CDA1/ApoE dKO mice as well as polyuria, polydipsia, renal hypertrophy, hyperfiltration, and increased urinary albumin excretion (Table 2 and Supplemental Table 2). However, albuminuria in the diabetic CDA1/ApoE dKO mice was lower than albuminuria observed in diabetic ApoE KO mice (P<0.05) (Table 2). The kidney/body weight ratio of the diabetic CDA1/ApoE dKO mice was also modestly lower than seen in diabetic ApoE KO mice (P<0.05) (Table 2).
Table 2.
Metabolic data for ApoE KO and CDA1/ApoE dKO mice with and without diabetes for 20 weeks
| Genotype | ApoE KO | ApoE/CDA1 dKO | ||
|---|---|---|---|---|
| Nondiabetic (n=25) | Diabetic (n=26) | Nondiabetic (n=14) | Diabetic (n=9) | |
| Plasma glucose (mmol/L) | 13.8±0.7 | 27.6±1.2a | 13.6±0.9 | 27.8±1.1a |
| Glycated Hb (%) | 4.1±0.1 | 15.4±0.7a | 4.2±0.1 | 14.5±0.9a |
| Nondiabetic (n=11) | Diabetic (n=12) | Nondiabetic (n=10) | Diabetic (n=7) | |
| Plasma cholesterol (mmol/L) | 8.9±0.9 | 19.7±1.3a | 10.4±0.8 | 22.3±2.5a |
| Body weight (g) | 30.6±0.6 | 24.5±0.7a | 32.8±0.8b | 22.3±0.6a |
| Kidney/body weight (%) | 0.63±0.02 | 0.96±0.03a | 0.60±0.02 | 0.82±0.03a,c |
| Nondiabetic (n=16) | Diabetic (n=18) | Nondiabetic (n=11) | Diabetic (n=14) | |
| Albuminuria (μg/d) | 95.4±11.7 | 263.2±25.1d | 56.9±8.9b | 147.8±25.6a,c |
| Nondiabetic (n=16) | Diabetic (n=18) | Nondiabetic (n=11) | Diabetic (n=6) | |
| Urine volume (ml/d) | 1.0±0.1 | 22.1±2.2a | 0.6±0.1b | 21.7±1.0a |
| Nondiabetic (n=6) | Diabetic (n=10) | Nondiabetic (n=9) | Diabetic (n=8) | |
| Creatinine clearance (ml/min per m2) | 18.0±1.8 | 38.0±4d | 16.5±0.8 | 39.1±3.0a |
Data are shown as mean ± SEM.
P<0.001 versus nondiabetic.
P<0.05 versus nondiabetic ApoE KO.
P<0.05 versus diabetic ApoE KO.
P<0.05 versus nondiabetic.
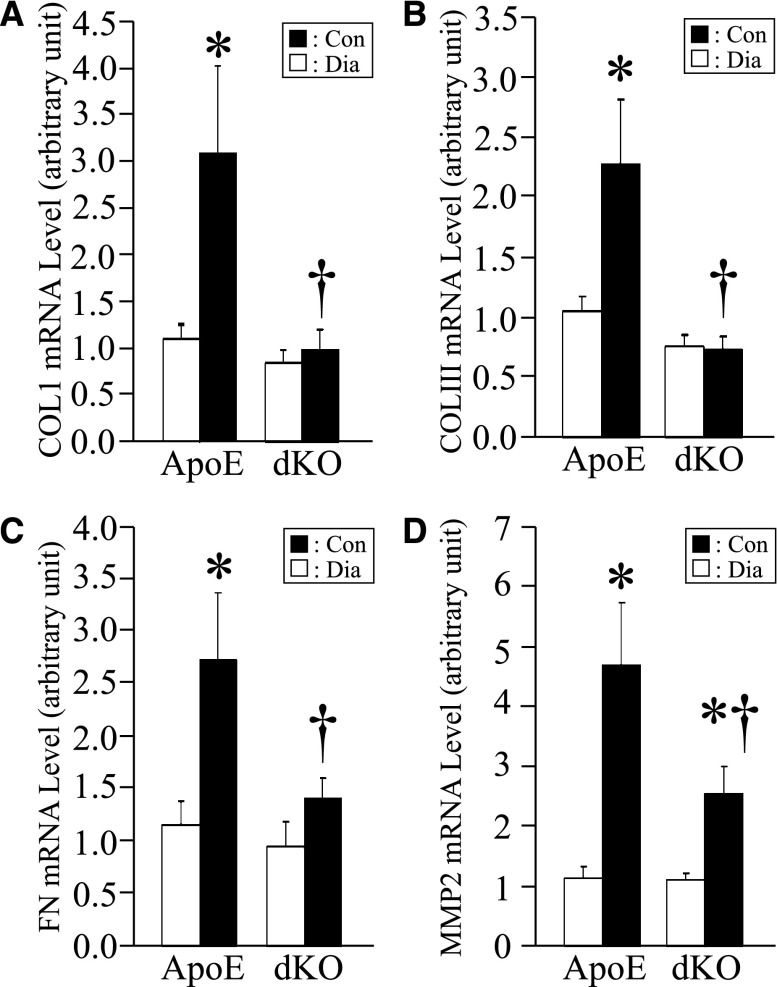
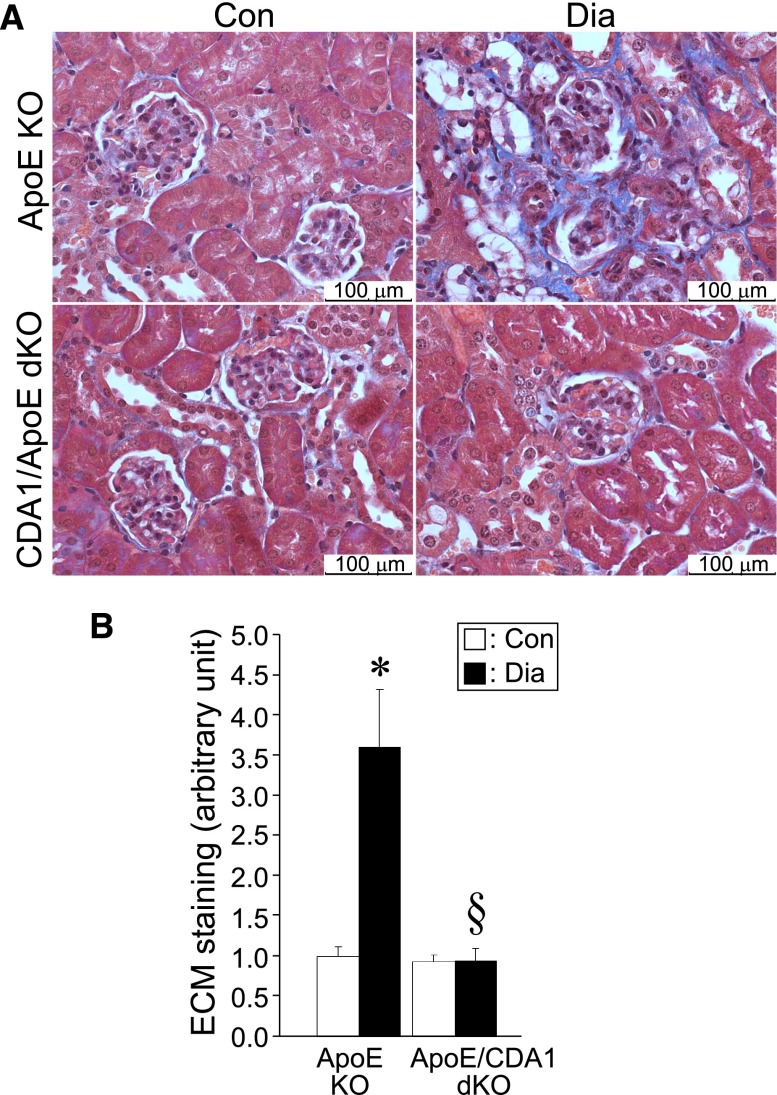
As observed in C57BL6 mice, the expression levels of TGF-β and its target genes, including collagens I and III as well as fibronectin and matrix metalloproteinase-2 (MMP2), were increased in diabetic ApoE KO mice, and this effect was attenuated in diabetic CDA1/ApoE dKO mice (Figure 3). Similarly, increased immunohistochemical staining of collagens III and IV (Figure 4) as well as ECM accumulation, which was determined by Trichrome staining (Figure 5), associated with the induction of diabetes in ApoE KO mice was attenuated in diabetic CDA1/ApoE dKO mice.
Figure 3.
CDA1 deficiency in ApoE KO mice attenuates diabetes-associated increase in renal gene expression for collagens I, III, and fibronectin as well as the TGF-β target gene MMP2. ApoE KO and CDA1/ApoE dKO mice were rendered diabetic for 20 weeks by STZ injections or injected with buffer to serve as nondiabetic controls. Renal mRNA levels for (A) collagens I and (B) III, (C) fibronectin (FN), and (D) MMP2, determined by real-time RT-qPCR in these mice, are shown as mean ± SE (n=8–9/group). *P<0.05 versus Con; †P<0.05 versus WT Dia.
Figure 4.
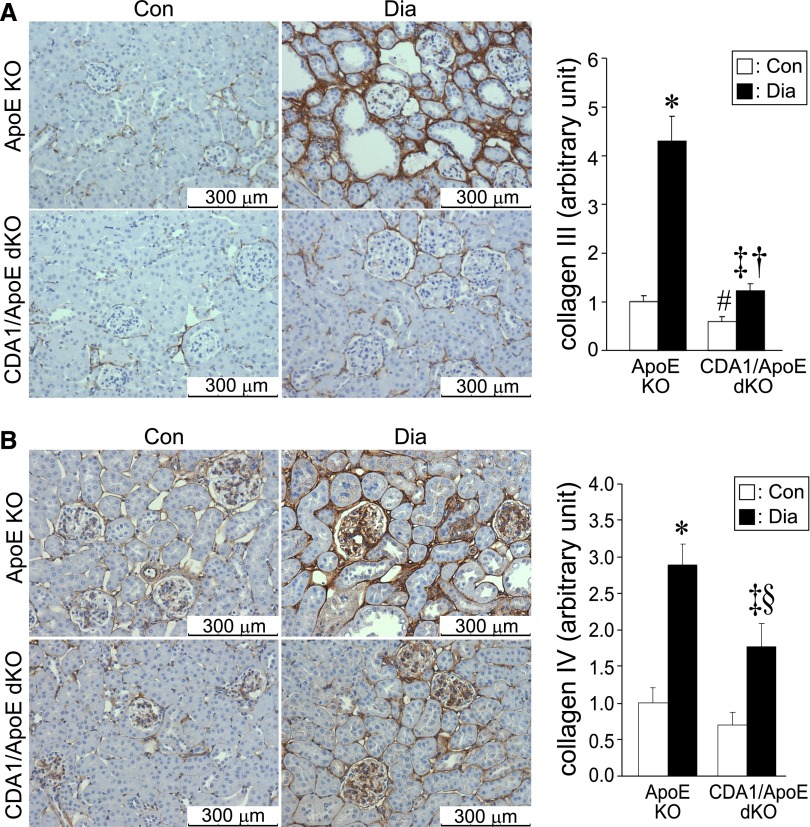
CDA1 deficiency in ApoE KO mice attenuates diabetes-associated increase in protein expression of collagens III and IV. ApoE KO and CDA1/ApoE dKO mice were rendered diabetic for 20 weeks by STZ injections or injected with buffer to serve as nondiabetic controls. Representative images of immunohistochemical staining (brown color) for (A) collagens III and (B) IV of the paraffin-embedded kidney sections of these mice are shown. Scale bar, 300 μm. Original magnification, ×200. Quantification of the staining is shown in the bar graph as mean ± SE (n=8–9/group). *P<0.001 and ‡P<0.05 versus Con; †P<0.001 and §P<0.05 versus ApoE Dia; #P<0.05 versus ApoE KO control group.
Figure 5.
CDA1 deficiency in ApoE KO mice attenuates diabetes-associated increase in accumulation of ECM. (A) Masson’s Trichrome staining for ECM accumulation in kidneys of 20-week Dia and Con ApoE KO and CDA1/ApoE dKO mice is shown in blue, and (B) quantification of the staining is shown in the bar graph as mean ± SE (n=8–12/group). *P<0.05 versus Con; §P<0.05 versus ApoE Dia.
In diabetic ApoE KO mice, there was an increase in collagen III expression, particularly in the tubulointerstitial region that was attenuated in CDA1/ApoE dKO mice (Figure 4A). With respect to collagen IV, diabetes was associated with an increase in both glomerular and tubulointerstitial collagen IV expression. CDA1 gene deletion in these diabetic ApoE KO mice was associated with reduced collagen IV immunostaining (Figure 4B). Consistent with effects on both collagens III and IV, the increase in ECM accumulation as assessed by Trichrome staining in diabetic ApoE KO mice was not observed in the CDA1/ApoE dKO mice, and this finding was particularly evident in the tubulointerstial area (Figure 5).
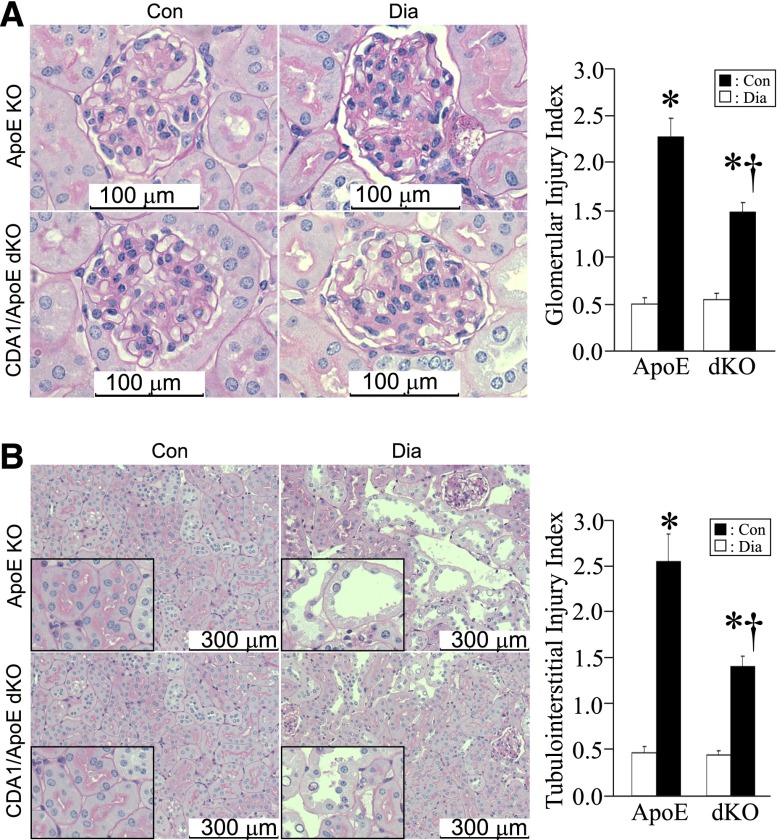
The renal structural changes associated with diabetes in ApoE KO mice, which were reflected by the increased glomerular and tubulointerstitial injury indices, were also attenuated in CDA1/ApoE dKO mice (Figure 6).
Figure 6.
Diabetes-associated pathologic changes are attenuated in CDA1/ApoE dKO mice. (A and B) Paraffin-embedded kidney sections (2-μm thickness) of 20-week Dia and Con ApoE KO and CDA1/ApoE dKO mice were stained by periodic acid–Schiff stain. Representative images of (A) glomeruli and (B) tubulointerstitial area are shown. Original magnification, ×400 in A and ×200 in B. (B) Parts of the pictures, for tubular injuries, are enlarged and shown as insets (boxed areas). The pathologic injury is shown for (A) glomerular injury index and (B) tubulointerstitial injury index as mean ± SE (n=9–12/group). *P<0.001 versus Con; †P<0.05 versus ApoE Dia.
CDA1 Deficiency Attenuates TGF-β–Associated Signaling Pathways In Vivo Involving Smad3 and Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase in Mouse Kidney
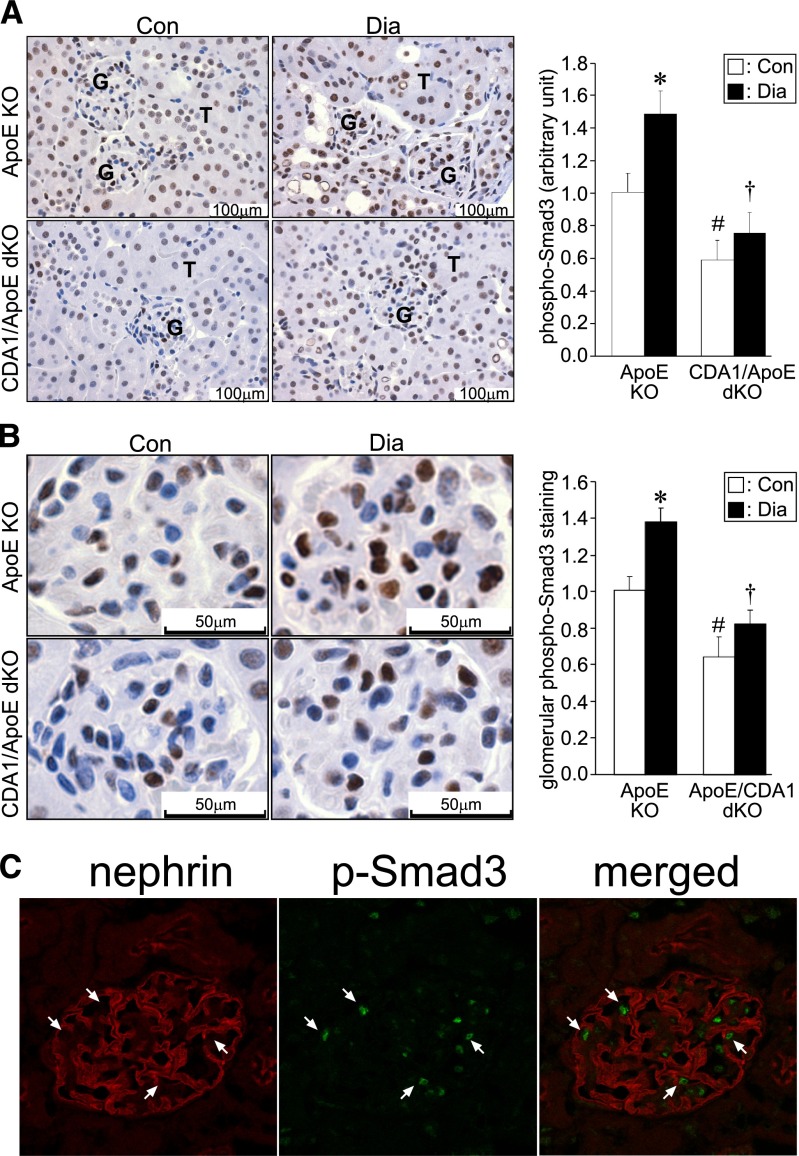
Because CDA1 seems to play a significant role in enhancing TGF-β signaling in vitro,19,20 it was hypothesized that the diabetes-induced activation of TGF-β signaling pathways, including Smad3 and extracellular signal-regulated kinase 1/2(p44/42) [ERK1/2(p44/42)] mitogen-activated protein kinase, would be attenuated in CDA1 KO mice. To examine this postulate, we assessed the activated form of Smad3 in the kidneys of these mice by immunohistochemical staining using an antibody specific for phospho-Smad3 (Ser423/425). Nuclear localization of phospho-Smad3 in both tubular cells (Figure 7A) and podocytes within glomeruli (Figure 7B) of both ApoE KO and CDA1/ApoE dKO mice was observed. Quantification of the staining showed that the basal level of phospho-Smad3 was ∼40% lower when CDA1 was deleted (P<0.05) (Figure 7A). Diabetes increased phospho-Smad3 levels by ∼1.5-fold in the ApoE KO mice (P<0.05), but no such increase was seen in diabetic CDA1/ApoE dKO mice, resulting in significantly lower levels of phospho-Smad3 in this dKO group compared with diabetic ApoE KO mice (P<0.001) (Figure 7A). Quantification of phospho-Smad3 within glomeruli alone showed a similar result (Figure 7B). A confocal image showed that most of the phospho-Smad3 staining-positive cells were also positive for nephrin staining, a well validated podocyte marker (Figure 7C).
Figure 7.
CDA1 deficiency attenuates diabetes-associated increase in TGF-β signaling in vivo. Immunohistochemical staining of phospho-Smad3 (C-terminal Ser-X-Ser phosphorylation site Ser423/425) on paraffin-embedded kidney sections from Con and 20-week Dia ApoE KO and CDA1/ApoE dKO mice. (A) Representative images of the phospho-Smad3 staining (brown color) are shown, with glomerulus (G) and tubule (T) indicated. Scale bar, 100 μm. Original magnification, ×400. (B) Representative images of glomerular phospho-Smad3 staining. Scale bar, 50 μm. Original magnification, ×400. Quantification of the staining is shown in bar graphs (right panels) as mean ± SE (n=10–12/group). *P<0.05 versus Con; #P<0.05 versus ApoE Con; †P<0.001 versus ApoE Dia. (C) Confocal microscopy images showing staining of nephrin (red) and phospho-Smad3 (p-Smad3; green) as well as a merged image (merged) on a frozen section of kidney from a 20-week Dia ApoE KO mouse. Arrow indicates the cells that are double positive for nuclear staining of phospho-Smad3 and membrane staining of nephrin.
TGF-β Fails to Stimulate Expression of ECM Genes in Kidney Cells Isolated from CDA1 KO Mice Because of Impaired TGF-β Signaling
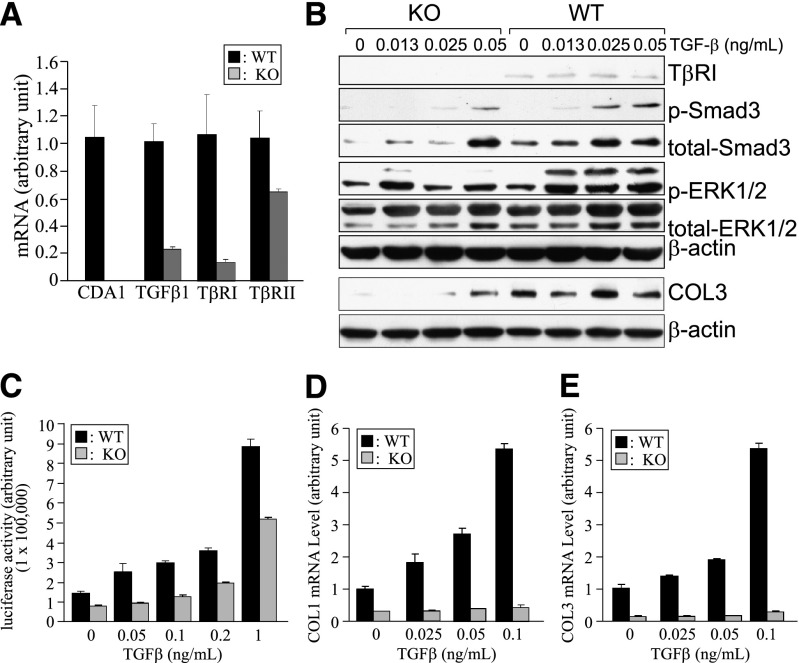
To directly examine the effect of CDA1 deficiency on TGF-β–stimulated signaling with its profibrotic effect in mouse kidney cells, we carried out a series of experiments using primary kidney cells isolated from CDA1 WT and KO mice. The absence of intact CDA1 mRNA in the cultured cells from CDA1 KO mice was confirmed by quantitative RT-PCR (RT-qPCR), showing an undetectable level of CDA1 (Figure 8A). mRNA levels of TGF-β1 and TβRI were reduced by ∼80%, and TβRII gene expression was reduced by >30% in CDA1 KO cells compared with WT cells (Figure 8A). Western blot analysis identified TβRI protein in WT cells, whereas it was undetectable in the CDA1 KO cells (Figure 8B).
Figure 8.
Deletion of CDA1 in kidney cells impairs TGF-β signaling, leading to failure of TGF-β to stimulate its target genes, such as collagens I and III. Kidney cells were isolated from CDA1 WT (black columns) and KO (gray columns) mice at the age of 6 weeks and cultured as described in Concise Methods. (A) mRNA levels of CDA1, TGF-β1, TβRI, and TβRII are shown as arbitrary units (mean ± SE) as determined by real-time RT-qPCR in CDA1 WT and KO kidney cells. (B) Western blotting analysis shows TβRI, p-Smad3 (Ser423/425), total Smad3, phospho-ERK1/2 (Thr202/Tyr204; p-ERK1/2), total ERK1/2, and β-actin in primary kidney cells isolated from CDA1 WT and KO mice, which were incubated with TGF-β at specified concentrations for 45 minutes. The same set of samples was analyzed by Western blotting under a nonreducing condition to detect collagen III, with β-actin serving as the loading control. (C) Luciferase activity (normalized against cotransfected β-galactosidase activity) of the Smad3-specific reporter construct, p(CAGA)12-Luc, in primary kidney cells of CDA1 WT and KO mice stimulated by TGF-β1 at specified concentrations for 48 hours. Data are shown as mean ± SE (n=6). (D and E) Cultured primary kidney cells isolated from CDA1 WT and KO mice were incubated with TGF-β at specified concentrations for 48 hours. mRNA levels of collagen I (COL1) and III (COL3) were determined by real-time RT-qPCR, and data are shown as mean ± SE (n=6).
TGF-β1 treatment for 45 minutes dose-dependently stimulated phosphorylation of Smad3 (Ser423/425), and this increase in Smad3 phosphorylation was significantly attenuated in CDA1 KO cells (Figure 8B). TGF-β1 also stimulated ERK1/2(p44/42) phosphorylation (Thr202/Tyr204) in the WT cells, and this effect was also attenuated in CDA1 KO cells (Figure 8B). Consistent with the reduced expression levels of these signaling molecules, the protein level of collagen III was found to be lower in CDA1 KO than WT cells (Figure 8B). The attenuation of TGF-β signaling in the CDA1 KO cells was further shown by measuring luciferase activity of the Smad3-specific reporter construct p(CAGA)12-Luc (luciferase reporter construct containing a repeat of 12 CAGA elements). TGF-β1 failed to activate the luciferase activity of this construct at low concentrations (0.05 and 0.1 ng/ml) in CDA1 KO cells, whereas TGF-β1 at these concentrations stimulated luciferase activity in a dose-dependent manner in CDA1 WT cells (Figure 8C). The stimulation of the p(CAGA)12-Luc reporter construct with TGF-β1 treatment at higher concentrations (0.2–1 ng/ml) was attenuated by ∼50% in CDA1 KO cells (Figure 8C). As a result of impaired TGF-β signaling, TGF-β1 failed to stimulate the expression of target genes, such as collagens I and III, in CDA1 KO cells, whereas these genes were stimulated two- to fivefold by TGF-β1 in a dose-dependent manner in CDA1 WT cells (Figure 8, D and E).
CDA1 Expression Is Elevated in Kidneys from Subjects with DN and Nondiabetic Renal Fibrosis
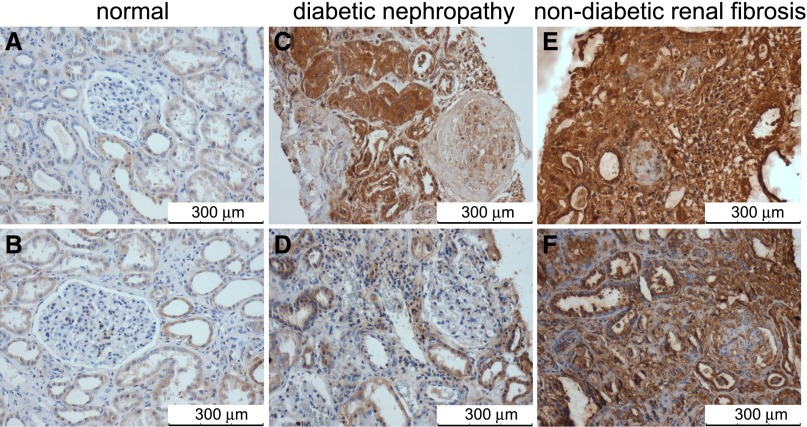
To assess if CDA1 upregulation observed in diabetic rodents is also seen in humans, human renal biopsy samples were evaluated. CDA1 is predominantly expressed in tubular cells in healthy kidney tissue with minimal glomerular staining (Figure 9, A and B). By contrast, immunohistochemical staining for CDA1 was increased in kidneys from individuals with diabetic kidney disease (Figure 9, C and D) and individuals with nondiabetic sclerotic renal disease (Figure 9, E and F). Notably, CDA1 immunostaining was markedly enhanced in sclerosed glomeruli from both diabetic and nondiabetic individuals. In association with increased staining of CDA1, an increase in phospho-Smad3 staining was observed in sclerotic human kidney biopsy specimens (Supplemental Figure 1).
Figure 9.
CDA1 protein levels are elevated in human kidneys with renal fibrosis. CDA1 proteins were shown by brown color in immunohistochemical staining of human kidney sections using specific anti-CDA1 antibodies (1:3000 dilution), which has been previously described.19 Scale bar, 300 μm. Original magnification, ×200. Sections are shown from the normal part of kidney tissues surgically removed from a patient with (A) a renal clear cell carcinoma or (B) a renal chromophobic cellular tumor, (C) a 39-year-old man with type 2 diabetes and end stage kidney disease, (D) a 56-year-old woman with type 1 diabetes and stage 3 CKD, (E) a 45-year-old man with focal segmental glomerulosclerosis, and (F) a 24-year-old woman with chronic interstitial nephritis and glomerulosclerosis.
Discussion
Enhancement of TGF-β signaling has been considered to be a key culprit in the pathogenesis and progression of DN, mediating the basement membrane thickening and increased production and accumulation of ECM within the glomerular mesangium and tubulointerstitium.10–12 TGF-β signaling can be considered a final common pathway activated by many factors associated with the diabetic milieu that promote DN, including high glucose,23 angiotensin II,24,25 and advanced glycation end products.26 Consequently, safely blocking the pathologic actions or activities of TGF-β represents an important priority for the prevention and management of DN. In the present study, we show that genetic deletion of CDA1, a key enhancer of TGF-β expression and activity,19,20 had no adverse effects on mouse health and attenuated the effects of diabetes on albuminuria, renal injury, and matrix accumulation in the kidney. When the cellular level of CDA1 is decreased by either genetic deletion, which was shown in this study, or siRNA knockdown, which was previously described,19,20 TGF-β signaling is attenuated but not totally blocked. Thus, these findings strengthen the postulate that CDA1 is a potential molecular target to reduce the pathologic levels of TGF-β activity in diabetes, leading ultimately to retardation of DN.
We originally identified CDA1 as the novel target for low titer autoantibodies found in patients with discoid lupus erythematosus.27 Recent studies have identified direct binding of CDA1 by its N terminus to SIRT6 (Sirtuin 6), a histone deacetylase known to be involved in inflammatory signaling and cell cycle regulation.28 Indeed, recent studies have shown that SIRT6 overexpression is able to antagonize TGF-β–mediated senescence in human bronchial epithelial cells.29 The mechanism by which CDA1 deletion attenuates TGF-β signaling in the kidney is a matter of ongoing investigation.
We hypothesize that the observed renoprotection in CDA1 KO mice was, at least in part, mediated by reduced gene expression of TGF-β receptors in the kidney (Figures 1 and 8), resulting in a functionally attenuated response to exogenous TGF-β, which was reflected by attenuated Smad3 and ERK1/2 activation. Notably, CDA1 deficiency reduces the expression of both TGF-β type I and II receptors, which are essential for TGF-β–induced effects on the cells. This in vivo observation is consistent with our previous in vitro findings. In HK-2 (human proximal tubule cell line) cells, alteration in cellular levels of CDA1 by using both overexpression and siRNA knockdown approaches modulated phosphorylation and activation of Smad3 as well as gene expression of TGF-β1 and TβRI.19 These effects of CDA1 were attenuated as a result of inhibition of Smad3 activity using an Smad3-specific inhibitor.19 These findings suggest that Smad3 mediates the effect of CDA1 on TGF-β signaling through an autoinduction mechanism, which was previously reported for the TGF-β pathway.30 However, the deletion of CDA1 does not eliminate TGF-β1 signaling. This finding may be one reason why CDA1 KO mice are viable and fertile without an obvious abnormal phenotype, whereas deletions of TGF-β1 or the TGF-β type I receptor genes are lethal in mice.18
We observed that CDA1 deficiency attenuated TGF-β–stimulated Smad3 phosphorylation in the primary kidney cells from these mice (Figure 8B). This finding is consistent with our previous findings using an siRNA approach to knockdown CDA1 in various cell types.19,20 However, the protein level of total Smad3 was lower in the primary kidney cells isolated from the CDA1 KO mice than cells isolated from WT mice, and TGF-β treatment seemed to further increase the protein level of Smad3 (Figure 8B). These latter findings are different from our previous observation in different cells, where CDA1 deficiency with siRNA knockdown, albeit resulting in attenuation of Smad3 phosphorylation, did not change or even tend to increase levels of total Smad3.19,20 Nevertheless, the level of phospho-Smad3 is an important parameter, because it is phospho-Smad3 that mediates the downstream signaling of TGF-β and not the inactive form of Smad3.
Overall, the effect of CDA1 deficiency in diabetic mice seems to be primarily related to a reduction in TGF-β signaling, resulting in a reduction in ECM accumulation. CDA1 deficiency did not prevent other renal changes, including polyuria, renal hypertrophy, and hyperfiltration, which is consistent with a lack of effect of this protein on glucose control. The effect of CDA1 deficiency on diabetes-associated albuminuria was variable in the two animal models examined. There was no difference in urinary albumin excretion after 20 weeks of diabetes between CDA1 WT and KO mice (Table 1). This finding is consistent with the previous reports on targeting TGF-β or its signaling molecules in experimental diabetes using various approaches, including studies with TGF-β neutralizing antibodies,11 or Smad3 KO mice.31 However, in the accelerated model of DN (ApoE KO mice) used in our study, CDA1 deficiency was shown to attenuate diabetes-associated albuminuria but not totally prevent it (Table 2). The underlying mechanism for this differential effect in these two models remains to be clarified. However, the presence of glomerulosclerosis in (susceptible) diabetic ApoE KO mice and a much less prominent fibrotic phenotype in diabetic C57BL6 mice may provide one explanation as to why an essentially antifibrotic agent may have differential effects on diabetes-associated albuminuria. Furthermore, it is noted that the renal mRNA levels of CDA1 were increased approximately fourfold after 20 weeks of diabetes in ApoE KO mice, which was previously reported by us,19 whereas we detected only an approximately twofold increase in renal CDA1 gene expression in C57Bl6 mice in this study (Figure 1). Whether this difference in the degree of CDA1 upregulation in response to diabetes is responsible for the observed differences in the effect of CDA1 deficiency on proteinuria in C57Bl6 and ApoE KO mice is not yet fully elucidated. Another possibility is that the renoprotective effect of CDA1 deletion is more readily detectable in a more advanced model of nephropathy, which is represented by the diabetic ApoE KO mouse model.
In summary, CDA1 deficiency was shown to attenuate TGF-β signaling and block TGF-β–stimulated gene expression of ECM molecules in the kidney cells from CDA1 KO mice. CDA1 expression is increased in the diabetic kidney in both humans (Figure 9) and rodents.19 Renal CDA1 expression is also increased in humans with the sclerotic form of nondiabetic renal disease. Taken together, our findings validate CDA1 as a potential molecular target to prevent and/or retard DN and other sclerotic renal disorders associated with TGF-β–mediated fibrogenesis.
Concise Methods
Antibodies and Reagents
Antibodies to CDA1 have been previously described.19,20,27 Commercially available antibodies were purchased, including antibodies to phospho-Smad3 (Ser423/425; 44–246G; Invitrogen), total Smad3 (51–1500; Invitrogen), phospho- and total ERK1/2 (Thr202/Tyr204; 9102 and 9106; Cell Signaling Technology, Danvers, MA), and collagens III and IV (SouthernBiotech, Birmingham, AL). Antiphospho-Smad3 (Ser423/425; ab52903) for immunohistochemical staining was purchased from Abcam, Cambridge, United Kingdom.
Generation of CDA1 KO and CDA1/ApoE dKO Mice
The CDA1 encoding gene, Tspyl2, was targeted by a construct through homologous recombination, which resulted in insertion of LoxP sites flanking exons 2–5 and a neoexpression cassette in an opposite direction within intron 1. Targeted C57BL6 mouse embryonic stem cells identified by Southern blotting and checked for the absence of random integration were then injected into blastocysts to generate chimera mice. Targeted offspring of the chimera mice were identified by coat color and then confirmed by Southern blotting, and they were subsequently crossed with a global cre mouse to generate a global CDA1 KO mouse. Deletion of exons 2–5 in the CDA1 KO mouse was further confirmed by sequencing of the PCR-amplified genomic DNA fragment and cDNA derived from the kidney tissues. The CDA1 KO mouse was crossed with an ApoE KO mouse, which was also on the C57BL6 background, to generate a CDA1/ApoE dKO strain.
Diabetes Model
Male WT, CDA1 KO, ApoE KO, or CDA1/ApoE dKO mice at the age of 6 weeks were intraperitoneally injected with STZ at a dose of 55 mg/kg body wt daily for 5 days to be rendered diabetic or injected with buffer to serve as nondiabetic controls. Blood glucose levels were monitored weekly for 5 weeks after STZ injections to confirm the diabetic status of these mice. Animals were housed on a 12-hour light/dark cycle with free access to water and standard mouse chow, and they were killed after 20 weeks of diabetes for collection of plasma and kidney tissues. Urine samples were collected in metabolic cages (Iffa Credo, L'Arbresele, France) for 24 hours before the end of the experiment. The animal study was approved according to the international guidelines, including the “Principles of Laboratory Animal Care” (National Institutes of Health, 1985) and the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes” (National Health and Medical Council of Australia, 2004). Methods for the measurement of glycated Hb and creatinine in blood and urine by HPLC (as recommended by the Animal Models of Diabetic Complications Consortium) as well as plasma glucose levels have been described previously.32,33 Albumin in urine was measured using the Mouse Albumin ELISA Quantitation Set (Bethyl Laboratories Inc., Montgomery, TX).
Gene-Specific mRNA Quantification by Real-Time RT-PCR
Relative mRNA levels of specific genes were determined by real-time RT-qPCR (7500 Fast Real-Time PCR; Applied Biosystems, Foster City, CA) after reverse transcription of total RNA into cDNA with primers and probes purchased from Applied Biosystems (Applied Biosystems) as previously described.19,20 Endogenous 18S ribosome RNA (Taqman; Applied Biosystems) was concurrently determined in the reaction and used as an internal control to normalize samples. The results were shown as fold changes (arbitrary unit) relative to control samples. The primers and probes were ordered from Applied Biosystems, and the sequences are shown in Supplemental Table 2.
Immunohistochemical Staining and Masson’s Trichrome Staining
Paraffin sections of mouse kidney samples were stained for collagens III and IV using specific antibodies (1:200 dilution; SouthernBiotech), and ECM was stained by Masson’s Trichrome kit as described previously.19 For phospho-Smad3 staining, the sections were heated in 10 mM citrate buffer in a microwave oven for 12 minutes with medium-low power to retrieve the epitopes before staining using a specific antibody (1:150 dilution) to phospho-Smad3 (Ser423/425; Abcam, Cambridge, United Kingdom). The positive staining signals were quantified by analyzing >10 views per section in the cortex using Image-Pro Plus (Media Cybernetics, Bethesda, MD). Renal ECM was determined by Masson’s Trichrome staining kit (AMT.K) from Australian Biostain P/L (Traralgon, Victoria, Australia) as previously described.19
Paraffin sections of human kidney tissues were obtained from either biopsy samples from subjects with CKD or a normal part of kidney surgically removed from subjects with a kidney tumor. CDA1 proteins were shown in brown color by immunohistochemical staining of human kidney sections using specific rabbit anti-CDA1 antibodies (1:3000 dilution), which has been previously described.19 The staining specificity of the antibodies was shown by a negative control without primary antibody (data not shown), and the antibody specificity had been previously shown by antigen absorption.19
Immunofluorescence Double Staining
Fresh frozen tissue sections were fixed in 4% paraformaldehyde for 30 minutes before being washed in PBS and blocked with 5% FCS/PBS containing 0.05% TWEEN20. A rabbit antibody to phospho-Smad3 (1 in 50 dilution; Ab52903; Abcam) and a guinea pig antibody to nephrin (1 in 50 dilution; BP5030; Acris) were incubated at room temperature for 1 hour and then visualized using secondary antibodies to rabbit IgG (raised in chicken) conjugated with Alexafluor 488 and guinea pig IgG (raised in goat) conjugated with Alexafluor 568 (both 1 in 200 dilution), respectively, after 30 minutes of incubation at room temperature. Sections were mounted using Fluorsave Reagent (345789; Millipore) and imaged using the Nikon A1r Plus Japan Confocal microscope.
Evaluation of Renal Pathologic Changes
Paraffin-embedded sections of mouse kidney with a thickness of 2 μm were stained by Periodic acid–Schiff method, and the morphologic changes reflecting the pathologic injuries in glomeruli and tubulointerstitial space were evaluated in a blinded fashion in a semiquantified manner as previously described.34 Briefly, 20 glomeruli per section were scored for the severity of sclerotic injury, with 0 for an intact glomerulus, 1 for less than 25%, 2 for 25%–50%, 3 for 50%–75%, and 4 for >75% of a glomerulus damaged. The tubulointerstitial injury index was assessed by evaluating amount and severity of tubule dilation, atrophy, and loss of tubular cells. Twenty views of a kidney section were photographed (magnification, ×200) and scored with 0 for no injury, 1 for <25%, 2 for 25%–50%, 3 for 50%–75%, and 4 for >75% of the proportion with tubulointerstitial injury.
Isolation and Culture of Primary Mouse Kidney Cells
Mice from 6- to 8-week-old female CDA1 WT and KO were culled by 0.3 ml intraperitoneal injection of Lethabarb. Kidneys were removed using sterile technique under cold condition. After washing in PBS, kidneys were sieved by a 100-μm cell strainer (352360; BD Falcon, Franklin Lakes, NJ) using PBS as flow-through media. The sieved-through cells were collected and pelleted by centrifugation at 4°C. The cells were washed with PBS and then cultured in RPMI 1640 medium containing 16% inactivated FCS (Invitrogen, Carlsbad, CA), l-glutamine (2503; GIBCO, Grand Island, NY), and antibiotic antimycotic (A5955; Sigma-Aldrich, St. Louis, MO) at 37°C with 5% CO2. Cells of second generation were used for all studies.
Luciferase Assay
Primary kidney cells from both CDA1 WT and KO mice were seeded in six-well plates. Cells at approximately 80% confluency were cotransfected with DNA containing 4 μg/well p(CAGA)12-Luc and 0.5 μg/well pCMV-LacZ (cytomegalovirus promoter driven beta-galactosidase plasmid) using Lipofectamine 2000 (11668–019; Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Cells were allowed to recover in RPMI-1640 containing 2% FCS overnight. The next day, cells were treated with TGF-β1 at specified concentrations for 30 hours. Luciferase and β-galactosidase activities were determined using the corresponding assay kits from Promega, Madison, WI (E1501 and E2000, respectively).
Data Analyses
Data are expressed as mean ± SEM unless otherwise specified. Differences in the mean among groups were analyzed using two-way ANOVA followed by pairwise comparisons between groups using the Newman–Keuls post hoc t test. A difference of P<0.05 was considered to be statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. Kylie Gilbert and Ms. Samantha Sacca for their assistance with animal studies and Mr. Edward Grixti for his assistance with HPLC for analysis of plasma and urine samples. We acknowledge the facilities and scientific and technical assistance of Monash Micro Imaging, Monash University, for acquiring confocal microscopy images.
This work was funded by the National Health and Medical Research Council of Australia and the Juvenile Diabetes Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010060/-/DCSupplemental.
References
- 1.Foley RN, Collins AJ: End-stage renal disease in the United States: An update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Rychlík I, Locatelli F, Halimi S: End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 34: 795–808, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Jim B, Ziyadeh FN: Diabetic nephropathy and transforming growth factor-beta: Transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23: 532–543, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC, 3rd: From fibrosis to sclerosis: Mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57: 1439–1445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME: Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest 108: 1853–1863, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH: Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int 65: 116–128, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Mason RM, Wahab NA: Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 14: 1358–1373, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns WC, Twigg SM, Forbes JM, Pete J, Tikellis C, Thallas-Bonke V, Thomas MC, Cooper ME, Kantharidis P: Connective tissue growth factor plays an important role in advanced glycation end product-induced tubular epithelial-to-mesenchymal transition: Implications for diabetic renal disease. J Am Soc Nephrol 17: 2484–2494, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Houlihan CA, Akdeniz A, Tsalamandris C, Cooper ME, Jerums G, Gilbert RE: Urinary transforming growth factor-beta excretion in patients with hypertension, type 2 diabetes, and elevated albumin excretion rate: Effects of angiotensin receptor blockade and sodium restriction. Diabetes Care 25: 1072–1077, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ: Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int 73: 705–715, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S: Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90: 770–774, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni AB, Karlsson S: Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143: 3–9, 1993 [PMC free article] [PubMed] [Google Scholar]
- 15.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ: Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121: 1845–1854, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Larsson J, Goumans MJ, Sjöstrand LJ, van Rooijen MA, Ward D, Levéen P, Xu X, ten Dijke P, Mummery CL, Karlsson S: Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J 20: 1663–1673, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, Gress RE, Hines KL, Tian H, Karlsson S: Immune dysregulation in TGF-beta 1-deficient mice. J Immunol 153: 1936–1946, 1994 [PubMed] [Google Scholar]
- 18.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T: Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu Y, Wu T, Dai A, Pham Y, Chew P, de Haan JB, Wang Y, Toh BH, Zhu H, Cao Z, Cooper ME, Chai Z: Cell division autoantigen 1 enhances signaling and the profibrotic effects of transforming growth factor-β in diabetic nephropathy. Kidney Int 79: 199–209, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Pham Y, Tu Y, Wu T, Allen TJ, Calkin AC, Watson AM, Li J, Jandeleit-Dahm KA, Toh BH, Cao Z, Cooper ME, Chai Z: Cell division autoantigen 1 plays a profibrotic role by modulating downstream signalling of TGF-beta in a murine diabetic model of atherosclerosis. Diabetologia 53: 170–179, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Lassila M, Jandeleit-Dahm K, Seah KK, Smith CM, Calkin AC, Allen TJ, Cooper ME: Imatinib attenuates diabetic nephropathy in apolipoprotein E-knockout mice. J Am Soc Nephrol 16: 363–373, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lassila M, Seah KK, Allen TJ, Thallas V, Thomas MC, Candido R, Burns WC, Forbes JM, Calkin AC, Cooper ME, Jandeleit-Dahm KA: Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: Role of advanced glycation end products. J Am Soc Nephrol 15: 2125–2138, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY: Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int 63: 2010–2019, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Vita J, Sánchez-López E, Esteban V, Rupérez M, Egido J, Ruiz-Ortega M: Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation 111: 2509–2517, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Rupérez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M: Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 108: 1499–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY: Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: Implications for diabetic renal and vascular disease. FASEB J 18: 176–178, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Chai Z, Sarcevic B, Mawson A, Toh BH: SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J Biol Chem 276: 33665–33674, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Polyakova O, Borman S, Grimley R, Vamathevan J, Hayes B, Solari R: Identification of novel interacting partners of Sirtuin6. PLoS One 7: e51555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K: Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Fraser D, Phillips A: ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. Am J Pathol 169: 1282–1293, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A, Ziyadeh FN, Lee EY, Pyagay PE, Sung SH, Sheardown SA, Laping NJ, Chen S: Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol 293: F1657–F1665, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Dunn SR, Qi Z, Bottinger EP, Breyer MD, Sharma K: Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int 65: 1959–1967, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA: Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57: 2461–2469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly DJ, Wilkinson-Berka JL, Allen TJ, Cooper ME, Skinner SL: A new model of diabetic nephropathy with progressive renal impairment in the transgenic (mRen-2)27 rat (TGR). Kidney Int 54: 343–352, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.