Abstract
The genetic factors underlying the pathogenesis of lupus nephritis associated with systemic lupus erythematosus are largely unknown, although animal studies indicate that nuclear factor (NF)-κB is involved. We reported previously that a knockin mouse expressing an inactive form of ABIN1 (ABIN1[D485N]) develops lupus-like autoimmune disease and demonstrates enhanced activation of NF-κB and mitogen-activated protein kinases in immune cells after toll-like receptor stimulation. In the current study, we show that ABIN1[D485N] mice develop progressive GN similar to class III and IV lupus nephritis in humans. To investigate the clinical relevance of ABIN1 dysfunction, we genotyped five single-nucleotide polymorphisms in the gene encoding ABIN1, TNIP1, in samples from European-American, African American, Asian, Gullah, and Hispanic participants in the Large Lupus Association Study 2. Comparing cases of systemic lupus erythematosus with nephritis and cases of systemic lupus erythematosus without nephritis revealed strong associations with lupus nephritis at rs7708392 in European Americans and rs4958881 in African Americans. Comparing cases of systemic lupus erythematosus with nephritis and healthy controls revealed a stronger association at rs7708392 in European Americans but not at rs4958881 in African Americans. Our data suggest that variants in the TNIP1 gene are associated with the risk for lupus nephritis and could be mechanistically involved in disease development via aberrant regulation of NF-κB and mitogen-activated protein kinase activity.
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by an abnormal immune response leading to autoantibody production, immune complex formation, T cell activation, and inflammatory cytokine release.1 Multiple factors contribute to the immune response in SLE, including genetic, epigenetic, immunoregulatory, environmental, and hormonal factors.1 Lupus nephritis (LN) occurs in about 50% of patients with SLE and is a major cause of morbidity and mortality.2 The incidence of LN varies among different ethnic groups, suggesting that genetic factors play an important role in the pathogenesis. Patients with African ancestry are at increased risk for LN.3 Immunosuppressive treatment is effective in only about 50% of patients with LN,4 and that therapy is associated with undesirable short- and long-term adverse effects. Thus, identifying the molecular mechanisms responsible for the pathogenesis of LN is necessary to define more specific diagnostic and therapeutic targets. However, the complex interactions of genetic risks, environmental factors, and molecular events that contribute to the development of LN are only beginning to be defined.
The transcription factor nuclear factor-κB (NF-κB) regulates the expression of hundreds of genes that control cell proliferation and survival, the cellular stress response, innate immunity, and inflammation. Dysregulation of NF-κB activity is associated with many human diseases, especially those involving chronic inflammation, and recent studies suggest that NF-κB plays a role in the incidence and severity of LN as well.5–9 Immunohistochemistry-based studies have shown enhanced glomerular and tubular expression of NF-κB, NF-κB regulatory proteins, and NF-κB target proinflammatory cytokines in renal biopsy specimens from patients with LN compared with normal controls and patients with minimal-change disease.8,9 Another report found that pharmacologic inhibition of NF-κB reduced the development of autoantibodies and renal impairment in SLE-susceptible FcγRIIb-deficient mice.5 Treatment of spontaneous SLE–developing SWRxNZB mice with a flavonoid, apigenin, inhibited NF-κB–mediated events in T cells and suppressed serum IgG levels, resulting in delayed appearance of nephritis.6
NF-κB is activated by a variety of immune, inflammatory, and stress stimuli through cytokine and toll-like receptors (TLRs) and regulated through a complex interplay of proteins (recently reviewed).10 In resting cells, NF-κB is sequestered in the cytoplasm in an inactive state by binding to inhibitor of κB (IκB) proteins.11,12 Following activation, the IκB is phosphorylated, polyubiquitinated through Lys48 linkages, and then degraded by the proteasome. This releases an active NF-κB complex to translocate to the nucleus and drive target gene expression. In the canonical NF-κB pathway, phosphorylation of IκB is mediated by the IκB kinase (IKK) complex, which consists of α, β, and γ subunits.13 IKKγ is the regulatory subunit also referred to as NF-κB essential modulator (NEMO). Activation of IKK is mediated by TGF-β–activated kinase 1 (TAK1), and recruitment of TAK1 to IKK is regulated by an integral complex of proteins that is assembled through protein-protein interactions to lysine 63-linked polyubiquitin chains.14 NEMO binding to head-to-tail linked linear polyubiquitin chains or the linear ubiquitin assembly complex also activates the canonical NF-κB pathway.15–17
The ubiquitin-editing protein, A20, and ubiquitin-binding protein, A20 binding inhibitor of NF-κB 1 (ABIN1) have important inhibitory roles in NF-κB signaling.18,19 ABIN1 binds to lysine 63-linked and linear polyubiquitin chains and contains the same ubiquitin-binding domain as NEMO that facilitates binding to other regulatory proteins, such as TRAF2/6, RIP1, and IRAK1.16,20–22 It is not clear how ABIN1 inhibits NF-κB activity, but two possible mechanisms have been proposed. One is that ABIN1 binding competes with NEMO binding to proteins required for activation of IKK, and the second is that ABIN1 recruits A20 to the IKK regulatory complex,23 where A20 disrupts the interactions needed for IKK activation by removing lysine 63- or linear polyubiquitin moieties from regulatory proteins.24 A20 has also been reported to mediate proteasomal degradation of ubiquitin processing proteins that mediate IKK activation.25
We previously reported that ABIN1[D485N] transgenic mice with disrupted ABIN1 lysine 63 and linear polyubiquitin binding have enhanced NF-κB and mitogen-activated protein kinase (MAPK) signaling in B cells, bone marrow–derived macrophages, and dendritic cells after stimulation with TLR agonists and developed an SLE-like autoimmune disease with enlarged spleens and lymph nodes and autoantibodies in the serum.26 Crossing ABIN1[D485N] mice with mice deficient for the TLR/IL-1R adaptor protein MyD88 suppressed the autoimmunity, indicating that a function for ABIN1 in autoimmunity is TLR/IL-1R-dependent. In the present study, we show that ABIN1[D485N] mice develop diffuse proliferative GN similar to class III and IV human LN. We also found that two SNPs in TNIP1, the gene encoding human ABIN1, have a strong association with LN. Our data suggest that variants in the TNIP1 gene could be mechanistically involved in LN via disrupted regulation of NF-κB and MAPK activity.
Results
Renal Pathophysiology of ABIN1[D485N] Knockin Mice
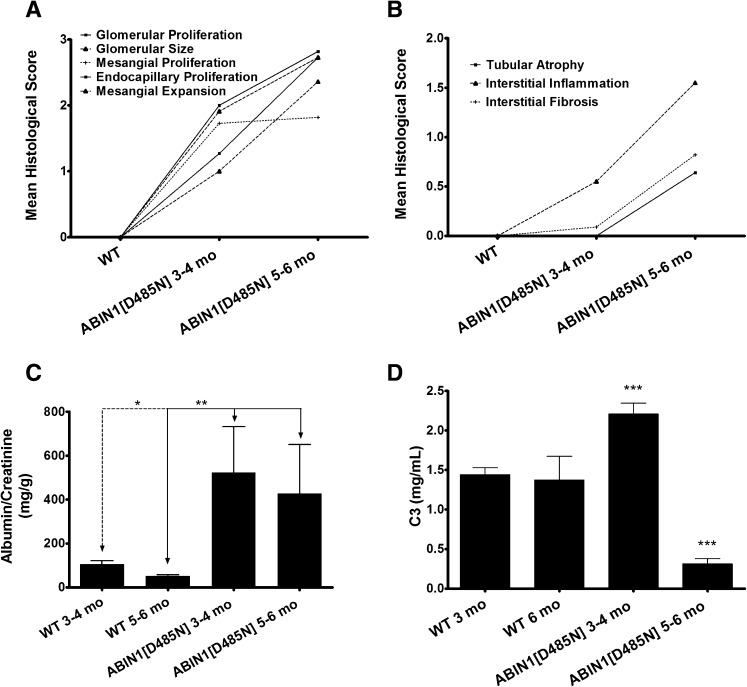
We reported previously that ABIN1[D485N] mice develop lupus-like autoimmunity.26 The aim of the present study was to completely characterize the renal abnormalities in these mice. Table 1 presents the evaluation of the histologic grading of glomerular changes in wild-type (WT) and ABIN1[D485N] mice at 3–4 months and 5–6 months of age. The scoring system used for histologic grading is described in the Concise Methods. Glomerular size, mesangial and endocapillary proliferation, and mesangial expansion in ABIN1[D485N] mice were all significantly increased by 3–4 months. By 5–6 months ABIN1[D485N] mice demonstrated a significantly progressive increase in glomerular size, endocapillary proliferation, and mesangial expansion compared with 3- to 4-month-old ABIN[D485N] mice (Figure 1A). All mice at 3–4 months of age demonstrated glomerular hypercellularity, with 50% showing severe (3+) changes. Hypercellularity was focal in two mice and diffuse in nine. At 5–6 months all mice showed diffuse hypercellularity, and all but one were graded severe (3+). An increase in mesangial matrix was seen in 8 of 11 mice at 3–4 months but was only mildly increased in 3 of those 8 mice. At 5–6 months, all but one mouse demonstrated a moderate to severe increase in mesangial matrix. Crescents were observed in only two mice at 5–6 months, and glomerular sclerosis was seen in four mice.
Table 1.
Glomerular, interstitial, and tubular pathology assessment in WT and ABIN1[D485N] kidneys
| Variable | Glomerular Size | Glomerular Proliferation | Mesangial Proliferation | Endocapillary Proliferation | Mesangial Expansion | Glomerular Fibrosis | Crescents | Tubular Atrophy | Interstitial Inflammation | Interstitial Fibrosis | Total Pathology Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT 3–6 mo | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| [D485N] 3–4 mo | 1.91 (0.16)a,b | 2 (0.19)a | 1.73 (0.19)a | 1.27 (0.27)c | 1 (0.13)a | 0 (0)d | 0 (0)d | 0 (0)d | 0.55 (0.16)e | 0.09 (0.09)d | 8.55 (0.88)a |
| [D485N] 5–6 mo | 2.73 (0.19)a,b/e,f | 2.82 (0.18)a,e | 1.82 (0.18)a,d | 2.73 (0.19)a,c | 2.36 (0.28)a,c | 0.09 (0.09)d | 0.09 (0.09)d | 0.64 (0.24)d | 1.55 (0.25)a,e | 0.82 (0.26)d,e | 15.64 (1.32)a,c |
Data are mean pathologic score (SEM). The numbers were derived using a scoring system described in the Concise Methods.
P<0.001.
P values for ABIN1[D485N] versus WT mice.
P<0.005.
P=NS.
P<0.05.
P values for ABIN1[D485N] 3–4 months versus 5–6 months.
Figure 1.
ABIN1[D485N] knockin mice develop progressive GN. (A and B) Graphical representations of the data in Table 1 showing that ABIN1[D485N] mice develop progressive glomerular and interstitial injury compared with WT mice. (C) Urine albumin-to-creatinine ratio of 3- to 4-month-old and 5- to 6-month-old ABIN1[D485N] and WT mice (total n=82). (D) Serum complement factor 3 (C3) levels for the same groups. All P values were calculated using the Mann-Whitney rank-order test.
Table 1 and Figure 1B show that there was a significant amount of interstitial inflammation in ABIN1[D485N] mice at 3–4 months of age, while little or no tubular atrophy or interstitial fibrosis was observed. By 5–6 months, interstitial inflammatory cell infiltration, tubular atrophy, and interstitial fibrosis had significantly increased (Figure 1B). Mild interstitial inflammation was seen in only 4 of 11 mice at 3–4 months. By 5–6 months, all mice had some degree of interstitial inflammation, which was moderate or severe in 8 of 11 mice. Mild, patchy tubular atrophy was seen in <50% of mice in each age group. A sparse amount of tubulointerstitial fibrosis was seen in 2 of 11 mice at 3–4 months, and mild to moderate tubulointerstitial fibrosis was observed in 6 of 11 mice at 5–6 months. Histologic injury did not differ between male and female mice.
Figure 1C shows that the urine albumin-to-creatinine ratio was significantly elevated in 3- to 4-month-old ABIN1[D485N] mice compared with WT mice. Urine albumin excretion did not increase further in 5- to 6-month-old ABIN[D485N] mice. Surprisingly, serum C3 was significantly higher in 3- to 4-month-old ABIN1[D485N] animals compared with WT mice, but in concordance with severity of human LN, C3 was markedly decreased in the 5- to 6-month-old ABIN1[D485N] mice (Figure 1D). NF-κB–dependent C3 inductions have been reported.27–29 Thus, elevated serum C3 at 3–4 months could result from a combination of enhanced NF-κB activity and minimal kidney C3 deposition at this age in ABIN1[D485N] mice (not shown).
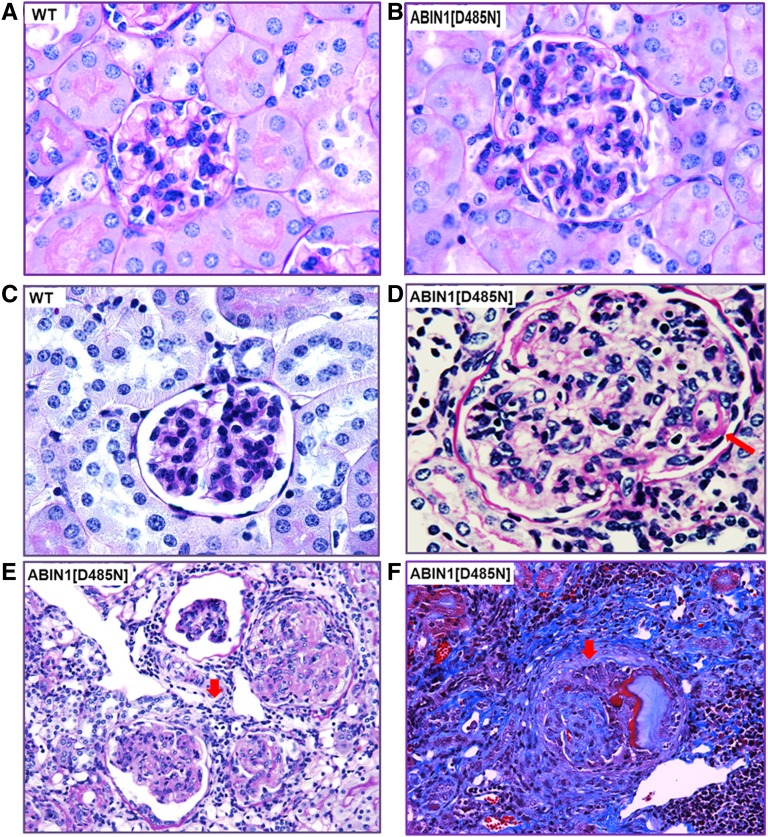
Figures 2 and 3 show representative examples of the histologic findings depicted in Table 1 and Figure 1. Figure 2B shows a glomerulus from an ABIN1[D485N] mouse at 4 months of age demonstrating mild mesangial and endocapillary hypercellularity with little or no mesangial matrix expansion or interstitial inflammatory infiltrate, and Figure 2A shows a glomerulus from a WT mouse for comparison. Figure 2C shows a glomerulus from a WT 6-month-old mouse. Figure 2, D–F, shows the more severe glomerular changes seen in 5- to 6-month-old ABIN[D485N] mice. Figure 2D shows a glomerulus with moderate mesangial and endocapillary cell proliferation and a thickened capillary loop wall typical of “wire loops” (arrow). Figure 2E shows an example of severe glomerular hypercellularity, marked mesangial matrix expansion, and severe interstitial cell infiltration (arrow). Figure 2F shows a glomerulus with crescent formation (arrow), glomerular sclerosis, and interstitial fibrosis.
Figure 2.
ABIN1[D485N] mouse kidneys display pathologic features of proliferative immune-mediated GN. (A) 100× magnification of a periodic acid-Schiff (PAS)–stained kidney section from a 3- to 4-month-old WT mouse. (B) Comparative 100× PAS image shows that at 3–4 months, the ABIN1[D485N] mouse kidneys display mesangial hypercellularity, matrix expansion, and capillary loop thickening compared with WT mouse kidneys. (C) 100× magnification of PAS-stained kidney section from a 5- to 6-month-old WT mouse. (D) Comparative 100× PAS image shows that at 5–6 months the ABIN1[D485N] mouse kidneys display severe mesangial hypercellularity, matrix expansion, and “wire loops” (arrow) compared with WT mouse kidneys. (E) 40× magnification PAS image showing examples of glomerular injury and extensive interstitial immune cell infiltration (arrow) observed in 5- to 6-month-old ABIN1[D485N] mouse kidneys. (F) 40× magnification of Masson trichrome staining showing examples of tubulointerstitial fibrosis, glomerular fibrosis, and crescent formation (arrow) and immune cell infiltration (lower right corner) in 5- to 6-month-old ABIN1[D485N] mouse kidneys.
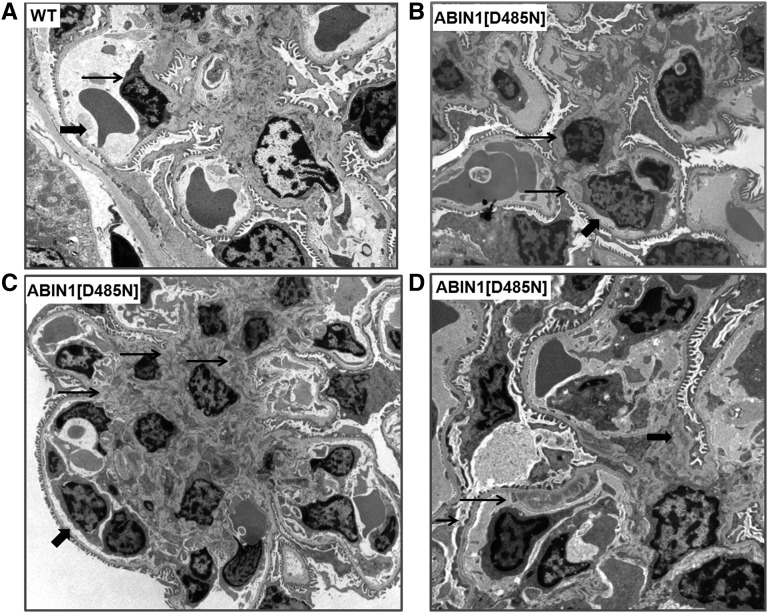
Figure 3.
ABIN1[D485N] mouse glomeruli display mesangial and subendothelial deposits. (A) TEM image of WT mouse kidney (large arrow, erythrocyte; small arrow, endothelial cell). (B) Minimal mesangial expansion and immune deposits (small arrows) in a TEM image of a 4-month-old ABIN1[D485N] mouse kidney. Podocyte foot processes are intact. The large arrow points out a monocyte in a capillary. (C) Extensive mesangial expansion and immune deposits (small arrows) in a TEM image of a 6-month-old ABIN1[D485N] mouse kidney. The large arrow points out a monocyte in a capillary. (D) Example of subendothelial immune deposits (long small arrow), areas of podocyte effacement (short small arrow), and mesangial deposits (large arrow) in a 6-month-old ABIN1[D485N] mouse kidney.
Figure 3A shows a transmission electron micrograph (TEM) from a 6-month-old WT mouse. Figure 3B shows a TEM from a 4-month-old ABIN1[D485N] mouse demonstrating moderate mesangial expansion and small electron-dense deposits in the mesangium (small arrows). Podocyte foot processes are intact. The large arrow points out a monocyte in a capillary. TEM in Figure 3C shows extensive mesangial expansion and mesangial deposits in a 6-month-old ABIN1[D485N] mouse kidney (small arrows). The large arrow points out a monocyte in a capillary. Figure 3D shows a TEM from a 6-month-old ABIN1[D485N] mouse demonstrating subendothelial (long, small arrow) and mesangial (large arrow) electron-dense deposits and focal foot process effacement (short, small arrow).
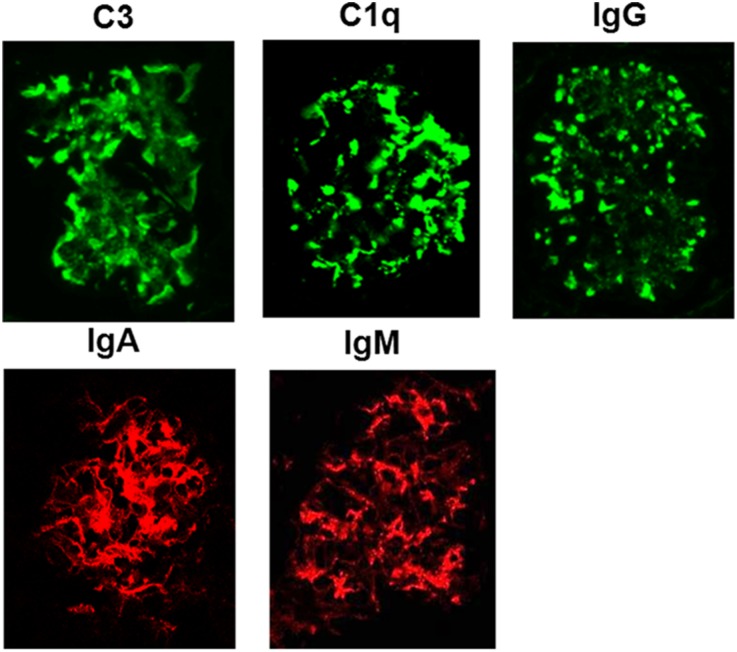
Figure 4 shows immunofluorescence images of 5- to 6-month-old ABIN1[D485N] kidneys demonstrating IgG, IgA, IgM, C3, and C1q deposition along capillary loops and in the mesangium. Immunofluorescence from 3- to 4-month-old ABIN1[D485N] mouse kidneys showed mild deposition of immunoglobulin and complement within the mesangium (not shown).
Figure 4.
Deposits in ABIN1[D485N] glomeruli contain complement factors and immunoglobulins. The panels show examples of the immunofluorescence staining that was observed for the different labeled complement factors and immunoglobulin subtypes in kidneys from 5- to 6-month-old ABIN1[D485N] mice. No significant immunofluorescence was observed for same-age WT mice.
Our results show that loss of ABIN1 function in NF-κB activity is associated with the development of proteinuria and proliferative GN with features typical of class III and IV human LN. Those changes become progressively worse between 3 and 6 months, at which time they are associated with low serum C3 levels. It should also be noted that there were no significant differences for any of the observed phenotypes between male and female ABIN1[D485N] mice. From our animal data, we postulated that genetic alterations in ABIN1 leading to dysregulation of NF-κB and MAPK activation could be involved in the development of LN. This hypothesis led us to evaluate single nucleotide polymorphisms (SNPs) in the gene for ABIN1, TNIP1, in patients who have SLE with and without LN.
Association of TNIP1 SNPs and Human LN
To investigate clinical relevance for ABIN1 dysfunction in human LN, five SNPs in TNIP1 previously associated with autoimmune diseases, including SLE, were genotyped in a total of 16,999 individuals of European, African American, Asian, Gullah, and Hispanic ancestry.30 After applying quality control assessment, we studied 15,864 individuals consisting of 3089 SLE cases with LN, 4308 SLE cases without LN, 1097 SLE cases with unknown LN status, and 7492 healthy controls (Materials and Methods; Table 2). To evaluate evidence for genetic association, we performed single-marker logistic regression analysis adjusting for sex and global ancestry estimates (Materials and Methods). Comparing SLE cases with LN to SLE cases without LN (case/case analysis) showed strong associations surpassing the Bonferroni-corrected significant threshold of P<0.01 at rs7708392 in persons of European ancestry (odds ratio [OR], 1.22; P=3.66×10−4) and rs4958881 in African Americans (P=8.47×10−3, OR = 1.22) (Table 3). Both of these variants are in noncoding regions of TNIP1.31,32 No SNPs reached the Bonferroni-corrected significant threshold in other populations, which concurs with published data using lupus as the phenotype.30 Analysis of SLE cases with LN versus healthy controls confirmed the validity of our case-only analysis (rs7708392 in persons of European ancestry: OR, 1.44 [P=1.82×10−11]; rs4958881 in African Americans: OR, 1.20 [P=4.43×10−3]). Overall, our results indicate that polymorphisms in the region of TNIP1 are associated with LN in persons of European ancestry and African Americans.
Table 2.
Sample summary following quality control adjustments
| Population | Samples (n) | Healthy Controls (n) | SLE Cases with LN (n) | SLE Cases without LN (n) | SLE Cases with Unknown LN Status (n) | Men (n) | Women (n) |
|---|---|---|---|---|---|---|---|
| European American | 7427 | 3491 | 1129 | 2161 | 646 | 1495 | 5932 |
| African American | 3338 | 1811 | 709 | 704 | 234 | 695 | 2643 |
| Asian | 2525 | 1260 | 529 | 610 | 126 | 253 | 2272 |
| Gullah | 275 | 123 | 70 | 79 | 3 | 33 | 242 |
| Hispanic | 2299 | 807 | 652 | 754 | 88 | 207 | 2092 |
| Total | 15,864 | 7492 | 3089 | 4308 | 1097 | 2683 | 13,181 |
Table 3.
Association results comparing SLE cases with LN versus SLE cases without LN in humans with particular polymorphisms in the TNIP1 gene
| SNP | BP (hg19) | Allelesa | European Ancestry | African American | Asian | Hispanic | Gullah | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAFb | OR (95% CI)c | P Value | MAFb | OR (95% CI)c | P Value | MAFb | OR (95% CI)c | P Value | MAFb | OR (95% CI)c | P Value | MAFb | OR (95% CI)c | P Value | |||
| rs2233287 | 150440097 | G/A | 0.110 | 1.21 (1.03 to 1.4) | 0.02 | 0.204 | 1.1 (0.91 to 1.32) | 0.32 | NA | NA | NA | 0.094 | 0.95 (0.74 to 1.21) | 0.66 | 0.225 | 0.85 (0.48 to 1.51) | 0.59 |
| rs4958881 | 150450236 | T/C | 0.140 | 1.16 (1.01 to 1.34) | 0.04 | 0.457 | 1.22 (1.05 to 1.42) | 8.47E-03 | 0.098 | 1.01 (0.77 to 1.31) | 0.139 | 0.95 (0.76 to 1.17) | 0.63 | 0.482 | 0.99 (0.62 to 1.57) | 0.95 | |
| rs7708392 | 150457485 | G/C | 0.284 | 1.22 (1.1 to 1.37) | 3.66E-04 | 0.789 | 1.21 (1 to 1.47) | 0.06 | 0.204 | 1.03 (0.84 to 1.27) | 0.49 | 1.13 (0.97 to 1.32) | 0.12 | 0.138 | 1.24 (0.6 to 2.59) | 0.56 | |
| rs999556 | 150473674 | A/G | 0.312 | 0.98 (0.88 to 1.1) | 0.74 | 0.47 | 1.07 (0.92 to 1.24) | 0.38 | 0.771 | 1.03 (0.85 to 1.25) | 0.488 | 1.07 (0.92 to 1.24) | 0.39 | 0.469 | 0.81 (0.51 to 1.3) | 0.39 | |
| rs17728338 | 150478318 | G/A | 0.063 | 0.94 (0.77 to 1.16) | 0.56 | 0.093 | 1.02 (0.78 to 1.32) | 0.89 | 0.096 | 1.09 (0.83 to 1.44) | 0.042 | 1.07 (0.75 to 1.53) | 0.71 | 0.109 | 2.15 (0.93 to 4.93) | 0.07 | |
MAF, minor allele frequency; OR, odds ratio; CI, confidence interval; NA, not applicable.
Major/minor.
Minor allele frequency
The odds ratio was calculated with respect to the minor allele.
Discussion
In this report, we show that a knockin mouse expressing an ABIN1 mutant, ABIN1[D485N], with impaired negative regulation of NF-κB developed progressive, proliferative GN with histologic features of class III and IV human LN, and two SNPs of the ABIN1 gene, TNIP1, are significantly associated with human LN. A role for NF-κB in the incidence and severity of LN was suggested previously by the correlation of enhanced NF-κB protein expression in disease kidney sections or reduction of disease phenotypes in animal models after administration of compounds that inhibit NF-κB signaling.5–9 Although the mechanisms regulating those effects of NF-κB on LN have not been determined, our data provide a potential mechanism for NF-κB dysregulation in some patients with LN.
K63 and linear polyubiquitin conjugation mediates several protein-protein interactions required for activation of the IKK complex and hence NF-κB transcription.20,33–35 ABIN1 contain a polyubiquitin binding domain that is also present in NEMO, termed UBAN (Ubiquitin-Binding domain in ABIN proteins and NEMO).36 The UBAN in ABIN1 contains a highly conserved 472–480 amino acid region in humans and 485–495 in mice.36 The Asp-to-Asn mutation at this conserved residue 485 (mouse) or 472 (human) of the UBAN renders ABIN1 incapable of binding recombinant Lys63 polyubiquitin chains and polyubiquitinated NF-κB activators from cell lysates.15,26,36–38 We previously reported that ABIN1[D485N] knockin mice have enhanced NF-κB signaling in B cells and bone marrow–derived macrophages and developed an SLE-like autoimmune disease, with enlarged spleens and lymph nodes, elevated levels of pathogenic immunoglobulins, and antinuclear antibodies in the serum as early as 4 months of age.26 The present report shows that mice develop proteinuria by 3–4 months of age, hypocomplementemia by 5–6 months, and renal histologic abnormalities (including focal and diffuse glomerular hypercellularity involving both endocapillary and mesangial cells, increased mesangial matrix accumulation, “wire loop” thickening of glomerular capillary walls, immunoglobulin [IgG, IgA, and IgM] and complement deposition in a mesangial and capillary loop pattern, and mesangial and subendothelial electron dense deposits by electron microscopy). Those findings suggest that ABIN1[D485N] knockin mice provide a model of human SLE, including the development of class III/IV LN. Our model indicates that SLE and LN can be initiated by disruption of ABIN1 NF-κB inhibitory function, at least in part, in B cells. Another recent report showed that ABIN1-deficient mice develop progressive lupus-like autoimmune phenotypes and GN.39 In addition to NF-κB activation, we also showed in a previous report that JNK and p38 MAPK signaling was enhanced after stimulation with TLR agonists in B cells, bone marrow–derived macrophages, and dendritic cells isolated from ABIN1[D485N] mice.26 A role for enhanced MAPK signaling in the development of GN has been suggested in several reports.40–44 Stambe et al. showed enhanced active p-p38 MAPK in glomeruli, tubules, and myofibroblast and infiltrated neutrophils and macrophage in kidney biopsy specimens from patients with different types of proliferative GN, including class III/IV LN, and found that elevated p-p38 MAPK correlated with renal dysfunction and histopathology.41 The same group showed in a separate study that administration of a specific p38 MAPK inhibitor prevented renal injury and renal function loss from anti–glomerular basement membrane (GBM)-induced GN in rats.40 Another report showed that proteinuria and glomerular cell proliferation induced by anti-GBM was dependent on JNK activity in bone marrow macrophages.44 Taken together, the present study and the previous reports support the hypothesis that loss of ABIN1 K63 and linear polyubiquitin binding leading to increased NF-κB and MAPK activity participates in the development of SLE and LN.
Variants in the ABIN1 gene TNIP1 have been reported in patients with SLE, suggesting a role for ABIN1 in human autoimmune disease.30,31,45 Gateva et al. showed an association for a TNIP1 variant (rs7708392) in European-ancestry patients with SLE from the United States and Sweden.31 Han et al. also reported a TNIP1 variant (rs10036748) in a Chinese Han SLE population.45 This SNP also showed significant association with European population, and the frequency (77%) was much higher in the European population than in the Chinese population (26%).45 The associations of those TNIP1 variants were also replicated in Japanese (rs7708392) and Chinese Han (rs10036748) populations.46,47 There was a stronger association for rs7708392 with LN in the Japanese population.46 A replicate study of rs7708392 and rs10036748 in a southwest Chinese SLE population found a weak association, but as for the Japanese population, a significant association for rs7708392 with LN in their SLE population was apparent.48 Consistent with those previous reports, the current study found association (OR, 1.22; P=3.66×10−4) for rs7708392 when United States patients of European ancestry with SLE and LN versus those without LN, but this association was not significant in African, Asian, or Hispanic American cohorts. The discord for the finding in our Asian cohort and the reported finding in southwest Chinese and Japanese populations with regards to LN association may be explained by the composition of our Asian population of 1012 total patients, which was 90% Korean. The present report also identified association for another TNIP1 variant (rs4958881) (OR, 1.22; P=8.47×10−3) in African American patients with SLE and LN versus those without LN. rs4958881 was previously reported to be associated with systemic sclerosis, but not SLE.32 Additionally, ABIN1 expression was decreased in systemic sclerosis skin lesions and in dermal fibroblasts from patients with systemic sclerosis compared with controls, and transgenic expression of ABIN1 abrogated matrix protein expression induced by inflammatory cytokines in fibroblast from patients with systemic sclerosis.32 This suggests that the rs4958881 variant could result in lower renal expression of ABIN1 in LN, leading to increased mesangial cell matrix production typically seen in class III and IV LN in humans49 and found in the ABIN1[D485N] knockin mice.
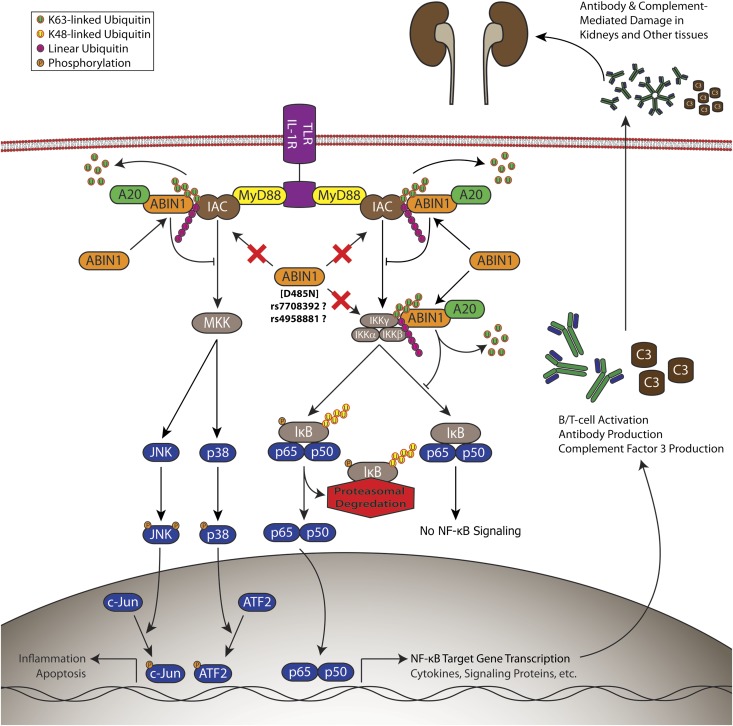
In summary, our data suggest that mutations in ABIN1 represent a new genetic basis for LN through the novel pathophysiologic mechanism of aberrant regulation of NF-κB and MAPK activity (Figure 5). This is supported by knockin mice expressing a mutation of ABIN1 with loss of NF-κB and MAPK inhibitory function and a strong association of a novel TNIP1 polymorphism and LN in an SLE patient population with a higher incidence of LN. Our findings support an association between polymorphisms in TNIP1 and the development of LN. This mouse model closely replicates the human disease and, therefore, potentially serves as an effective tool to study diagnostic and therapeutic strategies for LN.
Figure 5.
Disrupted ABIN1 polyubiquitin binding contributes to the development of lupus nephritis via aberrant regulation of NF-κB and mitogen-activated protein kinase activity. Proinflammatory gene activation is mediated by NF-κB, c-JUN, and ATF2 through TLRs and IL-1R by activation of the upstream IKK complex or MAPK kinase (MKK) activation of JNK or p38 MAPK. IKK and MKKs are activated by an inflammatory activating complex consisting of IRAKs, TRAFs, RIPs, and TAK1, among others. ABIN1 binds to K63-linked and linear polyubiquitin chains and inhibits NF-κB, c-JUN, and ATF2 by interaction with these moieties on components of the inflammatory activating complex or IKKγ. Inhibition of activators of MKKs or IKK is also facilitated by ABIN1 recruitment of the de-ubiquinating protein A20. ABIN1 mutation that disrupts these inhibitory functions contributes to LN.
Concise Methods
Animals
The ABIN1[D485N] mice were generated on a 129SvJxC57BL/6 background26 and then backcrossed eight generations to C57BL/6 for the present studies. Heterozygous mice were used to generate ABIN1[D485N] homozygous and littermate WT control mice for all the experiments. All animals were maintained in specific pathogen-free conditions consistent with European Union and United Kingdom regulations. All work was performed under a United Kingdom Home Office project license that was awarded after recommendation by the University of Dundee Ethical Review Committee.
Renal Histology
Kidneys were examined by light microscopy, direct immunofluorescence, and electron microscopy. For light microscopy kidneys were fixed in 10% neutral buffered formalin and embedded in paraffin; 4-micron sections were stained with periodic acid-Schiff reagent, hematoxylin and eosin, or Masson trichrome. Glomerular, interstitial, and tubular pathology was assessed by evaluation of a complete cross-section of an entire kidney in a blinded manner. Glomerular damage was assessed by semiquantitative scoring of size, cellularity, mesangial matrix expansion, crescent formation, and sclerosis on a 0 (normal), 1+ (mild abnormality), 2+ (moderate abnormality), and 3+ (severe abnormality) scale. The semiquantitative scoring of interstitial inflammatory cell infiltration, interstitial fibrosis, and tubular atrophy was based on the percentage of interstitium area affected: 0, normal; 1+, <25% of interstitial cross-section affected; 2+, 25%–50% of interstitium affected; 3+, >50% of interstitium affected in a single cross-section of an entire kidney. An activity index was calculated as the sum of individual scores for glomerular size, glomerular hypercellularity, cellular crescents, mesangial expansion, and interstitial inflammation. We also determined whether the lesions were focal (involving <50% of the glomeruli) or diffuse (involving >50% of the glomeruli).
For immunofluorescence, 4-micron frozen sections were first washed in PBS, 3×5 minutes each. The blocking solution was applied for 30 minutes at room temperature. The primary antibody was then added for 2 hours at room temperature. The sections were washed for 5 minutes in PBS three times. FITC primary antibody (C3, Cedar Lanes Laboratory; IgG, Sigma Aldrich; C1q, Hycult Biotech) or unlabeled primary antibody (IgA, Invitrogen; IgM, Invitrogen) followed by florescent secondary antibody (Alexa Flour 555, Invitrogen) were used. After antibody incubation, slides were washed in PBS 3×5 minutes and then viewed and imaged with confocal microscopy.
For electron microscopy, cortical tissue was minced to 1 mm3 and fixed in 3% glutaraldehyde. They were postfixed in 1% OsO4 for 60 minutes and dehydrated through graded alcohols and propylene oxide. Fixed tissue blocks were embedded in eponate resin. Thick (1-µm) sections were prepared to evaluate the orientation and presence of glomeruli. Thin sections were mounted on 200 mesh naked copper grids and stained with lead citrate and uranyl acetate (4% in absolute alcohol).
Urine Albumin and Creatinine Measurement
Urine was collected from WT and ABIN1[D485N] mice at 3–4 and 5–6 months of age using a simple technique outlined by Kurien and Scofield.50 Samples were centrifuged at 15,000g for 3 minutes at 4°C to pellet and remove any cellular debris. Urine albumin was determined using the Mouse Albumin ELISA Quantification Set from Bethyl Laboratories (#E90–134, Montgomery, TX) per the manufacturer’s guidelines. Urine creatinine measurements were obtained using the QuantiChrom Creatinine Assay Kit from BioAssay Systems (#DICT-500, Hayward, CA), which uses the well characterized Jaffe method.
Serum C3 Measurement
Serum was collected from ABIN1[D485N] and WT mice at ages 3–4 months and 5–6 months. C3 measurements were performed using a 1:25,000 dilution of mouse serum and measurement with the Mouse C3 ELISA Kit from GenWay Biotech (#40–374–130047) per the manufacturer’s guidelines. All samples were tested in duplicate and the average was reported.
SNP Analysis
Participants
A total of 16,999 independent case and control samples were collected from multiple sites as part of the Large Lupus Association Study 2, with institutional review board approval from each institution, and processed at the Oklahoma Medical Research Foundation (OMRF) under the auspices of the OMRF institutional review board. Only individuals who signed informed consent forms were included in the study. All SLE cases met the revised 1997 American College of Rheumatology for classification of SLE.51 Among SLE cases, those with LN fulfilled the renal criterion of (1) persistent proteinuria >0.5 g per day (24 hours) or persistent >3+ if quantification was not performed or (2) presence of urinary cellular casts.51 For additional information on the study participants, see the Supplemental Material.
Genotyping and Quality Control
The custom-design Illumina iSelect platform at OMRF was used to genotype the five SNPs in TNIP1 previously reported to be associated with systemic sclerosis (rs4958881, rs2233287),32 SLE (rs7708392),31 psoriasis (rs999556, rs17728338),52,53 and psoriatic arthritis (rs17728338, one of the psoriasis SNPs).54 In addition, 347 ancestral-informative markers spanning the genome were genotyped. After application of quality controls by excluding samples that exhibited low call rates (<90%) and extreme heterozygosity (>5 SDs from the mean), revealed discrepancies between reported sex and genetic data, or were determined to be a duplicate or cryptic related to another sample (the proportion of alleles shared identical by descent >0.4), and after removing extreme population outliers based on global ancestry estimation and principal component analysis (calculated using the ancestral-informative markers in the ADMIXMAP55,56 and EIGENSTRAT57 programs, respectively), as described in other Large Lupus Association Study 2 reports,58,59 a final data set of 15,864 unrelated patients was obtained (Table 2).
Association Analysis
Single-marker association analyses were calculated using the logistic regression function in PLINK, version 1.07.60 The additive genetic model was applied while adjusting for sex and global ancestry estimates (African, European, and East Asian).59,61,62 The Bonferroni-corrected P value threshold was set to P<0.01 based on multiple tests of five SNPs.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank the following individuals for contributing samples genotyped in this study: S. D'Alfonso (Italy), R. Scorza (Italy), P. Junker and H. Laustrup (Denmark), M. Bijl (The Netherlands), E. Endreffy (Hungary), C. Vasconcelos and B.M. da Silva (Portugal), A. Suarez and C. Gutierrez (Spain), I. Rúa-Figueroa (Spain), and C. Garcilazo (Argentina). For the Asociación Andaluza de Enfermedades Autoimmunes (AADEA) collaboration: N. Ortego-Centeno (Spain), J. Jimenez-Alonso (Spain), E. de Ramon (Spain), and J. Sanchez-Roman (Spain). For the collaboration on Hispanic populations enriched for Amerindian-European admixture: M. Cardiel (Mexico), I.G. de la Torre (Mexico), M. Maradiaga (Mexico), J.F. Moctezuma (Mexico), E. Acevedo (Peru), C. Castel and M. Busajm (Argentina), and J. Musuruana (Argentina). Other participants from the Argentine Collaborative Group are H.R. Scherbarth, P.C. Marino, E.L. Motta, S. Gamron, C. Drenkard, E. Menso, A. Allievi, G.A. Tate, J.L. Presas, S.A. Palatnik, M. Abdala, M. Bearzotti, A. Alvarellos, F. Caeiro, A. Bertoli, S. Paira, S. Roverano, C.E. Graf, E. Bertero, C. Guillerón, S. Grimaudo, J. Manni, L.J. Catoggio, E.R. Soriano, C.D. Santos, C. Prigione, F.A. Ramos, S.M. Navarro, G.A. Berbotto, M. Jorfen, E.J. Romero, M.A. Garcia, J.C. Marcos, A.I. Marcos, C.E. Perandones, A. Eimon, and C.G. Battagliotti.
D.W.P. was supported by National Institutes of Health grants DK176743, AR063124, and AI103980 and Juvenile Diabetes Research Foundation international grant 1-2011-588; P.C., by the United Kingdom Medical Research Council; K.R.M., by a Merit Review from the Department of Veterans Affairs and National Institutes of Health grant AI103980; P.M.G., by National Institutes of Health grants AR158959, AI063274, and GM103456; B.P.T., by National Institutes of Health grant AR043814 and the Alliance for Lupus Research; G.S.G., by National Institutes of Health grants UL1RR029882 and P60AR062755; I.A., by National Institutes of Health grant P20GM103456; J.B.H., by National Institutes of Health grants AI024717, AI083194, AR049084, and PR094002 and the U.S. Department of Veterans Affairs; R.P.K., by National Institutes of Health grants AR33062, AR 49084, AI083194, and TR000165; R.R.-G., by National Institutes of Health grants AR002138, AR30692, AR 49084, and RR025741; and S.-C.B., by Korea Healthcare Technology R&D project, Ministry for Health & Welfare, and Republic of Korea A121983.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013020148/-/DCSupplemental.
References
- 1.Tsokos GC: Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME: Lupus nephritis: A critical review. Autoimmun Rev 12: 174–194, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Buhaescu I, Covic A, Deray G: Treatment of proliferative lupus nephritis—a critical approach. Semin Arthritis Rheum 36: 224–237, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Kalergis AM, Iruretagoyena MI, Barrientos MJ, González PA, Herrada AA, Leiva ED, Gutiérrez MA, Riedel CA, Bueno SM, Jacobelli SH: Modulation of nuclear factor-kappaB activity can influence the susceptibility to systemic lupus erythematosus. Immunology 128[Suppl]: e306–e314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang HK, Ecklund D, Liu M, Datta SK: Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther 11: R59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L, Sinniah R, Hsu SI: Renal cell apoptosis and proliferation may be linked to nuclear factor-kappaB activation and expression of inducible nitric oxide synthase in patients with lupus nephritis. Hum Pathol 37: 637–647, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Sinniah R, Hsu SI: In situ glomerular expression of activated NF-kappaB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy. Virchows Arch 448: 172–183, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zheng L, Sinniah R, Hsu SI: Pathogenic role of NF-kappaB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J Histochem Cytochem 56: 517–529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oeckinghaus A, Hayden MS, Ghosh S: Crosstalk in NF-κB signaling pathways. Nat Immunol 12: 695–708, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S: Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev 9: 2723–2735, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Jacobs MD, Harrison SC: Structure of an IkappaBalpha/NF-kappaB complex. Cell 95: 749–758, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Karin M: How NF-kappaB is activated: The role of the IkappaB kinase (IKK) complex. Oncogene 18: 6867–6874, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Adhikari A, Xu M, Chen ZJ: Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26: 3214–3226, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K: Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol 11: 123–132, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, Randow F, Wakatsuki S, Dikic I: Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell 136: 1098–1109, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, Koschny R, Komander D, Silke J, Walczak H: Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell 36: 831–844, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Vereecke L, Beyaert R, van Loo G: The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol 30: 383–391, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Verstrepen L, Carpentier I, Verhelst K, Beyaert R: ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol 78: 105–114, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD: Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol 28: 3538–3547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laplantine E, Fontan E, Chiaravalli J, Lopez T, Lakisic G, Véron M, Agou F, Israël A: NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J 28: 2885–2895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windheim M, Stafford M, Peggie M, Cohen P: Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination of IL-1 receptor-associated kinase 1 to facilitate NEMO binding and the activation of IkappaBalpha kinase. Mol Cell Biol 28: 1783–1791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, Formisano S, Vito P, Leonardi A: ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in inhibiting NF-kappaB. J Biol Chem 281: 18482–18488, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS: Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J 378: 727–734, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shembade N, Ma A, Harhaj EW: Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327: 1135–1139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanda SK, Venigalla RK, Ordureau A, Patterson-Kane JC, Powell DW, Toth R, Arthur JS, Cohen P: Polyubiquitin binding to ABIN1 is required to prevent autoimmunity. J Exp Med 208: 1215–1228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon MR, Parikh AA, Pritts TA, Fischer JE, Cottongim S, Szabo C, Salzman AL, Hasselgren PO: Complement component C3 production in IL-1beta-stimulated human intestinal epithelial cells is blocked by NF-kappaB inhibitors and by transfection with ser 32/36 mutant IkappaBalpha. J Surg Res 82: 48–55, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Oeckinghaus A, Ghosh S: The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1: a000034, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castellano G, Cappiello V, Fiore N, Pontrelli P, Gesualdo L, Schena FP, Montinaro V: CD40 ligand increases complement C3 secretion by proximal tubular epithelial cells. J Am Soc Nephrol 16: 2003–2011, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Adrianto I, Wang S, Wiley GB, Lessard CJ, Kelly JA, Adler AJ, Glenn SB, Williams AH, Ziegler JT, Comeau ME, Marion MC, Wakeland BE, Liang C, Kaufman KM, Guthridge JM, Alarcón-Riquelme ME; BIOLUPUS and GENLES Networks, Alarcón GS, Anaya JM, Bae SC, Kim JH, Joo YB, Boackle SA, Brown EE, Petri MA, Ramsey-Goldman R, Reveille JD, Vilá LM, Criswell LA, Edberg JC, Freedman BI, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martín J, Merrill JT, Niewold TB, Pons-Estel BA, Scofield RH, Stevens AM, Tsao BP, Vyse TJ, Langefeld CD, Harley JB, Wakeland EK, Moser KL, Montgomery CG, Gaffney PM. Association of two independent functional risk haplotypes in TNIP1 with systemic lupus erythematosus. Arthritis Rheum 64:3695–3705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark G, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jönsen A, Bengtsson AA, Rantapää-Dahlqvist S, Baechler EC, Brown EE, Alarcón GS, Edberg JC, Ramsey-Goldman R, McGwin G, Jr, Reveille JD, Vilá LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Rönnblom L, Criswell LA, Syvänen AC, Behrens TW, Graham RR: A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41: 1228–1233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allanore Y, Saad M, Dieudé P, Avouac J, Distler JH, Amouyel P, Matucci-Cerinic M, Riemekasten G, Airo P, Melchers I, Hachulla E, Cusi D, Wichmann HE, Wipff J, Lambert JC, Hunzelmann N, Tiev K, Caramaschi P, Diot E, Kowal-Bielecka O, Valentini G, Mouthon L, Czirják L, Damjanov N, Salvi E, Conti C, Müller M, Müller-Ladner U, Riccieri V, Ruiz B, Cracowski JL, Letenneur L, Dupuy AM, Meyer O, Kahan A, Munnich A, Boileau C, Martinez M: Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet 7: e1002091, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD: Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected]. Nat Cell Biol 8: 398–406, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ: Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22: 245–257, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I: SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471: 637–641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner S, Carpentier I, Rogov V, Kreike M, Ikeda F, Löhr F, Wu CJ, Ashwell JD, Dötsch V, Dikic I, Beyaert R: Ubiquitin binding mediates the NF-kappaB inhibitory potential of ABIN proteins. Oncogene 27: 3739–3745, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Oshima S, Turer EE, Callahan JA, Chai S, Advincula R, Barrera J, Shifrin N, Lee B, Benedict Yen TS, Woo T, Malynn BA, Ma A: ABIN-1 is a ubiquitin sensor that restricts cell death and sustains embryonic development. Nature 457: 906–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heyninck K, Kreike MM, Beyaert R: Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett 536: 135–140, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Wu R, High AA, Slaughter CA, Finkelstein D, Rehg JE, Redecke V, Häcker H: A20-binding inhibitor of NF-κB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein β activation and protects from inflammatory disease. Proc Natl Acad Sci U S A 108: E998–E1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stambe C, Atkins RC, Tesch GH, Kapoun AM, Hill PA, Schreiner GF, Nikolic-Paterson DJ: Blockade of p38alpha MAPK ameliorates acute inflammatory renal injury in rat anti-GBM glomerulonephritis. J Am Soc Nephrol 14: 338–351, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Stambe C, Nikolic-Paterson DJ, Hill PA, Dowling J, Atkins RC: p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: Correlation with renal injury. J Am Soc Nephrol 15: 326–336, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K: Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 16: 2690–2701, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sheryanna A, Bhangal G, McDaid J, Smith J, Manning A, Foxwell BM, Feldmann M, Cook HT, Pusey CD, Tam FW: Inhibition of p38 mitogen-activated protein kinase is effective in the treatment of experimental crescentic glomerulonephritis and suppresses monocyte chemoattractant protein-1 but not IL-1beta or IL-6. J Am Soc Nephrol 18: 1167–1179, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ikezumi Y, Hurst L, Atkins RC, Nikolic-Paterson DJ: Macrophage-mediated renal injury is dependent on signaling via the JNK pathway. J Am Soc Nephrol 15: 1775–1784, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, Zhang XJ: Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41: 1234–1237, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki A, Ito S, Furukawa H, Hayashi T, Goto D, Matsumoto I, Kusaoi M, Ohashi J, Graham RR, Matsuta K, Behrens TW, Tohma S, Takasaki Y, Hashimoto H, Sumida T, Tsuchiya N: Association of TNFAIP3 interacting protein 1, TNIP1 with systemic lupus erythematosus in a Japanese population: A case-control association study. Arthritis Res Ther 12: R174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He CF, Liu YS, Cheng YL, Gao JP, Pan TM, Han JW, Quan C, Sun LD, Zheng HF, Zuo XB, Xu SX, Sheng YJ, Yao S, Hu WL, Li Y, Yu ZY, Yin XY, Zhang XJ, Cui Y, Yang S: TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 are associated with clinical features of systemic lupus erythematosus in a Chinese Han population. Lupus 19: 1181–1186, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Zhong H, Li XL, Li M, Hao LX, Chen RW, Xiang K, Qi XB, Ma RZ, Su B: Replicated associations of TNFAIP3, TNIP1 and ETS1 with systemic lupus erythematosus in a southwestern Chinese population. Arthritis Res Ther 13: R186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M, International Society of Nephrology Working Group on the Classification of Lupus Nephritis. Renal Pathology Society Working Group on the Classification of Lupus Nephritis : The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Kurien BT, Scofield RH: Mouse urine collection using clear plastic wrap. Lab Anim 33: 83–86, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR, Collaborative Association Study of Psoriasis : Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 41: 199–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun LD, Cheng H, Wang ZX, Zhang AP, Wang PG, Xu JH, Zhu QX, Zhou HS, Ellinghaus E, Zhang FR, Pu XM, Yang XQ, Zhang JZ, Xu AE, Wu RN, Xu LM, Peng L, Helms CA, Ren YQ, Zhang C, Zhang SM, Nair RP, Wang HY, Lin GS, Stuart PE, Fan X, Chen G, Tejasvi T, Li P, Zhu J, Li ZM, Ge HM, Weichenthal M, Ye WZ, Zhang C, Shen SK, Yang BQ, Sun YY, Li SS, Lin Y, Jiang JH, Li CT, Chen RX, Cheng J, Jiang X, Zhang P, Song WM, Tang J, Zhang HQ, Sun L, Cui J, Zhang LJ, Tang B, Huang F, Qin Q, Pei XP, Zhou AM, Shao LM, Liu JL, Zhang FY, Du WD, Franke A, Bowcock AM, Elder JT, Liu JJ, Yang S, Zhang XJ: Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat Genet 42: 1005–1009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowes J, Orozco G, Flynn E, Ho P, Brier R, Marzo-Ortega H, Coates L, McManus R, Ryan AW, Kane D, Korendowych E, McHugh N, FitzGerald O, Packham J, Morgan AW, Bruce IN, Barton A: Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann Rheum Dis 70: 1641–1644, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM: Control of confounding of genetic associations in stratified populations. Am J Hum Genet 72: 1492–1504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, McKeigue PM: Design and analysis of admixture mapping studies. Am J Hum Genet 74: 965–978, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D: Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38: 904–909, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Lessard CJ, Adrianto I, Kelly JA, Kaufman KM, Grundahl KM, Adler A, Williams AH, Gallant CJ, Anaya JM, Bae SC, Boackle SA, Brown EE, Chang DM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park SY, Petri MA, Pons-Estel BA, Ramsey-Goldman R, Reveille JD, Song YW, Stevens AM, Tsao BP, Vila LM, Vyse TJ, Yu CY, Guthridge JM, Bruner GR, Langefeld CD, Montgomery C, Harley JB, Scofield RH, Gaffney PM, Moser KL, Marta E. Alarcón-Riquelme on behalf of the BIOLUPUS and GENLES Networks : Identification of a systemic lupus erythematosus susceptibility locus at 11p13 between PDHX and CD44 in a multiethnic study. Am J Hum Genet 88: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adrianto I, Wen F, Templeton A, Wiley G, King JB, Lessard CJ, Bates JS, Hu Y, Kelly JA, Kaufman KM, Guthridge JM, Alarcón-Riquelme ME, Anaya JM, Bae SC, Bang SY, Boackle SA, Brown EE, Petri MA, Gallant C, Ramsey-Goldman R, Reveille JD, Vila LM, Criswell LA, Edberg JC, Freedman BI, Gregersen PK, Gilkeson GS, Jacob CO, James JA, Kamen DL, Kimberly RP, Martin J, Merrill JT, Niewold TB, Park SY, Pons-Estel BA, Scofield RH, Stevens AM, Tsao BP, Vyse TJ, Langefeld CD, Harley JB, Moser KL, Webb CF, Humphrey MB, Montgomery CG, Gaffney PM, BIOLUPUS and GENLES Networks : Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat Genet 43: 253–258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MW, Patterson N, Lautenberger JA, Truelove AL, McDonald GJ, Waliszewska A, Kessing BD, Malasky MJ, Scafe C, Le E, De Jager PL, Mignault AA, Yi Z, De The G, Essex M, Sankale JL, Moore JH, Poku K, Phair JP, Goedert JJ, Vlahov D, Williams SM, Tishkoff SA, Winkler CA, De La Vega FM, Woodage T, Sninsky JJ, Hafler DA, Altshuler D, Gilbert DA, O’Brien SJ, Reich D: A high-density admixture map for disease gene discovery in african americans. Am J Hum Genet 74: 1001–1013, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T: A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Hum Mutat 29: 648–658, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.