Abstract
Over the past decade, enormous progress has been made in both the technical approaches and the scientific information available for studying the human genome. Therefore, increasingly, scientists have begun to address not just single-gene disorders but complex disorders. The limiting factor in most of such studies remains appropriate, well-focused detailed phenotyping of the complex disorders under study, with careful ascertainment of subjects with the specific disorder, as well as healthy control subjects.
In this brief Discovery article, the focus is on a limited number of studies—primarily from our own laboratory, as well as a few selected studies from other laboratories—that directly address the topic of the possible role of a specific, functional, polymorphism of a specific human gene (the μ-opioid receptor gene), stress responsivity, and specific addictions. Over the last decade, there have been innumerable laboratory-based studies showing in great detail that response to stress and stressors contributes to the acquisition and persistence of, as well as relapse to, self-administration of a drug of abuse, precisely as we had hypothesized would happen in humans, more than 30 years ago. Studies from our laboratory, as well as that of Koob and a few others, have been reviewed by Koob and Kreek twice over this past decade.1,2 Also, 4 years ago, we reviewed diverse contributions made over 30 years from our laboratory.3 In addition to reviewing much of the work on stress response in animal models from my laboratory and those of others,1–3 we have also reviewed what is known about human molecular genetics of specific addictive diseases and provided both viewpoints and perspectives of the relative importance of findings.4–8
In the basic clinical research of our laboratory, we have especially focused on further dissecting the role of specific components of the stress-responsive hypothalamic–pituitary–adrenal (HPA) axis in humans in the setting of specific addictive diseases to determine the potential impact of different components of this axis on the acquisition and persistence of, and relapse to, addictions. At the same time, we worked to determine how chronic use of drugs of abuse may alter each of these components, or, alternatively, to identify alterations which existed before exposure to a drug of abuse on a genetic basis.9–16 The sequence of these events at this time cannot be determined. We also have conducted studies in subjects during successful treatment of addictions to determine whether normalization occurs during chronic effective treatment. Further, for our research in animals, models have been developed mimicking human patterns of drug abuse, addiction, and treatments.
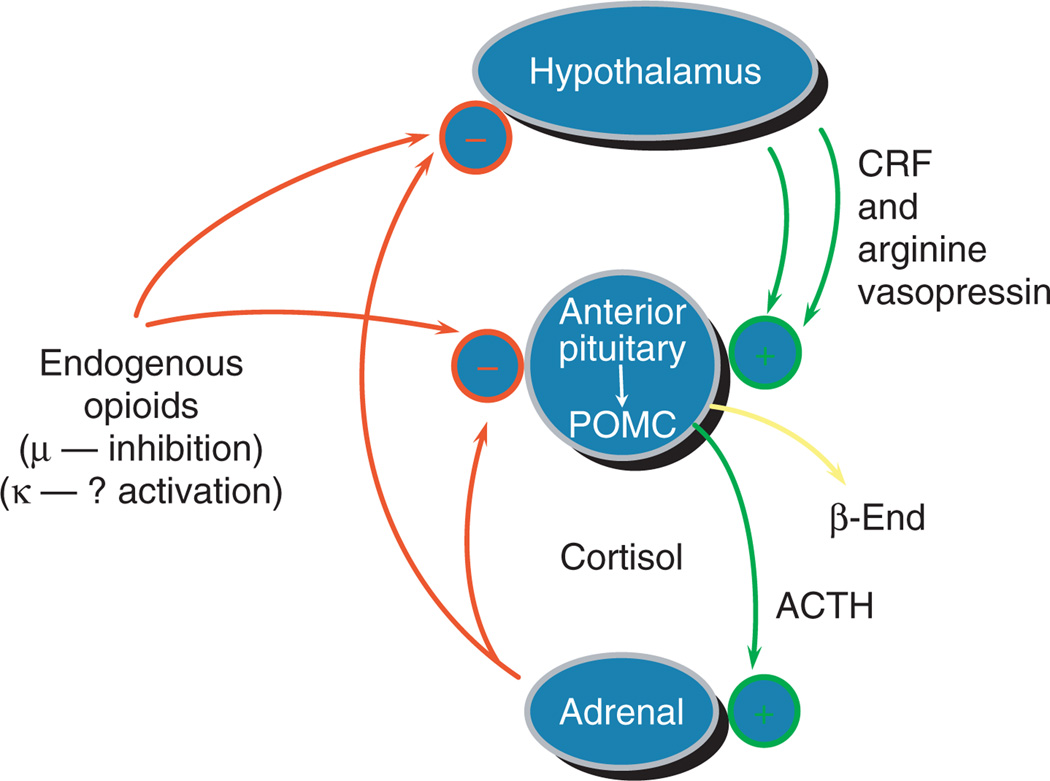
We and others have shown that the μ-opioid receptor tonically inhibits the HPA stress-responsive axis (reviewed in refs. 1–5,8). Thus, while we have continued to analyze each component of the HPA axis and continued to determine how each may be altered by chronic use of drugs of abuse, we have also started to elucidate whether altered function is due to some intrinsic, and probably some functional, genomic differences prior to any exposure to a drug of abuse. Our earliest studies, which documented the physiological effects, as well as the safety and effectiveness, of long-term methadone maintenance treatment, were conducted from 1964 to 1973. In addition, other studies using increasingly sophisticated technologies between 1973 and 1998 have addressed the role of the stress-responsive HPA axis in addictions and in treatment (reviewed in refs. 1–4). The human stress-responsive HPA axis is pictured in a simplified diagram (Figure 1).
Figure 1.
Hypothalamic–pituitary–adrenal axis and the endogenous opioid system have interrelated roles in the biology of addictive diseases. Reprinted from ref. 5. ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor.
As part of our research on the molecular genetics of specific addictive diseases in humans, we have also been interested in functional genomics—specifically in gene variants, which we or others have shown or in the future may show to be functionally different from the prototype. We then can determine whether these variants are involved in altering physiology of known processes in mammalian biology. Further, since in our initial genetics research the focus has been, in large part, on studying genes that are known to be altered by, or targets of, specific drugs of abuse, any findings of functional differences can be immediately related to the biological role of that gene in specific addictions.
Beginning with our earliest work, initiated in 1995 in collaboration with Lei Yu, after our first successful cloning of the μ-opioid receptor, we had agreed to first look for gene variants in the coding region of the μ-opioid receptor, the primary site of action of opiates, but also documented by our group, and now others, to be altered by chronic “binge”-pattern cocaine exposure17 (reviewed in refs. 1–3). We had decided to study possible functional alterations of any variant resulting in amino acid changes in regions of the μ-opioid receptor peptide, which could be of potential importance. In our first study, we found two gene variants of high allelic frequency (10.5 and 6.6%, respectively, in combined ethnic groups in the New York City population), each of which alters an amino acid in the N-terminus.17 One of these, the A118G polymorphism, is at a site of putative glycosylation. The μ-opioid receptor peptide resulting from this A118G variant has a change in the amino acid at position 40, from asparagine to aspartic acid, which may alter the physio-chemical properties of this receptor.
In appropriate molecular cellular constructs, we found that most of the exogenous and endogenous opioids bind similarly to the receptor resulting from the prototype gene compared to the receptor resulting from the variant allele. However, when the longest of the endogenous opioids, β-endorphin, was studied, there was a threefold shift to the left, indicating greater binding affinity to the receptor resulting from the polymorphism. In further studies using molecular cellular constructs, we found that one of the major signal transduction mechanisms, the G protein–activated inwardly rectifying potassium channel, was significantly enhanced when β-endorphin was used as a ligand to bind to the receptor resulting from the variant, compared to the prototype.17 However, as with binding, no differences were found when other ligands were used.
Based on these findings and many of our findings in basic clinical research or related laboratory-based research discussed above, we hypothesized that, since the μ-opioid receptor is central to the stress-responsive HPA axis, and further, since this axis has been shown to be of importance for addictive diseases, the A118G polymorphism might be associated with specific addictive diseases—in particular, opiate addiction and alcoholism.8,17 Further, given the diverse roles of β-endorphin and the μ-opioid receptor in many physiological functions, we hypothesized that the pathophysiology of addiction might be altered by the presence of one or two copies of this variant.8,17 We also hypothesized that response to an antagonist-type medication directed at the μ-opioid receptor for the treatment of alcoholism, which has the primary action of removing β-endorphin from μ receptors, that is, naltrexone or nalmefene treatment, might be significantly more effective in persons with one or two copies of the polymorphism, since our collaborative studies with O’Malley, as well as our related laboratory-based studies, have shown that human alcoholics, and also cocaine self-administering rodents, seek modest activation of the hypothalamic–pituitary stress-responsive axis (reviewed in refs. 1,3).
We conducted further studies in collaboration with Heilig and his group at the Karolinska Institute in Stockholm, Sweden.18,19 We chose central Sweden because of the minimal admixture of that population, with ~80% of Swedes having long Swedish heritage. In the first study, we found that the A118G variant is very significantly associated with the presence of opiate addiction (with an odds ratio of 2.72).18 Further, in collaboration with the statistical group of Ott, it was determined that the attributable risk of developing alcoholism due to this one variant alone in Swedes with Swedish heritage was 21%.18 In a second study of alcoholics in central Sweden, again in collaboration with Heilig, we again found a highly significant association of this A118G variant with opiate addiction.19 Further, the odds ratio for developing alcoholism when this variant was present was 1.92, and the attributable risk due to genotypes with the 118G allele was 11.1%.19 However, other studies, particularly those of populations with admixtures of ethnic-cultural heritage, or studies with less stringent phenotyping, have not found an association of the A118G variant with opiate addiction or, alternatively, with alcoholism (reviewed in refs. 6,7).
We had hypothesized that the presence of the 118G allele would alter normal physiology, and, in fact, proposed the term “physiogenetics,” meaning changes in physiology due to a gene variant, in parallel with the long-established term of “pharmacogenetics,” indicating differences in response to a medication due to a gene variant.8 While we were conducting basic clinical research studies to determine whether one could document a significant difference in healthy individuals with the variant, a group from Johns Hopkins, led by Wand et al., reported a profound difference in the objectively measured activation of the stress-responsive HPA axis in a small number of individuals with one copy of the variant compared to the prototype receptor.20 Shortly thereafter, a second paper appeared, from the group of Kranzler and colleagues, which corroborated these findings, showing an association between cortisol response to disinhibition of the HPA axis by μ-opioid receptor antagonism and the A118G variant of the μ-opioid receptor.21 More recently, a more extensive study has been reported by the group of Wand and colleagues, where again they documented a difference in objectively measured HPA axis stress responsivity in individuals with one or two copies of the 118G allele.22 In recent studies from our laboratory, we found altered levels of basal serum cortisol in healthy subjects with the 118G allele of the μ-opioid receptor gene, when subjects were studied in a stress-minimized clinical research setting.23
Further, Oslin and colleagues at University of Pennsylvania, Yale, and University of Connecticut, collaborated to invite back for further study all persons who had participated in one of the clinical trials using naltrexone to manage alcoholism.24 About one in six of the original participants in those studies voluntarily returned to the research setting and signed informed consents for specific genetics studies. When these subjects were all genotyped, and when the outcomes of the naltrexone trial were reanalyzed using the original criteria showing evidence of improvement in alcoholism—that is, primarily, decreased number of days of alcohol consumption and decreased amounts of alcohol consumed—it was found that the majority of those who had had a favorable response were compliant subjects receiving naltrexone and who had one or two copies of the 118G variant.24 Further studies are currently in progress in a collaboration between National Institutes of Health and National Institute on Alcohol Abuse and Alcoholism with Goldman and Anton at University of South Carolina to address the question of whether presence of the 118G allele increases the likelihood of successful naltrexone treatment outcome.
Subsequently, many studies have appeared documenting further associations of this μ-opioid receptor variant with specific pharmacodynamic responses and with associations to specific diseases, pharmacodynamic responses to medications, as well as physiological indices (reviewed in ref. 4). Also, further molecular studies have been reported.4 In our laboratory, we found that stable expression of variant vs. the prototype receptors were characterized by significant differences in the levels of cell surface binding capacity (Bmax), forskolin-induced cyclic adenosine monophosphate accumulation, and also agonist-induced accumulation of cyclic adenosine monophosphate (EC50).25 However, changes in transiently expressed variant receptors included only minor differences in cell surface binding capacity and no differences in cyclic adenosine monophosphate (EC50).25 Other groups, including notably that of Sadée, have shown again that mRNA levels resulting from the 118G allele may be significantly lower than from the prototype 118A (reviewed in ref. 4). Many other specific molecular and cellular studies have been reported documenting the functionality of the A118G polymorphism (reviewed in ref. 4).
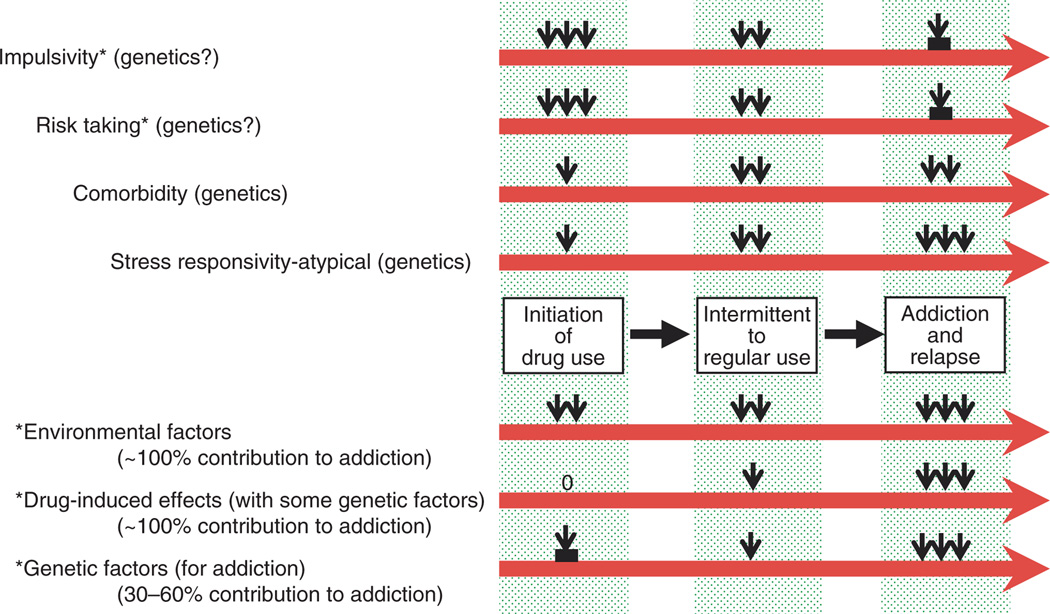
Many other findings have been made by our laboratory and others, some of which include the discovery of polymorphisms of many genes including the dynorphin-opioid receptor peptide and the κ-opioid receptor gene which may be, or which now have been shown to be, functional (e.g., refs. 26–29). In time it will be of great interest to determine how each of these functional polymorphisms may be associated with objective processes of human physiology and also the pathophysiology of specific diseases, including specific addictive diseases (Figure 2). In the future, treatments may be able to be targeted with specific human molecular genetic polymorphisms taken into consideration for more favorable or enhanced therapeutic effectiveness.30
Figure 2.
Hypothetical construct of the relative roles of specific personality traits (impulsivity and risk taking), psychiatric comorbidity, and stress responsivity, in the initiation of, and early progression to, intermittent and regular use of a drug of abuse, and further, the relative roles of environmental factors, specific drug-induced effects, and specific gene variants in the further progression from regular drug use to addiction, and relapse to addiction. Reprinted from ref. 5.
ACKNOWLEDGMENTS
The author has no involvement, financial or otherwise, that might potentially bias her work. Support for this article has been provided by National Institute of Drug Abuse grant numbers K05-DA00049 and P60-DA-05130; National Institute on Mental Health 5R01-MH079880-02 (MJK); NIH UL1RR024143 and the National Center for Research Resources (Coller) (contents are solely the responsibility of the author and do not necessarily represent the official view); and the New York State Office of Alcoholism and Substance Abuse Services, grant number C003189 (MJK).
Footnotes
CONFLICT OF INTEREST
The author declared no conflict of interest.
References
- 1.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 3.Kreek MJ, Schlussman SD, Bart G, LaForge KS, Butelman ER. Evolving perspectives on neurobiological research on the addictions: celebration of the 30th anniversary of NIDA. Neuropharmacology. 2004;47(suppl. 1):324–344. doi: 10.1016/j.neuropharm.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Mol. Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- 5.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk-taking, stress responsivity, and vulnerability to drug abuse and addiction. Nat. Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 6.Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol. Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate and cocaine addiction. Neuromolecular Med. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- 8.LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur. J. Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- 9.Aouizerate B, et al. Glucocorticoid negative feedback in methadone maintained former heroin addicts with ongoing cocaine dependence: dose-response to dexamethasone suppression. Addict. Biol. 2006;11:84–96. doi: 10.1111/j.1369-1600.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 11.Schluger JH, Bart G, Green M, Ho A, Kreek MJ. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology. 2003;28:985–994. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- 12.Schluger JH, Borg L, Ho A, Kreek MJ. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 13.Schluger JH, et al. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol. Clin. Exp. Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 14.Bart G, Schluger JH, Borg L, Ho A, Bidlack J, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- 15.Bart G, Borg L, Schluger JH, Green M, Ho A, Kreek MJ. Suppressed prolactin response to dynorphin A1–13 in methadone-maintained versus control subjects. J. Pharmacol. Exp. Ther. 2003;306:581–587. doi: 10.1124/jpet.103.050682. [DOI] [PubMed] [Google Scholar]
- 16.Kreek MJ, Schluger J, Borg L, Gunduz M, Ho A. Dynorphin A1–13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: gender differences and implications for modulations of dopaminergic tone in the treatment of addictions. J. Pharmacol. Exp. Ther. 1999;288:260–269. [PubMed] [Google Scholar]
- 17.Bond C, et al. Single nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bart G, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol. Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bart G, et al. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- 20.Wand GS, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- 22.Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- 23.Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- 24.Oslin DW, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 25.Kroslak T, LaForge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J. Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 26.Yuferov V, et al. Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics. 2004;14:793–804. doi: 10.1097/00008571-200412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Höllt V. An allelic variation in the human prodynorphin gene promoter alters stimulus induced expression. J. Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- 28.Williams TJ, et al. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict. Biol. 2007;12:496–502. doi: 10.1111/j.1369-1600.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 29.Dahl JP, et al. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;139:106–108. doi: 10.1002/ajmg.b.30238. [DOI] [PubMed] [Google Scholar]
- 30.Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]