Abstract
The transcription factor TFIID, composed of the TATA box-binding protein (TBP) and 14 TBP-associated factors (TAFs), plays a key role in the regulation of gene expression by RNA polymerase II. The structure of yeast TFIID, as determined by electron microscopy and digital image analysis, is formed by three lobes, labelled A–C, connected by thin linking domains. Immunomapping revealed that TFIID contains two copies of the WD-40 repeat-containing TAF5 and that TAF5 contributes to the linkers since its C- and N-termini were found in different lobes. This property was confirmed by the finding that a recombinant complex containing TAF5 complexed with six histone fold containing TAFs was able to form a trilobed structure. Moreover, the N-terminal domain of TAF1 was mapped in lobe C, whereas the histone acetyltransferase domain resides in lobe A along with TAF7. TBP was found in the linker domain between lobes A and C in a way that the N-terminal 100 residues of TAF1 are spanned over it. The implications of these data with regard to TFIID function are discussed.
Keywords: electron microscopy, histone acetyltransferase, image analysis, immunolabelling, TBP, 3-D model, TFIID, TAFs
Introduction
In eukaryotes, transcription of protein-coding genes requires the formation of a preinitiation complex (PIC) containing the TATA box-binding protein (TBP) and the five basal transcription factors TFII-A, -B, -E, -F and -H (Buratowski et al, 1989; Hampsey and Reinberg, 1999). In vitro, the PIC positions precisely RNA polymerase II, which can then accurately transcribe DNA from a TATA box-containing promoter (Orphanides et al, 1996). However, such a transcription system poorly reflects the in vivo situation since it often fails to respond to activators and only inefficiently transcribes TATA-less promoters and/or chromatinized DNA templates. In this respect, cellular TBP is not found in a free form, but instead is stably incorporated into multiple complexes that carry some of the above-mentioned missing functions. In Saccharomyces cerevisiae, TBP and 14 TBP-associated factors (TAFs) form the general transcription factor TFIID, which is 20–50 times more active than TBP for activated transcription in vitro (Sanders et al, 2002). The in vivo importance of TFIID is demonstrated by the observations that all, but one, yeast TAF-encoding genes are essential for viability and that the subunit composition of TFIID is highly conserved throughout evolution (Tora, 2002).
TFIID is instrumental in core promoter recognition through the binding of TBP to the TATA box and also because several TAFs interact with promoter sequences such as the initiator (INR) or the downstream promoter element (Verrijzer et al, 1994; Oelgeschlager et al, 1996; Burke and Kadonaga, 1997). In this respect, TAF1 and TAF2, the largest subunits of TFIID, were found to contribute to promoter recognition in vivo by targeting preferentially the INR sequences (Purnell et al, 1994; Sypes and Gilmour, 1994; Chalkley and Verrijzer, 1999). TAF1 was also reported to interact with TBP and to regulate negatively its binding to the TATA box through a TAND domain placed in the N-terminal region of TAF1 (Bai et al, 1997; Kotani et al, 1998; Liu et al, 1998; Banik et al, 2001). Furthermore, TAF1 post-transcriptionally modifies histones and general transcription factors through several enzymatic functions (reviewed in Wassarman and Sauer, 2001). Metazoan TAF1 contains a Ser/Thr kinase activity that phosphorylates TFIIFα and TFIIA in vitro (Dikstein et al, 1996; Solow et al, 2001). In addition, an acetyltransferase (AT) domain mapping to the C-terminal portion of TAF1 is able to acetylate free and nucleosomal histones in vitro as well as the transcription factors TFIIEβ and TFIIF (Mizzen et al, 1996; Imhof et al, 1997).
Based on interaction studies between recombinant TFIID subunits, it was suggested that TAF1 could serve as a scaffold for TFIID assembly since a Drosophila TFIID subcomplex comprising TAF2, 4, 5, 6, 9, 11 and 12 could be assembled on TAF1 (Chen et al, 1994). However, TAF5 is also a good candidate for organizing the TFIID complex since it forms a stable subcomplex with TAF6 and TAF9 (Tao et al, 1997). In vitro experiments further showed that TAF5 binds strongly to TAF1, TAF7 and TAF11, and with lower affinity to TAF12, TAF13 and TBP (Dubrovskaya et al, 1996). The C-terminal end of TAF5 contains multiple WD-40 repeats, an evolutionary conserved structural motif whose exact function is not understood in TAF5 but which has been shown in other systems to form a propeller-like platform dedicated to protein interactions (Dynlacht et al, 1993; Dubrovskaya et al, 1996; Smith et al, 1999).
A three-dimensional (3-D) model of yTFIID was recently solved at 3.5 nm resolution from electron microscopy images of isolated particles (Leurent et al, 2002). In this model, the TFIID subunits are organized as a molecular clamp composed of three lobes linked by thin connections. An immunolabelling approach, used to position nine TAFs containing histone fold domains (HFD), showed that these HFD-TAFs were distributed in each of the three lobes of yTFIID and raised the question as to which subunits form the structural linkers between the three domains and are responsible for the clamp shape. In this report, we mapped TBP, TAF1, TAF5 and TAF7 within the yTFIID complex. The results show that in contrast to previous suggestions, not only TAF1 but also TAF5 play a key structural role by bridging the three TFIID lobes and thus forming a molecular scaffold on which other TAFs can assemble. Furthermore, we demonstrate that the N-terminal domain of TAF1 is spanned over TBP that is itself located between the two lobes A and C. Moreover, we have colocalized the AT domain of TAF1 with TAF7 in the native TFIID, in good agreement with previous interaction experiments.
Results
Immunolocalization of TBP
In order to locate the still unmapped yTFIID subunits in the native complex, we employed an immunolabelling approach using subunit-specific antibodies (Leurent et al, 2002). The endogenous TFIID was incubated with a five-fold molar excess of a TBP-specific polyclonal antibody (pAb), and 1464 electron microscopy images of labelled complexes were recorded and analysed (n1 in Table I). A subset of 607 images representing a unique orientation of TFIID with a characteristic triangular shape (Figure 1A and n2 in Table I) was extracted and further analysed. To demonstrate binding specificity, the antibody interaction sites were plotted onto a map of the TFIID view composed of nine sectors obtained by dividing each lobe (A, B or C) into three sectors of similar size (Figure 1B and Table I). Sector 1 bound antibodies with highest frequency (33.7%), and this value was found to be significant since it was greater than the average background binding frequency of the eight other sectors (8.3%) by 3.2 sigmas (Figure 1B and Table I). To determine more accurately the site where antibodies contacted TFIID, the images were partitioned according to the peripheral density variation, which reflects the presence of the antibody. Finally, a density difference map was calculated between the unlabelled and the labelled class averages in order to locate the tip of the antibody when superimposed on TFIID. Using this approach, anti-TBP IgGs were found to bind to two sites, separated by 4–5 nm, one in lobe A and the other in lobe C, suggesting that TBP is located within the linker domain between the two lobes (Figure 1C and D). The detection of two antibody-binding sites could reflect (i) two different, predominant epitopes recognized by the pAb population, (ii) two TBP molecules present per TFIID complex or (iii) two distinct positions of TBP in the complex. In order to discriminate between these possibilities, a yeast strain expressing an N-terminally HA-tagged TBP was generated and used for the purification of TFIID. Upon incubation with monoclonal anti-HA antibodies, a single specific labelling site was detected in lobe CI (Figure 1E), a result that argues in favour of the presence of a single copy of TBP in the TFIID complex located in the connecting region between domains A and C, consistent with previous genetic and immunological analyses of the subunit composition of the complex (Sanders et al, 2002).
Table 1.
Labelling statistics
| yTAFs labelled | No. of images |
Lobe A |
Lobe B |
Lobe C |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n1 | n2 | Sector 1 | Sector 2 | Sector 3 | Sector 4 | Sector 5 | Sector 6 | Sector 7 | Sector 8 | Sector 9 | |
| TBP | 1464 | 607 | 3.2 | 0.9 | 0.8 | 1.3 | 0.8 | 1.2 | 0.8 | 0.8 | 0.9 |
| HA-TBP | 908 | 599 | 1.1 | 5.9 | 0.3 | 1 | 0.5 | 1.5 | 0.6 | 6.7 | 8.1 |
| Ab1-TAF1 | 1144 | 420 | 6.0 | 15.1 | 8.8 | 0.5 | 0 | 0.9 | 1.4 | 0.9 | 4.9 |
| Ab2-TAF1 | 1180 | 590 | 0.4 | 0.2 | 0.7 | 1.3 | 2 | 0.7 | 0.3 | 3.4 | 0.2 |
| Ab3-TAF1 | 286 | 126 | 0.4 | 0.4 | 0.7 | 0.6 | 2 | 0.9 | 0.5 | 5.1 | 0.9 |
| Ab4-TAF1 | 729 | 422 | 0.5 | 1.8 | 5.2 | 0.2 | 0.7 | -0.6 | -0.3 | -0.8 | -1.3 |
| N-term TAF5 | 888 | 248 | -0.8 | -1 | 0.8 | -0.8 | 1 | 0.3 | 5.6 | -0.8 | 1.3 |
| C-term TAF5 | 639 | 202 | 0.2 | 3.6 | 3.9 | 1.7 | 3.4 | 0.9 | 0.2 | 0.7 | 0.7 |
| TAF7 |
750 |
164 |
0.1 |
1.0 |
5.7 |
0.4 |
1.3 |
1.3 |
0.4 |
1.0 |
1.0 |
| For each antibody used to detect the position of TBP or yTAFs, the number of recorded images (n1) and the number of trilobed views (n2) are indicated. Each lobe was divided into three sectors as shown in Figure 1B. The values reported for each sector represent deviations from the average binding frequency (in sigma folds). Bold characters represent values higher than 3 sigmas. | |||||||||||
Figure 1.
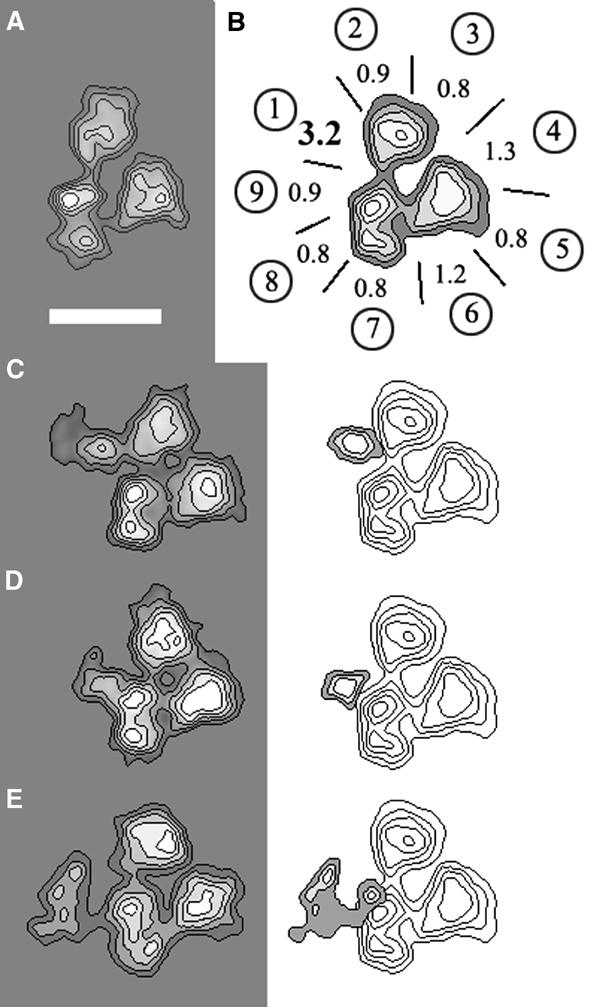
Immunolabelling of TBP. (A) Characteristic trilobed view of TFIID that is used throughout this report to identify the antibody-labelled site, represented with isodensity contour levels. (B) Mapping of the TBP-specific pAb-binding site at the outer contour of the trilobed TFIID view. Each lobe A, B and C is divided into three sectors labelled as in Table I (A 1,2,3; B 4,5,6; C 7,8,9). The values indicated for each sector represent deviations from the average binding frequency (in sigma folds). (C, D) Average images of TFIID molecules specifically labelled with the anti-TBP pAb (left panel) and density difference map with an unlabelled TFIID (right panel). (E) Average image of TFIID molecules in which the N-terminally HA-tagged TBP is labelled with an anti-HA antibody (left panel) and difference map with an unlabelled TFIID molecule (right panel). The bar represents 15 nm.
Mapping of yTAF1
Four different antibody probes were used to map the functional domains of yTAF1 within the yTFIID complex (Figure 2A). The first probe was a purified pAb raised against the entire yTAF1 subunit (Ab1-TAF1). These antibodies specifically bound to an extended region in lobe A and part of lobe C (>than 75% of the total labelling mapped to less than 40% of the contour). Sector 2 showed the highest labelling frequency (29%), and sectors 9, 1 and 3 also showed high antibody-binding scores (13, 15 and 19%, respectively), whereas the average background antibody-binding frequency of the five remaining sectors was 4.6%. The analysis of the peripheral density variation revealed that Ab1-TAF1 bound to several sites encompassing the whole of lobe A and a region of lobe C close to the connection between A and C (Figure 2B). These multiple binding sites of the pAb probably delineate the spread of yTAF1 in the yTFIID complex and suggest that yTAF1 is an extended protein, localized mainly in lobe A, but which could contribute to the linking domain between lobes A and C. This idea is supported by the observation that yTAF1 is extremely sensitive to proteolysis (data not shown). To map the N-terminus of yTAF1, which has been shown to interact in vitro with TBP, a pAb directed against the first 100 amino acids of yTAF1 was used (Ab2-TAF1). In this case, only sector 8 was labelled (Table I), and the analysis of the peripheral density variation revealed antibodies bound to lobes CI and CII (Figure 2C). The position of the N-terminus of TAF1 was confirmed using an anti-HA antibody (Ab3-TAF1) to label the N-terminally HA-tagged TAF1 (Sanders and Weil, 2000). In this experiment, only lobe CII was specifically labelled (Table I and Figure 2D). In order to map the position of the C-terminal part of TAF1, a yeast strain was constructed that carried an HA tag at the C-terminus of TAF1. Immunolabelling experiments with monoclonal anti-HA antibodies showed a significant labelling of sector 3, demonstrating that the C-terminal end of TAF1 was located in lobe A thus orienting TAF1 in the complex (Figure 2E). Altogether, these experiments show that TAF1 has an extended conformation and connects the two lobes A and C. The C-terminal part lies in lobe A and at least the first 100 residues are located in lobe C. In this situation, the N-terminal region of TAF1 is spanned over TBP consistent with the fact that the TAF1 TAND binds TBP.
Figure 2.

Immunolabelling of TAF1 domains. (A) Schematic representation of the structural and functional features of yTAF1. In (B–E), the stars below the inset indicate the portion of TAF1 recognized by the antibodies used. (B) Average images of TFIID molecules specifically labelled with a pAb raised against full-length TAF1 (Ab1-TAF1). Several classes of images differing by the position of the antibody-binding site were found and likely correspond to distinct epitopes (left panel). The density difference maps between the various labelled classes and the unlabelled TFIID were aggregated into a single diagram for clarity (upper right panel). The lower right panel summarized the results obtained with Ab1-TAF1 by mapping the labelled sites on the previously obtained 3-D model of TFIID (Leurent et al, 2002). (C) Average images of TFIID molecules specifically labelled with a pAb raised against the first 100 amino acids of TAF1 (Ab2-TAF1) are depicted in the left panel. The aggregated density difference maps are shown in the right panel and indicate that lobe C is labelled in different regions. (D) Average image of TFIID molecules HA-tagged on the N-terminus of TAF1 and labelled with an anti-HA antibody (Ab3-TAF1). The difference map (right panel) indicates that a single site is labelled in lobe CII. (E) Average image of TFIID molecules HA-tagged on the C-terminus of TAF1 and labelled with an anti-HA antibody (Ab4-TAF1). The difference map (right panel) indicates that a single site is labelled in lobe A.
Immunolocalization of TAF7
Biochemical experiments indicate that TAF7 interacts with the C-terminal part of TAF1. In order to test this hypothesis, we mapped the position of TAF7 within TFIID by using subunit-specific pAbs. The TFIID molecules were incubated with the TAF7-specific antibodies and the immune-complexes were analysed as described above. The labelling statistics clearly indicate that the antibodies bind preferentially to sector 3 within lobe A (Table I) and are substantiated by the noise-free views of the labelled TFIID complexes and the density difference map with the unlabelled molecules (Figure 3). This experiment shows that TAF7 is located close to the C-terminus of TAF1, which is in good agreement with recent genetic and biochemical data (Yatherajam et al, 2003; Singh et al, submitted).
Figure 3.
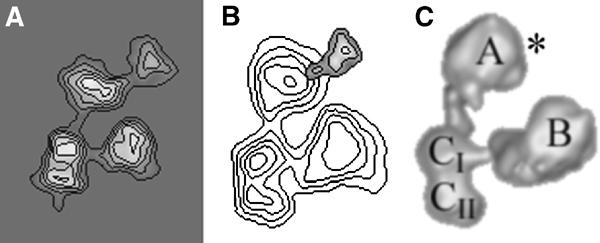
Immunolabelling of TAF7 with a subunit-specific pAb. (A) Average images of yTFIID molecules specifically labelled with a pAb raised against yTAF7. (B) Statistically significant difference map between the labelled and the unlabelled yTFIID molecules. (C) Position of the labelled sites on the 3-D model of TFIID highlighted by an asterisk.
Domain mapping of yTAF5
As TAF1 was only found to bridge lobes A and C, further experiments were performed to investigate how the three TFIID lobes are connected. Biochemical and genetic evidence indicates that the large 90 kDa, WD-40-containing TAF5 is present as two copies in TFIID and could therefore also be implicated in linking the three TFIID lobes (Sanders et al, 2002). In order to map the C-terminal region of TAF5, which contains the WD-40 repeats, a yeast strain was generated in which the C-terminus of TAF5 was fused to a calmodulin-binding protein–protein A tag (TAP tag) in an HA-TAF1 background (Figure 4A). The TFIID complex was purified via standard methods using HA-specific antibodies so as to leave intact the 30 kDa TAP tag on TAF5. The purified TAP-tagged TFIID appeared less homogeneous than the wild-type TFIID, and the complexes had a slight tendency to dissociate, suggesting that the insertion of a large protein moiety at the C-terminus of TAF5 decreases the stability of TFIID. There is however no reason to believe that the molecular organization of the complex is modified, since the yeast strain grows normally and the purified complex has a characteristic subunit composition. Despite this lower stability, characteristic trilobed particles were identified and a data set of 903 molecular images was collected and analysed. The average trilobed view (Figure 4B) closely resembled the shape of the wild-type TFIID molecule. In an attempt to locate the additional mass generated by the TAP tag, a density difference map was calculated between the tagged and the native TFIID. This calculation revealed two additional densities of similar size, one in lobe A and the other in lobe B (Figure 4B, right panel), whose size is consistent with that of the tag, confirming that TAF5 is present in two copies in TFIID. To further demonstrate that these densities correspond to the added TAP tag and do not arise from a local reorganization of TFIID, the complex was incubated with a nonspecific anti-rabbit antibody to target the protein A moiety of the tag. Large amounts of labelled complexes were observed, most of which labelled either lobe A or B (Figure 4C) and in some cases both lobes (data not shown). This experiment confirms that (i) the additional densities detected in the tagged TFIID correspond to the TAP tag, (ii) TAF5 is present as two copies in TFIID as shown previously (Sanders et al, 2002) and (iii) the C-termini of these two TAF5 molecules are located in lobes A and B, respectively.
Figure 4.

Labelling of the N- and C-terminal ends of TAF5. (A) Schematic representation of the structural features of TAF5 showing the WD-40 repeats and the 30 kDa tag for tandem affinity purification (TAP) that was introduced at the C-terminus of TAF5. (B) Average image of TFIID molecules in which TAF5 was fused to the TAP tag (left panel) and statistically significant density difference map with wild-type TFIID molecules (right panel). The difference map shows two additional densities located in lobes A and B. (C) Average images of TFIID containing TAP-tagged TAF5 subunits. The TAP tag was localized with an antibody to reveal the protein A moiety (left panels) and difference maps with nonlabelled TFIID molecules (right panels). Two distinct labelled particles were obtained that were labelled either in lobe B (upper panel) or in lobe A (lower panel), thus confirming the position of the TAP tag placed at the C-terminus of TAF5. (D) Average images of yTFIID molecules specifically labelled with a pAb raised against the first 18 residues of TAF5 (left panel) and corresponding difference map (right panel).
In order to locate the N-terminal part of TAF5, we raised antibodies against residues 1–18 of yTAF5; these antibodies were incubated with TFIID. The antibodies specifically interacted with lobe C (Table I), and the difference map between the labelled and the unlabelled TFIID complex showed that the N-terminal region of TAF5 is located in lobe CII (Figure 4D). The labelling did not reveal two distinct sites, one for each TAF5 molecule, suggesting either that the N-termini of the molecules are closer to each other than the method can resolve or that one of the two N-termini of TAF5 is hidden in the complex. Altogether, these results show that TAF5 is present as two copies in the complex, that the C-terminal part of the subunit is located in lobes A and B and suggest that the N-terminal ends of both copies are located in lobe C. Thus, TAF5 appears to be organized in a symmetric way and could form the linking regions between lobes A and C and between lobes B and C.
Analysis of a recombinant TAF-containing complex
To further investigate the putative architectural role of TAF5, different combinations of recombinant mammalian TAFs were coexpressed in Sf9 cells using a baculovirus expression system. The coexpression of six HFD-containing TAFs, which form pairwise interactions (TAF4/12, TAF6/9 and TAF8/10), both together with TAF5 gave rise to a stable complex that could be immunoprecipitated with an anti-TAF10 antibody and was found to migrate as a homogeneous complex with an apparent molecular weight of about 800 kDa on a size exclusion column (Figure 5A). Western blot analysis confirmed that the major bands observed in silver-stained SDS–PAGE correspond to the expected TAF molecules (Figure 5B). When TAF5 is left out of the coexpression experiment, the only subunit co-immunoprecipitated with TAF10 is its heterodimerization partner TAF8 (Gangloff et al, 2001), demonstrating that in the absence of TAF5 no large TAF10-containing complex is formed (Figure 5C). When fraction 14 from the size exclusion purification was analysed by electron microscopy, a homogeneous population of particles was observed in which three major domains were visible (Figure 5D). The image analysis of 1250 molecular images revealed a characteristic trilobed organization reminiscent of the architecture of TFIID (Figure 5E). The two external lobes A and C were smaller in the seven-TAF recombinant complex than in TFIID, likely reflecting the smaller subunit composition of the seven-TAF complex. Although it cannot be concluded from these observations that the TAF distribution is identical in the recombinant and in the WT complexes, the present data show that the seven-TAF complex, in which TAF5 is the only subunit without an HFD, is organized as three lobes connected by thin linkers.
Figure 5.

Analysis of a recombinant complex containing seven TAFs. (A) Seven TAFs (hTAF4, 5, 6, 9, 10, 12 and mTAF8) were coexpressed in Sf9 cells and the TAF10-containing complexes were purified using a TAF10-specific antibody. The bound complexes were eluted and separated by gel filtration on a Superdex 200 column. (A) SDS–PAGE analysis and silver staining of the collected fractions showing the formation of a homogeneous complex in fraction 14 with an estimated molecular weight of 800 kDa. The elution of molecular weight standards is indicated (top). (B) Western blot analysis showing that the seven TAFs coelute in the peak fractions of the Superdex 200 gel filtration profile. (C) Western blot analysis of anti-TAF10 immunoprecipitation (IP) experiments. Lane 1, coexpression of the seven TAFs; lane 2, TAF5 is missing in the coexpression; lane 3, TAF10 is missing in the coexpression. (D) Electron microscopic observation of the peak fraction 14 reveals a homogeneous population of complexes in negative stain. (E) Image analysis of the seven-TAF complex reveals a TFIID-like trilobed structure. The bar represents 50 nm in (D) and 13.5 nm in (E).
Discussion
The present immunoelectron microscopy experiments localize TBP, TAF7 as well as multiple distinct domains of TAF1 and TAF5 within the 3-D model of yTFIID. The location of these subunits, in addition to the previously mapped HFD-containing subunits (Leurent et al, 2002), illuminates the complex molecular architecture of TFIID (Figure 6).
Figure 6.
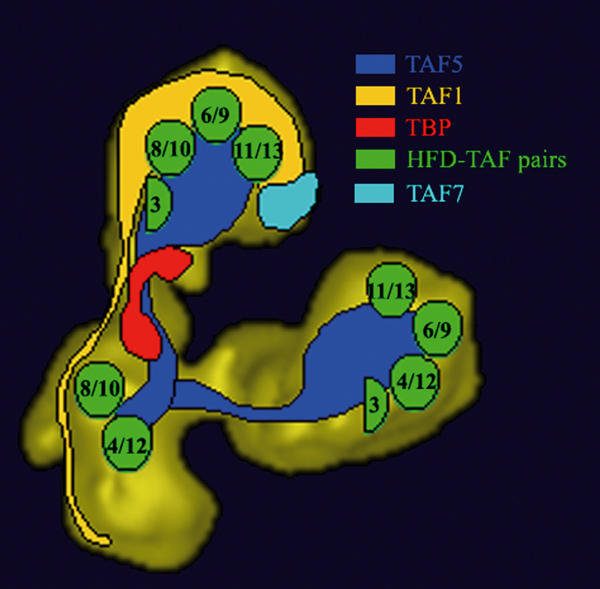
Summary of the immunolabelling experiments. Proposed location of TAFs within the 3-D model of yTFIID. The location of histone fold-containing TAF pairs is schematically represented by green circles as determined previously (Leurent et al, 2002). The extended TAF1 and TAF5 subunits are schematically represented to highlight their presence in two lobes, Note that the size and shape of the coloured areas does not directly correspond to polypeptide mass.
TAF5 contributes a scaffold functionality to TFIID
Our results characterize the structural scaffold underlying the trilobed structure found in human and yeast TFIID (Andel et al, 1999; Brand et al, 1999; Leurent et al, 2002) and identify the subunits involved in the interlobe connections. TAF1 and TAF5 are good candidates to form the linker regions since for both subunits, parts of the same polypeptide were found in two adjacent lobes of TFIID. However, TAF1 can only contribute to the A/C linker since it is absent from lobe B whereas TAF5 maps in all three lobes.
The experiments show that TFIID contains two copies of TAF5 since two distinct C-termini were found, one in lobe A and the other in lobe B. This stoichiometry of TAF5 is consistent with the subunit quantification of immunoprecipitated TFIID, with results showing the simultaneous incorporation of TAF5 and Myc-tagged TAF5 molecules into yTFIID (Sanders et al, 2002) and with the finding that Schizosaccharomyces pombe incorporates two TAF5 homologues, spTAF5 (TAF72) and spTAF5b (TAF73), into the same TFIID complex (Mitsuzawa et al, 2001). The detection of a single site for the N-termini in lobe CII is most likely due to the proximity of the two ends, which were not resolved. However, we cannot rule out the possibility that one of the TAF5 N-termini is hidden in the complex and thus is not accessible to the antibody. The location of the C- and N-termini of the two TAF5 molecules argues in favour of a symmetric arrangement of the molecules, each of which contributes to the formation of an interlobe linker. The importance of TAF5 in bridging the TFIID lobes is supported by the structure of the recombinant complex formed upon coexpression of TAF5 together with six HFD-TAFs. The trilobed architecture of this seven-TAF assembly indicates that the TAFs missing in this recombinant complexes, and particularly TAF1 and TAF2, are not essential to bridge the lobes. Although TAF5 is the only subunit shown to connect all lobes, we cannot formally exclude that some of the HFD-TAFs also contribute to the linker domain or that additional TFIID subunits stabilize or reinforce the links between the lobes.
The WD-40 motif present in the C-terminus of TAF5 was described as being involved in protein–protein interactions (Smith et al, 1999) and could therefore play a role in the assembly of the TAFs in lobes A and B. Temperature-sensitive mutations in the WD-40 repeats of yTAF5 were found to cause broad transcription defects, and their analysis established the importance of these motifs in maintaining the integrity of both the TFIID and SAGA complexes (Durso et al, 2001). When shifted to nonpermissive temperature, TFIID complexes purified from these mutants often showed reduced levels of TAF1 that we show here to reside partly in lobe A. This study also revealed lower amounts of several HFD-TAFs (TAF3, TAF9 and TAF6), TFIID subunits that have been mapped to lobes A and B (Leurent et al, 2002). These biochemical and genetic experiments are thus fully consistent with the proposed location of the subunits in the complex. The proposal that the WD-40 repeats play a role in TFIID assembly apparently contradicts a previous report that stated that the C-terminal part of hTAF5 is not required for incorporation into the TFIID complex (Dubrovskaya et al, 1996). This study showed that the N-terminal half of TAF5 can be incorporated into TBP-containing complexes whereas the C-terminal part fails to do so and was therefore believed to contact no other TFIID components. The interpretation of these results was flawed since at that time the trilobed architecture of TFIID and the presence of two TAF5 copies was not suspected. With our present knowledge, the results of Dubrovskaya et al (1996) are totally consistent with our model, which predicts that the C-terminal part of TAF5 cannot be incorporated into TFIID since its N-terminal ‘dimerization' domain is missing. On the other hand, the N-terminal part of TAF5 is predicted to dimerize with a full-length endogenous TAF5 molecule and thus be incorporated into a partial TFIID complex from which one lobe would be truncated.
Architecture of TAF-containing complexes: a tale of symmetry
The importance of TAF5 in forming the trilobed structure of TFIID is reinforced by the fact that TAF5, along with TAF6, 9, 10 and 12, is shared with the SAGA complex whose homologue in humans, the TFTC complex, was shown to have a similar multilobed structure (Grant et al, 1998; Wieczorek et al, 1998; Brand et al, 1999). The proposed symmetric arrangement of TAF5 within TFIID raises two intriguing questions: (i) how is asymmetry introduced into the native TFIID and SAGA/TFTC complexes and (ii) which signals decide whether TFIID or SAGA is assembled.
The TAF6–9 pair adopts the same symmetric position as the C-termini of TAF5 and could thus interact with the WD-40 repeats as predicted by analysis of mutants (Durso et al, 2001). A specific event has to introduce asymmetry and direct the assembly towards the recruitment of TFIID- or SAGA-specific subunits since the distribution of the remaining common subunits does not obey the two-fold symmetry. To trigger the specialization of lobe A versus lobe B, we propose that a subset of TAFs interacts with the TAF5-containing subcomplex in a way to prevent its symmetric growth. TAF1 has an extended, directional arrangement within the TFIID where it spans both lobes A and C thus providing the potential for directing a specific and nonsymmetric organization of TFIID by creating a unique environment in each lobe. An interaction between TAF1 and TAF5, consistent with their spatial overlap, was described by several groups (Dubrovskaya et al, 1996; Tao et al, 1997; Yatherajam et al, 2003). Accordingly, deletion analysis of yTAF1 showed that the removal of residues 208–303 impairs cell growth, and co-immunoprecipitation studies revealed that this variant, although capable of ‘normal' interaction with TAF7 and TBP, fails to assemble a holo-TFIID complex indicating that this region is important for TFIID assembly/stability (Bai et al, 1997; Mencia and Struhl, 2001).
Furthermore, TAF1 interacts with a large number of TFIID components that reinforce the asymmetry of the complex. TAF1 contacts TAF10 and TAF12, which are present as two copies in TFIID but do not obey two-fold symmetry (Jacq et al, 1994; Kirschner et al, 2002; Kobayashi et al, 2003). These biochemical and genetic data are consistent with our mapping results showing that TAF1 and TAF10 colocalize in lobes A and C, thus providing two possible interaction sites, and that the N-terminal ends of TAF1 and TAF12 are both located in lobe CII. TAF1 was also shown to contact several non-HFD TFIID subunits that are present as single copies in the complex: TBP has several binding sites within the N-terminus of TAF1; hTAF2 was reported to interact with hTAF1 in Sf9 cells (Chalkley and Verrijzer, 1999) and transfection experiments in COS cells showed that TAF7 interacts with TAF1 (Lavigne et al, 1996). The latter interaction is supported by co-immunoprecipitation studies with a family of TAF1 deletion showing that C-terminal deletions all decrease the association of TAF7 with TAF1 (Singh et al, submitted). All these interactions are consistent with the proposed location of these subunits mostly in lobe A of the native TFIID (this report and Leurent et al, 2002).
Positions of TBP and TAF1 define the primary DNA-binding interface
The major DNA-binding subunit, TBP, was found to reside in the thin linker region between lobes A and CI. Our results are consistent with a previous study locating human TBP in domain CI at the top of the cavity and facing the groove where the promoter DNA was suggested to bind (Andel et al, 1999). Residing in the linking domain, TBP would almost be surrounded by solvent, which would provide a high accessibility for interaction with additional transcription factors consistent with the description of manifold TBP interaction partners.
Several lines of evidence indicate that TAF1 could also directly contact specific sequence elements of the core promoter DNA and may thereby act as a promoter selectivity factor (Shen and Green, 1997). Yeast deletion mutants showed that the C-terminus of TAF1 is instrumental in promoter recognition either by directly contacting the DNA through its basic residues or through an indirect mechanism involving TFIIA (Ranish et al, 1999; Mencia and Struhl, 2001). Our observations indicate that the C-terminus of TAF1 is located in lobe A where TFIIA was found to bind (Andel et al, 1999) and support the hypothesis that lobe A is likely to contact DNA. The function of lobe A in binding directly or indirectly to DNA is reinforced by the observation that several subunits located in lobe A were shown to interact with transcriptional activators (Lavigne et al, 1999; Munz et al, 2003).
Our labelling experiments show that lobe A contains the C-terminal end of TAF1, including the conserved AT domain, and that TAF7 resides in close proximity to that part of TAF1. This is consistent with a report showing that hTAF7 interacts and inhibits the acetyltransferase activity of TAF1 (Gegonne et al, 2001). The positioning of lobe A close to DNA may assist the AT domain to exert its function to acetylate either histones as was shown in vitro for human TFIID (Brand et al, 1999) or the transcription factors TFIIEβ and TFIIF (Imhof et al, 1997).
YTAF1 and the autoinhibitory model
The position of the N-terminal part of yTAF1 is in good agreement with the location of TBP in the A/C linker domain. Several lines of evidences have shown that the N-terminal region of TAF1 interacts with TBP and may modulate its binding to the TATA box, resulting in a repressed basal transcriptional activity (Kokubo et al, 1998; Kotani et al, 1998; Liu et al, 1998). A region binding TBP with nanomolar affinity was mapped between residues 2 and 115 in yTAF1 and was shown to be of physiological importance for cell growth (Bai et al, 1997; Banik et al, 2001). In Drosophila, the N-terminal TAND region was dissected into subdomain I (residues 11–77) and subdomain II (residues 82–156), and were predicted to bind to the concave and the convex surfaces of TBP, respectively (Kokubo et al, 1994; Nishikawa et al, 1997). An NMR-generated structure of Drosophila subdomain I was solved in the presence of yeast TBP, and showed that the N-terminal TAND I domain of dTAF1 mimics the minor groove surface of the TATA box, a result that could explain its inhibitory effect on promoter recognition (Liu et al, 1998). Our results clearly show that the N-terminus of TAF1 is stretched over TBP, thus suggesting that the autoinhibitory model is plausible in the context of the native TFIID. However, while the isolated peptide adopts a compact structure, our results indicate that the N-terminal region of HA-tagged yTAF1 has to be very extended. The N-terminus, visualized with anti-HA antibody, is placed in domain CII at the tip of lobe C, whereas pAbs produced against the 100-residue-long N-terminal portion yTAF1 decorate the outer surface of domain CI and the CI/CII junction over a distance of 9 nm. The large distance covered by the first 100 residues suggests that, in the native TFIID, the TBP-binding subdomain I is not folded, as observed by NMR, but may wrap around domain C. This observation opens the possibility that yTAF1 TAND I domain may adopt different conformations possibly regulated by a dedicated mechanism (Kotani et al, 2000). Furthermore, the location of TBP does not overlap with the labelling pattern of the first 100 residues of TAF1 (Ab3-TAF1), indicating that TBP resides at some distance from the extreme N-terminus of yTAF1 and interacts more probably with subdomain II of yTAF1. However, we cannot exclude that a particular form of TFIID was selected during the immunolabelling, either for accessibility reasons or because the binding of the antibody was more readily detectable.
Future experiments will address more directly the position of the N-terminus of TAF1 in controlled conditions by making use, for example, of a large tag such as the one employed to map the C-terminus of TAF5. Such experiments, possibly performed in cryo-electron microscopy to preserve the hydration of the specimen, will help to better understand whether the TAND domain can adopt various positions and whether these are affected by the binding of TFIIA or other cofactors.
Materials and methods
Yeast strains and yeast TFIID purification
The yeast strains used to purify TFIID containing an HA tag on the N- or C-terminus of TAF1 or on TBP have been described, and the purification protocol was identical for all strains (Sanders and Weil, 2000). The majority of contaminating proteins in the HA peptide eluate and the eluting HA peptides were eliminated during the Uno-S ion exchange chromatography. The subunit composition and purity of the three TFIID preps were practically indistinguishable when analysed by SDS–PAGE/silver or Sypro stain. Yeast TFIID containing TAF5-TAP and HA-TAF1 was purified using the same protocol except that the 12CA5 anti-HA mAb-sepharose beads were preincubated with 5 mg of protein A (Sigma) per 1 ml beads for 1 h prior to immunoaffinity chromatography. This modification was necessary to prevent nonspecific binding between the IgG-binding protein A domains from the TAP-tagged TAF5 and the anti-HA antibody crosslinked to the beads.
Purification of the recombinant TAF complex
Sf9 cells were simultaneously infected with seven different baculoviruses that express His-hTAF4, His-hTAF5, Flag-hTAF6, His-mTAF8, hTAF9, hTAF10 and His-hTAF12, respectively. All constructs used to generate the baculoviruses have been described previously (Dubrovskaya et al, 1996) except for TAF8. To generate this vector, the coding sequence of the mouse cDNA was amplified by PCR and inserted in frame into the XhoI and BglII restriction sites of the pAcSG His NT-A baculovirus transfer vector (Pharmingen).
Cells were lysed 48 h postinfection in a buffer containing protease inhibitors and 400 mM KCl, 20 mM Tris–HCl (pH 7.9), 20% glycerol and 2 mM DTT by three cycles of freeze-thawing. From the extracted soluble material, TAF10-containing complexes were isolated by immunoprecipitation with the 23TA-1H8 monoclonal antibody attached to protein G–sepharose beads (Wieczorek et al, 1998). Bound complexes were eluted from the antibody by incubation with 2 mg/ml of the corresponding epitope peptide. The eluted material was concentrated on a Nanosep OMEGA30K ultrafiltration centrifuge column (Pall Filtron). The proteins were separated on a SUPERDEX 200 size exclusion chromatography column in the SMART FPLC system (Pharmacia). Fractions of 50 μl were collected and analysed by Western blotting and silver staining. A pAb was raised against TAF8 by immunizing rabbits with the peptide FPDPHTYIKTPTYREPVSD conjugated to ovalbumin (Georgieva et al, 2000).
Electron microscopy, immunoelectron microscopy and image processing
The purified yTFIID fractions were diluted to a concentration of 20 μg/ml in buffer A (20 mM Tris–HCl (pH 7.4), 150 mM NaCl and 20% glycerol). A volume of 10 μl of this preparation was placed on a 10 nm thick carbon film previously treated by a glow discharge in air. After 2 min of adsorption, the grid was negatively stained with a 2% (w/v) uranyl acetate solution. The images were formed on a Philips CM120 Transmission Electron Microscope operating at 100 kV with a LaB6 filament. Areas covered with individual molecules were recorded under low-dose condition (less than 20 electrons/Å2) at a nominal magnification of × 35,000 on a Pelletier cooled slow scan CCD camera (Model 794, Gatan, Pleasanton). The image processing was performed using the IMAGIC software package (van Heel et al, 1996) (Image Science Software, Berlin, Germany).
Unless otherwise stated, all immunolabelling experiments were performed using purified TFIID HA-tagged at the N-terminus of TAF1. Specific rabbit pAbs recognizing TAF1, TAF7 and TBP were generated, purified and characterized as described previously (Sanders et al, 1999; Sanders and Weil, 2000). To generate the anti-TAF5 rabbit pAbs, a peptide corresponding to residues 1–18 was synthesized and used for immunization. For immunoelectron microscopy a 3- to 5-fold molar excess of antibodies was incubated for 30 min at 20°C with purified TFIID at a final protein concentration of 20 μg/ml. The relative amounts of TFIID and of antibodies were adjusted by electron microscopy inspection of the incubation mixture. The statistical analysis of the immune complexes was performed as described previously (Leurent et al, 2002). Briefly, labelled trilobed views were collected and sorted according to their orientation. Distinct features introduce asymmetry in TFIID and allow an unambiguous discrimination of the six possible pseudosymmetric positions. The elongated shape and the separation into two subdomains makes lobe C clearly different from lobes A and B and prevents any in-plane confusion. The asymmetry is reinforced by the relative distances between the lobes, lobe B is closer to lobe C than is lobe A and by the position of lobe A collinearly with the long axis of lobe C whereas lobe B is positioned perpendicular to the long axis of lobe C. The opening of the clamp between lobes A and B is not a discriminating feature because slight variations in staining or in orientation may cause lobes A and B to overlap. As a result of this asymmetry, the two upside-down orientations were always separated into two different classes during the classification step showing that they cannot be mixed up. The antibody-binding specificity was evaluated by dividing the contour of the molecular view into nine equivalent sectors (three for each lobe) and plotting the antibody-binding site of each labelled complex into one of these sectors. The binding was judged as specific when one or more sectors bound antibodies significantly more frequently (average+3 sigma) than the average binding frequency of background sectors.
Acknowledgments
We thank M Richardot for generating the mTAF8 baculovirus expression vector, I Kolb-Cheynel for help in baculovirus expression, G Duval for polyclonal rabbit antibody production and M Schatz (Image Science Software, Berlin, Germany) for customizing the IMAGIC software package. This work was supported by the Ligue Contre le Cancer, the Institut National de la Santé et de la Recherche Médicale, the Centre National pour la Recherche Scientifique, the Hôpital Universitaire de Strasbourg (HUS), the Association pour la Recherche sur le Cancer, the Font National de La Science (FNS) ACI (to PS and LT), the European Community (grants HPRN-CT-2000-00087 and HPRN-CT-2000-00088 to LT and RTN2-2001-00026 to PS), the AICR (grant 03-084 to LT) and the NIH (grant GM 52461 to PAW).
References
- Andel F, Ladurner AG, Inouye C, Tjian R, Nogales E (1999) Three-dimensional structure of the human TFIID–IIA–IIB complex. Science 286: 2153–2156 [DOI] [PubMed] [Google Scholar]
- Bai Y, Perez GM, Beechem JM, Weil PA (1997) Structure–function analysis of TAF130: identification and characterization of a high-affinity TATA-binding protein interaction domain in the N terminus of yeast TAF(II)130. Mol Cell Biol 17: 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik U, Beechem JM, Klebanow E, Schroeder S, Weil PA (2001) Fluorescence-based analyses of the effects of full-length recombinant TAF130p on the interaction of TATA box-binding protein with TATA box DNA. J Biol Chem 276: 49100–49109 [DOI] [PubMed] [Google Scholar]
- Brand M, Leurent C, Mallouh V, Tora L, Schultz P (1999) Three-dimensional structures of the TAFII-containing complexes TFIID and TFTC. Science 286: 2151–2153 [DOI] [PubMed] [Google Scholar]
- Buratowski S, Hahn S, Guarente L, Sharp PA (1989) Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56: 549–561 [DOI] [PubMed] [Google Scholar]
- Burke TW, Kadonaga JT (1997) The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev 11: 3020–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley GE, Verrijzer CP (1999) DNA binding site selection by RNA polymerase II TAFs: a TAF(II)250–TAF(II)150 complex recognizes the initiator. EMBO J 18: 4835–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Attardi LD, Verrijzer CP, Yokomori K, Tjian R (1994) Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell 79: 93–105 [DOI] [PubMed] [Google Scholar]
- Dikstein R, Ruppert S, Tjian R (1996) TAFII250 is a bipartite protein kinase that phosphorylates the base transcription factor RAP74. Cell 84: 781–790 [DOI] [PubMed] [Google Scholar]
- Dubrovskaya V, Lavigne A-C, Davidson I, Acker J, Staub A, Tora L (1996) Distinct domains of hTAFII100 are required for functional interaction with transcription factor TFIIFβ (RAP30) and incorporation into the TFIID complex. EMBO J 15: 3702–3712 [PMC free article] [PubMed] [Google Scholar]
- Durso RJ, Fisher AK, Albright-Frey TJ, Reese JC (2001) Analysis of taf90 mutants displaying allele-specific and broad defects in transcription. Mol Cell Biol 21: 7331–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht BD, Weinzierl RO, Admon A, Tjian R (1993) The dTAFII80 subunit of Drosophila TFIID contains beta-transducin repeats. Nature 363: 176–179 [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Sanders SL, Romier C, Kirschner D, Weil PA, Tora L, Davidson I (2001) Histone folds mediate selective heterodimerization of yeast TAF25 with TFIID components yTAF47 and yTAF65 and with SAGA component ySPT7. Mol Cell Biol 21: 1841–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegonne A, Weissman JD, Singer DS (2001) TAFII55 binding to TAFII250 inhibits its acetyltransferase activity. Proc Natl Acad Sci USA 98: 12432–12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva S, Kirschner DB, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L (2000) Two novel Drosophila TAF(II)s have homology with human TAF(II)30 and are differentially regulated during development. Mol Cell Biol 20: 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PA, Schieltz D, Pray-Grant MG, Steger DJ, Reese JC, Yates JR, Workman JL (1998) A subset of TAF(II)s are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94: 45–53 [DOI] [PubMed] [Google Scholar]
- Hampsey M, Reinberg D (1999) RNA polymerase II as a control panel for multiple coactivator complexes. Curr Opin Genet Dev 9: 132–139 [DOI] [PubMed] [Google Scholar]
- Imhof A, Yang XJ, Ogryzko VV, Nakatani Y, Wolffe AP, Ge H (1997) Acetylation of general transcription factors by histone acetyltransferases. Curr Biol 7: 689–692 [DOI] [PubMed] [Google Scholar]
- Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L (1994) Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell 79: 107–117 [DOI] [PubMed] [Google Scholar]
- Kirschner DB, vom Baur E, Thibault C, Sanders SL, Gangloff YG, Davidson I, Weil PA, Tora L (2002) Distinct mutations in yeast TAF(II)25 differentially affect the composition of TFIID and SAGA complexes as well as global gene expression patterns. Mol Cell Biol 22: 3178–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Miyake T, Kawaichi M, Kokubo T (2003) Mutations in the histone fold domain of the TAF12 gene show synthetic lethality with the TAF1 gene lacking the TAF N-terminal domain (TAND) by different mechanisms from those in the SPT15 gene encoding the TATA box-binding protein (TBP). Nucleic Acids Res 31: 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo T, Swanson MJ, Nishikawa JI, Hinnebusch AG, Nakatani Y (1998) The yeast TAF145 inhibitory domain and TFIIA competitively bind to TATA-binding protein. Mol Cell Biol 18: 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo T, Yamashita S, Horikoshi M, Roeder RG, Nakatani Y (1994) Interaction between the N-terminal domain of the 230-kDa subunit and the TATA box-binding subunit of TFIID negatively regulates TATA-box binding. Proc Natl Acad Sci USA 91: 3520–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Banno K, Ikura M, Hinnebusch AG, Nakatani Y, Kawaichi M, Kokubo T (2000) A role of transcriptional activators as antirepressors for the autoinhibitory activity of TATA box binding of transcription factor IID. Proc Natl Acad Sci USA 97: 7178–7183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Miyake T, Tsukihashi Y, Hinnebusch AG, Nakatani Y, Kawaichi M, Kokubo T (1998) Identification of highly conserved amino-terminal segments of dTAFII230 and yTAFII145 that are functionally interchangeable for inhibiting TBP–DNA interactions in vitro and in promoting yeast cell growth in vivo. J Biol Chem 273: 32254–32264 [DOI] [PubMed] [Google Scholar]
- Lavigne AC, Mengus G, Gangloff YG, Wurtz JM, Davidson I (1999) Human TAF(II)55 interacts with the vitamin D(3) and thyroid hormone receptors and with derivatives of the retinoid X receptor that have altered transactivation properties. Mol Cell Biol 19: 5486–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne AC, Mengus G, May M, Dubrovskaya V, Tora L, Chambon P, Davidson I (1996) Multiple interactions between hTAFII55 and other TFIID subunits. Requirements for the formation of stable ternary complexes between hTAFII55 and the TATA-binding protein. J Biol Chem 271: 19774–19780 [DOI] [PubMed] [Google Scholar]
- Leurent C, Sanders S, Ruhlmann C, Mallouh V, Weil PA, Kirschner DB, Tora L, Schultz P (2002) Mapping histone fold TAFs within yeast TFIID. EMBO J 21: 3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ishima R, Tong KI, Bagby S, Kokubo T, Muhandiram DR, Kay LE, Nakatani Y, Ikura M (1998) Solution structure of a TBP–TAF(II)230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell 94: 573–583 [DOI] [PubMed] [Google Scholar]
- Mencia M, Struhl K (2001) Region of yeast TAF 130 required for TFIID to associate with promoters. Mol Cell Biol 21: 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuzawa H, Seino H, Yamao F, Ishihama A (2001) Two WD repeat-containing TATA-binding protein-associated factors in fission yeast that suppress defects in the anaphase-promoting complex. J Biol Chem 276: 17117–17124 [DOI] [PubMed] [Google Scholar]
- Mizzen CA, Yang XJ, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, Workman J, Wang L, Berger SL, Kouzarides T, Nakatani Y, Allis CD (1996) The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell 87: 1261–1270 [DOI] [PubMed] [Google Scholar]
- Munz C, Psichari E, Mandilis D, Lavigne AC, Spiliotaki M, Oehler T, Davidson I, Tora L, Angel P, Pintzas A (2003) TAF7 (TAFII55) plays a role in the transcription activation by c-Jun. J Biol Chem 278: 21510–21516 [DOI] [PubMed] [Google Scholar]
- Nishikawa J, Kokubo T, Horikoshi M, Roeder RG, Nakatani Y (1997) Drosophila TAF(II)230 and the transcriptional activator VP16 bind competitively to the TATA box-binding domain of the TATA box-binding protein. Proc Natl Acad Sci USA 94: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschlager T, Chiang CM, Roeder RG (1996) Topology and reorganization of a human TFIID–promoter complex. Nature 382: 735–738 [DOI] [PubMed] [Google Scholar]
- Orphanides G, Lagrange T, Reinberg D (1996) The general transcription factors of RNA polymerase II. Genes Dev 10: 2657–2683 [DOI] [PubMed] [Google Scholar]
- Purnell BA, Emanuel PA, Gilmour DS (1994) TFIID sequence recognition of the initiator and sequences further downstream in Drosophila class II genes. Genes Dev 8: 830–842 [DOI] [PubMed] [Google Scholar]
- Ranish JA, Yudkovsky N, Hahn S (1999) Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev 13: 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Garbett KA, Weil PA (2002) Molecular characterization of Saccharomyces cerevisiae TFIID. Mol Cell Biol 22: 6000–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Klebanow ER, Weil PA (1999) TAF25p, a non-histone-like subunit of TFIID and SAGA complexes, is essential for total mRNA gene transcription in vivo. J Biol Chem 274: 18847–18850 [DOI] [PubMed] [Google Scholar]
- Sanders SL, Weil PA (2000) Identification of two novel TAF subunits of the yeast Saccharomyces cerevisiae TFIID complex. J Biol Chem 275: 13895–13900 [DOI] [PubMed] [Google Scholar]
- Shen WC, Green MR (1997) Yeast TAF(II)145 functions as a core promoter selectivity factor, not a general coactivator. Cell 90: 615–624 [DOI] [PubMed] [Google Scholar]
- Singh MV, Bland CE, Weil PA Molecular genetic characterization of a Taf1p domain essential for yeast TFIID assembly. Submitted [DOI] [PMC free article] [PubMed]
- Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24: 181–185 [DOI] [PubMed] [Google Scholar]
- Solow S, Salunek M, Ryan R, Lieberman PM (2001) Taf(II) 250 phosphorylates human transcription factor IIA on serine residues important for TBP binding and transcription activity. J Biol Chem 276: 15886–15892 [DOI] [PubMed] [Google Scholar]
- Sypes MA, Gilmour DS (1994) Protein/DNA crosslinking of a TFIID complex reveals novel interactions downstream of the transcription start. Nucleic Acids Res 22: 807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Guermah M, Martinez E, Oegelschlager T, Hasegawa S, Takada R, Yamamoto T, Horikoshi M, Roeder RG (1997) Specific interactions and potential functions of human TAFII100. J Biol Chem 272: 6714–6721 [DOI] [PubMed] [Google Scholar]
- Tora L (2002) A unified nomenclature for TATA box binding protein (TBP)-associated factors (TAFs) involved in RNA polymerase II transcription. Genes Dev 16: 673–675 [DOI] [PubMed] [Google Scholar]
- van Heel G, Harauz G, Orlova EV (1996) A new generation of the IMAGIC image processing system. J Struct Biol 116: 17–24 [DOI] [PubMed] [Google Scholar]
- Verrijzer CP, Yokomori K, Chen JL, Tjian R (1994) Drosophila TAFII150: similarity to yeast gene TSM-1 and specific binding to core promoter DNA. Science 264: 933–941 [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Sauer F (2001) TAF(II)250: a transcription toolbox. J Cell Sci 114: 2895–2902 [DOI] [PubMed] [Google Scholar]
- Wieczorek E, Brand M, Jacq X, Tora L (1998) Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature 393: 187–191 [DOI] [PubMed] [Google Scholar]
- Yatherajam G, Zhang L, Kraemer SM, Stargell LA (2003) Protein–protein interaction map for yeast TFIID. Nucleic Acids Res 31: 1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]


