Summary
Nesfatin-1 was discovered in 2006 and introduced as a potential novel anorexigenic modulator of food intake and body weight. The past years have witnessed increasing evidence establishing nesfatin-1 as a potent physiological inhibitor of food intake and body weight and unraveled nesfatin-1's interaction with other brain transmitters to exert its food consumption inhibitory effect. As observed for other anorexigenic brain neuropeptides, nesfatin-1 is also likely to exert additional, if not pleiotropic, actions in the brain and periphery. Recent studies established the prominent expression of the nesfatin-1 precursor, nucleobindin2 (NUCB2) in the stomach and pancreas, where nesfatin-1 influences endocrine secretion. This review will highlight the current experimental state-of-knowledge on the effects of NUCB2/nesfatin-1 on food intake, body weight and glucose homeostasis. Potential implications in human obesity will be discussed in relation with evidence of changes in circulating levels of NUCB2/nesfatin-1 in disease states, the occurrence of genetic NUCB2 polymorphisms – and in contrast to several other hormones – the independence of leptin signaling known to be blunted under conditions of chronically increased body weight.
Keywords: body weight, food intake, hypothalamus, NUCB2, nesfatin-1, obesity, satiation, satiety, X/A-like cells
Introduction
Discovery of nesfatin-1
In 2006, Mori and his group identified a gene in brain medulloblastoma (HTB185) and 3T3-L1 adipocyte cells that was highly responsive to troglitazone, a ligand for the peroxisome proliferator-activated receptor (PPAR)γ [1]. Detailed analysis of this gene showed correspondence to the gene encoding nucleobindin2 [1]. Nucleobindin2 (NUCB2) consists of a 24-amino acid (aa) N-terminal signal peptide followed by 396 aa with a highly conserved sequence across mammalian and non-mammalian vertebrates indicative of its phylogenetic relevance [2].
Processing of NUCB2: nesfatin-1, nesfatin-2 and nesfatin-3
The NUCB2 precursor protein is possibly post-translationally cleaved by the enzyme pro-hormone convertase (PC)-1/3 into the N-terminal nesfatin-1 (aa 1-82), nesfatin-2 (aa 85-163) and the C-terminal nesfatin-3 (aa 166-396) [1]. Until now, several biological actions have been identified for nesfatin-1 [3], whereas none have been described for nesfatin-2 and nesfatin-3. Mori et al.'s initial report showed the occurrence of the 9.7 kDa peptide nesfatin-1 in rat hypothalamic extracts and a pooled sample of cerebrospinal fluid monitored by a competitive ELISA for nesfatin-1 using ab24 [1] as well as in human plasma using a nesfatin-1 specific sandwich-type ELISA assay [4]. Although nesfatin-1 has been reported to be present in human cerebrospinal fluid [5], all subsequent studies using the commercially available Phoenix NUCB2/nesfatin-1 antibody for Western blot analysis detected only the full length NUCB2 in the rat hypothalamus, pituitary, pancreas, gastric mucosa and adipose tissue and goldfish brain [6-10], while synthetic nesfatin-1 used as a positive control was detectable in the same Western blot analysis as a 10 kDa band [9, 10]. These discrepant results may be related to differences between antibodies used in the initial (affinity purified Ab24 nesfatin-1 antibody) and subsequent (Phoenix NUCB2/nesfatin-1 antibodies) reports. However, it remains unclear why the mature processed nesfatin-1 form is not detectable in most tissue samples analyzed so far. Of note, it cannot be ruled out that the unprocessed protein may be the active native form as full length NUCB2 exerts a similar food intake reducing effect as nesfatin-1 when injected into the rat 3rd brain ventricle [1].
It is also important to highlight that except the initial report on the distribution of nesfatin-1 [1], all subsequent immunohistochemical studies did not distinguish between full length NUCB2 and nesfatin-1 since the antibodies used (Phoenix rabbit anti-rat nesfatin-1 and Gunma University rabbit anti-nesfatin-1 antibody) were raised against the full length nesfatin-1 and also recognize NUCB2 [6, 11-14]. Therefore, immunohistochemical studies performed with these antibodies should refer to NUCB2/nesfatin-1 based on their cross-reactivity between NUCB2 and nesfatin-1. Specific detection of nesfatin-1 will require the use of the initially developed Ab24 nesfatin-1 antibody [1] or the recently developed sandwich-type human nesfatin-1 ELISA assay using N-terminal (sequence Val-Pro-Ile-Asp-Ile-Asp-Lys-Cys) and C-terminal (Cys-His-His-Val-Arg-Thr-Lys-Leu-Asp-Glu-Leu) antibodies without cross-reaction with full length NUCB2, nesfatin-2 or nesfatin-3 as reported in human plasma [4].
Central nervous system actions of nesfatin-1 to reduce food intake
Brain distribution of NUCB2/nesfatin-1 and co-localization with other transmitters
Initially, NUCB2 mRNA and protein expression have been identified in the rat brain in hypothalamic nuclei implicated in the regulation of food intake including the supraoptic nucleus (SON), lateral hypothalamic area, arcuate nucleus and paraventricular nucleus (PVN) [1]. Subsequent studies corroborated and extended these findings by showing the distribution of NUCB2/nesfatin-1 in various additional regions/nuclei of the brain, namely the insular cortex, central amygdaloid nucleus, periventricular nucleus, tuberal hypothalamic area, dorsomedial hypothalamic nucleus, Edinger-Westphal nucleus, the medullary raphe nuclei, ventrolateral medulla (VLM), locus coeruleus (LC), cerebellum, dorsal motor nucleus of the vagus nerve (DMV), nucleus of the solitary tract (NTS) and preganglionic sympathetic and parasympathetic neurons of the spinal cord in rats [6, 11-13, 15-17], mice [14] and pigs [18]. Interestingly, in mice, a novel brain nucleus was identified which strongly expressed NUCB2/nesfatin-1 immunoreactivity that was named the intermediate dorsomedial area of the hypothalamus [14]. Overall, the pattern of brain NUCB2/nesfatin-1 distribution encompasses stress-related circuitries and autonomic regulatory centers. This strongly suggests additional stress or autonomic related biological actions outside of the initially reported regulation of ingestive behavior as recently reviewed [19, 20].
Following central mapping studies, several groups provided a detailed phenotypic characterization of brain NUCB2/nesfatin-1 containing neurons. NUCB2/nesfatin-1 immunoreactivity was colocalized with several other peptides and neurotransmitters known to be involved in the regulation of food intake, namely urocortin 1 (~90%), melanin-concentrating hormone (MCH, ~80%), pro-opiomelanocortin (POMC, ~60-80%), cocaine-and amphetamine-regulated transcript (CART, ~70%), α-melanocyte-stimulating hormone (α-MSH, ~60%), %), vasopressin (~50%), neuropeptide Y (NPY, ~40%), oxytocin (~40%), growth hormone-releasing hormone (GHRH, ~30%), corticotropin-releasing factor (CRF, ~20%), thyrotropin-releasing hormone (TRH, ~20%), somatostatin or neurotensin (~10%) and serotonin [6, 11, 12, 15, 16, 21, 22]. The extensive overlap of NUCB2/nesfatin-1 with other transmitters provides neuroanatomical support for the pleiotropic relevance of nesfatin-1 in addition to food intake regulation [3].
Characteristics of nesfatin-1's central anorexigenic action
The initial report showed that full length NUCB2 or mature nesfatin-1 injected through a chronically implanted cannula into the third brain ventricle dose-dependently reduce the dark phase food intake in freely fed rats [1]. Thereafter, a number of independent groups corroborated and expanded these findings and showed that nesfatin-1 injected into the brain ventricles at different levels (lateral or 4th) at doses ranging from 5 to 20 pmol inhibited the dark phase food intake in rodents [21, 23-29] or goldfish [7, 30]. When nesfatin-1 was injected at a low dose (5 pmol) into the rat lateral brain ventricle (intracerebroventricular, icv), the reduction of food intake had a delayed onset with a robust peak reduction of 87% during the third hour post injection. The suppression was long lasting and observed over 6 h and up to 48 h at a higher dose (25 pmol) [23, 28]. Similarly, in mice the icv injection of nesfatin-1 induces an anorexigenic action that was apparent at 2 h after administration and lasted for 8 h [27]. However, when injected into the third or fourth brain ventricle or into the cisterna magna, the nesfatin-1 induced reduction of nocturnal feeding occurred during the first hour post injection in rats [1, 23]. This rapid onset contrasts with the icv injection indicating a difference in nesfatin-1's kinetic and/or mechanisms of action in the forebrain versus the midbrain/hindbrain. It is to note that the food intake suppressive effect of nesfatin-1 was not related to an overall alteration of locomotor activity [1, 23, 28] or changes in grooming including scratching, licking and washing [23] pointing towards a direct modulation of feeding behavior.
Insight into hypothalamic sites responsive to nesfatin-1 came from microinjection studies of the peptide directly into different nuclei. When microinjected into the PVN, there was a pronounced reduction of dark phase food intake during the 1st to 5th h post injection [21]. This finding was reproduced following injection of nesfatin-1 into the lateral hypothalamic area although higher doses were required, whereas injection into the ventromedial hypothalamus or subfornical organ had no effect [31, 32]. Therefore, existing data along with the greatest number of Fos-labeled neurons induced by icv injection of nesfatin-1 (5 pmol) occurring in the PVN [32] point towards the PVN as a primary responsive forebrain site for nesfatin-1 to reduce food intake, while responsive sites within midbrain/hindbrain nuclei remain to be localized.
Recent studies provided additional insight into the alterations of food intake microstructure underlying the anorexigenic action using an automated monitoring system that allows for recording of the nocturnal ingestion of standard chow every second in undisturbed mice. Nesfatin-1 injected acutely icv at a dose of 0.3 nmol under short isoflurane anesthesia reduces the cumulative dark phase food intake in freely fed mice which resulted from the decrease in both, the size of the meal (as a characteristic for satiation) and the meal frequency associated with the prolongation of the inter-meal intervals (as a characteristic for satiety) [24]. Similar to full length nesfatin-11-82, the icv injection of nesfatin-130-59 prolonged inter-meal intervals and reduced meal frequency of nocturnal feeding in mice, however, the meal size was not altered (Table 1) [33]. By contrast, the N- and C-terminal fragments, nesfatin-11-29 and nesfatin-160-82 respectively had no effect when tested under the same conditions [33]. These studies identified the 30 aa mid fragment nesfatin-130-59 as the main active core exerting the food inhibitory action of nesfatin-1. The induction of satiety by icv nesfatin-130-59 versus satiety and satiation by nesfatin-1 may be due to additional binding sites at the receptor outside of the active core that are only targeted by full length nesfatin-1. However, one has to keep in mind that it is currently not known whether the processing of nesfatin-1 to nesfatin-130-59 occurs endogenously.
Table 1.
Alteration of food intake microstructure by intracerebroventricular injection of nesfatin-1 and nesfatin-130–59 in mice.
| Characteristic | Nesfatin-1 (0.3 nmol) | Nesfatin-130–59 (0.3 nmol) | ||
|---|---|---|---|---|
| Effect | Comment | Effect | Comment | |
| Acute onset | no | No alteration of first meal | no | No alteration of first meal |
| Delayed action | yes | Onset in 3rd h post injection | yes | Onset in 3rd h post injection |
| Long lasting | yes | Reduction of 12-h cumulative food intake | yes | Reduction of 24-h cumulative food intake |
| Satiation | yes | Reduction of meal size | no | No effect on meal size |
| Satiety | yes | Prolongation of inter-meal intervals | yes | Prolongation of inter-meal intervals |
Other central actions of nesfatin-1 with potential influence on food intake
Anxiogenic behavior
Although lower doses (5 pmol) injected icv specifically reduce feeding [1, 23], higher doses (25-80 pmol) induce anxiety-like behaviors assessed by a heightened startle response and decreased time spent in the open arms of the elevated plus maze in rats [25, 34] which may also contribute to the sustained anorexigenic effect observed at higher doses.
Sleeping behavior
Disturbance of sleep profoundly affects food intake, resulting in the development of obesity [35]. Several secreted molecules regulating sleep, such as MCH and orexin, have been identified [36]. It is particularly interesting that NUCB2/nesfatin-1 neurons are co-localized with neurons expressing MCH, but not orexin, in the lateral hypothalamic area. As recent observations have shown that endogenous nesfatin-1 associated with MCH plays an important role in the regulation of sleep [37, 38], the mechanisms underlying nesfatin-1-induced anorexia with respect to sleeping behavior will become clearer in the future.
Drinking behavior
In addition to its anorexigenic action, icv injected nesfatin-1 at doses ranging from 160 to 540 pmol reduces water consumption under various stimulated conditions: after injection of angiotensin II, following a hypertonic challenge or after 18 h of water restriction [39]. Although food and drinking consumptive behaviors are intrinsically linked [40], the antidipsogenic effect is separated from the anorexigenic action of nesfatin-1 and required doses superior to 60 pmol injected icv in rats [25]. While these studies emphasize that nesfatin-1 is able to control both thirst and hunger independently, the increased anorexigenic potency of nesfatin-1 at higher dose may encompass the compromised ratio of food to water intake. By contrast, recent studies showed that nesfatin-1 microinjected into the subfornical organ at a low dose of 5 pmol elicits a dipsogenic effect independently of the angiotensinergic pathways and in the absence of food. Moreover, icv injected nesfatin-1 at a similar dose induces a prominent activation of circumventricular organs [32]. These data support a potential important role of nesfatin-1 in fluid balance.
Gastric emptying
Nesfatin-1 injected icv inhibits gastric emptying in mice [14] and rats [23] and reduces gastroduodenal motility in mice [27]. Several peptides regulating food intake also affect digestive functions such as gastric emptying and thereby may negatively impact on food consumption by increasing vagal afferent activity linked with gastric distension [41, 42]. Whether the gastric stasis associated with icv injection of nesfatin-1 is a contributing factor to the sustained anorexigenic action of nesfatin-1 deserves further considerations.
Gastric acid secretion
Besides the effect on gastric motility, nesfatin-1 acts in the brain to influence gastric secretion. When injected into the fourth brain ventricle, nesfatin-1 inhibited the vagally mediated stimulation of gastric acid secretion by 2-deoxy-D-glucose-, while basal or pentagastrin stimulated gastric acid secretion was not affected [29]. These data indicate that the anorexigenic effect of centrally injected nesfatin-1 is integrated with the inhibition of gastric digestive processes as observed for other brain peptides [43].
Interaction of nesfatin-1 with brain transmitters regulating food intake
Pharmacological and molecular approaches were used to investigate the functional relevance of the extensive co-localization of brain NUCB2/nesfatin-1 with several neuropeptides and transmitters established to influence food intake. Early on, the anorexigenic effect of nesfatin-1 was established to be independent from leptin signaling as evidenced by the retained food intake suppression in leptin receptor deficient Zucker rats [1, 21]. Moreover, leptin did not alter the expression level of hypothalamic NUCB2 mRNA [1].
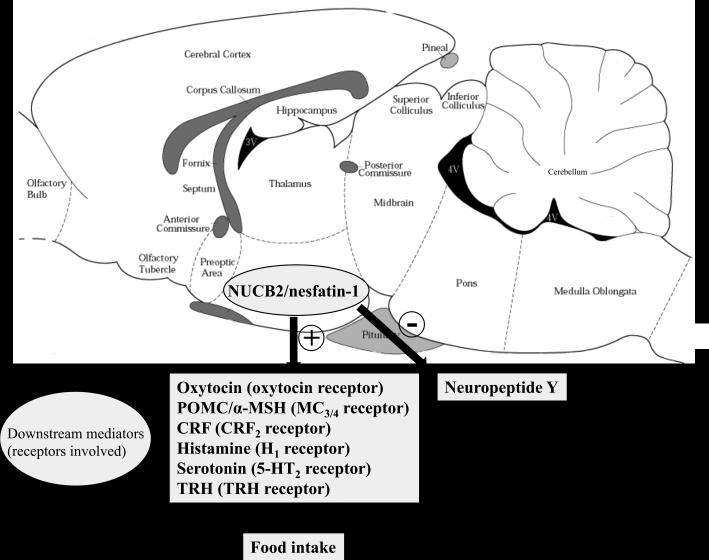
On the other hand, nesfatin-1 shows a downstream interplay with oxytocin and melatonin signaling. When injected into the 3rd brain ventricle, nesfatin-1 activates oxytocin containing neurons in the PVN as shown by increased Fos expression as well as Ca2+ influx into these neurons [21]. Moreover, nesfatin-1 stimulated the release of oxytocin from PVN slices in vitro [21] and the 4th ventricular injection of the oxytocin receptor antagonist H4928 blocked nesfatin-1's anorexigenic action in vivo in rats [21, 26]. Further downstream of oxytocin, POMC in the hindbrain is likely to play a role in the mediation of nesfatin-1's food intake inhibitory effect based on the observations that oxytocinergic nerve terminals in the NTS originating from the PVN [44] are in close proximity to POMC containing neurons and that oxytocin stimulates the release of POMC in the NTS [21]. POMC is proteolytically cleaved into several peptides including α-MSH which is the endogenous ligand of the melanocortin 3/4 receptor [45]. Additional studies showed that blockade of the α-MSH-melanocortin 3/4 receptor with the antagonist, SHU9119 injected icv or into the 3rd ventricle completely blocked the food intake suppressive effect of nesfatin-1 injected icv or into the 3rd ventricle [1, 25]. In addition, 3rd ventricular injection of α-MSH up-regulates NUCB2 mRNA expression in the PVN [1]. Whether this feed-forward feedback is also part of the mechanism underlying the long lasting reduction of food intake induced by central NUCB2/nesfatin-1 is yet to be investigated. In summary, convergent evidence points towards a downstream mediation of nesfatin-1's anorexigenic action by the activation of a hypothalamic-pontine oxytocin-POMC-α-MSH-melanocortin3/4 receptor signaling pathway (Fig. 1).
Figure 1.
Upstream pathways stimulating central NUCB2/nesfatin-1 signaling and downstream mediators involved in the brain nesfatin-1 injection-induced reduction of food intake. Symbols: +, stimulation; −, inhibition; ↑, increase. Abbreviations: 5-HT2 receptor, serotonin receptor 2; α-MSH, alpha-melanocyte stimulating hormone; CRF2 receptor, corticotropin releasing factor receptor 2; H1 receptor, histamine receptor 1; MC3/4 receptors, melanocortin receptors 3 and 4; NUCB2, nucleobindin2; POMC, proopiomelanocortin, TRH, thyrotropin releasing hormone.
In addition to oxytocin and α-MSH, the CRF2 receptor signaling system, well established to reduce food intake [46, 47], was implicated in the food intake regulatory action of nesfatin-1. The peptide injected into the 3rd brain ventricle in rats increases the CRF content in the PVN [48]. When administered directly onto PVN neurons in vitro, nesfatin-1 increases the excitability of CRF positive neurons [49]. In addition, nesfatin-1 injected icv increases plasma levels of ACTH and corticosterone, while in vitro nesfatin-1 did not alter ACTH release from cultured pituitary cells [50]. Collectively, these data provide conclusive evidence that nesfatin-1 stimulates the hypothalamic-pituitary-adrenal axis through CRF release from the PVN. In addition, pharmacological studies showed that blockade of CRF2 receptors by icv injection of the selective peptide CRF2 antagonist, astressin2-B abolished (Fig. 1) and 3rd ventricle injection of the CRF1/CRF2 antagonist, α-helical CRF9-41 blunted nesfatin-1's anorexigenic effect [23, 48]. By contrast, when nesfatin-1 was injected into the cisterna magna, astressin2-B did not influence the suppression of dark phase feeding in rats [23] supporting a primary role of POMC-melanocortin3/4 receptor as downstream pathway in the brainstem [1, 25].
Additionally, hypothalamic histamine and serotonin (5-HT) are likely to contribute to modulate the anorexigenic effect of nesfatin-1 via interaction with the H1 and 5-HT2C receptors, respectively [48, 51]. Injection of nesfatin-1 into the 3rd brain ventricle in rats increases the turnover of hypothalamic histamine which seems to be part of a feed forward loop as histamine increases NUCB2/nesfatin-1 in the PVN but not in the lateral hypothalamus and VMH [48]. In line with these findings, mice lacking the H1 receptor or rats with blunted hypothalamic histamine signaling due to inhibition of histidine decarboxylase by icv injection of α-fluoromethyl histidine showed a reduced food intake suppressive effect following 3rd ventricular injection of nesfatin-1 (Fig. 1) [48]. The histaminergic pathways may be downstream of hypothalamic CRF. Indeed, the injection of α-helical CRF9-41 into the 3rd brain ventricle reduced the nesfatin-1 induced stimulation of histamine turnover in the PVN along with its anorexigenic effect [48]. The role of serotonergic pathways in nesfatin-1's food intake inhibitory action has been established in studies showing that intraperitoneal (ip) injection of the 5-HT1B/2C agonist, m-chlorophenylpiperazine (mCPP) up-regulates hypothalamic NUCB2 mRNA resulting in the reduction of food intake, whereas mice lacking the 5-HT2C displayed a reduction of hypothalamic NUCB2 and POMC and an increased food intake [51]. These data suggest a physiological negative tone of serotonin-5-HT2C-NUCB2/nesfatin-1 on food consumption that needs to be further confirmed (Fig. 1).
Limited investigation also indicated that the hypothalamic tripeptide thyrotropin-releasing hormone (TRH) may be implicated in the anorexigenic effect of nesfatin-1 as TRH mRNA expression in the PVN is up-regulated following 3rd ventricular injection of nesfatin-1, and a TRH antibody injected into the 3rd ventricle blunted the nesfatin-1 induced reduction of feeding (Fig. 1) [48].
Besides the involvement of several anorexigenic pathways, nesfatin-1 can also influence the activity of neurons containing the well-established orexigenic brain peptide NPY. Administration of nesfatin-1 directly onto arcuate neurons in vitro results in the hyperpolarization of NPY positive neurons [52] which is likely to promote an anorexigenic effect (Fig. 1). It is important to note that the food intake suppressive effect of nesfatin-1 is predominantly observed during the dark phase in ad libitum fed animals [1, 21, 23, 25, 48], whereas during the light phase in fasted rodents, inconsistent data were reported [23, 25]. Such a specificity towards the nocturnal feeding suggests that central injection of nesfatin-1 is targeting brain transmitters specifically involved in driving the physiological food consumption occurring in the dark photoperiod in rodents [53-55]. Alternatively, as recent evidence established that NUCB2/nesfatin-1 rises selectively in the PVN in synchrony with feeding suppression in the early light phase [56], this may render the hypothalamus less responsive to exogenous administration of nesfatin-1 during the light phase.
Interaction between NUCB2/nesfatin-1 and metabolic factors
Recent in vitro studies demonstrated a direct effect of meal-evoked metabolic factors, glucose and insulin, on PVN NUCB2/nesfatin-1 neurons [57]. Using changes in cytosolic calcium concentration monitored by ratiometric fura-2 fluorescence imaging, it was found that 58.2% of glucose-responsive and 62.5% of insulin-responsive neurons were immunoreactive for NUCB2/nesfatin-1 [57]. Furthermore, nesfatin-1 differentially modulates glucosensing neurons in the hypothalamus as assessed by microinjection. In the PVN nesfatin-1 excited most of the glucose-inhibited neurons and inhibited most of the glucose-excited neurons [31]. By contrast, nesfatin-1 induced the stimulation of glucose-excited neurons in the ventromedial hypothalamus, and the inhibition of most of the glucose-inhibited neurons in the lateral hypothalamus [31]. Other studies showed that icv injected nesfatin-1 increases the number of Fos positive neurons in the hypothalamic nuclei involved in glucose homeostasis including the arcuate, PVN and SON [32, 58]. Nesfatin-1's involvement in glucose homeostasis and insulin signaling was further supported by the demonstration that third ventricular injection of nesfatin-1 inhibits the hepatic glucose production, increases insulin signaling through the phosphorylation of insulin receptor (InsR)/insulin receptor substrate-1 (IRS-1)/AMP-dependent protein kinase (AMPK)/Akt kinase (Akt)/target of rapamycin complex (TORC) 2 in the liver and promotes muscle glucose uptake in both standard and high fat diet fed rats [59].
Conversely, nesfatin-1 is regulated by glucose homeostasis as injection of insulin or 2-deoxyglucose activated NUCB2/nesfatin-1 immunoreactive neurons in the arcuate nucleus, PVN, lateral hypothalamic area, dorsal NTS and DMV [60]. This activation is likely to directly impact on gastrointestinal glucose regulatory effector sites based on the observation that NUCB2/nesfatin-1 immunoreactive neurons of the DMV project to the stomach, liver and pancreas [60]. It will have to be delineated to what extent the additional function of nesfatin-1 to regulate hypothalamic glucose sensing and insulin sensitivity contributes to its anorexigenic property.
Physiological roles of hypothalamic NUCB2/nesfatin-1
Earlier studies provided evidence for a physiological role of NUCB2/nesfatin-1 in the regulation of food intake based on the increased food intake when endogenous brain NUCB2/nesfatin-1 signaling was blocked by 3rd ventricular injection of an anti-nesfatin-1 antibody (Ab24) acutely or of an anti-NUCB2 antisense oligonucleotide for 10 days in rats [1]. In a consistent manner, 3rd ventricular injection of an anti-nesfatin-1 antibody neutralizing endogenous nesfatin-1 also produces hyperphagia [1, 21]. However, in other studies when hypothalamic NUCB2/nesfatin-1 was knocked down using chronic icv delivery of anti-NUCB2 morpholino oligonucleotides for 7 days in young female rats or for 2 days in male rats, no changes in daily food intake was observed although hypothalamic NUCB2 content was reduced by 75% in female and 29% in male rats [8, 39]. These divergent findings may be related to compensatory actions of other anorexigenic brain transmitters or differences in treatment conditions (third ventricle versus lateral ventricle 10 days versus two days) or sex (male versus female).
The regulation of hypothalamic NUCB2/nesfatin-1 mRNA and/or protein in relation with the feeding status or the nocturnal pattern of eating is also consistent with a physiological role of this pathway. In particular, convergent studies reported the reduction of NUCB2 mRNA and protein in the SON and PVN under conditions of fasting and the restoration of these levels following re-feeding in rats [1, 8, 12] and goldfish [7] (Table 2). In addition, there are circadian changes of NUCB2 mRNA levels in the PVN with an increase during the early light phase associated with the inhibition of feeding and low levels maintained during the dark phase, the eating period. The physiological significance was demonstrated by the elevation of light but not dark phase food intake in rats with PVN-selective knockdown of NUCB2 mRNA by shRNA or immunoneutralization of PVN NUCB2/nesfatin-1 by anti-nesfatin-1-IgG [56]. Although the exact cellular mechanisms controlling the responsiveness of NUCB2/nesfatin-1 to acute changes in metabolic status remain largely unknown, these data indicate a tight inverse relationship with the drive to eat.
Table 2.
Regulation of NUCB2/nesfatin-1.
| Condition | Effect | Reference |
|---|---|---|
| Circadian rhythm | Increase of NUCB2 mRNA expression in the PVN during the light phase, decrease during the dark phase | [56] |
| Fasting | Decrease of NUCB2 mRNA in PVN, SON and NUCB2/nesfatin-1 in circulation, restoration after re-feeding | [1, 7, 8, 12, 23] |
| Body weight | Decrease of circulating NUCB2/nesfatin-1 in anorexic and increase in obese subjects | [5, 10, 93] |
| Cholecystokinin ip | Activation of NUCB2/nesfatin-1 positive neurons in the PVN and NTS | [23, 62] |
| Desacyl ghrelin ip | Activation of NUCB2/nesfatin-1 positive neurons in the arcuate nucleus | [17] |
| Inhibition of mTOR by rapamycin | Reduction of gastric NUCB2 mRNA and protein expression in vivo and in vitro | [100] |
| Glucose | Release of NUCB2/nesfatin-1 from pancreatic islets | [77] |
Abbreviations: ip, intraperitoneal; mTOR, mammalian target of rapamycin; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus of the hypothalamus; SON, supraoptic nucleus of the hypothalamus.
Of interest, brain NUCB2/nesfatin-1 neurons are activated by gut peptides inhibiting food intake or experimental stress conditions associated with a decrease in food intake. For instance, the well-established satiety hormone, cholecystokinin (CCK) [61] injected ip activated NUCB2/nesfatin-1 neurons in the hypothalamus and brainstem [23, 62] which may indicate a role of nesfatin-1 in the downstream signaling of CCK (Table 2). Similarly, NUCB2/nesfatin-1 expressed in the arcuate nucleus is selectively activated by the ip injection of desacyl ghrelin plus ghrelin which may play a role in the desacyl ghrelin-induced reduction of ghrelin-stimulated food intake (Table 2) [17]. Similarly, several stressors including psychological/physical (restraint) [22, 50, 58, 63, 64], immunological (intraperitoneal injection of low doses of lipopolysaccharide) [65], chemical (mycotoxin, hypoglycemia) [18, 60] and visceral (abdominal surgery) [66] activate NUCB2/nesfatin-1 expressing cells as indicated by the occurrence of c-Fos in NUCB2/nesfatin-1 positive cells mainly in the hypothalamus (SON, PVN), midbrain (LC) and brainstem (NTS, VLM, raphe pallidus) (Table 3) [19]. These stressors are also known to inhibit food intake [18, 67-69] and the activation of NUCB2/nesfatin-1 in these nuclei may play a role in the mediation/modulation of the stress response including the anorexigenic effect. However, the identification of the NUCB2-nesfatin-1 receptor(s) and the development of selective antagonists will be required to assess and ascertain the cause-effect relationship between activation of hypothalamic NUCB2-nesfatin-1 neurons under these conditions and the decrease in food intake.
Table 3.
Activation of brain nesfatin-1 immunoreactive neurons by different stressors.
| Brain nucleus [References] | Psychological | Immunological | Chemical | Visceral | |
|---|---|---|---|---|---|
| Restraint | LPS | Mycotoxin | Hypoglycemia | Surgery | |
| [22, 50, 58, 63, 64] | [65] | [18] | [60] | [66] | |
| Amygdala | n.i. | n.i. | yes | n.i. | n.i. |
| Supraoptic nucleus | yes | yes | yes | n.i. | yes |
| Arcuate nucleus | no | yes | yes | yes | no |
| PVN | yes | yes | yes | yes | yes |
| Anterior parvicellular PVN | yes | n.i. | n.i. | n.i. | yes |
| Lateral magnocellular PVN | yes | n.i. | n.i. | n.i. | yes |
| Medial magnocellular PVN | yes | n.i. | n.i. | n.i. | yes |
| Medial parvicellular PVN | yes | n.i. | n.i. | n.i. | yes |
| Locus coeruleus | yes | n.i. | no/yes | n.i. | yes |
| Rostral raphe pallidus | yes | n.i. | no/yes | n.i. | yes |
| Dorsal raphe nucleus | yes | n.i. | no/yes | n.i. | n.i. |
| Edinger-Westphal nucleus | yes | n.i. | n.i. | n.i. | yes |
| Ventrolateral medulla | yes | n.i. | yes | n.i. | yes |
| NTS | yes | yes | yes | yes | yes |
LPS, lipopolysaccharide; n.i., not investigated; NTS, nucleus of the solitary tract; PVN, paraventricular nucleus.
Effects of peripheral nesfatin-1 on food intake and body weight
Peripheral distribution of NUCB2/nesfatin-1
Subsequent to reports of NUCB2/nesfatin-1 expression throughout the brain, NUCB2/nesfatin-1 immunoreactivity was also detected in peripheral tissues, namely the stomach, adipose tissue, pancreas, pituitary gland and testis of rat and goldfish [7, 9, 10, 70, 71]. Surprisingly, the expression of NUCB2 mRNA as assessed by RT-qPCR was found to be 10-times higher in the rat gastric mucosa than in the brain [9]. The gastric cells within the oxyntic mucosa expressing NUCB2/nesfatin-1 immunoreactivity had an endocrine phenotype [9] and are regulated by the mammalian target of rapamycin (mTOR) as recently reviewed (Table 2) [72]. Double labeling immunohistochemistry unraveled that NUCB2/nesfatin-1 immunosignals were primarily co-localized in the cytoplasm of cells expressing ghrelin, indicating the co-expression of these two peptides in gastric X/A-like cells [9]. In rats, ghrelin and NUCB2/nesfatin-1 are – although expressed in the same cell – present in separate pools of vesicles [9]. This finding was recently extended to humans where ghrelin and NUCB2/nesfatin-1 were also co-localized in the gastric oxyntic mucosa of severely obese patients [73]. However, one has to note that ghrelin and NUCB2/nesfatin-1 in humans seem to be localized in the same cytoplasmic vesicles [73]. Whether this differential expression reflects a species difference or metabolic conditions (lean vs. obese) warrants further investigation in other species and healthy human subjects. In the pancreas of rats, β-cells express both insulin and NUCB2/nesfatin-1 with a different subcellular localization in rats [70]. These data support a differential regulation and release of ghrelin and NUCB2/nesfatin-1 in the rat stomach and insulin and NUCB2/nesfatin-1 in the rat pancreas.
The wide peripheral distribution of NUCB2/nesfatin-1 in endocrine cells of the gut, pituitary and testis along with the expression in adipose tissue strongly points towards a release of this peptide and a humoral action to influence feeding and other metabolic actions. This hypothesis is supported by the observation that NUCB2/nesfatin-1 can cross the blood-brain barrier bidirectionally by a non-saturable mechanism [74, 75] making it a likely candidate for bidirectional brain-gut interactions.
Effect on food intake
In light of the prominent expression of NUCB2/nesfatin-1 in the stomach in the same cells as ghrelin, a peripheral effect of nesfatin-1 on food intake has been assumed. However, compared to the brain much less data have been obtained so far and they are less consistent. One group showed an anorexigenic effect following injection of a large dose of full length nesfatin-1 and mid fragment nesfatin-124-53 at the beginning of the dark phase in ad libitum fed outbred mice, an effect that was independent of leptin signaling [76]. This finding was recently extended to Fischer 344 rats where continuous peripheral infusion of nesfatin-1 at a dose of 100 μg/kg body weight/d decreased cumulative food intake compared to controls [77]. However, this effect was not observed in other studies when nesfatin-1 was injected intraperitoneally at similarly high doses in C57Bl/6 mice [24] and Sprague-Dawley rats [23]. Likewise, in goldfish the robust anorexigenic effect of nesfatin-1 readily observed following icv application was not reproduced upon peripheral injection even at 102-3 higher doses that reduce food intake by only 18% [7].
The mechanisms through which peripherally injected nesfatin-1 acts involve vagal afferent pathways based on the findings that nesfatin-1 activates Ca2+ influx in primary cultured nodose ganglion neurons in vitro [78] and that chemical vagal de-afferentiation by capsaicin abolishes the food intake suppressing effect of peripherally injected nesfatin-124-53 in mice [79]. Taken together, existing data clearly point towards a central site of action for nesfatin-1's anorexigenic effect and the action and role of peripheral nesfatin-1 is yet to be ascertained. However, it is to note that fasting for 24 h decreases circulating plasma levels of NUCB2/nesfatin-1 which were restored upon re-feeding for 12 h in rats (Table 2) [23] indicative of a regulation of release by nutritional status.
Implication in glucose control
Expression in the pancreas
Besides the prominent expression in the stomach, convergent reports delineated NUCB2/nesfatin-1's protein expression mainly in pancreatic islets co-localized with insulin in β-cells in rodents and humans [70, 71, 80-82].
Implication in insulin signaling and glucose control
Functional in vitro studies using rat or mouse isolated islets or cultured MIN cells demonstrated that nesfatin-1 stimulates the expression of pre-proinsulin mRNA expression and increases the glucose-induced insulin release through stimulation of calcium influx involving L-type channels [77, 81, 83]. Another study using isolated mouse islets or INS-1 (832/13) cells did not observe an increase in insulin secretion but showed a stimulation of glucagon release [82]. However, the data on insulin action hold true in vivo where intravenous injection of nesfatin-1 reduces blood glucose levels in hyperglycemic db/db mice indicating an insulinotropic effect [84]. These effects were associated with an increased basal and insulin-induced glucose uptake of adipocytes in rats [77]. Extending these findings, NUCB2/nesfatin-1 is locally released from rat pancreatic islets following stimulation with glucose (Table 2) [70, 77] supporting a regulatory action of nesfatin-1 in response to increasing glucose levels. However, in healthy human subjects, an oral glucose tolerance test does not alter circulating NUCB2/nesfatin-1 levels [4, 85] suggesting a rather local/paracrine mode of action of NUCB2/nesfatin-1 within the endocrine pancreas.
Regulation under condition of diabetes mellitus
The expression of NUCB2 in the endocrine pancreas is regulated by the glycemic state with a reduced protein expression in type 2 diabetic Goto-Kakizaki rats compared to normoglycemic Wistar rats [70] and in human islets of type 2 diabetic subjects obtained from an islet transplantation center [82]. Likewise, the circulating levels of NUCB2/nesfatin-1 show an inverse relationship with circulating glucose in Goto-Kakizaki rats [70], a finding confirmed in type 2 diabetic human subjects [85]. Since reduced NUCB2/nesfatin-1 plasma levels are already observed in normoglycemic subjects with insulin resistance, the regulatory interaction is likely to occur predominantly with insulin [86]. In addition, NUCB2/nesfatin-1 levels in breast milk and serum of mothers with gestational diabetes and cord blood of babies that were large for gestational age were lower compared to healthy subjects and newborns appropriate for gestational age, respectively [87-89]. Collectively, existing experimental and clinical studies are consistent with lower NUCB2/nesfatin-1 levels as an underlying mechanism contributing to the impaired glucose tolerance under conditions of diabetes. However, two recent studies reported increased plasma NUCB2/nesfatin-1 levels in Chinese patients with newly diagnosed type 2 diabetes mellitus or impaired glucose tolerance compared to healthy controls [90] and in Chinese newborns that were small for gestational age and displayed a greater homeostasis model assessment-insulin resistance index compared to infants appropriate for gestational age [91]. The reasons for these divergent results are not clear and should take into account potential specific racial and genetic factors.
Correlation of NUCB2/nesfatin-1 with body weight and implication as a new drug target in the treatment of obesity
Changes of NUCB2/nesfatin-1 under conditions of chronically altered body weight
Obese Tsumura Suzuki diabetic mice, a polygenic model of obesity, display decreased hypothalamic NUCB2 mRNA and protein levels compared to non-diabetic controls leading to the speculation of an underlying role for hypothalamic NUCB2/nesfatin-1 deficiency in the hyperphagia observed in these mice [92]. In humans, the cerebrospinal fluid (CSF)/plasma NUCB2/nesfatin-1 ratio is negatively associated with body mass index (BMI) and body fat mass due to a strong increase of plasma NUCB2/nesfatin-1 with rising BMI, whereas CSF NUCB2/nesfatin-1 levels were only moderately increased [5]. In addition, NUCB2/nesfatin-1 plasma levels are decreased in female patients with anorexia nervosa [93] and increased under conditions of obesity in a mixed population of patients [5, 10] resulting in a positive correlation with BMI (Table 2) [10, 93]. In line with these findings, gastric NUCB2/nesfatin-1 protein expression was increased with increasing BMI in a population of male and female obese patients undergoing bariatric surgery that may be related to a compensatory feedback regulatory mechanism to stimulate nesfatin-1's anorexigenic signaling [73]. Conversely, a decrease in plasma nesfatin-1 levels in non-morbid obese type 2 diabetes mellitus patients was observed 12 months after undergoing bariatric surgery which was positively correlated with the decrease of BMI [94]. The elevation of gastric NUCB2/nesfatin-1 protein expression in obese patients and the reduction of circulating levels after bariatric surgery may suggest that the stomach contributes prominently to the circulating levels of NUCB2/nesfatin-1. However, another study reported a negative correlation of plasma nesfatin-1 levels with BMI in non-obese males [4]. Whether gender differences (females versus males), different assessment methods (ELISA recognizing NUCB2 and nesfatin-1 versus sandwich-type ELISA recognizing only nesfatin-1), or the body weight range (normal weight versus underweight and obesity) account for these differences will have to be further investigated.
Genetic variants of NUCB2 in obesity
Genetic variants of the NUCB2 gene may play a role in the susceptibility for or protection against the development of obesity. So far, three single nucleotide polymorphisms were identified to be associated with obesity when investigating a large population of 1049 obese and 315 normal weight male and female Caucasian subjects [95]. Interestingly, this association only held true when investigating male subjects [95] possibly pointing towards a sex difference in the NUCB2/nesfatin-1-modulated susceptibility to develop obesity. In another cohort of 471 obese children and adolescents seven sequence variants of the NUCB2 gene were observed [96]. However, this did not result in differences in plasma NUCB2/nesfatin-1 levels from those observed in body weight matched controls. It may be speculated that the mutation impairs the signaling primarily in the brain where NUCB2/nesfatin-1 is prominently involved in appetite regulation.
Pharmacological approaches targeting nesfatin-1 to treat obesity
The observation that NUCB2/nesfatin-1's anorexigenic action is independent of leptin signaling in rats [1, 21] and mice [76] nourishes hope for NUCB2/nesfatin-1 to become a relevant anti-obesity target, since leptin resistance is a common feature in obesity [97]. New routes and durations of application will have to be tested such as acute intranasal [98] and continuous peripheral application [77] to show the effect on food intake and body weight in order to approach the goal of testing NUCB2/nesfatin-1 as drug treatment option in the battle against obesity.
Future endeavors to fill our gaps of knowledge
Identification and characterization of the yet unknown nesfatin-1 receptor
So far, receptors through which NUCB2/nesfatin-1 interacts have eluded attempts to molecular identification. Many efforts to identify a receptor specific to nesfatin-1 have failed so far (Mori, unpublished observations). The existing indirect evidence supports a signaling through a Gi/o-protein-coupled receptor [11, 99] and nesfatin-1 in vitro evokes Ca2+ influx via L- and P/Q type Ca2+ channels in cultured rat hypothalamic neurons [11, 21], T-type channels in DMV neurons [29], N-type channels in mouse vagal afferent nodose ganglion neurons [78] and via L-type Ca2+ channels in pancreatic endocrine β-cells [83]. Additional evidence for the mediation of nesfatin-1's effects through a cell surface Gi/o protein-coupled receptor came from a recent report showing that full length nesfatin-1 as well as the mid fragment of nesfatin-1 increased cAMP response element (CRE) reporter activity in the mouse NB41A3 neuroblastoma cell line, an effect abolished by an L-type Ca2+ channel blocker [99]. Moreover, radiolabeled 125I-nesfatin-1 binds to these cells as well as the mouse hypothalamus [99] suggesting the expression of the nesfatin-1 receptor on these cells.
Cellular release
Immunohistochemical characterization of NUCB2/nesfatin-1 cells in the brain conclusively showed immunosignals in the cytoplasm of the cell body and primary dendrites, whereas no staining was detected in axons and nerve terminals [1, 6, 11-14]. This subcellular expression pattern in brain neurons may indicate an intracellular signaling pathway rather than a function as a secreted peptide. Nonetheless, also cell bodies and primary dendrites are able to release peptide products. In addition, in the PVN nesfatin-1 immunoreactivity was demonstrated in secretory granules by the use of electron microscopy supporting the action as a secreted peptide [21]. However, no data are available so far to support the existence of nesfatin-1 immunoreactivity on the surface of cells or in a membrane protein complex. In addition to these findings in the brain, in the periphery NUCB2/nesfatin-1 is clearly located in secretory vesicles in rat [9] and human [73] endocrine cells supporting a hormonal or paracrine mode of action. Lastly, the soluble fraction of gastric mucosal and pancreatic protein homogenates stained for NUCB2/nesfatin-1 showed a reduction of 3 kDa compared to full length NUCB2 which may point towards the release of full-length protein into a secretory vesicular compartment of the endocrine cells by cleavage of a 3 kDa signal sequence [9].
Investigation of the physiological role of nesfatin-1 using NUCB2/nesfatin-1 knockout mice
So far, animals with genetic manipulations of NUCB2/nesfatin-1 have not yet been established and reported in the literature. However, in several groups work is in progress to generate a conditional knockout mouse model. In the near future it is likely that these new advances will uncover new insight into the physiological functions of NUCB2/nesfatin-1 in vivo.
Conclusion
Conclusive experimental evidence supports a central action of nesfatin-1 as a physiological anorexigenic peptide, while the action of peripheral NUCB2/nesfatin-1 to influence feeding behavior is less clear in rodents. Another important function which is receiving growing interest is the involvement of nesfatin-1 expressed in endocrine cells of the pancreas in the regulation of glucose homeostasis through its insulinotropic action in both experimental and clinical studies. NUCB2/nesfatin-1 levels are altered under conditions of obesity in rodent hypothalamus and in blood of human subjects. This, combined with nesfatin-1's anorexigenic action independently of leptin, opened new venues to target this peptide as a promising approach in the drug treatment of obesity and type 2 diabetes mellitus. Important breakthrough will come from the identification of the NUCB2/nesfatin-1 receptor allowing the subsequent development of stable and highly selective pharmacological tools to interfere with this signaling pathway.
Acknowledgments
Supported by: German Research Foundation STE 1765/3-1 (A.S.) and Charité University Funding UFF 89-441-176 (A.S.), NIH R01 DK-33061, NIH Center Grant DK-41301 (Animal Core), VA Research Career Scientist (Y.T.), VA Merit Grant (Y.T.).
Footnotes
Conflict of interest statement
A.S., M.M. and Y.T. have nothing to disclose. No conflicts of interest exist.
References
- 1.Oh-I S, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 2.Mohan H, Unniappan S. Phylogenetic Aspects of Nucleobindin-2/Nesfatin-1. Curr Pharm Des. 2013 doi: 10.2174/138161281939131127124149. [DOI] [PubMed] [Google Scholar]
- 3.Stengel A, Taché Y. New Developments On NUBC2/Nesfatin-1. Curr Pharm Des. 2013 [PubMed] [Google Scholar]
- 4.Tsuchiya T, Shimizu H, Yamada M, et al. Fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males. Clin Endocrinol (Oxf) 2010;73:484–490. doi: 10.1111/j.1365-2265.2010.03835.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS. Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese humans: evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J Clin Endocrinol Metab. 2011;96:E669–673. doi: 10.1210/jc.2010-1782. [DOI] [PubMed] [Google Scholar]
- 6.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez R, Kerbel B, Chun A, Unniappan S. Molecular, cellular and physiological evidences for the anorexigenic actions of nesfatin-1 in goldfish. PLoS One. 2010;5:e15201. doi: 10.1371/journal.pone.0015201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Galiano D, Navarro VM, Roa J, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30:7783–7792. doi: 10.1523/JNEUROSCI.5828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stengel A, Goebel M, Yakubov I, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–238. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramanjaneya M, Chen J, Brown JE, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–3180. doi: 10.1210/en.2009-1358. [DOI] [PubMed] [Google Scholar]
- 11.Brailoiu GC, Dun SL, Brailoiu E, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–5094. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 12.Kohno D, Nakata M, Maejima Y, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 13.Goebel M, Stengel A, Wang L, Lambrecht NW, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–246. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goebel-Stengel M, Wang L, Stengel A, Taché Y. Localization of nesfatin-1 neurons in the mouse brain and functional implication. Brain Research. 2011;1396:20–34. doi: 10.1016/j.brainres.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fort P, Salvert D, Hanriot L, et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–181. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Inhoff T, Stengel A, Peter L, et al. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides. 2010;31:257–262. doi: 10.1016/j.peptides.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inhoff T, Mönnikes H, Noetzel S, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaige S, Bonnet MS, Tardivel C, et al. c-Fos immunoreactivity in the pig brain following deoxynivalenol intoxication: focus on NUCB2/nesfatin-1 expressing neurons. Neurotoxicology. 2013;34:135–149. doi: 10.1016/j.neuro.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Emmerzaal TL, Kozicz T. Nesfatin-1: implication in stress and stress-associated anxiety and depression. Curr Pharm Des. 2013 doi: 10.2174/138161281939131127125042. [DOI] [PubMed] [Google Scholar]
- 20.Yosten GL, Samson WK. Cardiovascular and antidipsogenic effects of nesfatin-1. Curr Pharm Des. 2013 doi: 10.2174/138161281939131127142720. [DOI] [PubMed] [Google Scholar]
- 21.Maejima Y, Sedbazar U, Suyama S, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317C:92–99. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 23.Stengel A, Goebel M, Wang L, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goebel M, Stengel A, Wang L, Taché Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides. 2011;32:36–43. doi: 10.1016/j.peptides.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–1647. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atsuchi K, Asakawa A, Ushikai M, et al. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport. 2010;21:1008–1011. doi: 10.1097/WNR.0b013e32833f7b96. [DOI] [PubMed] [Google Scholar]
- 28.Konczol K, Pinter O, Ferenczi S, et al. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes (Lond) 2012;36:1514–1521. doi: 10.1038/ijo.2012.2. [DOI] [PubMed] [Google Scholar]
- 29.Xia ZF, Fritze DM, Li JY, et al. Nesfatin-1 inhibits gastric acid secretion via a central vagal mechanism in rats. Am J Physiol Gastrointest Liver Physiol. 2012;303:G570–577. doi: 10.1152/ajpgi.00178.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerbel B, Unniappan S. Nesfatin-1 suppresses energy intake, co-localises ghrelin in the brain and gut, and alters ghrelin, cholecystokinin and orexin mRNA expression in goldfish. J Neuroendocrinol. 2012;24:366–377. doi: 10.1111/j.1365-2826.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Dong J, Jiang ZY. Nesfatin-1 influences the excitability of glucosensing neurons in the hypothalamic nuclei and inhibits the food intake. Regul Pept. 2012;177:21–26. doi: 10.1016/j.regpep.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Moreau JM, Ciriello J. Nesfatin-1 induces Fos expression and elicits dipsogenic responses in subfornical organ. Behav Brain Res. 2013;250C:343–350. doi: 10.1016/j.bbr.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 33.Stengel A, Goebel-Stengel M, Wang L, Kato I, Mori M, Taché Y. Nesfatin-1(30-59) but not the N- and C-terminal fragments, nesfatin-1(1-29) and nesfatin-1(60-82) injected intracerebroventricularly decreases dark phase food intake by increasing inter-meal intervals in mice. Peptides. 2012;35:143–148. doi: 10.1016/j.peptides.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl) 2008;201:115–123. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- 35.Gonnissen HK, Adam TC, Hursel R, Rutters F, Verhoef SP, Westerterp-Platenga MS. Sleep duration, sleep quality and body weight: Parallel developments. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai T. Hypothalamic neuropeptides implicated in the regulation of sleep/wakefulness states. Brain Nerve. 2012;64:629–637. [PubMed] [Google Scholar]
- 37.Jego S, Salvert D, Renouard L, et al. Tuberal hypothalamic neurons secreting the satiety molecule Nesfatin-1 are critically involved in paradoxical (REM) sleep homeostasis. PLoS One. 2012;7:e52525. doi: 10.1371/journal.pone.0052525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vas S, Adori C, Konczol K, et al. Nesfatin-1/NUCB2 as a Potential New Element of Sleep Regulation in Rats. PLoS One. 2013;8:e59809. doi: 10.1371/journal.pone.0059809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yosten GL, Redlinger L, Samson WK. Evidence for a role of endogenous nesfatin-1 in the control of water drinking. J Neuroendocrinol. 2012;24:1078–1084. doi: 10.1111/j.1365-2826.2012.02304.x. [DOI] [PubMed] [Google Scholar]
- 40.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 41.Taché Y, Garrick T, Raybould H. Central nervous system action of peptides to influence gastrointestinal motor function. Gastroenterology. 1990;98:517–528. doi: 10.1016/0016-5085(90)90849-v. [DOI] [PubMed] [Google Scholar]
- 42.Cuomo R, D'Alessandro A, Andreozzi P, Vozzella L, Sarnelli G. Gastrointestinal regulation of food intake: do gut motility, enteric nerves and entero-hormones play together? Minerva Endocrinol. 2011;36:281–293. [PubMed] [Google Scholar]
- 43.Martinez V, Taché Y. Bombesin and the brain-gut axis. Peptides. 2000;21:1617–1625. doi: 10.1016/s0196-9781(00)00293-x. [DOI] [PubMed] [Google Scholar]
- 44.Kirchgessner AL, Sclafani A. PVN-hindbrain pahway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol Behav. 1988;42:517–528. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 46.Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 47.Tabarin A, Diz-Chaves Y, Consoli D, et al. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotoh K, Masaki T, Chiba S, et al. Nesfatin-1, corticotropin-releasing hormone, thyrotropin-releasing hormone, and neuronal histamine interact in the hypothalamus to regulate feeding behavior. J Neurochem. 2013;124:90–99. doi: 10.1111/jnc.12066. [DOI] [PubMed] [Google Scholar]
- 49.Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol. 2008;20:245–250. doi: 10.1111/j.1365-2826.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- 50.Konczol K, Bodnar I, Zelena D, et al. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int. 2010;57:189–197. doi: 10.1016/j.neuint.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Nonogaki K, Ohba Y, Sumii M, Oka Y. Serotonin systems upregulate the expression of hypothalamic NUCB2 via 5-HT2C receptors and induce anorexia via a leptin-independent pathway in mice. Biochem Biophys Res Commun. 2008;372:186–190. doi: 10.1016/j.bbrc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu B, Kalra PS, Farmerie WG, Kalra SP. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology. 1999;140:2868–2875. doi: 10.1210/endo.140.6.6789. [DOI] [PubMed] [Google Scholar]
- 54.Taheri S, Sunter D, Dakin C, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett. 2000;279:109–112. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 55.Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ. Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology. 2002;143:3905–3915. doi: 10.1210/en.2002-220150. [DOI] [PubMed] [Google Scholar]
- 56.Sedbazar U, Maejima Y, Nakata M, Mori M, Yada T. Paraventricular NUCB2/nesfatin-1 rises in synchrony with feeding suppression during early light phase in rats. Biochem Biophys Res Commun. 2013;434:434–438. doi: 10.1016/j.bbrc.2013.03.090. [DOI] [PubMed] [Google Scholar]
- 57.Gantulga D, Maejima Y, Nakata M, Yada T. Glucose and insulin induce Ca2+ signaling in nesfatin-1 neurons in the hypothalamic paraventricular nucleus. Biochem Biophys Res Commun. 2012;420:811–815. doi: 10.1016/j.bbrc.2012.03.079. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida N, Maejima Y, Sedbazar U, et al. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2010;2:775–784. doi: 10.18632/aging.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M, Zhang Z, Wang C, et al. Nesfatin-1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced insulin resistance. Diabetes. 2012;61:1959–1968. doi: 10.2337/db11-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonnet MS, Djelloul M, Tillement V, et al. Central NUCB2/Nesfatin-1-expressing neurones belong to the hypothalamic-brainstem circuitry activated by hypoglycaemia. J Neuroendocrinol. 2013;25:1–13. doi: 10.1111/j.1365-2826.2012.02375.x. [DOI] [PubMed] [Google Scholar]
- 61.Dockray GJ. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2012;19:8–12. doi: 10.1097/MED.0b013e32834eb77d. [DOI] [PubMed] [Google Scholar]
- 62.Noetzel S, Stengel A, Inhoff T, et al. CCK-8S activates c-Fos in a dose-dependent manner in nesfatin-1 immunoreactive neurons in the paraventricular nucleus of the hypothalamus and in the nucleus of the solitary tract of the brainstem. Regul Pept. 2009;157:84–91. doi: 10.1016/j.regpep.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. Stress-related changes in the activity of cocaine- and amphetamine-regulated transcript and nesfatin neurons in the midbrain non-preganglionic Edinger-Westphal nucleus in the rat. Neuroscience. 2010;170:478–488. doi: 10.1016/j.neuroscience.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1 expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stengel A, Goebel-Stengel M, Wang L, et al. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol Motil. 2011;23:e294–308. doi: 10.1111/j.1365-2982.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stengel A, Goebel M, Wang L, Reeve JR, Jr., Taché Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: Potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calvez J, Fromentin G, Nadkarni N, et al. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104:675–683. doi: 10.1016/j.physbeh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol. 2010;204:255–263. doi: 10.1677/JOE-09-0254. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643–648. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 72.Li Z, Mulholland M, Zhang W. Regulation of Gastric Nesfatin-1/NUCB2. Curr Pharm Des. 2013 doi: 10.2174/138161281939131127143306. [DOI] [PubMed] [Google Scholar]
- 73.Stengel A, Hofmann T, Goebel-Stengel M, et al. Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese patients. Histochem Cell Biol. 2013;139:909–918. doi: 10.1007/s00418-013-1087-8. [DOI] [PubMed] [Google Scholar]
- 74.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu H, Oh-I S, Hashimoto K, et al. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez R, Perry RL, Gao X, et al. Nutrient responsive nesfatin-1 regulates energy balance and induces glucose-stimulated insulin secretion in rats. Endocrinology. 2011;152:3628–3637. doi: 10.1210/en.2010-1471. [DOI] [PubMed] [Google Scholar]
- 78.Iwasaki Y, Nakabayashi H, Kakei M, Shimizu H, Mori M, Yada T. Nesfatin-1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N-type channels. Biochem Biophys Res Commun. 2009;390:958–962. doi: 10.1016/j.bbrc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30:995–998. doi: 10.1016/j.peptides.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Mohan H, Unniappan S. Ontogenic pattern of nucleobindin-2/nesfatin-1 expression in the gastroenteropancreatic tissues and serum of Sprague Dawley rats. Regul Pept. 2012;175:61–69. doi: 10.1016/j.regpep.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez R, Reingold BK, Gao X, Gaidhu MP, Tsushima RG, Unniappan S. Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet beta- and MIN6 cells. J Endocrinol. 2011;208:R9–R16. doi: 10.1530/JOE-10-0492. [DOI] [PubMed] [Google Scholar]
- 82.Riva M, Nitert MD, Voss U, et al. Nesfatin-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res. 2011;346:393–405. doi: 10.1007/s00441-011-1268-5. [DOI] [PubMed] [Google Scholar]
- 83.Nakata M, Manaka K, Yamamoto S, Mori M, Yada T. Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca(2+) influx through L-type channels in mouse islet beta-cells. Endocr J. 2011;58:305–313. doi: 10.1507/endocrj.k11e-056. [DOI] [PubMed] [Google Scholar]
- 84.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 85.Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72–77. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Basar O, Akbal E, Koklu S, et al. A novel appetite peptide, nesfatin-1 in patients with non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 2012;72:479–483. doi: 10.3109/00365513.2012.699097. [DOI] [PubMed] [Google Scholar]
- 87.Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–2240. doi: 10.1016/j.peptides.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 88.Aslan M, Celik O, Celik N, et al. Cord blood nesfatin-1 and apelin-36 levels in gestational diabetes mellitus. Endocrine. 2012;41:424–429. doi: 10.1007/s12020-011-9577-8. [DOI] [PubMed] [Google Scholar]
- 89.Boutsikou T, Briana DD, Boutsikou M, et al. Cord blood nesfatin-1 in large for gestational age pregnancies. Cytokine. 2013;61:591–594. doi: 10.1016/j.cyto.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2012;120:91–95. doi: 10.1055/s-0031-1286339. [DOI] [PubMed] [Google Scholar]
- 91.Cheng YY, Zhao XM, Cai BP, Ma LN, Yin JY, Song GY. Nesfatin-1 in newborns: relationship with endocrine and metabolic and anthropometric measures. J Pediatr Endocrinol Metab. 2012;25:727–732. doi: 10.1515/jpem-2012-0095. [DOI] [PubMed] [Google Scholar]
- 92.Miyata S, Yamada N, Kawada T. Possible involvement of hypothalamic nucleobindin-2 in hyperphagic feeding in Tsumura Suzuki obese diabetes mice. Biol Pharm Bull. 2012;35:1784–1793. doi: 10.1248/bpb.b12-00505. [DOI] [PubMed] [Google Scholar]
- 93.Ogiso K, Asakawa A, Amitani H, et al. Plasma nesfatin-1 concentrations in restricting-type anorexia nervosa. Peptides. 2011;32:150–153. doi: 10.1016/j.peptides.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Lee WJ, Chen CY, Ser KH, et al. Differential influences of gastric bypass and sleeve gastrectomy on plasma nesfatin-1 and obestatin levels in patients with type 2 diabetes mellitus. Curr Pharm Des. 2013 doi: 10.2174/13816128113198880010. [DOI] [PubMed] [Google Scholar]
- 95.Zegers D, Beckers S, Mertens IL, Van Gaal LF, Van Hul W. Association between polymorphisms of the Nesfatin gene, NUCB2, and obesity in men. Mol Genet Metab. 2011;103:282–286. doi: 10.1016/j.ymgme.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Zegers D, Beckers S, de Freitas F, et al. Identification of mutations in the NUCB2/nesfatin gene in children with severe obesity. Mol Genet Metab. 2012;107:729–734. doi: 10.1016/j.ymgme.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 97.Small CJ, Bloom SR. Gut hormones as peripheral anti obesity targets. Curr Drug Targets CNS Neurol Disord. 2004;3:379–388. doi: 10.2174/1568007043336950. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu H, Oh IS, Okada S, Mori M. Nesfatin-1: An Overview and Future Clinical Application. Endocr J. 2009;56:537–543. doi: 10.1507/endocrj.k09e-117. [DOI] [PubMed] [Google Scholar]
- 99.Ishida E, Hashimoto K, Shimizu H, et al. Nesfatin-1 induces the phosphorylation levels of cAMP response element-binding protein for intracellular signaling in a neural cell line. PLoS One. 2012;7:e50918. doi: 10.1371/journal.pone.0050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Z, Xu G, Li Y, Zhao J, Mulholland MW, Zhang W. mTOR-dependent modulation of gastric nesfatin-1/NUCB2. Cell Physiol Biochem. 2012;29:493–500. doi: 10.1159/000338503. [DOI] [PMC free article] [PubMed] [Google Scholar]