Abstract
Rationale
A high-fat diet accompanied by hypertriglyceridemia increases an individual’s risk for developing atherosclerosis. An early event in this process is monocyte recruitment through binding to VCAM-1 upregulated on inflamed arterial endothelium. Diets high in polyunsaturated fatty acids (PUFAs) may provide athero-protection by ameliorating this effect.
Objective
We investigated the acute regulation of VCAM-1 expression in human aortic endothelial cells (HAEC) in response to triglyceride-rich lipoproteins (TGRL) isolated from subjects following consumption of a high-fat meal.
Methods and Results
Postprandial TGRL isolated from 38 subjects were categorized as pro- or anti-atherogenic according to their capacity to alter the inflammatory response of HAEC. Pro-atherogenic TGRL increased expression of VCAM-1, ICAM-1, and E-selectin by ~20% compared to stimulation with TNFα alone, while anti-atherogenic TGRL decreased VCAM-1 expression by ~20% while still upregulating ICAM-1. The relative atherogenicity of TGRL positively correlated with particle density of TG, ApoCIII, ApoE, and cholesterol. Ω3-PUFA mimicked the effect of anti-atherogenic TGRL by down-regulating VCAM-1 expression. TGRL exerted this differential regulation of VCAM-1 by reciprocally modulating expression and activity of the transcription factor IRF-1 and expression of microRNA 126 (miR-126). Overexpression or silencing of IRF-1 or miR-126 expression recapitulated the pro- or anti-atherogenic regulation of VCAM-1.
Conclusions
In response to a high-fat meal, TGRL bias the inflammatory response of endothelium via transcriptional and post-transcriptional editing of VCAM-1. Subjects with an anti-inflammatory response to a high-fat meal produced TGRL that was enriched in non-esterified fatty acids, decreased IRF-1 expression, increased miR-126 activity, and diminished monocyte arrest.
Keywords: Hypertriglyceridemia, triglyceride, fatty acid, atherosclerosis, endothelial dysfunction
INTRODUCTION
The composition of triglyceride-rich lipoproteins (TGRL) that circulate in blood after a meal reflects the content of the meal and how an individual metabolizes the constituent fatty acids (FA) and triglyceride (TG). TGRL composition determines particle size, density, receptor interactions, and time in circulation, which in turn directly influence the relative risk of atherogenesis.1 Hypertriglyceridemia (HTG) affects ~35% of the population and is directly associated with the growing epidemic of obesity and type 2 diabetes in Western society.2 Diets high in fat are associated with elevated serum triglycerides (sTG) and endothelial cell (EC) dysfunction, which translate into increased risk of cardiovascular disease.1, 3 Recent data show that HTG, when combined with visceral obesity, elicits a low level of systemic inflammation that may ultimately predispose an individual to coronary artery disease and myocardial infarction.4 Dietary supplementation with marine-derived omega-3 polyunsaturated fatty acids (PUFAs) including docosahexaenoic acid (DHA) has a dose-dependent effect on lowering sTG.2 Meals high in the ratio of saturated fatty acids (SFA) to PUFA affect the composition and distribution of VLDL5, enrich ApoCIII and ApoE levels in postprandial TGRL6, and amplify cell adhesion molecule (CAM) expression on inflamed EC.7 The relative inflammatory effects of TGRL on human aortic EC (HAEC) reflect its FA8 and apolipoprotein composition.9 A key question that remains is how heterogeneity in TGRL composition, which can vary with an individual’s diet and metabolism, affects the uptake by HAEC and correlates with endothelial inflammation and atherosclerosis.
Dysregulated expression of CAMs by EC and corresponding monocyte infiltration into the arterial wall are two early events in atherogenesis. In response to metabolic and inflammatory activation, vascular cell adhesion molecule-1 (VCAM-1) is upregulated on endothelium and bound by α4β1 integrins on activated monocytes as they arrest under arterial shear stress.10 To understand the inflammatory mechanisms by which metabolic dysfunction of endothelium can lead to atherogenesis, we have developed an ex vivo model using cultured HAEC.11 These studies revealed that TGRL isolated from human subjects after a moderately high-fat meal can prime either a pro- or anti-atherogenic state, defined as a net up- or down-regulation of VCAM-1 expression relative to stimulation with TNFα. We reported that modulation of VCAM-1 expression and monocyte recruitment to HAEC under shear flow is dependent on both the quality of the TGRL, which correlates directly with the subject’s body morphology and postprandial TG level, as well as the level of TNFα stimulation.12 Specifically, we demonstrated that TGRL particles from subjects with sTG above a threshold of 225 mg/dl and a waist circumference greater than 0.8 m were associated with enhanced VCAM-1 expression and the recruitment efficiency of monocytes, a harbinger of atherosclerosis.12, 13
Our objective for this study was to elucidate the mechanisms underlying regulation of VCAM-1 expression. TGRL was isolated from individuals following a high fat meal and its relative atherogenicity assessed by ex vivo analysis. We sought to elucidate the transcriptional mechanisms that could account for the selectivity in gene regulation of VCAM-1 versus ICAM-1 and E-selectin as a function of changes in postprandial particle content. The relative atherogenicity of TGRL mapped quantitatively to changes in particle TG density and non-esterified fatty acid (NEFA) content, while treatment with Ω3-PUFA reversed this effect. These findings reveal a mechanism for the specific regulation of VCAM-1 expression and monocyte adhesion in response to a high-fat meal through the reciprocal activity of interferon regulatory factor 1 (IRF-1) and the microRNA miR-126.
MATERIALS AND METHODS
An expanded Methods section is available in the online supplement
TGRL isolation, characterization and fatty acid analysis
Thirty-eight subjects were recruited according to Institutional Review Board-approved protocols at the University of California, Davis. Venous blood was collected after a 12 hr overnight fast, and again 3.5 hr after a standardized moderately high-fat meal (1,230 calories, 42% from fat, 32% from saturated fat).12, 13 TGRL (ρ<1.0063g/mL) were isolated by ultracentrifugation11 and the TG, ApoCIII, ApoE, cholesterol and ApoB composition analyzed by spectrophotometric assay (Polymedco Inc.). All components were normalized to ApoB concentration and converted to density per particle (one ApoB molecule per TGRL particle).2 TGRL aliquots were spiked with a suite of lipid class surrogates and extracted for analysis of total fatty acid (TFA) and NEFA concentrations. Lipid residues were converted to fatty acid methyl esters and quantified by GC-MS (Agilent 6890GC - 5973A MSD).
HAEC culture and treatment
HAEC (Genlantis) were cultured in Endothelial Growth Media-2 (Lonza) and treated with TNFα (0.3 ng/ml, R&D) alone or simultaneously with TGRL (10 mg/dl ApoB) or FAs for 4hr unless otherwise indicated.
Flow cytometry
HAEC were detached using enzyme-free cell dissociation buffer (Gibco), labeled with fluorescein-conjugated antibodies against human E-selectin, ICAM-1 or VCAM-1, and analyzed by FACScan flow cytometer (Becton Dickinson). 12
NF-κB and AP-1 activation
NF-κB and AP-1 activation were measured by ELISA targeting active phospho-p65 and phospho-c-Jun in the nuclear extract.
Quantitative PCR
miR-126 and miR-17-3p expression were quantified using TaqMan MicroRNA Cells-to-CT (Applied Biosystems) and normalized to small nuclear RNA, RNU6B. VCAM-1 expression was quantified by TaqMan assay.12
VCAM-1 reporter assay
5x105 HAEC were cotransfected with pGL3/hVCAM-1/-228 - +1214 and pGL4.75/hRluc/CMV at 10:1 using a 4D-Nucleofector system (Lonza). After 18 hr, cells were treated as described and activity measured using a dual luciferase reporter system (Promega).
Chromatin immunoprecipitation assay
Cells were treated with TNFα (3 ng/ml) alone or simultaneously with TGRL or DHA for 2 hr and chromatin was immunoprecipitated using ChIP-IT Express kit (Active Motif). IRF-1 binding VCAM-1 promoter was quantified by qPCR and normalized to input DNA.
Cell transfection
5x105 cells were transfected with 150 pmol siRNA (Santa Cruz Biotech), miR-126 inhibitor (Dharmcon), or 3 µg pCMV6-XL5/human Irf-1 (OriGene) using a 4D-Nucleofector system. After 48 hr (siRNA and miR-126 inhibitor) or 72 hr (plasmid), cells were treated as described followed by Western blot or flow cytometry analysis.
Monocyte adhesion assay
HAEC were grown in 24-well plates. 2x104 mononuclear cells isolated from whole blood using Lymphoprep (Accurate Chemical & Scientific Corp) were added to each well and allowed to settle for 7 minutes at 37°C. After 3 washes with PBS, adhered monocytes were identified by CD14-positive fluorescence on a Nikon microscope.
Statistical analysis
Data are presented as mean±SE. Multiple groups were analyzed by ANOVA with Dunnett’s posttest or two groups compared using Student’s t-test (GraphPad Prism). P<0.05 was considered significant. Correlations between groups were assessed using Pearson's correlation coefficient (r). Multivariate and non-parametric statistics were performed using the Excel Add-In imDEV.15
RESULTS
TGRL modulates TNFα-stimulated VCAM-1 expression as a function of subject lipoprotein particle composition
To investigate the effect of postprandial TGRL due to a high-fat meal on the inflammatory response of EC, we recruited 38 subjects exhibiting a broad range of body mass index (18–45 kg/m2), waist circumference (WC; 0.66–1.22 m), and fasting sTG (44–356 mg/dl) (Online Tables I and II). We examined the relative capacity of TGRL to modulate the inflammatory response of HAEC following 4 hours of low dose TNFα stimulation that corresponds to 0.3 ng/ml, the EC50 for CAM upregulation.11 A correlation of VCAM-1, ICAM-1, and E-selectin expression as a function of TGRL particle density of TG, ApoCIII, ApoE and cholesterol revealed the relative importance of these components in up- or down-regulation relative to cytokine stimulation alone. TG and cholesterol were the most enriched components in TGRL particles isolated from postprandial serum (Online Table III); however, an individual’s particle TG density yielded the most significant positive correlation in its capacity to alter VCAM-1 expression (Fig 1A, Pearson r = 0.6, p = 0.002). We observed that subjects with postprandial TG above a threshold density routinely increased expression of VCAM-1, while a second subset decreased expression compared to TNFα stimulation alone. Moreover, particle densities of ApoCIII, ApoE, and cholesterol also exhibited threshold values in modulating VCAM-1 expression (Figures 1B-D). In contrast, most subjects elicited a net upregulation of ICAM-1 (18.7% ± 2.4%) and E-selectin (16.9+ ± 11.8%) in response to TNFα and TGRL. This pro-inflammatory priming response of TGRL did not systematically correlate with particle composition (Online Figures I & II).
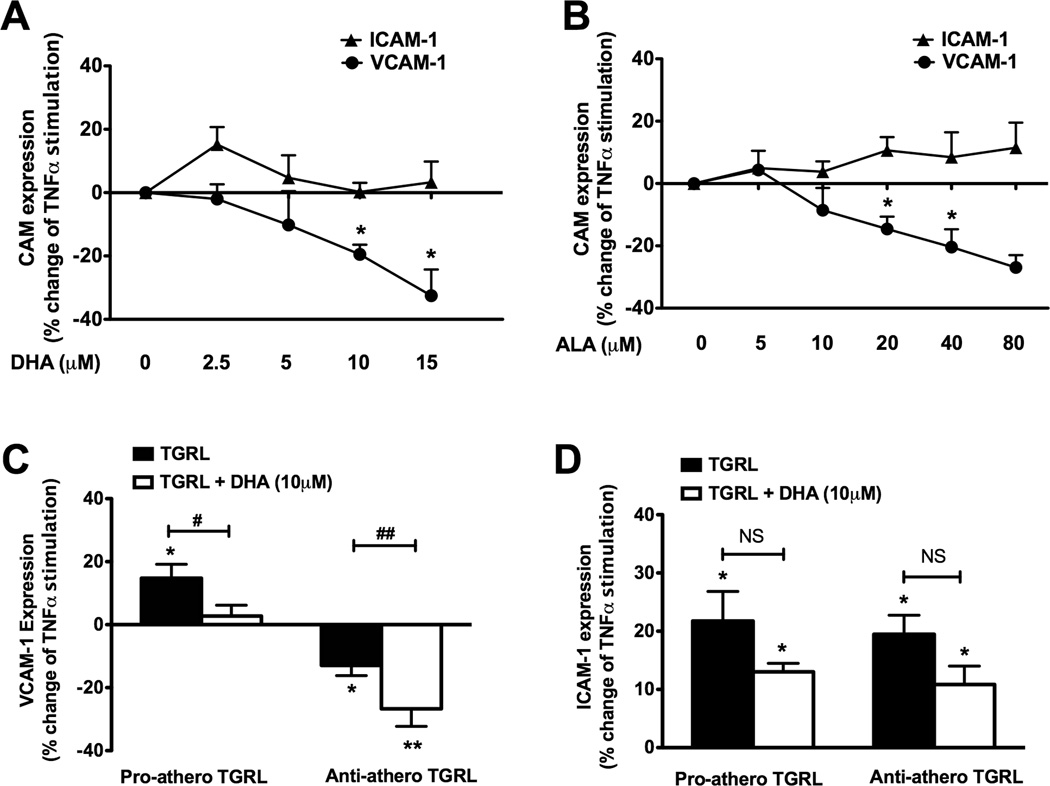
Figure 1. Atherogenicity of TGRL varies with postprandial particle composition.
TGRLs were analyzed for their composition using a clinical chemistry analyzer and each component was normalized to ApoB concentration in the sample to obtain a per particle density. TGRLs (10 mg/dl ApoB) were characterized for their atherogenicity, defined by the ability to positively (pro) or negatively (anti) modulate TNFα (0.3 ng/ml)-induced VCAM-1 expression in HAEC at 4 hr. Correlation of particle density for (A) TG, (B) ApoCIII, (C) ApoE, and (D) Cholesterol with the % change in VCAM-1 expression from TNFα. (E-H) Particle compositions for the same samples categorized by their ability to positively or negatively modulate VCAM-1 expression. (*P<0.05, **P<0.01, ***P<0.001 from paired TNFα; n=22–28).
Since VCAM-1 is upregulated in nascent atherosclerotic plaques and changes in receptor expression correlate directly with the extent of monocyte recruitment to inflamed endothelium,16 we refer to TGRL that enhanced TNFα-induced VCAM-1 membrane expression as pro-atherogenic and that which suppressed VCAM-1 expression as anti-atherogenic. We determined that the TGRL elicited its atherogenic activity at the level of transcription using quantitative RT-PCR and a VCAM-1 luciferase reporter.14 VCAM-1 promoter activity was increased by pro- but decreased by anti-atherogenic TGRL relative to that elicited by TNFα stimulation alone. This resulted in a significant difference in mRNA levels between the two groups (Online Figure III). TGRL particles that elicited the strongest pro-atherogenic response were those with the highest concentrations of TG, ApoCIII, ApoE and cholesterol compared to particles characterized as anti-atherogenic (Fig 1E-H). The difference in TG between the two groups was significant at a threshold density of 530 x 10−7 pg/particle, which corresponded to serum TG concentrations above 200 mg/dL (Online Fig IV). Above this density VCAM-1 expression was increased on average 16.2%, while below it was reduced by 18%. Pro-atherogenic TGRL contained ~2.6-fold higher TG content compared to anti-atherogenic (865 ± 98 x 10−7 pg/particle vs. 329 ± 34 x 10−7 pg/particle). In contrast, TGRL uniformly enhanced TNFα-induced ICAM-1 and E-selectin expression independent of TG and lipoprotein density (Online Figure I & II).
TGs contain a variety of glycerol-esterified fatty acids that can exert opposing effects on endothelial inflammation as a function of their level of saturation.17 We examined pro- and anti-atherogenic TGRL from 13 subjects for total and non-esterified fatty acid constituents (Online Table IV & Online Figure 4). The percentage of NEFA (1–2%) and relative abundance of each FA were consistent with observations from previous studies.8 A principle component analysis revealed discrete groupings of the pro-atherogenic and anti-atherogenic phenotypes. TGRL that had neutral effects on VCAM-1 expression segregated in a group between the pro- and anti-atherogenic phenotypes (Online Figure V). The relative abundance of NEFA provided the greatest discrimination between the samples. Online Table IV reveals that the TFA content was nearly equivalent between pro- and anti-atherogenic TGRL. However, NEFA SFAs, MUFAs, PUFAs and HUFAs showed 3-, 2-, 1-, and 0.7-fold enrichment in anti-atherogenic TGRL, respectively. Specifically, enriched in anti-atherogenic TGRL were the SFAs palmitic (C16:0) and stearic acid (C18:0), the MUFAs palmitoleate (C16:1n7), oleic acid (C18:1n9), vaccenic acid (C18:1n7); and the PUFA linoleic acid (C18:2n6). Arachidonic acid (C20:4n6) was also significantly lower in the anti-atherogenic TGRL. These results suggest that the specific fatty acid composition of the pro- versus anti-atherogenic TGRL accounts for the atherogenic bias of the cytokine-induced inflammatory response in HAEC.
Polyunsaturated fatty acid supplementation suppresses the atherogenic response to TGRL
Since Ω3-PUFAs have exhibited beneficial effects in lowering sTG and were present at moderate levels in subject’s TGRL, we examined αLA and DHA for their association with inflammatory VCAM-1 expression. Treatment with DHA significantly inhibited VCAM-1 upregulation, but not ICAM-1, in a dose-dependent manner yielding ~30% inhibition at 15µM DHA (Fig 2A). This inhibition was also observed for αLA that induced a significant anti-atherogenic effect in response to TNFα stimulation at 20µM (Fig 2B). In contrast, treatment with palmitic acid, the most abundant SFA in TGRL (Online Table IV) at ~10µM, did not alter TNFα–induced VCAM-1 or ICAM-1 upregulation (data not shown).
Figure 2. PUFAs suppress VCAM-1 expression and reverse the effects of pro-atherogenic TGRL.
VCAM-1 and ICAM-1 expression as % change from TNFα-stimulated (0.3 ng/ml) HAEC treated simultaneously with increasing concentrations of (A) DHA or (B) αLA for 4 hr. (*P < 0.05 from TNFα; n=5–9). Change in (C) VCAM-1 or (D) ICAM-1 expression from TNFα-stimulated HAEC treated with TGRL (10 mg/dl ApoB) with or without DHA (10 µM). (*P<0.05, **P<0.01 from paired TNFα) or by Student’s t-test (#P<0.05 and ##P<0.001 from TGRL. NS=not significant from TGRL; n=4–10).
We next assessed whether PUFA along with TGRL would alter its relative pro- or anti-atherogenic effect in response to cytokine stimulation. Treatment with DHA (10µM) abrogated the pro-atherogenic influence of TGRL by decreasing VCAM-1 expression to the level stimulated with TNFα alone (Fig 2C). Likewise, superposition of DHA with anti-atherogenic TGRL was additive in reducing VCAM-1 expression to below that of TNFα stimulation. As with TGRL treatment, DHA did not significantly reduce the net upregulation of ICAM-1 in response to TNFα stimulation (Fig 2D). Collectively, these data demonstrate that exogenously applied PUFA at levels found in human serum exert an anti-atherogenic effect that mimics that observed with the lower density TG particles, and that dietary FAs are a primary regulator of VCAM-1 production during inflammation.
TNFα-induced NFκB and AP-1 activity are not modulated by TGRL or PUFA
Since VCAM-1, ICAM-1, and E-selectin production depend on a conserved set of transcription factors that include NFκB and AP-114, 18 we measured their activation to gauge the priming activity of TGRL on TNFα stimulation. Neither TGRL nor DHA altered NFκB activity in unstimulated cells. TNFα increased NFκB activity, which was not significantly altered by the addition of pro- or anti-atherogenic TGRL or DHA (Fig 3A). Likewise, neither TGRL nor DHA altered the baseline or TNFα-induced activation of c-Jun (Fig 3B). These data reveal that TGRL did not augment activation of transcription factors that are common to VCAM-1 and ICAM-1, and motivated further analysis of distinct transcriptional mechanisms to explain the propensity for TGRL to differentially modulate VCAM-1.
Figure 3. TGRL and DHA do not affect basal or TNFα-induced NF-κB and AP-1 activity.
(A) Active NF-κB (phospho-p65) measured by ELISA in nuclear extract of HAEC treated for 30 min with DHA (10µM) or TGRL (10 mg/dl ApoB) alone or simultaneously with TNFα (0.3ng/ml). n=3–8. (B) Phosphorylated c-Jun expression measured by ELISA in nuclear extract of HAEC treated as in (A). n=3–9. *p<0.05; **p<0.01; ***p<0.001 vs. non-stimulated.
TGRL and PUFA modulate VCAM-1 transcription via an IRF-1-dependent mechanism
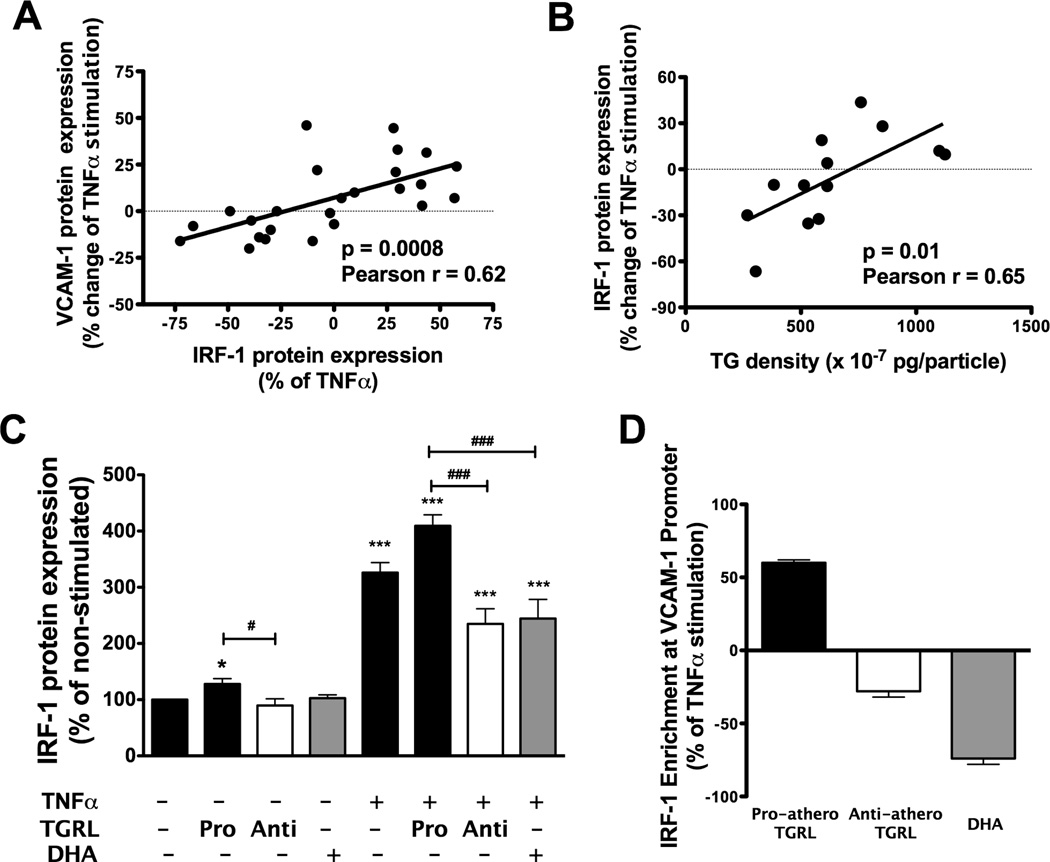
In order to account for the differential response in HAEC exerted by TGRL from our subject pool, we examined whether VCAM-1 expression was transcriptionally regulated at a motif that is distinct from ICAM-1. 14 We focused on IRF-1, since it has previously been reported that IRF-1 activity specifically modulates TNFα-induced VCAM-1 production.14,19,20 IRF-1 protein expression tightly correlated with the extent of pro- or anti-atherogenic modulation of TNFα-induced VCAM-1 expression (Pearson r = 0.6, p<0.001) (Fig 4A). This effect was attributed to the composition of TGRL as suggested by the positive correlation observed between particle TG density and cytokine-induced IRF-1 expression over a range from a 66% drop to a 43% increase from TNFα alone (Fig 4B, Pearson r = 0.65, p= 0.01). As was the case with VCAM-1 expression, IRF-1 levels also increased with particle density of ApoCIII, ApoE and cholesterol (Online Figure VI.). Although TGRL did not itself induce CAM expression, a priming effect of TGRL on IRF-1 was observed in the absence of TNFα. Pro-atherogenic TGRL enhanced basal expression of IRF-1 by 24%, while anti-atherogenic suppressed expression by 11% (Fig 4C). Following TNFα stimulation, pro-atherogenic TGRL enhanced IRF-1 expression by 26%, while anti-atherogenic decreased it by 28% relative (Fig 4C). Likewise, DHA significantly suppressed TNFα-induced IRF-1 expression to a level similar to that observed with anti-atherogenic TGRL (i.e. −25%). Though basal expression of VCAM-1 in unstimulated HAEC is undetectable, the effects of DHA and TGRL on modulating IRF-1 expression were quantifiable leading to the conclusion that they edit VCAM-1 expression primarily during transcription activated by TNFα stimulation.
Figure 4. IRF-1 expression varies with particle TG density and correlates directly with TGRL atherogenic potential.
HAEC were treated for 4 hr with TNFα (0.3ng/ml) alone or simultaneously with DHA (10 µM) or TGRL (10 mg/dl ApoB), characterized for its effect on VCAM-1 expression. (A) Correlation between TGRL modulation of TNFα-induced VCAM-1 expression and IRF-1 expression. Linear regression to data for n=25. (B) Correlation between TGRL modulation of TNFα-induced IRF-1 expression and particle TG density. n=13. (C) HAEC were treated for 4 hr with 10 µM DHA or pro- or anti-atherogenic TGRL in the absence or presence of TNFα. IRF-1 protein expression was detected using Western blot and expressed relative to non-stimulated HAEC. (*P< 0.05; ***P<0.001 from non-stimulated; # P<0.05; ### P<0.001; n = 5–14.) (D) HAEC were treated as described above except TNFα was applied at 3 ng/ml. Chromatin was immunoprecipitated at 2 hr. IRF-1 binding to VCAM-1 promoter was quantified by qPCR and normalized to input DNA (means ± SE from 2 independent experiments).
We next measured the binding of IRF-1 to the VCAM-1 promoter using a chromatin immunoprecipitation (ChIP) assay in order to compare the activity of pro- versus anti-atherogenic TGRL on VCAM-1 during transcription. IRF-1 binding was enriched ~50% relative to TNFα stimulation alone for pro-atherogenic TGRL (Fig 4D). In contrast, IRF-1 binding decreased by ~30% for anti-atherogenic TGRL and 74% in response to treatment with DHA, consistent with its capacity to diminish levels of IRF-1 and downregulate VCAM-1 expression. We confirmed that IRF-1 expression was suppressed to undetectable levels in the presence of siRNA targeting IRF-1, but not a scrambled control (Fig 5A). This resulted in a reduction of VCAM-1 by 33% and 23% when cells were treated with TNFα at 0.3 and 3 ng/mL, respectively. In contrast, ICAM-1 expression was not significantly altered by modulating IRF-1 levels (Fig 5B-C) Treatment with siRNA to IRF-1 brought VCAM-1 expression down by 25% from that due to TNFα stimulation with pro-atherogenic TGRL (Fig 5D). This level of inhibition was equivalent to that elicited by treatment with anti-atherogenic TGRL. Remarkably, blocking IRF-1 activity with IRF-1 siRNA nullified the difference between pro- versus anti-atherogenic TGRL in regulating TNFα-stimulated VCAM-1 expression.
Figure 5. TGRL-modulated VCAM-1 upregulation is IRF-1 dependent.
HAEC were transfected with human IRF-1 siRNA (siIRF-1) (A-D) or pCMV6-XL5/human Irf-1 cDNA (E-H). Scrambled siRNA (siCtrl) and empty plasmid vector (sham) were used as controls. After 24 hr (knockdown) or 72 hr (overexpression), cells were stimulated with different doses of TNFα for 4 hrs prior to Western blot detection of IRF-1 (A, E), and flow cytometric analysis of VCAM-1 (B, F) and ICAM-1 (C, G) surface expression. Significance was determined by paired t-student test. (*P<0.05 vs. control. n=4). Flow cytometric analysis of VCAM-1 surface expression (D, H) Post-transfection, cells were treated with TNFα (0.3ng/mL) alone or plus TGRL (10mg/dl ApoB) for 4 hr (*P<0.05, **P<0.01 vs. siCtrl or sham; # P<0.05, ##P<0.01 pro- vs. anti-atherogenic TGRL group with the same transfection. NS, P>0.05. n=4–6).
To evaluate the extent to which over-expression of IRF-1 increased inflammatory VCAM-1 expression, HAEC were transfected with a cDNA plasmid that elevated its expression in response to TNFα stimulation by 50% at 0.3 ng/ml and 61% at 3 ng/mL, without affecting ICAM-1 expression (Fig 5E-G). The resulting amplification of VCAM-1 expression was comparable to that elicited by pro-atherogenic TGRL (Fig 5H). Thus, overexpression of IRF-1 reversed the anti-atherogenic effect of TGRL on VCAM-1. Together these data demonstrate the relative potency and specificity of IRF-1 in modulation of VCAM-1 transcription in response to TNFα and TGRL.
Post-transcriptional modulation of VCAM-1 expression via miR-126
We hypothesized that the relative level and activity of miR-126 could in part account for the specificity of TGRL in modulating VCAM-1 production, since it is reported to suppress its expression post-transcriptionally without affecting TNFα-induced transcription or transcript stability. 21 Measurement of miR-126 induced by treatment with individual TGRLs revealed an inverse correlation with its capacity to modulate TNFα-induced VCAM-1 expression (Pearson r = −0.7, p<0.004) (Fig 6A). Expression of miR-126 was not altered by stimulation with TNFα alone, or treatment with TGRL alone, over culture duration up to 4h (data not shown). However, in response to cytokine superposed with pro-atherogenic TGRL the expression of miR-126 was decreased by 27% relative to TNFα, while anti-atherogenic TGRL and DHA increased miR-126 expression by 20% and 30%, respectively (Fig 6B). Furthermore, DHA treatment elicited a dose-dependent increase in miR-126 and a corresponding inhibition in VCAM-1 expression in response to TNFα stimulation (Fig 6C). We examined the response in cells transfected with an antisense inhibitor to miR126, which elicited a 17% increase in VCAM-1 from control (Online Figure VIII). The specificity of miR-126 in modulating VCAM-1 was examined by measuring expression of miR-17-3p, a specific modulator of ICAM-1 translation.22 Treatment with either pro- or anti-atherogenic TGRL each decreased the expression of miR-17-3p by 20%, which correlated inversely with the observed 20% net increase in ICAM-1 in response to cytokine stimulation. Co-treatment with DHA and TNFα had no effect on miR-17-3p expression (Fig 6D). Taken together, these data reveal a post-transcriptional mechanism for editing cytokine-induced VCAM-1 expression in which miR-126 acts in a reciprocal manner to IRF-1 by increasing its expression in response to anti-atherogenic TGRL or PUFA treatment of HAEC.
Figure 6. miR-126 expression correlates inversely with TGRL atherogenic potential.
(A) Correlation between TGRL modulation of TNFα-induced VCAM-1 expression and miR-126 expression at 1 hr. Linear regression for n=15. (B) miR-126 expression in HAEC treated for 1hr with TNFα (0.3 ng/ml) alone or simultaneously with DHA (10µM) or TGRL (10 mg/dl ApoB). (C) Change in VCAM-1 and miR-126 expression relative to TNFα-stimulated HAEC treated simultaneously with increasing concentrations of DHA for 1 hr (miR-126) or 4 hr (VCAM-1). (D) miR-17-3p expression in HAEC treated as in (B). (*P < 0.001 from TNFα; n=3–5).
Monocyte adhesion to activated HAEC correlates with TGRL-modulated VCAM-1 expression
Monocyte arrest on HAEC is an early event in atherogenesis and dependent upon the relative expression of endothelial VCAM-1. 12 We quantified VCAM-1 dependent adhesion under a variety of conditions in which its expression was altered at the transcriptional and post-transcriptional level (Fig 7). Pro-atherogenic TGRL increased monocyte adhesion by ~20%, while anti-atherogenic TGRL and DHA decreased it by 20–30%. Similarly, silencing IRF-1 with siRNA decreased adhesion by ~30%. Conversely, both IRF-1 over-expression and miR-126 inhibition had a similar effect of increasing monocyte adhesion by ~30%. A striking finding was that VCAM-1 expression and monocyte recruitment were highly correlated regardless of the method of regulating VCAM-1 expression. A 1% change in VCAM-1 expression corresponded to 1.5% change in monocyte adhesion that prescribed a constant slope over a dose range of TNFα from 0.03–3 ng/ml (Fig 7). Together, these data demonstrate the functional importance of VCAM-1 regulation on monocyte adhesion in which expression was modulated physiologically by a subject’s TGRL or engineered at the transcriptional and post-transcriptional level via IRF-1 and miR126.
Figure 7. Monocyte adhesion to activated HAEC correlates with changes in VCAM-1 expression.
HAEC were treated with pro- or anti-atherogenic TGRL plus 0.3ng/mL TNFα, or transfected with IRF-1 siRNA or cDNA construct, or miR-126 inhibitor prior to TNFα stimulation. Monocyte adhesion was performed as described in Methods and plotted relative to the number arrested in response to 0.3ng/mL TNFα plus control transfection. Black dots represented 0.1ng/ml (−27%), 0.3ng/ml (0%), or 1.0ng/ml (25%) ng/ml TNFα, respectively. Ellipse centroid represents the mean value, the width and height correspond to the SEM of VCAM-1 expression or monocyte adhesion, respectively. (n=3–5 individual experiments for each treatment.)
DISCUSSION
We applied ex-vivo analysis of an individual’s TGRL following consumption of a high-fat meal to examine the molecular mechanisms that skew arterial endothelium toward atherogenicity, characterized by increased VCAM-1 expression and monocyte adhesion. A direct correlation was measured between the density of TG, cholesterol, ApoCIII and ApoE that constituted a subject’s TGRL and its atherogenic potential to alter the inflammatory response to TNFα. A remarkable finding was that TGRL from a subset of subjects exerted an anti-atherogenic effect by decreasing inflammatory expression of VCAM-1. In contrast, ICAM-1 and E-selectin expression were consistently upregulated with cytokine stimulation and TGRL, independent of its lipid or apolipoprotein content. These data reveal a distinct mechanism whereby the fatty acid composition of TGRL alters the kinetics of TNFα-induced VCAM-1 expression through fine-tuning the transcriptional and post-transcriptional regulators that also determine the efficiency of monocyte recruitment.
Previously, we reported that treatment of HAEC with TGRL isolated from the serum of fasting subjects did not alter the inflammatory response. 12 An intriguing question that arises is why individuals who consume an identical high fat meal produce TGRL that elicit distinct ex vivo endothelial responses.12 Circulating TGRL consist of chylomicrons and remnant particles synthesized by the gut and VLDL produced by processing in the liver. Its make up varies as a function of an individual’s level of carbohydrate intake, adipose tissue metabolism, and insulin-sensitivity. 23 Moreover, heterogeneity in TGRL may reflect genetic variation in apolipoproteins and lipoprotein lipase (LPL) that influence the rate and extent of particle clearance from the circulation.2
Our studies focused on native TGRL particles that were not subjected to lipolysis or oxidation following isolation. We previously reported that LDLR-mediated endocytosis by HAEC accounted for ~80% of native TGRL uptake that increased with stimulation by TNFα, but the extent of endocytosis did not vary significantly between donors. 12 Moreover, TGRL was not inherently inflammatory to endothelium, but rather modulated the inflammatory response in a manner that correlated with a subject’s triglyceride level and waist circumference.11, 12 Based on these findings, we reasoned that differences in TGRL particle composition accounted for the variation in inflammatory modulation.
We observed that a subject’s serum TG correlated closely with its density in freshly isolated TGRL particles, which above a threshold value of ~200 mg/dl elicited an atherogenic response. Triglycerides are rich in esterified fatty acids that can elicit differential inflammatory responses as a function of their relative abundance upon release by the action of LPL or other phospholipases in EC.8 For example, the balance between neutral and oxidized FA could affect production of reactive oxygen species and inflammation in EC, 8 or these lipolysis products can serve as peroxisome proliferator–activated receptor (PPAR) agonists that downregulate TNFα-induced VCAM-1 expression. 24 However, it does not appear that the total FA content of TGRL was responsible for the relative atherogenecity of a subject’s TGRL since it was equivalent between pro- and anti-atherogenic TGRL. In contrast, both saturated and unsaturated NEFA were enriched in anti-atherogenic TGRL, while pro-atherogenic particles showed ~65% enrichment in arachidonic acid (C20:4n6). Although NEFA only comprised 1–2% of the total fatty acid content detected in native TGRL, it is more likely to be directly available for uptake by EC in absence of LPL. Moreover, differences in NEFA content can result in activation of a variety of cellular responses through the action of PPARs 25 and Toll-like receptors (TLRs). 26 PPARs serve as regulators of metabolism and lipid-mediated inflammatory signaling since they bind FAs as ligands with different affinities and specificities. PPARα is the most promiscuous, binding both SFA and PUFA,25 and its agonist was reported to inhibit TNFα-induced VCAM-1 expression in human arterial EC.27 Similarly, FAs differentially affect TLR4 activation as a function of their relative saturation.26 Inflammatory effects of SFAs are mediated via TLR-4 and its deficiency can prevent insulin resistance and endothelial dysfunction.28 We conclude that a distinct distribution of FA composition is in part responsible for the ex vivo atherogenic effect of an individual’s TGRL on HAEC since we could statistically segregate the cohort of subjects into three discrete groups represented by pro-, anti-, and neutral-atherogenicity (Online Figure V).
Several differences in the NEFA content between pro- and anti-atherogenic TGRL are worth highlighting. As a marker for de novo lipogenesis, palmitoleate (C16:1n7), which registered higher levels in anti-atherogenic NEFA, is involved in conversion of glucose to fatty acids and elevated levels in plasma were associated with metabolic syndrome-resistant mice.29 Oleic acid (C18:1n9), also elevated in anti-atherogenic TGRL, can reduce the inflammatory effects of long-chain SFAs in HAECs through reducing cellular stearic acid incorporation and NF-κB activation.30 Another study indicated that stearic acid (C18:0), oleic acid (C18:1n9), or vaccenic acid (C18:1n7) reduced H2O2-induced pulmonary artery endothelial cell injury. In contrast, linolenic acid (C18:3n6) and eicosatrienoic acid (20:3n3), both trending lower in anti-atherogenic TGRL, enhanced H2O2-induced injury to PAEC.31 Our observations that higher levels of palmitoleate, oleic acid, vaccenic acid, and linoleic acid were associated with the beneficial effect of anti-atherogenic TGRL on endothelial inflammatory response were consistent with these findings. Similarly, we found anti-atherogenic TGRL contained lower level of arachidonic acid (C20:4n6), a well-known inflammatory intermediate. While SFA were previously shown to enhance the inflammatory response to cytokine in ECs and monocytes,26 the inflammatory effect of SFA is somewhat controversial. Consistent with other studies, we found that exogenously added palmitic acid, the most abundant SFA in TGRL lipolysis products,8 did not alter VCAM-1 or ICAM-1 upregulation relative to TNFα stimulation. 32 The findings that anti-atherogenic TGRL contained more palmitic and stearic acids than did pro-atherogenic TGRL suggest a lesser role of SFA in skewing the inflammatory response in HAEC.
Studies on cultured EC have shown that Ω3-PUFA suppresses the effects of cytokines33 and pro-inflammatory mediators. 17 In line with these results, we found that treatment of HAEC with dietary levels of the PUFAs DHA and αLA suppressed cytokine-induced VCAM-1 (but not ICAM-1) expression in a dose-dependent manner. Moreover, PUFA supplementation nullified the upregulation of VCAM-1 induced by pro-atherogenic TGRL, consistent with a reported role for Ω3-PUFA in the selective regulation of CAM expression. 33 While there was a trend for high αLA (p=0.07) in anti-atherogenic TGRL, similar levels of DHA and other Ω3-PUFA were observed in pro-atherogenic TGRL, suggesting that the beneficial effect of PUFA may be not simply attributed to the absolute level of Ω3-PUFA. Instead, the anti-atherogenic property may derive from a balance among different fatty acid species. For example, we found that pro-atherogenic TGRL displayed a higher ratio of AA(C20:4n6)+DTA (C22:4n6):EPA(C20:5n3)+DPA(C22:5n3)+DHA(C22:6n3), which is associated with metabolic syndrome. 34 In addition to fatty acids, we also observed an association between VCAM-1 expression and the postprandial concentration of cholesterol and apoCIII. This finding may partially account for the inflammatory effect of TGRL, given the potential association between high levels of serum cholesterol and apoCIII and inflammatory activation of EC. 8, 9
We sought to elucidate the transcriptional mechanisms underlying the selectivity in gene regulation of VCAM-1 versus ICAM-1 as a function of donor TGRL content. In response to cytokine stimulation, these adhesion receptors reach peak expression within six hours and are dependent on a conserved group of transcription factors that includes NF–κB and AP-1 14, 18 (Online Figure IX). There have been conflicting reports as to the capacity of DHA to inhibit TNFα-induced NF–κB activation.35, 36 We found that neither TGRL nor DHA modulated the cytokine-induced activation of NF–κB and AP-1 in HAEC treated with TNFα for 30 min, corresponding to maximum NF-κB assembly on the promoter region of VCAM-1. 18, 37, 38 Optimal VCAM-1 expression requires both constitutively bound SP-1, along with de novo production of IRF-1 by TNFα that acts cooperatively with NF–κB to induce a maximal response. 37, 38 Previous studies have reported that IRF-1-dependent regulation can either enhance or inhibit TNFα-induced VCAM-1 expression.14,19,20,37 Consistent with these studies, we observed that unstimulated HAEC expressed detectable levels of IRF-1, which increased steadily for up to 4 hrs following TNFα stimulation. 37 Our data are the first to demonstrate that TGRL alone can modulate levels in basal expression of IRF-1 and in this manner amplify or suppress cytokine-induced regulation of VCAM-1 transcription and expression. Analysis of IRF-1 activity was confirmed by ChIP assay that clearly implicated TGRL-induced changes in relative expression of IRF-1 that edited up or down mRNA levels relative to TNFα stimulation alone. Confirmation that IRF-1 was a specific regulator of VCAM-1 transcription was provided by the use of siRNA and cDNA constructs. By specifically inhibiting IRF-1 expression, we abrogated pro-atherogenic TGRL amplification of VCAM-1 expression. Conversely, overexpression of IRF-1 effectively increased VCAM-1 in the presence of TNFα and nullified the effect of anti-atherogenic TGRL. DHA supplementation mimicked the action of anti-atherogenic TGRL in a dose-dependent manner by diminishing IRF-1 expression and assembly on the VCAM-1 promoter. A previous report has shown that DHA inhibited IRF-1 expression in macrophages,39 but our data is the first to demonstrate DHA inhibition of IRF-1 expression in cultured EC.
miR-126 is constitutively expressed in quiescent EC and binds to a complementary target site of the UTR of VCAM-1 mRNA, resulting in inhibition of mRNA translation and suppression of de novo protein synthesis. 21 Consistent with previous reports, TNFα alone did not alter the basal expression of miR-126,21 nor did TGRL treatment alone. However, TNFα and TGRL combined to modulate miR-126 levels in a manner dependent upon TGRL atherogenicity. The mechanisms underlying regulation of miR-126 are not well understood, although recent studies have demonstrated important roles for transcription factors of the Ets family and KLF2.40 miR-126 expression in HAEC co-treated with TGRL and TNFα was inversely correlated with modulation in VCAM-1 expression. Moreover, DHA as well as anti-atherogenic TGRL significantly increased miR-126 in a dose-dependent fashion, while SFAs had no effect. The extent of up- or down-regulation of miR-126 expression was equivalent and reciprocal to that observed for IRF-1, producing a tight inverse correlation with subject’s TGRL (Online Figure VII). These data are the first to confirm that the anti-atherogenic effect of PUFAs occurs in part at the post-transcriptional level via the action of miR-126.
A critical demonstration of the functional significance of dietary modulation of VCAM-1 expression is the effect of TGRL on monocyte arrest. The efficiency of adhesion varied in direct proportion to changes in VCAM-1 protein expression induced by TGRL, IRF-1, and miR-126, over a range of expression elicited by TNFα dose. Together these data directly link modulation of IRF-1 and miR-126 in adhesion receptor specific regulation of VCAM-1 and monocyte adhesion to HAEC. Moreover, these data shed light on a mechanism by which PUFAs that lower circulating TG levels can modulate inflammation and possibly diminish an individual’s risk of arterial plaque formation.
In summary, we reveal a putative mechanism by which TGRL modulates VCAM-1 production in HAEC through independent regulation of miR-126 and IRF-1 expression as a function of its FA and apolipoprotein constituents. In this manner, TGRL regulates both transcriptional and post-transcriptional pathways that superpose to edit VCAM-1 expression both in health and disease.
Supplementary Material
Novelty and Significance.
What Is Known?
Diets high in fat are associated with hypertriglyceridemia and increased risk of cardiovascular disease.
Cholesterol and fatty acids transported by lipoprotein particles exacerbate systemic inflammation and can initiate plaque formation in arteries.
Genetics, diet, and lifestyle choices coalesce in determining the extent to which inflammation triggers atherosclerosis.
What New Information Does This Article Contribute?
Subjects consuming an identical high fat meal produced and circulated lipoproteins that segregated into subsets eliciting either a pro- or anti-inflammatory response in arterial endothelial cells.
A direct correlation was found between an increase in particle density of triglycerides and expression of VCAM-1 receptors that supported monocyte adhesion to endothelium, a harbinger or atherosclerosis.
We identified a molecular mechanism by which uptake of lipoproteins bias the inflammatory response of endothelium via transcriptional and post-transcriptional editing of VCAM-1.
The alarming correlation between obesity, serum triglycerides, and cardiovascular disease motivated this study to gauge the inflammatory capacity of circulating triglyceride rich lipoproteins (TGRL) following consumption of a high fat meal in a cohort of subjects ranging from low to high cardiovascular risk. A lab-on-a-chip approach was applied to quantify biomarker expression and monocyte adhesion ex vivo on a monolayer of aortic endothelium. Subjects whose TGRL contained elevated density of triglycerides and apolipoproteins and elicited a pro-atherogenic response in endothelium, were categorized as high cardiovascular disease risk. This response was characterized by enhanced VCAM-1 production through amplification of the transcriptional activity of IRF-1 and suppression of post-transcriptional activity via miR126. Conversely, anti-atherogenic TGRL was associated with downregulation of VCAM-1 expression on inflamed endothelium due to decreased IRF-1 and enhanced miR-126 activity. The latter subjects circulated particles with lower density of triglyceride and higher content of non-esterified fatty acids. Supplementation of native TGRL with the omega-3 fatty acid DHA decreased VCAM-1 and monocyte adhesion. This ex vivo strategy for quantifying the atherogenic potential of an individual’s lipoprotein particles provides a means for mechanistic studies of how dietary metabolites correlate with atherogenic changes induced by genotype, diet, and lifestyle.
Acknowledgments
SOURCES OF FUNDING
This study is supported by National Institute of Health grants HL082689 (SIS and AGP), HL077281 and HL079071 (AAK), the Department of Veterans Affairs Merit Award (AAK) and the USDA-Agricultural Research Service (ARS) project 5306-51530-019-00D (JWN). ARS is an equal opportunity employer.
Non-standard Abbreviations
- αLA
α–linolenic acid
- CAM
cell adhesion molecules
- DHA
docosahexaenoic acid
- FA
fatty acids
- HAEC
human aortic endothelial cells
- HTG
hypertriglyceridemia
- ICAM-1
intercellular adhesion molecule 1
- IRF-1
interferon regulatory factor 1
- pp-sTG
postprandial serum triglyceride
- TGRL
triglyceride-rich lipoprotein
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
- TG
triglycerides
- TNFα
tumor necrosis factor α
- VCAM-1
vascular cell adhesion molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–355. doi: 10.1046/j.1365-2796.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease: A scientific statement from the american heart association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 3.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 5.Chan JW, Motton D, Rutledge JC, Keim NL, Huser T. Raman spectroscopic analysis of biochemical changes in individual triglyceride-rich lipoproteins in the pre- and postprandial state. Analytical chemistry. 2005;77:5870–5876. doi: 10.1021/ac050692f. [DOI] [PubMed] [Google Scholar]
- 6.Jackson KG, Wolstencroft EJ, Bateman PA, Yaqoob P, Williams CM. Greater enrichment of triacylglycerol-rich lipoproteins with apolipoproteins e and c-iii after meals rich in saturated fatty acids than after meals rich in unsaturated fatty acids. The American journal of clinical nutrition. 2005;81:25–34. doi: 10.1093/ajcn/81.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Williams CM, Maitin V, Jackson KG. Triacylglycerol-rich lipoprotein-gene interactions in endothelial cells. Biochemical Society transactions. 2004;32:994–998. doi: 10.1042/BST0320994. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized ffas that induce endothelial cell inflammation. Journal of lipid research. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein ciii induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–687. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 10.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 11.Ting HJ, Stice JP, Schaff UY, Hui DY, Rutledge JC, Knowlton AA, Passerini AG, Simon SI. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circulation research. 2007;100:381–390. doi: 10.1161/01.RES.0000258023.76515.a3. [DOI] [PubMed] [Google Scholar]
- 12.Wang YI, Schulze J, Raymond N, Tomita T, Tam K, Simon SI, Passerini AG. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. American journal of physiology. Heart and circulatory physiology. 2011;300:H784–H791. doi: 10.1152/ajpheart.01036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. Cd11c/cd18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagia NM, Harii N, Meli AE, Sun X, Lewis CJ, Kohn LD, Goetz DJ. Phenyl methimazole inhibits tnf-alpha-induced vcam-1 expression in an ifn regulatory factor-1-dependent manner and reduces monocytic cell adhesion to endothelial cells. Journal of immunology. 2004;173:2041–2049. doi: 10.4049/jimmunol.173.3.2041. [DOI] [PubMed] [Google Scholar]
- 15.Grapov D, Newman JW. Imdev: A graphical user interface to r multivariate analysis tools in microsoft excel. Bioinformatics. 2012 doi: 10.1093/bioinformatics/bts439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libby P. Inflammation and cardiovascular disease mechanisms. The American journal of clinical nutrition. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. The American journal of clinical nutrition. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 18.Neish AS, Williams AJ, Palmer HJ, Whitley MZ, Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. The Journal of experimental medicine. 1992;176:1583–1593. doi: 10.1084/jem.176.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warfel JM, D'Agnillo F. Anthrax lethal toxin enhances tnf-induced endothelial vcam-1 expression via an ifn regulatory factor-1-dependent mechanism. Journal of immunology. 2008;180:7516–7524. doi: 10.4049/jimmunol.180.11.7516. [DOI] [PubMed] [Google Scholar]
- 20.Lechleitner S, Gille J, Johnson DR, Petzelbauer P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule 1 (cd106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. The Journal of experimental medicine. 1998;187:2023–2030. doi: 10.1084/jem.187.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. Microrna-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suarez Y, Wang C, Manes TD, Pober JS. Cutting edge: Tnf-induced micrornas regulate tnf-induced expression of e-selectin and intercellular adhesion molecule-1 on human endothelial cells: Feedback control of inflammation. Journal of immunology. 2010;184:21–25. doi: 10.4049/jimmunol.0902369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bard JM, Charles MA, Juhan-Vague I, Vague P, Andre P, Safar M, Fruchart JC, Eschwege E. Accumulation of triglyceride-rich lipoprotein in subjects with abdominal obesity: The biguanides and the prevention of the risk of obesity (bigpro) 1 study. Arterioscler Thromb Vasc Biol. 2001;21:407–414. doi: 10.1161/01.atv.21.3.407. [DOI] [PubMed] [Google Scholar]
- 24.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates ppar ligands: Evidence for an antiinflammatory role for lipoprotein lipase. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Molecular cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 26.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. The Journal of biological chemistry. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. Pparalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of toll-like receptor-4/nuclear factor-kappab pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey KA, Walker CL, Xu Z, Whitley P, Pavlina TM, Hise M, Zaloga GP, Siddiqui RA. Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. Journal of lipid research. 2010;51:3470–3480. doi: 10.1194/jlr.M010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart CM, Tolson JK, Block ER. Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. The American journal of physiology. 1991;260:L481–L488. doi: 10.1152/ajplung.1991.260.6.L481. [DOI] [PubMed] [Google Scholar]
- 32.Reissig D, Rassoul F, Salvetter J, Wagner O, Richter V. Effect of fatty acids on expression of endothelial leukocyte adhesion molecules. European journal of nutrition. 2003;42:224–227. doi: 10.1007/s00394-003-0408-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57:1328–1339. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nigam A, Frasure-Smith N, Lesperance F, Julien P. Relationship between n-3 and n-6 plasma fatty acid levels and insulin resistance in coronary patients with and without metabolic syndrome. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2009;19:264–270. doi: 10.1016/j.numecd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Goua M, Mulgrew S, Frank J, Rees D, Sneddon AA, Wahle KW. Regulation of adhesion molecule expression in human endothelial and smooth muscle cells by omega-3 fatty acids and conjugated linoleic acids: Involvement of the transcription factor nf-kappab? Prostaglandins, leukotrienes, and essential fatty acids. 2008;78:33–43. doi: 10.1016/j.plefa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang TM, Chen CJ, Lee TS, Chao HY, Wu WH, Hsieh SC, Sheu HH, Chiang AN. Docosahexaenoic acid attenuates vcam-1 expression and nf-kappab activation in tnf-alpha-treated human aortic endothelial cells. The Journal of nutritional biochemistry. 2011;22:187–194. doi: 10.1016/j.jnutbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Neish AS, Read MA, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with nf-kappa b as a transcriptional activator of vascular cell adhesion molecule 1. Molecular and cellular biology. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neish AS, Khachigian LM, Park A, Baichwal VR, Collins T. Sp1 is a component of the cytokine-inducible enhancer in the promoter of vascular cell adhesion molecule-1. The Journal of biological chemistry. 1995;270:28903–28909. doi: 10.1074/jbc.270.48.28903. [DOI] [PubMed] [Google Scholar]
- 39.Kielar ML, Jeyarajah DR, Penfield JG, Lu CY. Docosahexaenoic acid decreases irf-1 mrna and thus inhibits activation of both the irf-e and nfkappa d response elements of the inos promoter. Transplantation. 2000;69:2131–2137. doi: 10.1097/00007890-200005270-00030. [DOI] [PubMed] [Google Scholar]
- 40.Harris TA, Yamakuchi M, Kondo M, Oettgen P, Lowenstein CJ. Ets-1 and ets-2 regulate the expression of microrna-126 in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1990–1997. doi: 10.1161/ATVBAHA.110.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.