Abstract
Background
Oral corticosteroids (OCSs) are recommended for severe wheezing episodes in children. However, limited evidence supports this intervention in preschool children with outpatient wheezing illnesses.
Objective
We sought to investigate whether OCSs reduce symptom scores during acute lower respiratory tract illnesses (LRTIs) in preschool children with recurrent wheeze
Methods
We performed post hoc and replication analyses in 2 outpatient cohorts of children aged 1 to 5 years with episodic wheezing participating in clinical trials. We compared symptom scores during LRTIs that were or were not treated with OCSs, adjusting for differences in disease and episode severity covariates.We stratified episodes by severity by using a propensity model. The primary outcome was the area under the curve (AUC) of total symptom scores among the more severe episodes.
Results
Two hundred fifteen participants from the Acute Intervention Management Strategies trial experienced 798 acute LRTIs, 112 of which were defined as severe based on propensity scores. The AUCs of total symptom scores did not differ between the episodes that were (n = 70) and were not (n = 42) treated with OCSs (P = .46) nor was there an OCS treatment effect on individual symptom scores. Similar analyses of the Maintenance Versus Intermittent Inhaled Corticosteroids in Wheezing Toddlers trial, involving 278 participants with 133 severe LRTIs, confirmed the above findings (P =.46 for AUC of total symptoms score comparison).
Conclusion
In 2 separate cohorts of preschool children with episodic wheezing, OCS treatment during clinically significant LRTIs did not reduce symptom severity during acute LRTIs, despite asthma controller medication use during most episodes. These findings need to be confirmed in a prospective randomized controlled trial.
Keywords: Oral corticosteroids, episodic wheezing, preschool children
Preschool-aged children with recurrent episodes of wheezing experience significant morbidity during acute episodes of lower respiratory tract illnesses (LRTIs) but have minimal symptoms consistent with persistent asthma between these episodes.1 Although most preschool children exhibit episodic disease, this age group experiences disproportionately greater morbidity and health care use compared with school-aged children.2 Daily therapy with inhaled corticosteroids (ICSs) to prevent LRTIs from progressing to severe asthma exacerbations is recommended by asthma guidelines for preschool children with persistent disease or for those preschool children who required 2 or more courses of oral corticosteroids (OCSs) over 6 months.3 This approach reduces the likelihood of exacerbations requiring OCSs by approximately 35% but does not prevent them entirely.4 Therefore defining the appropriate treatment for these significant LRTIs is essential.
Asthma guidelines recommend treatment with OCSs3,5 for significant exacerbations poorly responsive to bronchodilators. However, despite substantial evidence for the efficacy of OCSs in asthma exacerbations in school-aged children and adolescents,6–9 evidence supporting this intervention in nonhospitalized preschool-aged children is limited.10 Moreover, recent studies11,12 and editorials13,14 question the benefit of OCSs for wheezing illnesses in this age group. Therefore studies of the effects of OCSs in acute LRTIs in young children are needed given the recent concerns about the lack of efficacy of this treatment in this age group and the potential side effects of OCS therapy.
The Childhood Asthma Research and Education Network evaluated different treatment approaches in preschool-aged children with severe intermittent wheezing in the Acute Management Intervention Strategies (AIMS) and Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers (MIST) clinical trials.15,16 We used these well-defined cohorts to perform post hoc analyses investigating whether OCS treatment, when administered to preschool children with severe intermittent wheezing during acute episodes of severe LRTIs, reduces illness burden, as reflected by daily symptom scores during the LRTIs.
METHODS
AIMS and MIST study designs, treatment, and participants
Detailed descriptions of the screening, recruitment, design, outcomes, and statistical analysis for the AIMS and MIST trials have been reported in detail elsewhere.15,16 Briefly, the AIMS trial15 was a multicenter, double-blind, randomized, placebo-controlled, double-dummy, parallel-group comparison of 3 treatment regimens used episodically at the early signs of respiratory tract illness (RTI) in children with recurrent episodes of moderate-to-severe wheezing: budesonide inhalation suspension (1 mg twice daily), montelukast (4 mg daily), or albuterol alone. Eligible children were 12 to 59 months of age and had experienced at least 2 episodes of wheezing in the context of an RTI within the past year, one of which must have been documented by a health care provider. In an effort to include children with prior moderate-to-severe wheezing episodes, children were required to have experienced either 2 urgent care visits for acute wheezing within the past year or 2 wheezing episodes for which systemic corticosteroids were prescribed or 1 episode requiring urgent care and 1 episode requiring systemic corticosteroids.
The MIST trial16 was a multicenter, double-blind, randomized, placebocontrolled trial comparing daily low-dose budesonide inhalation suspension (0.5 mgnightly) with intermittent high-dose budesonide inhalation suspension (1 mg twice daily for 7 days) starting at the early signs of an RTI. The MIST trial targeted children 12 to 53 months of age with recurrent wheezing and mandated a history of at least 4 episodes of wheezing in the past year (or ≥3 episodes if receiving a controller for ≥3 months) with at least 1 exacerbation requiring the use of systemic corticosteroids, an urgent care visit, or hospital-ization. Children with persistent asthma symptoms were not eligible to participate in either of these studies. These trials differed in the prevalence of risk factors for future asthma measured by using the modified Asthma Predictive Index (mAPI17): the AIMS trial included children with both positive (60%) and negative (40%) mAPI results, whereas the MIST trial included only children with positive mAPI results (100%).
In both studies prednisolone treatment was prescribed as a rescue treatment for significant LRTIs after consultation with a physician (by telephone or in person) and was based on specific predefined protocol criteria (see Table E1 in this article’s Online Repository at www.jacionline.org).15,16 The prednisolone regimen included a once-daily dose at 2 mg/kg/d for 2 days, followed by 1 mg/ kg/d for an additional 2 days, as previously used in randomized controlled trials (RCTs).4,15,16,18–21 Institutional review boards at all participating centers approved these protocols, and parents provided written informed consent; the trials were monitored by the Childhood Asthma Research and Education Network’s Data and Safety Monitoring Board.
Measurement of symptom scores during acute RTIs
RTI, as used in the AIMS and MIST trials,15,16 is a clinical descriptor of the constellation of parent-identified early upper and lower respiratory tract symptoms that precede and accompany significant wheezing episodes. Both trials included parent-reported symptom scores for cough, wheezing, trouble breathing, and interference with activity (each on a scale of 0–5, with 0 indicating no symptoms and 5 indicating severe symptoms) determined by using a validated questionnaire,15,16,22 and scores were recorded daily for 14 days from the start of each illness. The total symptom score (on a scale of 0–20) was calculated as the sum of all 4 individual scores.
General overview of the statistical analyses
Because of the post hoc nature of the analytic strategy, participants were not randomly assigned to treatment with OCSs because the decisions to use OCSs made during the trials were directed by the study protocols. This lack of randomization was associated with differences in relevant baseline diseaserelated patients’ characteristics and episode baseline severity between OCS-treated and untreated episodes, resulting in a potential indication bias, which confounds the estimate of the effect of OCS treatment.23 To adjust for the differences in baseline characteristics between OCS-treated and untreated episodes, we used propensity score methodology. This approach creates a state of “semi-randomization” in observational (ie, nonrandomized) studies or retrospective analyses and effectively adjusts the outcome for the effects of baseline confounding.23–25 Our propensity model included multiple relevant baseline covariates (patients’ characteristics and episode baseline severity) that might have affected the decision to initiate OCS therapy, which might have biased the outcome toward higher severity scores among the episodes treated with OCSs. The combination of these covariates is reflected by the propensity score, which represents the probability that each episode was treated with OCSs. Consequently, all outcome analyses were adjusted for this propensity score.
In addition, the propensity model was used to identify the more severe episodes for outcome comparisons. The propensity model (variables listed in Table I) was developed, and the outcomes were initially measured in the AIMS cohort. Then we applied the same model, without repeating the model development step, to the MIST cohort to confirm that the model effectively accounted for differences in baseline covariates in a separate cohort. Therefore all analyses in the MIST cohort were adjusted for the same covariates as in the AIMS cohort.
TABLE I.
Variables that comprise the propensity model to predict the probability of OCS treatment based on baseline covariates (AIMS cohort)
| Covariate | P value |
|---|---|
| Episode no. | .05 |
| Race (nonwhite) | <.01 |
| Age | .06 |
| Treatment with OCSs in past year | .27 |
| ED visit for wheezing in past year | <.01 |
| Physician’s office visit for wheezing in past year | <.01 |
| Positive skin test to aeroallergen | <.01 |
| Wheezing score >2 | .26 |
| Albuterol use >5 times over the past day | .43 |
| Trouble breathing score >3 | <.01 |
| Interference with activity score >1 | <.01 |
| Daytime cough score >6 | <.01 |
| Total symptoms score >9 | .11 |
| Drug arm | .62 |
| Center no. | .25 |
| Interaction terms | |
| Wheezing score >2 × albuterol use >5 | .02 |
| Wheezing score >2 × trouble breathing score >3 | .33 |
| Wheezing score >2 × interference with activity score >1 | .36 |
| Albuterol use >5 × trouble breathing score >3 | .59 |
The following baseline covariates from the AIMS trial were evaluated and were not included in the model because they did not reach statistical significance and did not increase the overall predictive power of the model: Asthma Predictive Index score, ethnicity, sex, baseline exacerbations, blood eosinophilia, and maternal asthma. ED, Emergency department.
Statistics: Propensity model
A repeated-measures logistic regression model was fitted to the daily indicator of prednisolone use (0/1) to estimate the propensity score. For the episodes in which prednisolone was administered, the model included days from the start of the LRTI until prednisolone was used, whereas for the episodes in which prednisolone was not administered, the model included days from the start of illness until day 2 (the median day on which prednisolone was started for the episodes that were actually treated with prednisolone). This “baseline” period used for model development was not included in area under the curve (AUC) calculations of outcome measurements. Numerous baseline and daily symptom variables were evaluated in this model to determine the best-fitted final propensity model (Table I). From the final propensity model, a propensity score was estimated for each day, and a maximum propensity score was then calculated for each episode. This maximum propensity score was log-transformed and used to adjust for the comparison of the episodes that were or were not treated with prednisolone with respect to the baseline and episode-specific baseline measurements. Comparison of baseline covariates between episodes that were or were not treated with OCSs was performed to demonstrate the efficacy of the propensity score adjustment for the comparison of nonrandomized groups in an observational study.
The propensity model was also used to define strata to help further classify the episodes based on the severity level. Therefore more and less severe episodes were defined based on the propensity score assigned for each episode. The exact cut point for defining the more severe episodes was identified by using sensitivity analyses, which evaluated the effect of multiple cut-point values on the kappa statistic, median values of the propensity score in each group, and sample size, and was determined to be a propensity score of 0.2. Subsequently, comparison of the episodes that were or were not treated with prednisolone was performed within each level of stratification (ie, the more and less severe episodes).
Statistics: Outcome measurements
We compared the AUCs of symptom scores during episodes of LRTIs that were treated with OCSs with the AUCs of symptom scores during episodes that were not treated with OCSs. The AUC was calculated until day 14 for each episode. The primary comparison was the effect of OCS use on total symptom score AUCs among the episodes identified by the propensity model as the more severe episodes.
The AUCs of the symptom scores were compared by using repeated-measures models, which included severity group (more or less severe episodes), OCS use (yes/no), and the severity by OCS interaction, with adjustment for the log-transformed maximum propensity score, center, treatment group, and episode number. From these repeated-measures models, least-squares means and SEs are reported for the comparison of those who received versus those who did not receive OCSs stratified by episode severity. Post hoc power calculations demonstrated 91% and 96% power to detect clinically relevant 35% and 40% reductions in symptom score AUCs (as seen in the AIMS trial15), respectively, with our sample size of 112 severe episodes in the AIMS cohort.
RESULTS
Pre-enrollment disease activity and episode baseline severity in the AIMS cohort
The AIMS cohort consisted of 215 participants with 798 episodes of acute LRTIs. OCSs were used in 152 (19%) episodes. Indicators of pre-enrollment disease activity and episode baseline severity at the onset of the episode were significantly higher in episodes that were treated with OCSs compared with those not treated with OCSs (Tables II and III, column 3), indicating a potential for an indication bias.
TABLE II.
Pre-enrollment disease activity (AIMS cohort) among episodes that were or were not treated with OCSs
| Covariate | Episodes that were not treated with OCSs (n = 646) |
Episodes that were treated with OCSs (n = 152) |
Wald statistic before propensity adjustment (Pvalue)* |
Wald statistic after propensity adjustmenty† (Pvalue)* |
|---|---|---|---|---|
| Demographics and AIMS study covariates | ||||
| Age (mo) | 30 (0.84) | 29 (0.84) | 4.3 (.04)* | 0.2 (.65) |
| Male sex (%) | 63 | 57 | 0.4 (.55) | 0.7 (.40) |
| Race (O/AA/W [%]) | 10/7/83 | 3/16/81 | 18.8 (<.001)* | 0.5 (.79) |
| Ethnicity (Hispanic [%]) | 17 | 16 | 4.4 (.04)* | 0.8 (.37) |
| Drug arm (LTRA, ICS, albuterol [%]) | 36/44/19 | 45/35/20 | 3.3 (.19) | 0.5 (.79) |
| Asthma history (past 12 mo before randomization) | ||||
| No. of physician’s office visits for wheezing | 4.04 (0.23) | 4.68 (0.57) | 0.9 (.34) | 0.01 (.92) |
| No. of ED visits for wheezing | 1.01 (0.16) | 0.96 (0.34) | 0.01 (.91) | 0.2 (.63) |
| ≥4 Wheezing exacerbations (%) | 70 | 77 | 7.2 (.01)* | 0.1 (.76) |
| Receive ≥1 course of OCS (%) | 60 | 74 | 7.6 (.01)* | 0.3 (.61) |
| Atopic characteristics (on randomization) | ||||
| Positive skin test response to aeroallergen (%) | 46 | 57 | 3.8 (.05) | 1.9 (.17) |
| Positive API response (%) | 61 | 72 | 8.5 (.01)* | 0.2 (.70) |
Data are presented before and after adjustment by the propensity model and expressed as means (SEs), except as noted. Repeated-measures models were used to adjust for multiple events within subjects. Propensity score adjustment was performed based on the natural log transformation (to improve normality) of the maximal score in each episode.
AA, African American; API, Asthma Predictive Index; ED, emergency department; LTRA, leukotriene receptor antagonist; O, other; W, white.
P < .05.
Statistics after propensity model adjustment were included to demonstrate effective adjustment of baseline covariate differences between the groups by using the propensity score.
TABLE III.
Initial severity scores (AIMS cohort) among episodes that were or were not treated with OCSs
| Covariate | Episodes that were not treated with OCSs (n = 646) |
Episodes that were treated with OCSs (n = 152) |
Wald statistic before propensity adjustment (Pvalue)* |
Wald statistic after propensity adjustment† (Pvalue)* |
|---|---|---|---|---|
| Total symptom score | 5.1 (0.19) | 8.1 (0.36) | 77.0 (<.001)* | 0.8 (.37) |
| Trouble breathing score | 1.0 (0.06) | 1.8 (0.11) | 58.9 (<.001)* | 0.9 (.35) |
| Interfere with activity score | 1.1 (0.06) | 2.0 (0.11) | 66.0 (<.001)* | 0.1 (.74) |
| Daytime cough score | 2.1 (0.06) | 2.8 (0.09) | 58.6 (<.001)* | 0.8 (.37) |
| Wheezing score | 1.0 (0.05) | 1.6 (0.10) | 39.0 (<.001)* | 0.1 (.70) |
| No. of albuterol treatments (past 24 h) | 3.2 (0.09) | 4.1 (0.20) | 20.6 (<.001)* | 0.0 (.99) |
Data are presented before and after adjustment by the propensity model and represented as means (SEs) of symptom scores from day 1 of the episode (start of acute illness kit) until OCSs were started (for those who received prednisone) and from day 1 through day 2 of the episode for those who did not receive OCSs (day 2 was the median for the day OCSs were started in the group who actually received OCSs). Symptom scores are on a scale of 0 to 5, except for the total symptom score, which is on a scale of 0 to 20. Repeated-measures models were used to adjust for multiple events within subjects. Propensity score adjustment was performed based on the natural log transformation (to improve normality) of the maximal score in each episode.
P < .05.
Statistics after propensity model adjustment were included to demonstrate effective adjustment of baseline covariate differences between the groups by using the propensity score.
Adjustment for indication bias and identification of severe LRTIs by using propensity modeling
A propensity model to predict OCS treatment was used to adjust for potential indication bias. The final predictors in this model are detailed in Table I. The propensity model created a state of semi-randomization,23,25 as reflected by an effective adjustment of differences in baseline covariates between the groups, with no residual significant differences in the baseline covariates between episodes treated or not treated with OCSs (Tables II and III, column 4).
We then used the propensity score to identify the more severe episodes to compare the primary outcome among episodes that are most severe and thus clinically relevant. One hundred twelve episodes were determined by the model as more severe episodes based on their propensity scores. The more severe episodes were significantly more likely to have been treated with OCSs compared with the less severe episodes (P < .001, χ2 test; κ = 0.44; 95% CI, 0.36–0.52; see Table E2 in this article’s Online Repository at www.jacionline.org). The median AUC of the total symptom score for the more severe episodes was 40 (Q1, 25.5; Q3, 55.0), with a maximal average score of 12.0, whereas the median AUC of the total symptom score for the less severe episodes was 13 (Q1, 5.5; Q2, 25.0), with a maximal average score of 6.8.
Sensitivity analyses revealed that the propensity model effectively adjusted for baseline differences between the episodes that were or were not treated with OCSs, even while stratifying based on episode severity level (ie, separate analysis for the more and the less severe episodes, see Tables E3 and E4 in this article’s Online Repository at www.jacionline.org).
Effect of OCS treatment on episode severity in the AIMS cohort
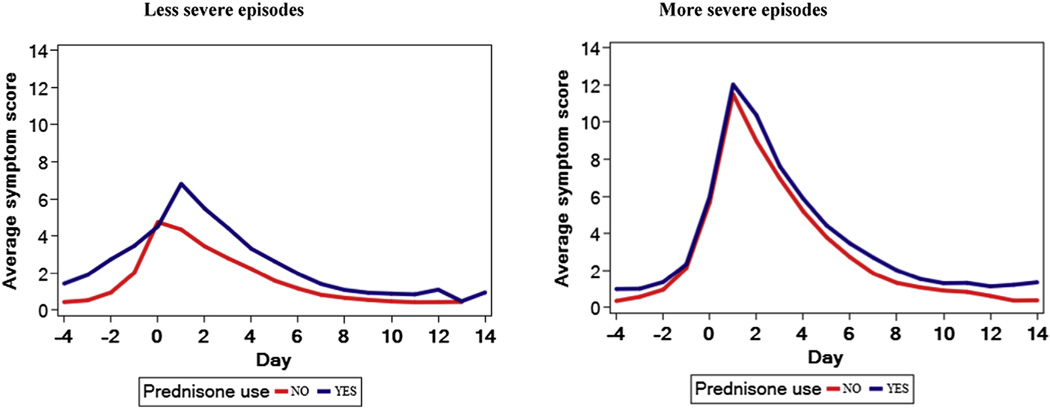
We compared the AUCs of total symptom scores during episodes of LRTIs that were treated with OCSs with those that were not treated with OCSs after adjustment for the maximum propensity score for each episode and stratifying by episode severity level (Fig 1 and Table IV). The AUCs of total symptom scores among the more severe episodes (defined as those episodes with propensity scores >0.20) did not differ between episodes that were or were not treated with OCSs (P =.46). OCS therapy was not associated with lower AUCs of any of the individual symptom scores: cough, wheeze, shortness of breath, and interference with activity. Among the less severe episodes (propensity score <0.20), total and individual symptom score AUCs were significantly greater for episodes that were treated with OCSs compared with those for episodes not treated with OCSs (Fig 1, Table IV, and see Fig E1 in this article’s Online Repository at www.jacionline.org).
FIG 1.
Comparison of total symptom scores in the AIMS cohort between episodes that were or were not treated with OCSs (corresponding to the comparison detailed in Table IV, without adjustment for propensity score). Figures of the secondary outcomes are available in this article’s Online Repository. Data are stratified based on episodes that met and did not meet severity criteria.
TABLE IV.
Comparison of AUCs of symptom scores between episodes that were or were not treated with OCSs (AIMS cohort)
| Outcome | Episode severity level* | No. | Episodes that were not treated with OCSs (n = 646) |
Episodes that were treated with OCSs (n = 152) |
Pvalue |
|---|---|---|---|---|---|
| AUC total symptom score | Less severe episodes | 686 | 3.9 (0.15) | 4.6 (0.29) | .01 |
| More severe episodes | 112 | 4.9 (0.32) | 5.1 (0.34) | .46 | |
| AUC cough score | Less severe episodes | 686 | 2.6 (0.10) | 3.0 (0.18) | .02 |
| More severe episodes | 112 | 3.1 (0.21) | 3.2 (0.21) | .83 | |
| AUC wheeze score | Less severe episodes | 686 | 1.4 (0.11) | 1.6 (0.18) | .05 |
| More severe episodes | 112 | 1.7 (0.25) | 2.0 (0.22) | .35 | |
| AUC trouble breathing score | Less severe episodes | 686 | 1.4 (0.10) | 1.8 (0.18) | .02 |
| More severe episodes | 112 | 1.8 (0.23) | 2.1 (0.20) | .15 | |
| AUC interference with activity score | Less severe episodes | 686 | 1.5 (0.11) | 2.3 (0.19) | <.01 |
| More severe episodes | 112 | 2.1 (0.20) | 2.1 (0.23) | .79 |
Data are stratified based on the episode’s severity: less severe episodes (n = 686) and more severe episodes (n = 112). The square root transformation was applied to all AUCs to improve the approximate normality of the AUC measurements. Data are represented as means (SEs) of the square root of the AUC. All comparisons were adjusted for maximum propensity score (natural log) along with correlation among repeated measurements.
Episode severity was defined based on a propensity score of greater than or less than 0.2.
We then performed additional sensitivity analyses to investigate the OCS treatment effect while using alternative propensity score cut points (0.18 and 0.22) to define more and less severe episodes, and found that OCS treatment did not reduce symptom severity during acute episodes (data not shown).
Confirmation of the propensity model and replication of the results in an independent cohort (MIST cohort)
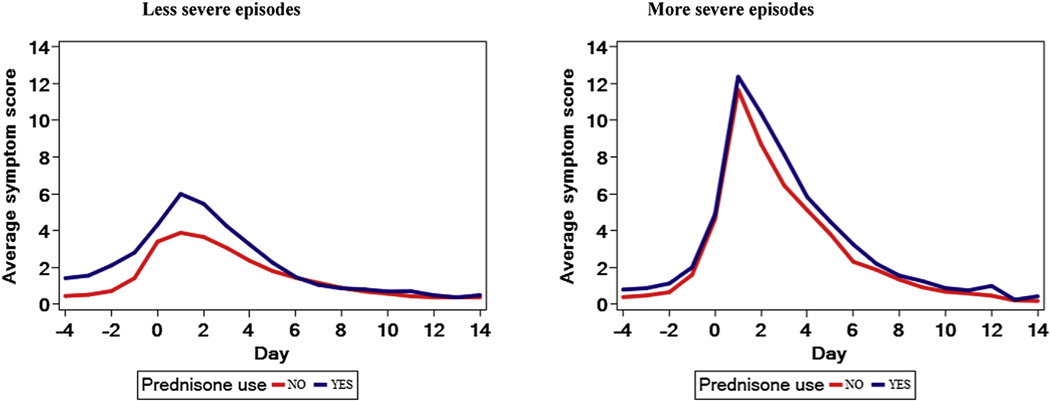
We validated the above findings using the MIST study cohort of 278 preschool children with high risk for persistence of asthma symptoms as reflected by positive mAPI results. This cohort experienced 746 acute illnesses, 161 (22%) of which were treated with OCSs. Similar to the AIMS trial analyses, indicators of pre-enrollment disease activity and the episode’s baseline severity score were significantly higher among episodes that were treated with OCSs (see Table E5 and E6, column 3, in this article’s Online Repository at www.jacionline.org). Adjustment of baseline covariates by using the propensity model developed in the AIMS trial led to elimination of these differences (see Table E5 and E6, column 4). One hundred thirty-three episodes were determined by the propensity model as more severe episodes based on their propensity score (see Table E7 in this article’s Online Repository at www.jacionline.org). Comparisons of AUC symptom scores during episodes of LRTIs that were treated with OCSs with those that were not treated with OCSs, after adjustment for the maximum propensity score for each episode, and while stratifying by episode severity level (Table V) provided qualitatively similar results to the results from the AIMS cohort. Among the more severe episodes, the AUCs of total symptom scores did not differ between episodes that were treated or were not treated with OCSs (P = .46), nor were there differences in the individual symptom score AUCs (Fig 2, Table V, and see Fig E2 in this article’s Online Repository at www.jacionline.org). Among the less severe episodes (ie, those with propensity scores <0.20), total and individual symptom score AUCs were significantly greater for episodes that were treated with OCSs compared with those of episodes not treated with OCSs (Fig 2, Table V, and see Fig E3).
TABLE V.
Comparison of AUCs of symptoms scores between episodes that received and did not receive OCSs (MIST cohort)
| Outcome | Episode severity level* | No. | Episodes that were not treated with OCSs (n = 585) |
Episodes that were treated with OCSs (n = 161) |
Pvalue |
|---|---|---|---|---|---|
| AUC total symptoms score | Less severe episodes | 613 | 4.4 (0.18) | 4.8 (0.24) | .18 |
| More severe episodes | 133 | 4.6 (0.36) | 4.9 (0.34) | .46 | |
| AUC cough score | Less severe episodes | 613 | 3.0 (0.11) | 3.2 (0.17) | .26 |
| More severe episodes | 133 | 3.1 (0.22) | 3.2 (0.22) | .83 | |
| AUC wheeze score | Less severe episodes | 613 | 1.5 (0.11) | 1.8 (0.14) | .01 |
| More severe episodes | 133 | 1.6 (0.23) | 2.1 (0.20) | .05 | |
| AUC trouble breathing score | Less severe episodes | 613 | 1.7 (0.11) | 1.9 (0.13) | .25 |
| More severe episodes | 133 | 1.9 (0.22) | 2.0 (0.19) | .76 | |
| AUC interference with activity score | Less severe episodes | 613 | 1.6 (0.11) | 1.8 (0.14) | .36 |
| More severe episodes | 133 | 1.5 (0.21) | 1.6 (0.20) | .53 |
Data are stratified based on the episode’s severity: less severe episodes (n = 613) and more severe episodes (n = 133). The square root transformation was applied to all AUCs to improve the approximate normality of the AUC measurements. Data are represented as means (SEs) of the square root of AUCs. All comparisons were adjusted for maximum propensity score (natural log) along with correlation among repeated measurements.
Episode severity was defined based on a propensity score of greater than or less than 0.2.
FIG 2.
Comparison of total symptoms scores in the MIST cohort between episodes that were or were not treated with OCSs (corresponding to the comparison detailed in Table V, without adjustment to maximal propensity). Figures of the secondary outcomes are available in this article’s Online Repository. Data are stratified based on episodes that met and did not meet severity criteria.
Effect of OCSs on the rate of resolution of respiratory symptoms
To assess the rate of resolution of respiratory symptoms in both cohorts, we calculated the time from peak of symptoms until symptoms reached pre-episode baseline levels. The baseline symptom level was defined as the mean of daily symptoms between days 14 and 7 before the beginning of the episode (ie, days−7 to −14). OCS treatment did not shorten the time to return to baseline symptoms (see Fig E3 and Table E8 in this article’s Online Repository at www.jacionline.org).
Response to OCS treatment based on atopic status of the child
Lack of response to OCSs was not modified by the presence or absence of atopic characteristics of the children, including peripheral blood eosinophilia (>4%), IgE levels (compared as median splits), positive skin test responses to aeroallergens, family history of asthma, and personal history of eczema (see Table E9 in this article’s Online Repository at www.jacionline.org).
DISCUSSION
The present post hoc analyses developed in the AIMS cohort15 and replicated in the MIST cohort16 of preschool children with severe intermittent wheezing with more than 1500 LRTIs suggest that OCSs do not reduce symptom severity during acute outpatient episodes of LRTI. These findings are strengthened by the quality of 2 well-characterized and relatively homogenous cohorts in which data were recorded daily and prospectively by parents and the use of well-defined protocol-driven criteria for the initiation of OCSs by a study physician.15,16 Furthermore, subgroups of children who might have been more likely to experience greater response to OCSs, such as those with asthma risk factors (positive mAPI result, personal eczema, and/or family history of asthma) did not appear to have greater benefit than those without such factors.
RCTs of the efficacy of OCSs in preschool children are few and report conflicting findings.10–12,26,27 Limitations of these studies include inclusion of multiple wheezing phenotypes,12,27 relatively small sample sizes and poor adherence to study medication and protocol in the outpatient studies,11,27 and episodes of relatively mild severity in both outpatient11,27 and inpatient12 studies. As such, we attempted to learn more about the role of OCSs in the treatment of LRTIs poorly responsive to short-acting β-agonists. We stratified our outcome measurements based on the severity of the episodes and chose the more severe episodes for primary comparisons because these episodes are more clinically relevant. This stratification also prevents a “dilution effect” by the less severe episodes since there is less opportunity to discern a treatment effect in the less severe episodes given that the more severe episodes accounted for only 13% of the total number of episodes. In contrast to the lack of an effect of OCSs among the more severe episodes, symptom score AUCs among the less severe episodes revealed statistically significant yet paradoxically greater values among episodes that were treated with OCSs, even after adjustment for baseline severity. OCSs were prescribed by a study physician based on predefined criteria, most often over the telephone, based on the parents’ descriptions of signs and symptoms during the episodes. However, we cannot exclude that parents who perceived their child’s symptoms as more severe provided clinical information in a way that prompted treatment with OCSs. We speculate that the higher symptom scores among the less severe episodes during which OCSs were used might be related to greater parental perception and reporting of symptom severity to the study physician, which prompted OCS treatment for episodes that might have been less severe than the parental description. In contrast, the more severe episodes consist of a more homogeneous group of episodes with a comparable level of symptoms in which parents’ perceptions might play less of a role in the decision to initiate OCS treatment.
Our findings are applicable only to the population that we investigated: preschool children with severe intermittent asthma who wheeze during viral upper respiratory tract infections, but are minimally symptomatic between these episodes. These findings are not applicable to older children or to preschool children with characteristics of persistent asthma.3 In addition, our findings apply to outpatient episodes and should not be extrapolated to episodes that require urgent, emergency, or hospital-based care. Indeed, 2 previous studies performed in the emergency department setting suggest some beneficial effects of OCSs in this age group.10,26 It might be that OCSs are an effective treatment in episodes with higher severity requiring emergency department visits; however, a recent, well-designed, randomized control trial revealed that OCSs were not superior to placebo in decreasing the duration of hospitalization in a heterogeneous group of preschool children hospitalized with wheezing.12
Previous studies have also demonstrated lack of efficacy of OCSs for severe wheezing exacerbations in preschool children.11,12 In contrast, studies in older children and adults consistently demonstrate clear benefits for OCS treatment in the setting of acute asthma.6–9 It is possible to speculate that the lack of an OCS effect in our analyses might have resulted from the study of a distinct preschool wheezing phenotype represented by intermittent wheezing that is usually induced by viral upper respiratory tract infections separated by symptom-free intervals1 rather than persistent asthma. Viral upper respiratory tract infections might be associated with neutrophilic airway inflammation,28–30 a pattern of inflammation that has been demonstrated to be less responsive to the therapeutic effects of corticosteroids.31 It is possible that a subgroup of patients with the phenotype of severe recurrent wheezing might have underlined eosinophilic airway inflammation either chronically, as demonstrated by Saglani et al32 in a highly selected group of 16 preschool children who experienced recurrent wheezing episodes unresponsive to ICSs, or acutely, which might benefit from OCS treatment. However, our sensitivity analyses did not suggest a differential response based on other features associated with allergic airway inflammation, including peripheral blood eosinophilia, increased serum IgE levels, allergic sensitization, or family history of atopy.
Although daily ICS treatment results in a significant reduction in the likelihood of exacerbations requiring OCSs,4,33 this approach does not prevent all wheezing episodes.4 The apparent disconnect in the effects of ICS treatment, either chronically4 or acutely,34 on episode prevention and our finding of an absence of the effect of OCSs on episode resolution might be a result of diminished corticosteroid responsiveness of episodes, which progress despite ICS administration, possibly because of corticosteroid-insensitive neutrophil-dominant airway inflammation; thus such episodes might not improve with the addition of systemic corticosteroids. The efficacy of OCSs might be dependent on the timing of OCS administration, and although we cannot exclude the possibility that earlier treatment with OCSs might have yielded a different (ie, a beneficial) effect, the lack of effect of parent-initiated OCS treatment at the first sign of an upper respiratory tract infection11 does not support this possibility.
A limitation of our study is that the original clinical trials were not designed to investigate the effect of OCS treatment. Overall, RCTs are the gold standard for interventional studies because the random treatment allocation minimizes the likelihood that treatment status will be confounded by baseline characteristics. In contrast, treatment “allocation” in retrospective analysis could be influenced by a variety of factors, including patients’ characteristics. This was indeed the case in our post hoc analyses: episodes that were treated with OCSs were associated with, on average, greater disease activity before enrollment, along with more severe respiratory symptoms at the beginning of the episode, compared with episodes that were not treated with OCSs. We used the well-established propensity model methodology23–25 to achieve a state of semi-randomization for OCS treatment in an effort to overcome this indication bias. This methodology is robust and widely implemented in nonrandomized studies.35 As recommended by the biostatistics literature,23,25 we verified that the model actually created a state of semi-randomization, as reflected by an effective adjustment of differences in baseline covariates between episodes that were or were not treated with OCSs. Moreover, the model was confirmed and the results were replicated in a second independent cohort. To adjust for a potential effect of study treatments on these analyses, all outcome measurements were adjusted for study treatment as part of the propensity model. Although our analyses have included adjustment for numerous disease- and episode-related factors, we cannot exclude the presence of unknown and unadjusted residual biases based on the post hoc nature of this study, nor can we definitely exclude the possibility that although OCSs did not lead to improvement in symptom scores, the initiation of an OCS might have stabilized episodes and prevented them from getting worse. Finally, the OCS regimen used in these 2 trials consisted of 4 days of OCSs.4,15,16,19–21 It is possible that longer courses (≥5 days) or higher doses of OCSs or earlier timing of initiation of treatment would have performed differently.
Overall, these findings suggest that the addition of OCSs during outpatient care of clinically significant RTIs in preschool children with severe intermittent wheezing, despite use of an asthma controller medication during most episodes, does not reduce episode severity, as measured by symptom scores. Although the immediate effect of these findings on clinical care and asthma guidelines for the treatment of acute LRTIs in preschool-aged children is tempered by the limitations inherent in post hoc analyses, these findings should serve as a strong impetus to conduct rigorous prospective RCTs to definitively establish the role of OCSs in reducing illness burden in preschool children with frequent wheeze.
Supplementary Material
Acknowledgments
Supported by grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, and 5U10HL064313 from the National Heart, Lung, and Blood Institute. Supported in part by Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences subaward KL2 TR000450 and by Colorado CTSA grant 1 UL1RR025780 from the National Center for Research Resources (NCRR)/National Institutes of Health (NIH). This study was carried out in part in the General Clinical Research Centers at Washington University School of Medicine (M01 RR00036), at National Jewish Health (M01 RR00051), and at the University of New Mexico (5M01 RR00997).
Abbreviations used
- AIMS
Acute Management Intervention Strategies
- AUC
Area under the curve
- ICS
Inhaled corticosteroid
- LRTI
Lower respiratory tract illnesses
- mAPI
Modified Asthma Predictive Index
- MIST
Maintenance and Intermittent Inhaled Corticosteroids in Wheezing Toddlers
- OCS
Oral corticosteroid
- RCT
Randomized controlled trial
- RTI
Respiratory tract illness
Footnotes
Disclosure of potential conflict of interest: A. Beigelman has received research support from the National Heart, Lung, and Blood Institute (NHLBI)/Washington University School of Medicine, an American College of Allergy, Asthma & Immunology (ACAAI) Young Investigator Award, a KL2 Award, and Washington University’s ICTS award and is employed by Washington University School of Medicine. T. S. King has received research support from the NHLBI. D. Mauger has received consultancy fees from GlaxoSmithKline and Sunovion. R. S. Zeiger has received research and travel support from the NHLBI; is on the GlaxoSmithKline pediatric steering committee; has received consultancy fees from AstraZeneca, Genentech, Sunovion, GlaxoSmithKline, MedImmune, Schering-Plough, Novartis, and NHLBI/ Penn State; and has received research support from Genentech, GlaxoSmithKline, Merck, Aerocrine, and MedImmune. R. C. Strunk has received research support from the NHLBI and has received consultancy fees from GlaxoSmithKline, Novartis, Merck, and AstraZeneca for work on various steering committees. H. W. Kelly has received research support from the NHLBI and has received consultancy fees from Novartis, Merck, GlaxoSmithKline, and AstraZeneca. F. D. Martinez has received consultancy fees from MedImmune; has received research support from the National Institutes of Health (NIH); and has received lecture fees and travel support from Abbott and Merck. R. F. Lemanske has received travel support and fees for participation in review activities from the NIH; has received consultancy fees from Merck, Sepracor, SA Boney and Associates, GlaxoSmithKline, the American Institute of Research, Genentech, and Double Helix Development; is employed by the University of Wisconsin School of Medicine and Public Health; has received research support from the NHLBI and Pharmaxis; has received lecture fees from the Michigan Public Health Institute, Allegheny General Hospital, the American Academy of Pediatrics, West Allegheny Health Systems, California Chapter 4 AAP, the Colorado Allergy Society, the Pennsylvania Allergy and Asthma Association, Harvard Pilgrim Health, the California Society of Allergy, the NYC Allergy Society, the World Allergy Organization, and the American College of Chest Physicians; has received payment for manuscript preparation from the American Academy of Allergy, Asthma & Immunology (AAAAI); and receives royalties from Elsevier and UpToDate. D. J. Jackson has received research support from the National Institutes of Health, AAAAI/ GlaxoSmithKline, and Pharmaxis and has received consultancy fees from Gilead. T. Guilbert has received research and travel support as well as fees for review activities from the NIH; is on the American Board of Pediatrics Pediatric Pulmonary Subboard; has received consultancy fees from MedImmune, Teva, MAP Pharmaceuticals, and GlaxoSmithKline; has received research support from the Centers for Disease Control and Prevention (CDC), the US Department of Health and Human Services (DHHS), Altus Pharmaceuticals, Inspire Pharmaceuticals, the NIH, UW Medical and Education Research Committee, Abbott Laboratories, Array Biopharma, Teva, Mylan, Forest Research Institute, Hoffman-LaRoche, GlaxoSmithKline and Development Limited, and MedImmune; has received lecture fees from Merck/Schering-Plough; receives royalties from UpToDate; and has received payment for the development of educational presentations from Teva. R. Covar has received consultancy fees from United Biosource and Merck and has received research support from GlaxoSmithKline. L. B. Bacharier has received research support from the NHLBI/NIH Childhood Asthma Research and Education Network; has received consultancy fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, Schering, and Cephalon; and has received lecture fees from Aerocrine, AstraZeneca, Genentech, GlaxoSmithKline, Merck, and Schering-Plough. K. Rivera-Spoljaric declares that she has no relevant conflicts of interest.
Clinical implications: The results of these post hoc analyses suggest that OCS treatment might not reduce symptom severity during acute LRTIs in preschool children with recurrent wheeze.
REFERENCES
- 1.Bacharier LB, Phillips BR, Bloomberg GR, Zeiger RS, Paul IM, Krawiec M, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–610. doi: 10.1016/j.jaci.2006.12.607. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24. [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program. Expert panel report III: guidelines for the diagnosis and management of asthma. Bethesda (MD): US Department of Health and Human Services; 2007. [Google Scholar]
- 4.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma (GINA) [Accessed February 13, 2013]; Available at: http://www.ginasthma.org.
- 6.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001(1) doi: 10.1002/14651858.CD002178. CD002178. [DOI] [PubMed] [Google Scholar]
- 7.Rowe BH, Edmonds ML, Spooner CH, Diner B, Camargo CA., Jr Corticosteroid therapy for acute asthma. Respir Med. 2004;98:275–284. doi: 10.1016/j.rmed.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Rachelefsky G. Treating exacerbations of asthma in children: the role of systemic corticosteroids. Pediatrics. 2003;112:382–397. doi: 10.1542/peds.112.2.382. [DOI] [PubMed] [Google Scholar]
- 9.Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007(3) doi: 10.1002/14651858.CD000195.pub2. CD000195. [DOI] [PubMed] [Google Scholar]
- 10.Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356. [PubMed] [Google Scholar]
- 11.Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362:1433–1438. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 12.Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 13.Bush A. Practice imperfect—treatment for wheezing in preschoolers. N Engl J Med. 2009;360:409–410. doi: 10.1056/NEJMe0808951. [DOI] [PubMed] [Google Scholar]
- 14.Grigg J. Role of systemic steroids in acute preschool wheeze. Arch Dis Child. 2010;95:491–492. doi: 10.1136/adc.2009.160994. [DOI] [PubMed] [Google Scholar]
- 15.Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, Jr, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122:1127–1135. doi: 10.1016/j.jaci.2008.09.029. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiger RS, Mauger D, Bacharier LB, Guilbert TW, Martinez FD, Lemanske RF, Jr, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilbert TW, Morgan WJ, Krawiec M, Lemanske RF, Jr, Sorkness C, Szefler SJ, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 19.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Lemanske RF, Jr, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santanello NC, Demuro-Mercon C, Davies G, Ostrom N, Noonan M, Rooklin A, et al. Validation of a pediatric asthma caregiver diary. J Allergy Clin Immunol. 2000;106:861–866. doi: 10.1067/mai.2000.110478. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulley SB, Browner WS, Grady DG, Newman TB. Designing clinical research. Philadelphia: Lippincott Williams & Wilkins; 2007. Propensity scores; pp. 139–140. [Google Scholar]
- 25.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Csonka P, Kaila M, Laippala P, Iso-Mustajarvi M, Vesikari T, Ashorn P. Oral prednisolone in the acute management of children age 6 to 35 months with viral respiratory infection-induced lower airway disease: a randomized, placebo-controlled trial. J Pediatr. 2003;143:725–730. doi: 10.1067/S0022-3476(03)00498-0. [DOI] [PubMed] [Google Scholar]
- 27.Grant C, Duggan A, DeAngelis C. Independent parental administration of prednisone in acute asthma: A double-blind, placebo-controlled, crossover study. Pediatrics. 1995;96:224–229. [PubMed] [Google Scholar]
- 28.Message SD, Johnston SL. Viruses in asthma. Br Med Bull. 2002;61:29–43. doi: 10.1093/bmb/61.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13:386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 31.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161:769–774. doi: 10.1164/ajrccm.161.3.9809071. [DOI] [PubMed] [Google Scholar]
- 32.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 33.Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with metaanalysis. Pediatrics. 2009;123:e519–e525. doi: 10.1542/peds.2008-2867. [DOI] [PubMed] [Google Scholar]
- 34.Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–353. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 35.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.