Abstract
Aims
Cardiovascular disease is the leading cause of death for individuals diagnosed with type II diabetes mellitus (DM). Changes in cardiac function, left ventricular wall thickness and fibrosis have all been described in patients and animal models of diabetes; however, the factors mediating increased matrix deposition remain unclear. The goal of this study was to evaluate whether cardiac fibroblast function is altered in a rat model of type II DM.
Main methods
Cardiac fibroblasts were isolated from 14 week old Zucker diabetic and lean control (LC) adult male rat hearts. Fibroblasts were examined for their ability to remodel 3-dimensional collagen matrices, their adhesion, migration and proliferation on collagen and changes in gene expression associated with collagen remodeling.
Key findings
Cardiac fibroblasts from diabetic animals demonstrated significantly greater ability to contract 3-dimensional collagen matrices compared to cardiac fibroblasts from LC animals. The enhanced contractile behavior was associated with an increase in diabetic fibroblast proliferation and elevated expression of α-smooth muscle actin and type I collagen, suggesting the transformation of diabetic fibroblasts into a myofibroblast phenotype.
Significance
Cardiac fibrosis is a common complication in diabetic cardiomyopathy which may contribute to the observed cardiac dysfunction associated with this disease. Identifying and understanding the changes in fibroblast behavior which contribute to the increased deposition of collagen and other matrix proteins may provide novel therapeutic targets for reducing the devastating effects of diabetes on the heart.
Keywords: Heart, Fibroblast, Extracellular matrix, Collagen, Diabetes
Introduction
In 2010 it was estimated that 1.9 million individuals ≥20 years of age were newly diagnosed with diabetes mellitus (DM) (CDC, 2011). The overwhelming majority of new DM cases, between 90 and 95%, are classified as type II diabetes. Type II diabetes, also known as adult onset diabetes, carries significant consequences for cardiac function. One major complication of diabetes is fibrosis, the excessive deposition of extracellular matrix (ECM) within tissues. Examination of human cardiac tissue from individuals with type II DM but no other cardiac disease risk factors (i.e. hypertension, coronary artery disease) demonstrated a significant increase in interstitial fibrosis compared to non-diabetic controls and revealed increased levels of a number of different collagens, including types I and III; however, only levels of type III collagen were significantly different between the two groups (Shimizu et al., 1993). Multiple animal models of type II DM also show similar changes in the cardiac ECM. A study using the Otsuka Long Evans Tokushima Fatty (OLETF) rat type II DM model found myocardial fibrosis and significant increases in collagen content in the pre-diabetic state (Mizushige et al., 2000). Additionally, a recent study in the Zucker Diabetic Fatty rat model by Baynes and Murray (2009) demonstrated that even after being diabetic for only 6 weeks (e.g. 16 weeks of age) significant changes in left ventricular (LV) chamber morphology and cardiac function were evident. While it is likely that increased fibrosis contributes to some of the observed functional changes in the diabetic myocardium, the mechanism behind diabetes-induced fibrosis is unclear.
Within the heart there are four resident cells types and of these the cardiac fibroblast, the most numerous cell type in the rat heart (Banerjee et al., 2007), is responsible for the production and maintenance of the cardiac ECM (Camelliti et al., 2005). Under physiological conditions, regulation of ECM homeostasis is a complex balance between biochemical factors, cell–matrix communication and mechanical forces. Under pathological situations, cardiac fibroblasts differentiate into myofibroblasts, sometimes referred to as “activated” fibroblasts, which are characterized by elevated expression of α-smooth muscle actin, enhanced contractile abilities, increased motility and collagen production (Porter and Turner, 2009; Brown et al., 2005; Gabbiani, 2003). While a number of studies have documented the accumulation of ECM, particularly collagens, in type II diabetic hearts, few studies have examined the effects of diabetes on cardiac fibroblasts.
The objective of this study was to analyze the cellular differences between normal and diabetic cardiac fibroblasts exploring the hypothesis that prolonged exposure to hyperglycemia in vivo alters cardiac fibroblast phenotype and function compared to fibroblasts isolated from a normal in vivo glycemic environment. By comparing collagen remodeling behavior using an in vitro 3D collagen contraction assay, changes in gene expression associated with cellular phenotype and differences in cellular function (adhesion, proliferation and migration) this study demonstrates that type II DM contributes to a cardiac myofibroblast phenotype.
Materials and methods
Cell isolation and culture
Lean control (LC; fa/) and Zucker Diabetic (ZDF; fa/fa) adult male rats were placed on a high fat diet (Purina 5008; Charles River, Charleston, SC) at 6 weeks of age with development of diabetes observed when blood glucose levels were greater than 300 mg/dl (~ 10 weeks of age). At 14 weeks of age, after exhibiting diabetes for 4 weeks, the animals were euthanized, hearts removed and cardiac fibroblasts isolated by enzymatic digestion using Liberase 3 (Roche Applied Sciences, Indianapolis, IN; now Liberase TM) as previously described (Stewart et al., 2010). Briefly, excised hearts were rinsed in sterile saline, minced, tissue transferred to 37 °C Liberase in Krebs–Henseleit buffer and incubated in a shaking water bath. Cells were collected by centrifugation and plated into flasks containing High Glucose Dulbecco'sModified Eagles Medium (DMEM) supplemented with 10% newborn calf serum, 5% fetal calf serum, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 10 µg/ml gentamicin (Life Sciences, Grand Island NY). All cells were used between passages 2 and 4 since previous studies have shown that cardiac fibroblasts maintain their phenotype in culture until at least passage 6 (Burgess et al., 2002). Previous studies have indicated that fibroblasts, when plated at low cell density under standard culture conditions, can differentiate into myofibroblasts (Swaney et al., 2005). To avoid this, all fibroblasts cultures were maintained at relatively high cell density (> 80% confluent). All cell assays described below were carried out using fibroblasts isolated from individual animals and all chemicals were purchased from Sigma Chemical Company (Saint Louis, MO) unless otherwise noted.
Collagen gel contraction
To assess the remodeling capacity of diabetic and non-diabetic fibroblasts, a 3-dimensional collagen gel contraction assay was used. This assay has been used to address a variety of factors which affect the remodeling behavior of fibroblasts (Wilson et al., 2011; Stewart et al., 2010; Sisco et al., 2008; Carver et al., 1995). Fibroblasts were removed from culture plates by incubation with trypsin/EDTA (0.25% trypsin, 1 mM ethylenediaminetetraacetic acid) and counted. A neutral collagen solution was prepared by combining bovine type I collagen (Advanced BioMatrix, Inc., San Diego, CA), 0.2 N 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid pH 9.0 and 10X alpha minimal essential medium (αMEM) on ice in a 8:1:1 volume ratio. Two hundred thousand fibroblasts were added to the neutral collagen solution and 1 ml of cell-gel solution was added to each well of a 24 well plate. The gels were allowed to polymerize for 1 h at 37 °C. After polymerization, media was added to each well and the gels were physically dissociated from the wells so that they were free-floating in the media. Gels were photographed at this time (t0) using a Canon EOS Rebel digital camera and at subsequent time intervals. Gel area was measured using ImageJ (National Institutes of Health, Bethesda, MD).
Fibroblast adhesion and migration assays
To characterize changes in cell behavior between diabetic and LC fibroblasts, cell adhesion and migration assays were carried out. For adhesion assays, 96 well plates were coated overnight with either bovine serum albumin (BSA; 1 mg/ml) or bovine type I collagen (1 mg/ml). Plates were rinsed with Moscona's saline and non-specific attachment blocked by treatment with 10 mg/ml BSA. To each coated well, 50,000 fibroblasts were added and a standard curve of fibroblasts ranging from 10,000–150,000 cells was also prepared. Cells were incubated for 1 h at 37 °C to allow time for adhesion and plates were rinsed with Moscona's to remove any non-attached cells. Attached cells were fixed with 5% glutaraldehyde followed by staining with 0.1% crystal violet and detection by absorbance at 570 nm.
Fibroblast migratory ability was examined using a scratch assay (Stewart et al., 2010; Liang et al., 2007). Wells in a 6-well plate were coated with 1 mg/ml bovine type I collagen, rinsed with Moscona's to remove unbound collagen then seeded with 500,000 fibroblasts, LC or Diabetic, and incubated until cells reached confluency. A scratch was made in the middle of each well using a 1000 µl pipet tip and wells were immediately washed with Moscona's to remove detached cells and culture media added. The scratched area was photographed at this time (t0) and at 4, 8, 12, 24 and 48 h with an Infinity 1 digital camera (Media Cybernetics, Silver Spring, MD). Migration of fibroblasts into the empty scratch was determined using ImageJ.
Fibroblast proliferation assay
Incorporation of BrdU was used to compare Diabetic and LC fibroblast proliferation. Two-well permanox chamber slides (Electron Microscopy Sciences, Hatfield, PA) were coated with bovine type I collagen (1 mg/ml) and non-coated slides were prepared as controls. Chamber slides were seeded with diabetic or LC fibroblasts at a final cell density of 5 × 104 cells per well and maintained in media for 24 h prior to the addition of BrdU. BrdU labeling solution (Roche) was added to culture media for the last 3 h of culture. After incubating in the labeling solution, fibroblasts were washed in Mosconas saline buffer and fixed using a 1:3 50 mM glycine and absolute ethanol solution (vol: vol ratio). Slides were rinsed and BrdU incorporation detected by immunofluorescence using a mouse anti-BrdU primary antibody (1:10) and anti-mouse IgG fluorescein-conjugated secondary antibody (1:10). All nuclei were visualized by staining with DAPI (5 µg/ml). BrdU and DAPI positive cells were visualized on a 510LSM confocal laser scanning microscope (Carl Zeiss, Thornwood, NY) with a 20X Achroplan water objective and the percentage of proliferating fibroblasts calculated. For each of three independent experiments five random image fields were analyzed per treatment with the number of BrdU positive cells and the total number of cells, evidenced by DAPI staining, counted.
RNA isolation and real time PCR
To quantify changes in gene expression, RNA was isolated from fibroblast-populated collagen gels using the TriZol Reagent (Invitrogen, Carlsbad, CA) and RNeasy Mini kit (Qiagen, Valencia, CA) as per manufacturer's recommendations. For real time PCR analysis, 100 ng of RNA was reverse-transcribed using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). Real time PCR was carried out using iQ SYBR Green supermix (Bio-Rad) and rat primers for the detection of alpha-smooth muscle actin (α-SMA; 5′-GGAGTGATGGTTGGAATGG-3′ and 5′-ATGATGCCGTGTTCTATCG-3′), type I collagen (5′-GCGAAGGCAACAGTCGATTC-3′ and 5′-CCCAAGTTCCGGTGTGACTC-3′), type III collagen (5′-TGATGATGAGCCACTAGACTG-3′ and 5′-CAGGAGCAGGTGTAGAAGG-3′) and acidic ribosomal binding protein (ARBP; 5′-TAGAGGGTGTCCGCAATG-3′ and 5′-GAAGGTGTAGTCAGTCTCC-3′) as the control gene. All primers were purchased from Integrated DNA Technologies (Coralville, IA). Resulting data was analyzed using the REST2009 program (Qiagen).
Quantification of collagen in tissue sections
Tissue was fixed in 2% paraformaldehyde, embedded in paraffin and sectioned at 5 µm for histological analysis. One section per rat with four rats per group (LC or Diabetic) was analyzed. Sections cut transversely through the left ventricle were stained with picrosirius red (Sigma, St. Louis, MO; Law et al., 2012) to visualize fibrillar collagen. For each section, fifteen random fields within the left ventricle were imaged, excluding perivascular regions, and the area in each image positive for collagen staining was determined using ImageJ software.
Statistical analysis
For each experiment, independent experiments were conducted using fibroblasts isolated from three or four different animals depending upon the assay. Student's T-test or Analysis of variance (ANOVA; Microsoft Excel) followed by Tukey's method of multiple comparisons using Minitab (Minitab Inc., State College, PA) was used to determine significant differences, defined as p<0.05, between experimental groups. Error bars represent±standard error of the mean.
Results
Diabetic fibroblasts exhibit greater capacity for 3D matrix remodeling
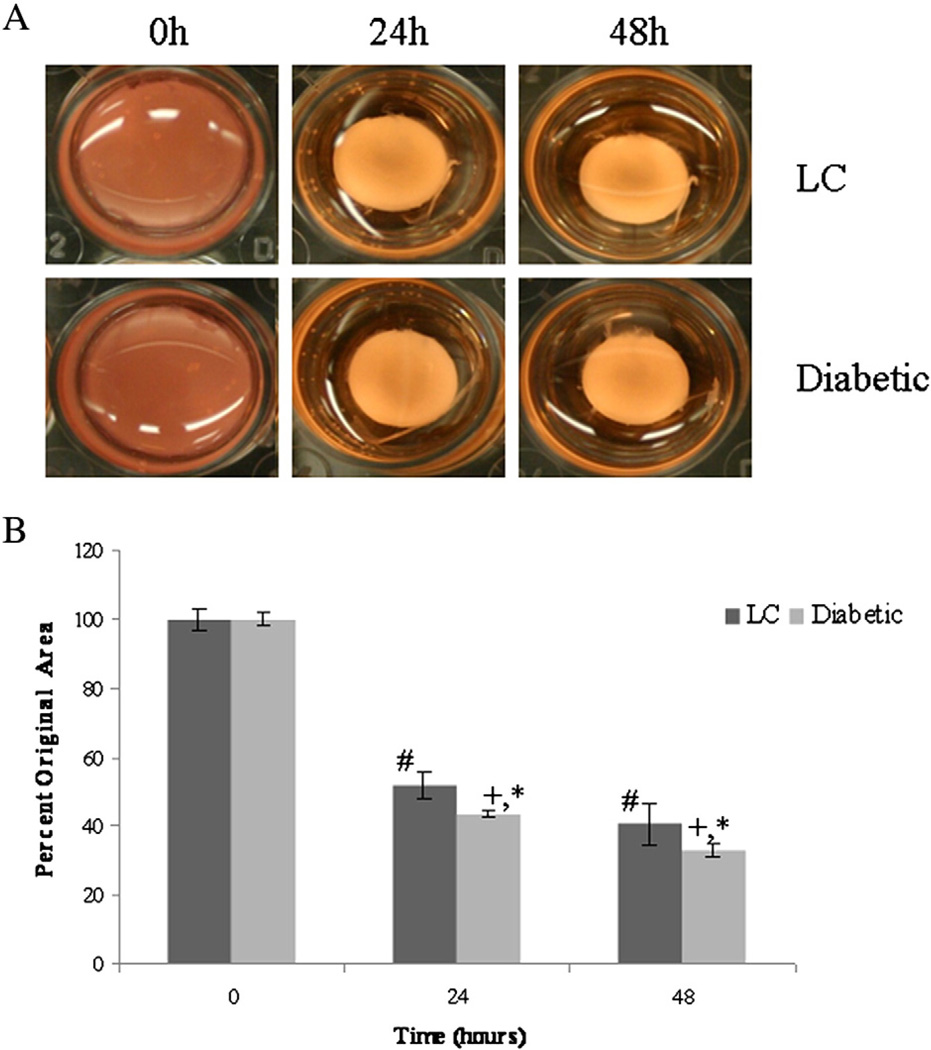
To examine the effect that diabetes has on the ability of cardiac fibroblasts to remodel a collagen matrix, fibroblasts isolated from LC and diabetic animals were embedded in 3-dimensional collagen gels and their ability to decrease gel diameter was measured. Both diabetic and LC fibroblasts demonstrated a significant ability to contract collagen gels over time as evidenced by decreased gel diameter (Fig. 1a). However, after 24 and 48 h of contraction, diabetic fibroblasts showed an enhanced ability to contract collagen gels, 43.7±0.9% and 33.3±1.9% original diameter respectively, compared to LC fibroblasts, 52.2±3.9% and 40.7±6.1% original diameter respectively (Fig. 1b).
Fig. 1.
Diabetic fibroblasts demonstrated increased collagen remodeling activity. Using a 3D collagen gel contraction assay, diabetic fibroblasts were able to physically contract collagen gels significantly more than LC fibroblasts at the same time points (p<0.05). A) Representative images of collagen gels over the experimental time course. B) Over the time course of the study, both diabetic and LC fibroblasts displayed significant contractile properties compared to their initial starting point (denoted # for LC and + for Diabetic). In addition, Diabetic fibroblasts demonstrated significantly increased contraction compared to LC fibroblasts after 24 and 48 h (indicated by *). Statistical significant was determine using an ANOVA where significance is defined as p<0.05. Sample size was n=4 and error bars are given as standard error of the mean.
Diabetic fibroblasts exhibit normal adhesion and migration
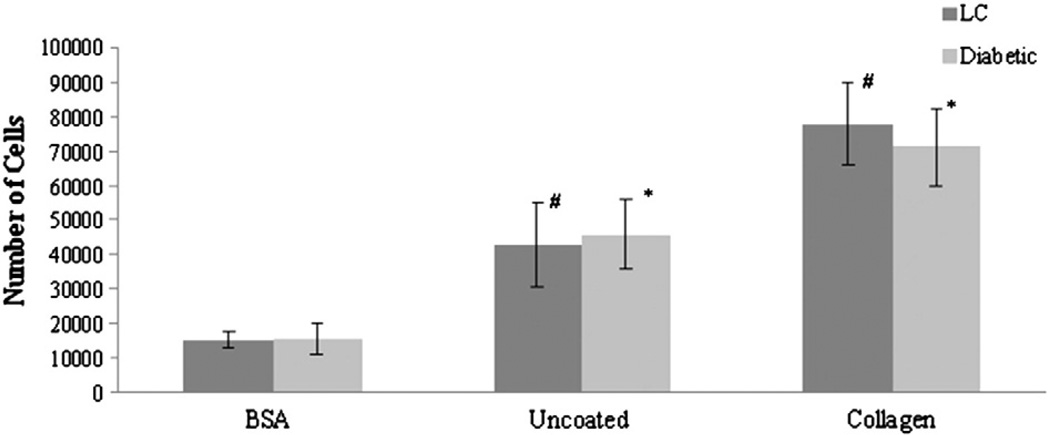
To evaluate potential alterations in attachment to collagen, adhesion assays were conducted in which LC and diabetic fibroblasts were plated onto BSA, uncoated (UC) or collagen-coated (Col) culture plates. As shown in Fig. 2, both LC and diabetic fibroblasts had increased adhesion in the presence of collagen compared to uncoated or BSA coated wells. While the difference in the number of attached LC or diabetic fibroblasts differed significantly (p<0.01) with substrate within the respective cell types, no significant differences in adhesion were observed between LC and diabetic fibroblasts on any of the substrates examined.
Fig. 2.
Diabetes does not alter fibroblast adhesion. Diabetic and LC cardiac fibroblasts were allowed to adhere to microtiter plates coated with BSA or collagen type I or wells that were left uncoated. No significant differences (p>0.05) in adhesion between LC and diabetic fibroblasts were measured on any of the substrates tested. Within the cell populations, LC (#) and diabetic fibroblasts (*) demonstrated significantly greater adhesion (p<0.01) when plated on uncoated or collagen coated wells as compared of the respective cell type plated on BSA. Statistical significant was determine using an ANOVA where significance is defined as p<0.05. Sample size was n=4 and error bars are given as standard error of the mean.
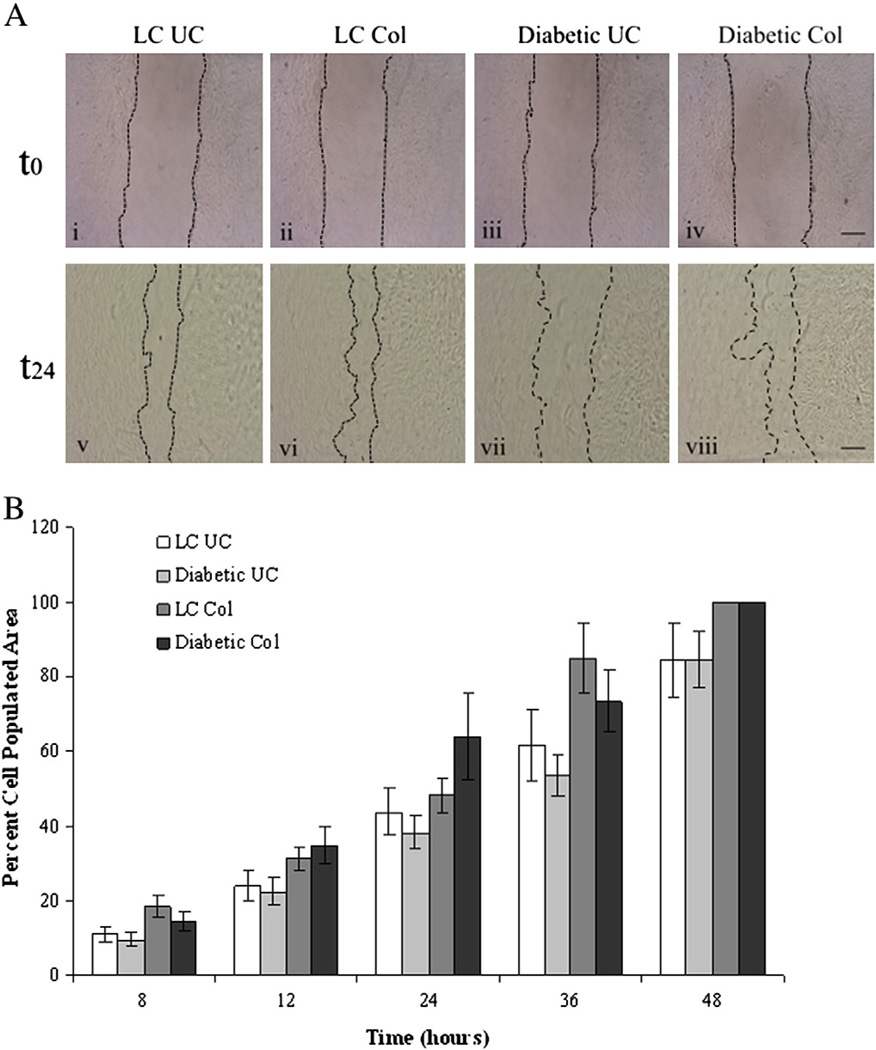
To examine potential differences in migratory ability of fibroblasts from diabetic and LC animals, fibroblasts were plated onto collagen coated and uncoated surfaces. Upon reaching confluency, a scratch was made in the cell layer and the time necessary for fibroblasts to repopulate the decellularized zone was determined. As shown in Fig. 3, both LC and diabetic fibroblasts demonstrated a significant increase (p<0.05) in the repopulation of the decellularized zone over time, indicating migration of cells into this area. In addition, LC and diabetic fibroblasts repopulated the decellularized region significantly faster when plated on collagen-coated substrates as compared to uncoated substrates (p<0.05), suggesting that these cells migrated faster when plated on collagen. By 24 h, fibroblasts plated on uncoated substrates covered less than half of the decellularized region (LC 43.8±6.2% and diabetic 38.2±4.5% area covered) where as cells plated onto collagen covered 48.3±4.6% and 63.8±11.5% for LC and diabetic fibroblasts respectively. By 48 h, both types of cells plated on collagen-coated substrates had completely repopulated the decellularized area compared with 84.4±9.9% area repopulated by LC fibroblasts and 84.6±7.5% area repopulated by diabetic fibroblasts. While substrate-dependent differences in migration were evident, no significant differences in migration between LC and diabetic fibroblasts were observed.
Fig. 3.
Fibroblast migration is unaffected by exposure to an in vivo diabetic environment. LC and diabetic fibroblasts were grown to confluency on uncoated or collagen coated wells and then a small scratch was made in the cell monolayer. Images were captured over time and compared to the original decellularized area to determine cell migration. A) Representative images of cell monolayers after initial scratch (t0) and the same sample 24 h later (t24) for LC and diabetic fibroblasts plated on uncoated (UC) or collagen-coated (Col) culture wells. Scale bar=200 µm. B) While LC and diabetic fibroblasts demonstrated significant increases (p<0.05) in cell occupied area over time, which was further enhanced on collagen coated substrates compared to uncoated substrates, no significant difference in migration was observed between LC and diabetic fibroblast migration regardless of substrate condition. Statistical significant was determine using an ANOVA where significance is defined as p<0.05. Sample size was n=3 and error bars are given as standard error of the mean. Note that no error bars are shown for the 48 h UC samples as these scratches were completely closed.
Type I collagen stimulates diabetic fibroblast proliferation
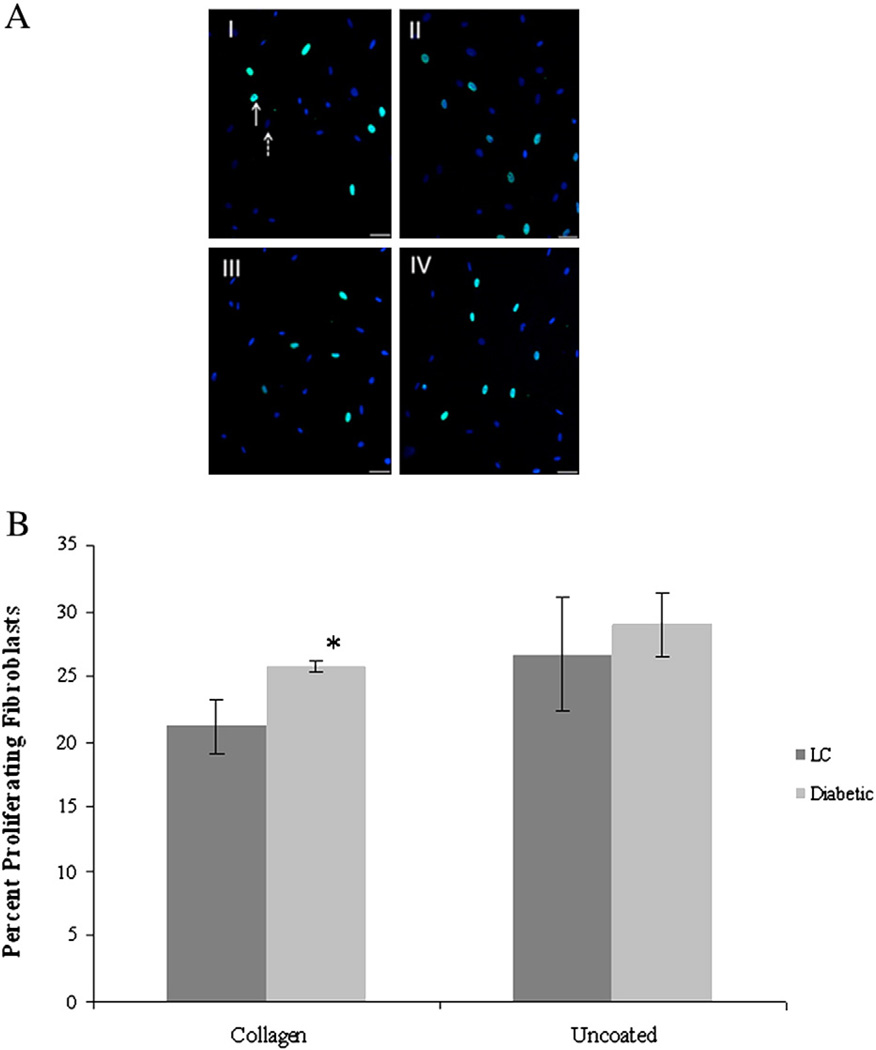
BrdU incorporation assays were used to assess the proliferative capacity of LC and diabetic fibroblasts on collagen matrices. Both cell types were cultured on either uncoated tissue culture plates or plates coated with type I collagen and 24 h later the percentage of proliferating cells was determined by dividing the number of BrdU positive cells by the total number of cells present as determined by DAPI staining (Fig. 4A). The diabetic fibroblasts showed significantly increased levels of proliferation (25.8±0.5% proliferating) when plated on type I collagen compared to LC fibroblasts (21.1±2.1% proliferating) plated on the same substrate (Fig. 4B).However, there was no measureable difference in proliferation of LC and diabetic fibroblasts plated on uncoated surfaces (Fig. 4B).
Fig. 4.
Type I collagen enhances diabetic fibroblast proliferation. Fibroblasts (LC and Diabetic) were plated on type I collagen or uncoated culture dishes and assayed for proliferation. A) Representative confocal images of BrdU (green) and DAPI (blue) showing proliferation of LC cells plated on uncoated dishes (I) or on collagen (III) and Diabetic fibroblasts on uncoated (II) or collagen coated (IV) dishes. Scale bar is 50 µm. Solid arrow indicates BrdU positive cell and dashed arrow indicates DAPI positive staining. B) While no differences were observed between the two cell types on uncoated surfaces, the presence of type I collagen resulted in a significant increase (*; p<0.05) in diabetic fibroblast proliferation compared to LC proliferation on collagen substrates. Sample size was n=3 with five random fields analyzed for each condition and error bars are given as standard error of the mean.
Diabetic fibroblasts express genes characteristic of a myofibroblast phenotype
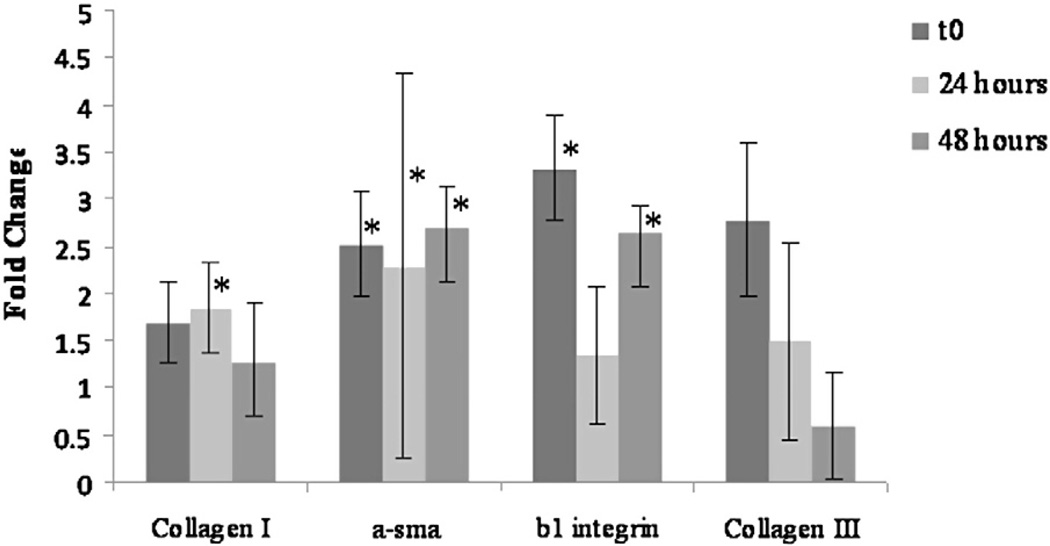
To examine the changes in gene expression associated with the observed increase in contractile behavior, RNA was isolated over time from 3D collagen gels seeded with either normal LC or diabetic fibroblasts and analyzed by real time PCR. At all time points examined, α-SMA expression (fold change T0=2.52; T24=2.28; T48=2.69) was significantly (p<0.05) increased in diabetic fibroblasts compared to normal LC fibroblasts (Fig. 5). In addition, collagen type I expression was slightly elevated in diabetic fibroblasts versus LC fibroblasts at all time points (fold change of T0=1.69, T24=1.84, T48=1.27); however, this increase was only significant (p<0.05) at the 24 hour time point. Examination of collagen type III expression demonstrated a decreasing trend in mRNA levels over time (fold change of T0=2.78, T24=1.49, T48=0.59); however, these changes were not statistically significant.
Fig. 5.
Expression of α-smooth muscle actin is increased in diabetic fibroblasts. RNA was isolated from 3D collagen gels containing either LC or diabetic fibroblasts and real-time PCR conducted to examine the expression of α-SMA, collagen types I and III and β1 integrin. Samples were normalized to acidic ribosomal binding protein (ARBP) and data expressed as fold change between diabetic and LC cultures. Statistical significance was determined using pairwise randomization test with p<0.05. α-SMA expression was significantly elevated at all time points in diabetic fibroblasts compared to control fibroblasts whereas collagen type I and β1 integrin expression levels varied over time and were only significantly different at 24 h (collagen I) or initially and 48 h later (β1 integrin). Collagen type III mRNA levels showed no significant change from LC controls. Sample size was n=3 and * indicates statistical significance compared to LC control at that time point.
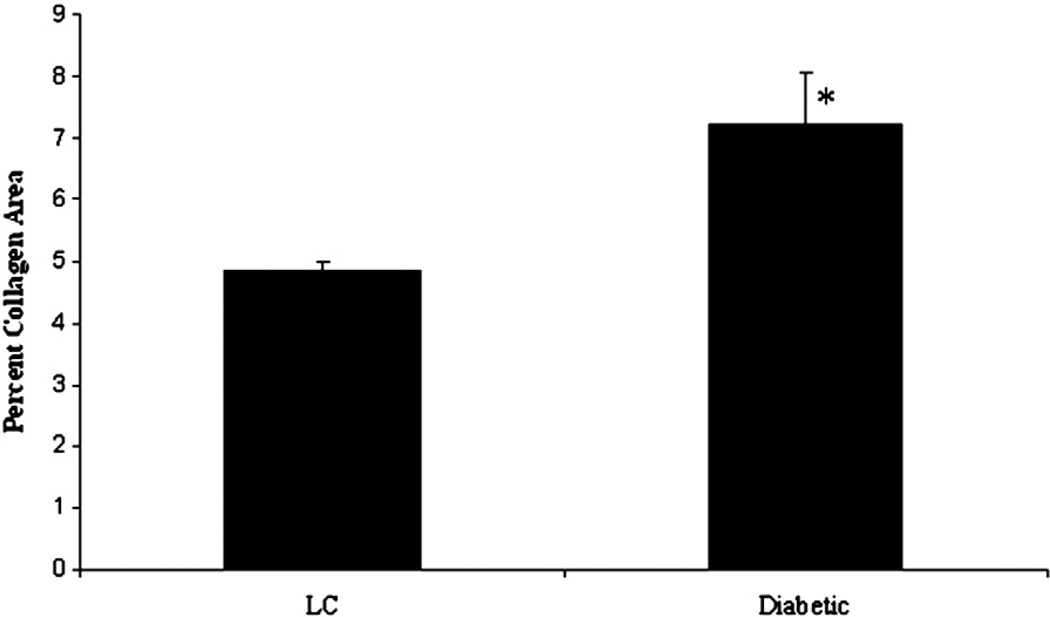
To determine if the observed in vitro changes in collagen type I gene expression were representative of the in vivo environment, sections of left ventricles (LV) from diabetic and lean control hearts were stained with picrosirius red and collagen content determined. As shown in Fig. 6, diabetic LVs showed a significant increase in ventricular area occupied by collagen (7.2%±0.81) compared to LV sections from lean control hearts (4.9%±0.14).
Fig. 6.
In vivo collagen content is increased in diabetic hearts. Sections were prepared from lean control (n=4) and diabetic (n=4) hearts and stained with picrosirius red to determine the left ventricular area occupied by collagen. Fifteen images were collected from each section and the area of collagen staining determined using ImageJ. Statistical significance was determined using a Student's T-test with p<0.05 representing a significant difference. Diabetic ventricles demonstrated a significant increase in collagen area, indicative of fibrosis, compared to lean control ventricles.
In addition to examining expression of genes associated with contractile function, expression of β1 integrin, a cell surface receptor which binds multiple extracellular matrix components including collagen, and has been previously shown to play a significant role in cardiac fibroblast mediated gel contraction (Carver et al., 1995), was examined. Expression of β1 integrin in diabetic fibroblasts was significantly increased (p<0.05) at T0 (3.33 fold increase) and T48 (2.63 fold increase) compared to normal LC fibroblasts (Fig. 5). No significant difference in β1 integrin expression was detected between diabetic and LC fibroblasts at the 24 hour time point (1.35 fold change).
Discussion
Several decades ago it was proposed that diabetes could have direct effects on the heart in the absence of confounding cardiovascular risk factors, a condition termed diabetic cardiomyopathy (Rubler et al., 1972). In addition to observed LV structural changes, fibrosis is also prevalent in type II diabetic hearts (Ban and Twigg, 2008; Aneja et al., 2008; Boudina and Abel, 2010). Work from Shimizu and colleagues examining human cardiac biopsy specimens demonstrated a significant increase in fibrosis in type II diabetic patients, with type III collagen being significantly elevated compared to the control group (Shimizu et al., 1993) and no changes in type I or type IV collagen observed. In type II DM hearts, the degree of fibrosis positively correlated with increased heart weight, and was proposed to contribute to decreased cardiac function observed in these patients (van Hoeven and Factor, 1990). Given the increased fibrosis observed in type II DM hearts and the role that fibroblasts play in the deposition of collagen, this study was designed to investigate the cellular differences between lean control and diabetic cardiac fibroblasts by comparing their collagen remodeling behavior, investigating cellular phenotype and changes in gene expression, and examining differences in cellular functions typically associated with fibroblasts.
To assess the remodeling capacity of diabetic and LC fibroblasts, cells were embedded in 3D collagen gels and allowed to physically contract the gels over a 48 hour period. The collagen gel contraction assay is a well accepted system for studying the remodeling behavior of cells and has been used to assess the role of a variety of factors, including growth factors, mechanical environment and cell surface receptors, in mediating cellular response to an in vivo like collagen environment (Dallon and Ehrlich, 2008; Baxter et al., 2008; Grinnell, 2000; Carver et al., 1995). As shown in Fig. 1, diabetic fibroblasts demonstrated an enhanced ability to contract collagen matrices compared to LC fibroblasts at both 24 and 48 h. These results are in agreement with those reported by Zhang et al. (2007). In that study, neonatal cardiac fibroblasts were cultured for 48 h in high glucose (25 mM glucose) media to mimic the hyperglycemic state associated with type II diabetes or low glucose (5.5 mM glucose) media for control cultures and their remodeling capacity evaluated. Fibroblasts exposed to high glucose conditions demonstrated a significant increase in collagen gel contraction compared to the low glucose controls. These results suggest that exposure to a diabetic environment, particularly elevated circulating glucose levels, can alter the ability of cardiac fibroblasts to remodel a collagen matrix.
To determine if exposure to a diabetic environment altered other fibroblast functions, and if any of these could contribute to the enhanced gel contraction, the ability of LC and diabetic fibroblasts to adhere, proliferate and migrate on a collagen substrate was examined. As shown in Figs. 2 and 3, while LC and diabetic fibroblasts demonstrated both an increase in adhesion and migration when plated on collagen compared to uncoated substrates, there was no significant difference between the two cell types (i.e. LC and diabetic). However, differences were observed with respect to fibroblast proliferation on collagen substrates. As shown in Fig. 4, diabetic fibroblasts demonstrated a significant increase in proliferation compared to LC fibroblasts when plated on collagen, indicating diabetic fibroblasts exhibit a pro-growth response when cultured on collagen compared to LC fibroblasts. These data are in contrast to what has previously been reported for neonatal cardiac fibroblasts treated with high glucose media. Neonatal fibroblasts demonstrated a significant decrease in both fibroblast migration and proliferation when exposed to high glucose media (Zhang et al., 2007). Other studies using dermal fibroblasts isolated from the Goto-Kakizaki (GK) rat, a spontaneous model for non-insulin dependent type II DM, demonstrated a decrease in DNA content after culture in high glucose conditions, indicative of reduced proliferation (Hehenberger et al., 1999). However, these discrepancies could be explained by the differences in the age of the cells which were used in the studies (the work presented herein examined adult cardiac fibroblasts while Zhang et al. used neonatal cardiac fibroblasts) or the tissue source of the fibroblasts (dermal versus cardiac). It is also possible, as speculated by Hehenberger et al. that other in vivo factors in type II DM may impact fibroblast function. Taken together these results indicate that the ability of fibroblasts to adhere to or migrate on collagen matrices was not a contributing factor in the altered contractile function of diabetic cardiac fibroblasts.
Remodeling of collagen matrices by cardiac fibroblasts is associated with TGFβ1 induced differentiation of fibroblasts into a myofibroblast phenotype, exhibiting enhanced contractile properties due to increased expression of α-SMA (Lijnen et al., 2003). Examination of α-SMA mRNA levels in contracted collagen gel-fibroblast (LC or diabetic) constructs revealed a significant increase in α-SMA expression in the diabetic fibroblasts compared to LC cells. These data indicate that the enhanced collagen remodeling of the diabetic fibroblasts are due to an increased expression of α-SMA, reflective of conversion to amyofibroblast phenotype. Furthermore, the observation that α-SMA expression was elevated at the T0 time point suggests that the isolated diabetic fibroblasts may contain a larger population of myofibroblasts than was present in the LC cultures, possibly a reflection of the influence of their in vivo environments. Alternatively, it has been demonstrated that in vitro culture conditions (serum concentration, rigid culture surface and cell density) can influence fibroblast differentiation into myofibroblasts (Swaney et al., 2005). Therefore the observed increase in α-SMA expression at T0 may reflect an initial response by the diabetic fibroblasts to their culture environment. However, beyond this initial point, the differences in α-SMA expression between diabetic and LC fibroblasts remain significant and are not likely due to culture environment, but changes in the cellular phenotype associated with collagen gel remodeling. Staining of heart sections from Spontaneously Diabetic Torii (SDT) rats for α-SMA and collagen revealed localization of myofibroblasts in areas with increased fibrosis (Jin et al., 2009), suggesting a role for fibroblasts/myofibroblasts in the increased collagen deposition seen in DM hearts. In our in vitro model, type I collagen mRNA expression was also elevated in gels contracted by diabetic fibroblasts and picrosirius red staining of sections from diabetic hearts demonstrated elevated collagen content compared to lean controls, recapitulating the in vivo observations of increased fibrosis observed in other studies. While no significant change in type III collagen mRNA expression was detected, it is important to note that the observed increase in collagen deposition via picrosirius red staining cannot be solely attributed to increases in collagen type I. Other in vitro studies treating non-diabetic cardiac fibroblasts with high concentrations of glucose (25 mM) have also shown increased type I collagen production (Singh et al., 2008; Zhang et al., 2007; Asbun et al., 2005), although none of these studies explored the role of the myofibroblast in this process.
While integrins have long been appreciated for their role in mediating the response of cardiac fibroblasts to changes in their extracellular environment (Ross and Borg, 2001) or facilitating collagen gel contraction (Carver et al., 1995), their role in type II DM associated fibrosis and matrix remodeling is unclear. Our data suggest that like α-SMA expression, β1 integrin is also up-regulated in diabetic fibroblasts prior to the initiation of collagen remodeling (gel contraction) and at the later stages of collagen remodeling. This observation is similar to what has been previously observed with fibroblasts cultured in high glucose media which demonstrated increased expression of α1β1 integrin (Zhang et al., 2007). In contrast to these observations, Yuen et al. reported that β1 levels were unchanged between controls and fibroblasts cultured on methyl-glyoxal modified collagen matrices (Yuen et al., 2010). Additional studies are needed to fully address the role that cardiac integrins play in mediating cellular response to diabetes.
Conclusion
The data presented herein represents one of the first examinations of the affect that a diabetic environment has on cardiac fibroblasts and clearly suggests a role for myofibroblasts in the fibrosis associated with type II DM. These results, in combination with other studies, suggest a role for the hyperglycemic diabetic environment in propagating the myofibroblast phenotype.
Acknowledgments
The authors would like to thank Cheryl Cook for her assistance with cardiac fibroblast isolation. This work was supported by grants from National Institutes of Health (HL083441 W.C.) and (HL097214 E.C.G.) and the South Carolina Center for Biomedical Research Excellence (COBRE) for Cardiovascular disease (D.B.M.).
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aneja A, Tang WHW, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: Insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Asbun J, Manso AM, Villarreal FJ. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: effects of losartan and vitamin E. Am J Physiol Heart Circ Physiol. 2005;288:H227–H234. doi: 10.1152/ajpheart.00340.2004. [DOI] [PubMed] [Google Scholar]
- Ban CR, Twigg SM. Fibrosis in diabetes complications: Pathogenic mechanisms and circulating and urinary markers. Vascul Health Risk Manage. 2008;4(3):575–596. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- Baxter SC, Morales MO, Goldsmith EC. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochem Biophys. 2008;51(1):33–44. doi: 10.1007/s12013-008-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes J, Murray DB. Cardiac and renal function are progressively impaired with aging in Zucker diabetic fatty type II diabetic rats. Oxid Med Cell Longev. 2009;2(5):328–334. doi: 10.4161/oxim.2.5.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy: causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Thearpeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc Pathol. 2002;11:78–87. doi: 10.1016/s1054-8807(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterization of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Carver W, Molano I, Reaves TA, Borg TK, Terracio L. Role of the a1 1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol. 1995;165:425–437. doi: 10.1002/jcp.1041650224. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control, Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Dallon JC, Ehrlich HP. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472–479. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast–collagen matrix contraction: Growth-factor signaling and mechanical load. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Hehenberger K, Hansson A, Heilborn JD, Abel-Haum SM, Ostensson CL, Brismar K. Impaired proliferation and increased L-lactate production of dermal fibroblasts in the GK-rat, a spontaneous model of non-insulin dependent diabetes mellitus. Wound Repair Regen. 1999;7:65–71. doi: 10.1046/j.1524-475x.1999.00065.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Takai S, Sugiyama T, Hayashi T, Fukumoto M, Oku H, et al. Long-term angiotensin II blockade may improve not only hyperglycemia but also age-associated cardiac fibrosis. J Pharmacol Sci. 2009;109:275–284. doi: 10.1254/jphs.08210fp. [DOI] [PubMed] [Google Scholar]
- Law BA, Levick SP, Carver WE. Alterations in cardiac structure and function in a murine model of chronic alcohol consumption. Microsc Microanal. 2012;18:453–461. doi: 10.1017/S1431927612000372. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta1 promotes contraction of collagen gels by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25(2):79–86. doi: 10.1358/mf.2003.25.2.723680. [DOI] [PubMed] [Google Scholar]
- Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, et al. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant pre-diabetic stage of a type II diabetic rat model. Circulation. 2000;101:899–907. doi: 10.1161/01.cir.101.8.899. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88(11):1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumarl T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Baker KM, Kumar R. Activation of the intracellular renin–angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- Sisco PN, Wilson CG, Mironova E, Baxter SC, Murphy CJ, Goldsmith EC. The effect of gold nanorods on tissue remodeling. Nano Lett. 2008;8:3409–3412. doi: 10.1021/nl802142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JA, Massey EP, Fix C, Zhu J, Goldsmith EC, Carver WE. Temporal alterations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res. 2010;340:117–126. doi: 10.1007/s00441-010-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and over-expression of adenylyl cyclase. Proc Natl Acad Sci. 2005;1:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848. [DOI] [PubMed] [Google Scholar]
- Wilson CG, Stone JW, Fowlkes V, Morales MO, Murphy CJ, Baxter SC, et al. Age-dependent expression of collagen receptors and deformation of type I collagen substrates by rat cardiac fibroblasts. Microsc Microanal. 2011;17:555–562. doi: 10.1017/S1431927611000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen A, Laschinger C, Talior I, Lee W, Chan M, Birek J, et al. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010;29:537–548. doi: 10.1016/j.matbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stewart JA, Kane ID, Massey EP, Cashatt DO, Carver WE. Effects of elevated glucose levels on interactions of cardiac fibroblasts with the extracellular matrix. In Vitro Cell Dev Biol Anim. 2007;43:297–305. doi: 10.1007/s11626-007-9052-2. [DOI] [PubMed] [Google Scholar]