Abstract
Uremic toxins are involved in a variety of symptoms in advanced chronic kidney disease. Especially, the accumulation of protein-bound uremic toxins in the blood of dialysis patients might play an important role in the development of cardiovascular disease. Serum concentration of protein-bound uremic toxins such as indoxyl sulfate, indoxyl glucuronide, indoleacetic acid, p-cresyl sulfate, p-cresyl glucuronide, phenyl sulfate, phenyl glucuronide, phenylacetic acid, phenylacetylglutamine, hippuric acid, 4-ethylphenyl sulfate, and 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF) in hemodialysis patients were simultaneously measured by liquid chromatography/tandem mass spectrometry. Serum levels of these protein-bound uremic toxins were increased in hemodialysis patients. Indoxyl sulfate, p-cresyl sulfate, and CMPF could not be removed efficiently by hemodialysis due to their high protein-binding ratios. Serum level of total indoxyl sulfate did not show any significant correlation with total p-cresyl sulfate. However, free indoxyl sulfate correlated with free p-cresyl sulfate, and reduction rate by hemodialysis of indoxyl sulfate correlated with that of p-cresyl sulfate. Serum levels of total and free indoxyl sulfate showed significantly positive correlation with those of indoxyl glucuronide, phenyl sulfate, and phenyl glucuronide. Serum levels of total and free p-cresyl sulfate showed significantly positive correlation with those of p-cresyl glucuronide, phenylacetylglutamine, and phenylacetic acid. Indoxyl sulfate and indoxyl glucuronide are produced from indole which is produced in the intestine from tryptophan by intestinal bacteria. p-Cresyl sulfate and p-cresyl glucuronide are produced from p-cresol which is produced in the intestine from tyrosine by intestinal bacteria. Thus, intestinal bacteria play an important role in the metabolism of protein-bound uremic toxins.
Keywords: indoxyl sulfate, indoxyl glucuronide, phenyl sulfate, p-cresyl sulfate, p-cresyl glucuronide, phenylacetylglutamine
UREMIC TOXINS
Uremic toxins are involved in a variety of symptoms in patients with stage-5 chronic kidney disease (CKD). More than ninety compounds have been considered to be uremic toxins.1) Uremic toxins include: 1) free water-soluble low-molecular-weight solutes with molecular weight less than 500 such as urea, 2) protein-bound solutes such as indoxyl sulfate, and 3) middle molecules with molecular weight more than 500 such as β2-microglobulin.1) Notably, protein-bound uremic toxins such as indoxyl sulfate and p-cresyl sulfate have emerged as important targets of therapeutic removal. Hemodialysis (HD) even with a high-flux membrane cannot efficiently remove the protein-bound uremic toxins because of their high albumin-binding property. The accumulation of these protein-bound uremic toxins in the blood of dialysis patients might play an important role in the development of uremic complications such as cardiovascular disease (CVD). Indoxyl sulfate is the most promising protein-bound uremic toxin as a biomarker of progression in CKD.2–5) Novel dialysis techniques or membranes should be developed to efficiently remove these protein-bound uremic toxins for the prevention and management of uremic complications.
ANALYSIS OF UREMIC TOXINS WITH LIQUID CHROMATOGRAPHY/MASS SPECTROMETRY (LC/MS)
Mass spectrometry (MS) has been successfully applied for the identification and quantification of uremic toxins.6–8) Based on MS analysis of uremic toxins, the pathogenesis of the uremic symptoms will be elucidated to prevent and manage the symptoms. LC/MS with electrospray ionization (ESI) can separate and identify highly polar, thermally labile and/or high-molecular weight mixture compounds.
Kikuchi et al.9) applied the metabolomic analysis of comprehensive small-molecular metabolites with liquid chromatography/tandem mass spectrometry (LC/MS/MS) and principal component analysis to identify uremic toxins accumulated in the serum of CKD rats. Indoxyl sulfate was demonstrated to be the first principal serum metabolite which differentiates CKD from normal, followed by phenyl sulfate, hippuric acid and p-cresyl sulfate. Then, they measured the serum levels of indoxyl sulfate, phenyl sulfate, hippuric acid and p-cresyl sulfate by the selected reaction monitoring (SRM) of LC/MS/MS, and demonstrated that these serum levels were markedly increased in CKD rats as compared with normal rats. As creatinine clearance decreased, the serum levels of the metabolites increased.
Further, Kikuchi et al.10) applied the metabolomic approach to search for uremic toxins as possible indicators of the effect of an oral sorbent AST-120. AST-120 composed of spherical porous carbon particles has superior adsorption ability for certain small-molecular-weight organic compounds known to accumulate in patients with CKD. Serum metabolites in normal and CKD rats before and after administration of AST-120 for 3 days were analyzed by LC/MS/MS and principal component analysis. Further, serum and urine levels of the indicators were quantified with SRM of LC/MS/MS. Indoxyl sulfate was the first principal serum metabolite, which could differentiate CKD from both normal and AST-120-administered CKD rats, followed by hippuric acid, phenyl sulfate and 4-ethylphenyl sulfate. CKD rats showed increased serum levels of indoxyl sulfate, hippuric acid, phenyl sulfate, 4-ethylphenyl sulfate and p-cresyl sulfate. Administration of AST-120 for 3 days to the CKD rats reduced the serum and urine levels of these metabolites. Thus, indoxyl sulfate is the best indicator of the effect of AST-120 in CKD rats. Hippuric acid, phenyl sulfate and 4-ethylphenyl sulfate are the additional indicators.
ANALYSIS OF PROTEIN-BOUND UREMIC TOXINS IN HD PATIENTS
Itoh et al.11) measured the serum levels of 12 uremic solutes in HD patients and healthy subjects. Serum levels of indoxyl sulfate, indoxyl glucuronide, indoleacetic acid, p-cresyl sulfate, p-cresyl glucuronide, phenyl sulfate, phenyl glucuronide, phenylacetic acid, phenylacetylglutamine, hippuric acid, and 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid (CMPF) except 4-ethylphenyl sulfate were significantly increased in HD patients compared with healthy subjects. All the 12 solutes showed protein-binding ratios more than 30%. Especially, indoxyl sulfate, p-cresyl sulfate, CMPF, and 4-ethylphenyl sulfate showed high protein-binding ratios (more than 95%) and low reduction rates by HD (less than 35%).
They used SRM of LC/MS/MS for the measurement of the protein-bound uremic solutes.11) Briefly, HPLC analysis of a sample (10 μL) was performed using gradient elution with a LC-10Avp LC system (Shimadzu, Kyoto, Japan) on an Atlantis dC18 column (2.1 mm×50 mm, 3 μm) (Waters, Milford, MA, USA) at 0.2 mL/min with the column maintained at 40°C and an auto-sampler maintained at 10°C. The gradient solution consisted of solvent A (5 mmol/L ammonium acetate solution) and solvent B (methanol). For negative ion (NI) mode, the elution solution was 20% B (A : B; 80 : 20, by volume) for 2 min followed by a linear gradient up to 60% B for the next 2 min and up to 95% for the next 3 min and returned to 20% B for 5 min. For positive ion (PI) mode, the elution solution was 20% B (A : B; 80 : 20, by volume) for 2 min and kept at 95% B for the next 5 min and returned to 20% B for 5 min. SRM method of LC/ESI-MS/MS was carried out using a triple quadrupole mass spectrometer (API4000, AB SCIEX, WayCarlsbad, CA, USA) equipped with an ESI source. For measurement of total serum concentration, a 20-μL serum sample of HD patients before and after HD and healthy male volunteers was diluted with 40 μL of distilled water. A 50-μL aliquot of serum was added into acetonitrile (200 μL) containing internal standard (stable-isotope-labeled compounds; indoxyl-d4 sulfate 100 ng/mL; indole-d5-3-acetic acid, 200 ng/mL; phenyl-d5-acetic acid, 100 ng/mL; hippuric acid-d5, 100 ng/mL) in Sirocco 96 well-protein precipitation plate (Waters, Milford, MA, USA), and the mixture was thoroughly mixed. The solution was filtered by applying a vacuum to remove protein precipitation, and eluents (40 μL) were diluted with 5 mmol/L ammonium acetate solution (120 μL) before LC/ESI-MS/MS analysis. For measurement of free form concentration, the protein-free fraction (25 μL) was added into 25 μL of acetonitrile containing internal standards (stable-isotope-labeled compounds) in a test tube, and the mixture was thoroughly mixed. The solution was centrifuged at 20,600 g for 10 min at 4°C, and upper layer was diluted with 5 mmol/L ammonium acetate solution (75 μL) before LC/MS/MS analysis.
CORRELATIONS BETWEEN SERUM LEVELS OF PROTEIN-BOUND UREMIC TOXINS IN HD PATIENTS
The serum levels of 12 protein-bound uremic toxins in HD patients measured by LC/MS/MS were reported previously.11) Table 1 shows Spearman rank correlations between serum levels of the uremic toxins in 19 HD patients. The etiology of end-stage renal disease in the HD patients includes chronic glomerulonephritis (n=8), diabetes mellitus (n=4), nephrosclerosis (n=3), polycystic kidney disease (n=2), bilateral nephrolithiasis followed by bilateral nephrectomy (n=1), and chronic glomerulonephritis after right renal cancer nephrectomy (n=1).11) Total indoxyl sulfate showed significantly positive correlations with total indoxyl glucuronide, total phenyl sulfate, total phenyl glucuronide, free indoxyl sulfate, free indoxyl glucuronide, and free phenyl sulfate, and showed significantly negative correlation with total indoleacetic acid. Free indoxyl sulfate showed significantly positive correlation with total indoxyl glucuronide, total p-cresyl sulfate, total p-cresyl glucuronide, total phenylacetic acid, total phenylacetylglutamine, free indoxyl glucuronide, free indoleacetic acid, free p-cresyl sulfate, free p-cresyl glucuronide, free phenyl sulfate, free phenyl glucuronide, free phenylacetic acid, free phenylacetylglutamine, and free hippuric acid.
Table 1. Spearman rank correlations between serum levels of uremic toxins in HD patients.
| T-IS | F-IS | T-PCS | F-PCS | |
|---|---|---|---|---|
| T-IS | 1.00 | — | — | — |
| T-IG | 0.67** | 0.73*** | 0.33 | 0.47* |
| T-IAA | −0.54* | −0.21 | 0.15 | 0.09 |
| T-PCS | 0.32 | 0.62** | 1.00 | — |
| T-PCG | 0.34 | 0.62** | 0.79*** | 0.81*** |
| T-PhS | 0.53* | 0.43 | 0.18 | 0.22 |
| T-PhG | 0.72* | 0.30 | −0.28 | −0.38 |
| T-PAA | 0.13 | 0.46* | 0.53* | 0.56* |
| T-PAGln | 0.42 | 0.78*** | 0.83*** | 0.92*** |
| T-HA | 0.22 | 0.41 | 0.41 | 0.44 |
| T-4EtPhS | −0.05 | 0.06 | 0.35 | 0.29 |
| T-CMPF | 0.02 | −0.15 | −0.40 | −0.36 |
| F-IS | 0.78*** | 1.00 | — | — |
| F-IG | 0.67** | 0.72*** | 0.30 | 0.44 |
| F-IAA | 0.15 | 0.59** | 0.63** | 0.71*** |
| F-PCS | 0.39 | 0.76*** | 0.95*** | 1.00 |
| F-PCG | 0.35 | 0.66** | 0.81*** | 0.85*** |
| F-PhS | 0.65** | 0.73*** | 0.43 | 0.54* |
| F-PhG | 0.38 | 0.52* | 0.31 | 0.39 |
| F-PAA | 0.23 | 0.53* | 0.59** | 0.61** |
| F-PAGln | 0.37 | 0.78*** | 0.87*** | 0.95*** |
| F-HA | 0.33 | 0.56* | 0.49* | 0.54* |
| F-4EtPhS | −0.04 | 0.27 | 0.62** | 0.63** |
| F-CMPF | 0.04 | 0.16 | 0.16 | 0.17 |
* p<0.05, ** p<0.01, *** p<0.001. T: total, F: free, total=protein-bound+free. IS: indoxyl sulfate, IG: indoxyl glucuronide, IAA: indoleacetic acid, PCS: p-cresyl sulfate, PCG: p-cresyl glucuronide, PhS: phenyl sulfate, PhG: phenyl glucuronide, PAA: phenylacetic acid, PAGln: phenylacetylglutamine, HA: hippuric acid, EtPhS: 4-ethylphenyl sulfate, CMPF: 3-carboxy-4-methyl-5-propyl-2-furanpropionic acid.
Total p-cresyl sulfate showed significantly positive correlations with total p-cresyl glucuronide, total phenylacetic acid, total phenylacetylglutamine, free indoleacetic acid, free p-cresyl sulfate, free p-cresyl glucuronide, free phenylacetic acid, free phenylacetylglutamine, free hippuric acid, and free 4-ethylphenyl sulfate. Free p-cresyl sulfate showed significantly positive correlations with total indoxyl glucuronide, total p-cresyl glucuronide, total phenylacetic acid, total phenylacetylglutamine, free indoleacetic acid, free p-cresyl glucuronide, free phenyl sulfate, free phenylacetylglutamine, free hippuric acid, and free 4-ethylphenyl sulfate.
Total CMPF showed significantly negative correlations only with free p-cresyl sulfate (−0.46, p<0.05) and free p-cresyl glucuronide (−0.49, p<0.05).
Figure 1 shows correlations between indoxyl sulfate and p-cresyl sulfate. There was no significant correlation between total indoxyl sulfate and total p-cresyl sulfate. However, free indoxyl sulfate showed significantly positive correlation with free p-cresyl sulfate. The reduction rate by HD of total indoxyl sulfate showed significantly positive correlation with that of total p-cresyl sulfate. Reduction rate, RR (%), was calculated according to the following equation: RR (%)=(Cpre−Cpost)/Cpre×100 where Cpre and Cpost are total serum concentrations before and after HD, respectively.
Fig. 1. Correlations between (A) total indoxyl sulfate (T-IS) and total p-cresyl sulfate (T-PCS), (B) free indoxyl sulfate (F-IS) and free p-cresyl sulfate (F-PCS), and (C) reduction rate of total indoxyl sulfate (RR-IS) and reduction rate of total p-cresyl sulfate (RR-PCS).
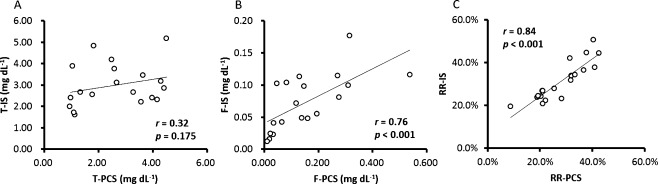
Figure 2 shows correlations between indoxyl sulfate and indoxyl glucuronide. Total and free indoxyl sulfate showed significantly positive correlations with total and free indoxyl glucuronide, respectively. The reduction rate of total indoxyl sulfate showed significantly positive correlation with that of total indoxyl glucuronide.
Fig. 2. Correlations between (A) total indoxyl sulfate (T-IS) and total indoxyl glucuronide (T-IG), (B) free indoxyl sulfate (F-IS) and free indoxyl glucuronide (F-IG), and (C) reduction rate of total indoxyl sulfate (RR-IS) and reduction rate of total indoxyl glucuronide (RR-IG).
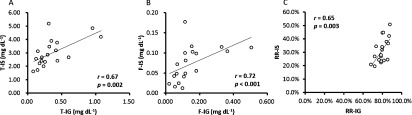
Figure 3 shows correlations between indoxyl sulfate and phenyl sulfate. Total and free indoxyl sulfate showed significantly positive correlations with total and free phenyl sulfate, respectively. The reduction rate of total indoxyl sulfate showed significantly positive correlation with that of total phenyl sulfate.
Fig. 3. Correlations between (A) total indoxyl sulfate (T-IS) and total phenyl sulfate (T-PhS), (B) free indoxyl sulfate (F-IS) and free phenyl sulfate (F-PhS), and (C) reduction rate of total indoxyl sulfate (RR-IS) and reduction rate of phenyl sulfate (RR-PhS).
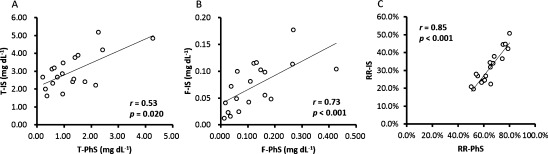
Figure 4 shows correlations between p-cresyl sulfate and p-cresyl glucuronide. Total and free p-cresyl sulfate showed significantly positive correlations with total and free p-cresyl glucuronide, respectively. The reduction rate of total p-cresyl sulfate showed significantly positive correlation with that of total p-cresyl glucuronide.
Fig. 4. Correlations between (A) total p-cresyl sulfate (T-PCS) and total p-cresyl glucuronide (T-PCG), (B) free p-cresyl sulfate (F-PCS) and free p-cresyl glucuronide (F-PCG), and (C) reduction rate of total p-cresyl sulfate (RR-PCS) and reduction rate of total p-cresyl glucuronide (RR-PCG).
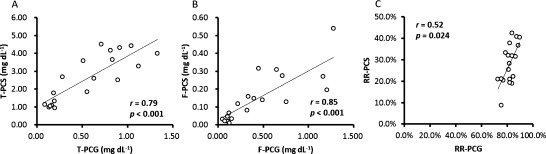
Figure 5 shows correlations between p-cresyl sulfate and phenylacetylglutamine. Total and free p-cresyl sulfate showed significantly positive correlations with total and free phenylacetylglutamine, respectively. The reduction rate of total p-cresyl sulfate showed significantly positive correlation with that of total phenylacetylglutamine.
Fig. 5. Correlations between (A) total p-cresyl sulfate (T-PCS) and total phenylacetylglutamine (T-PAGln), (B) free p-cresyl sulfate (F-PCS) and free phenylacetylglutamine (F-PAGln), and (C) reduction rate of total p-cresyl sulfate (RR-PCS) and reduction rate of total phenylacetylglutamine (RR-PAGln).
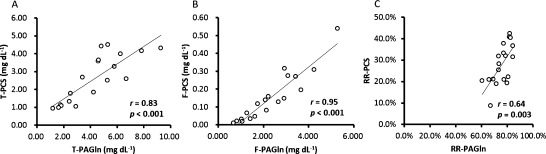
Thus, serum level of total indoxyl sulfate did not show any significant correlation with that of total p-cresyl sulfate. However, free indoxyl sulfate correlated with free p-cresyl sulfate, and reduction rate by HD of indoxyl sulfate correlated with that of p-cresyl sulfate. Serum levels of total and free indoxyl sulfate correlated with those of indoxyl glucuronide, phenyl sulfate, and phenyl glucuronide. Serum levels of total and free p-cresyl sulfate correlated with those of p-cresyl glucuronide, phenylacetylglutamine, and phenylacetic acid.
METABOLISM OF PROTEIN-BOUND UREMIC TOXINS
Aronov et al.12) has demonstrated by measuring serum levels in HD patients with or without colon that indoxyl sulfate, indoxyl glucuronide, p-cresyl sulfate, and phenyacetylglutamine were derived exclusively from colon. p-Cresyl glucuronide was also suggested to be derived from colon. However, hippuric acid, phenyl glucuronide or indoleacetic acid was not significantly derived from colon. Wikoff et al.13) reported by comparing serum levels in germ-free and conventional mice that indoxyl sulfate, p-cresyl sulfate, and phenyl sulfate were produced only in conventional mice but not in germ-free mice, demonstrating a critical role of intestinal bacteria in their production.
Indoxyl sulfate is derived from indole, which is produced in the intestine from tryptophan by intestinal bacteria.2,3) Indole is oxidized to indoxyl, and subsequently sulfated to produce indoxyl sulfate in the liver.5) Indoxyl glucuronide is also synthesized in the liver from indole through indoxyl. A high serum level of indoxyl sulfate in advanced CKD patients leads to the enhanced synthesis of indoxyl glucuronide.14) Oral sorbent (AST-120) administration suppresses the serum and urine levels of indoxyl sulfate and indoxyl glucuronide in the advanced CKD patients by adsorbing indole in the intestine.4,14) Indoxyl sulfate cannot be removed efficiently by HD because of its high albumin binding ratio.11) However, serum indoxyl glucuronide can be efficiently removed by HD.11,14) Indoleacetic acid is derived from tryptophan through indolepyruvic acid by transamination and decarboxylation. It is not clear why total indoxyl sulfate showed significantly negative correlation with total indoleacetic acid (Table 1). However, this might be related to competitive inhibition of albumin binding of indoleacetic acid by indoxyl sulfate, because indoxyl sulfate and indoleacetic acid share the same albumin binding site II.15)
p-Cresyl sulfate is derived from p-cresol, which is produced in the intestine from tyrosine by intestinal bacteria. p-Cresol is sulfated to produce p-cresyl sulfate in the intestinal wall.16) p-Cresyl glucuronide is produced from p-cresol in the liver and the intestinal wall.16,17) p-Cresyl sulfate cannot be removed by HD because of its high albumin binding ratio.11) However, serum p-cresyl glucuronide can be efficiently removed by HD.11,18)
Indoxyl sulfate and p-cresyl sulfate share the same albumin binding site II, for which they are competitive binding inhibitors,19) whereas CMPF binds to albumin binding site I.15) Indoxyl sulfate and p-cresyl sulfate might have a multi-compartmental distribution, which may affect reduction ratios.20) Total indoxyl sulfate and total p-cresyl sulfate were not associated, whereas free indoxyl sulfate and free p-cresyl sulfate were associated, and reduction rate by HD of indoxyl sulfate and p-cresyl sulfate correlated tightly.
Phenyl sulfate and phenyl glucuronide are derived from phenol, which is produced in the intestine from tyrosine by intestinal bacteria. Although phenyl sulfate and phenyl glucuronide are derived from tyrosine as p-cresyl sulfate, they are not correlated with p-cresyl sulfate, but rather correlated with indoxyl sulfate. In vitro, phenol tended to be produced by aerobic bacteria from tyrosine, whereas p-cresol was produced by anaerobic bacteria.21) Metabolic pathway including intestinal bacteria for the production of phenyl sulfate, phenyl glucuronide and/or phenol might have some relation to the production of indoxyl sulfate.
Production of phenylacetylglutamine by human tissue and microbial decarboxylation of phenylalanine to phenylethylamine with subsequent conjugation has been reported.22,23) Phenylacetic acid, derived from phenylalanine, is converted in the liver to phenylacetylglutamine.24) p-Cresyl sulfate was significantly correlated with phenylacetylglutamine and phenylacetic acid. Metabolic pathway including intestinal bacteria for the production of phenylacetylglutamine, phenylacetic acid and/or their precursors might have some relation to the production of p-cresyl sulfate.
Most of protein-bound uremic toxins are derived from the intestine, so they are called gastrointestinal tract (GI)-related uremic toxins. Further study is required to elucidate the intestinal bacteria-related metabolism for the production of these protein-bound uremic toxins in CKD patients.
References
- 1) R. Vanholder, R. De Smet, G. Glorieux, A. Argilés, U. Baurmeister, P. Brunet, W. Clark, G. Cohen, P. P. De Deyn, R. Deppisch, B. Descamps-Latscha, T. Henle, A. Jörres, H. D. Lemke, Z. A. Massy, J. Passlick-Deetjen, M. Rodriguez, B. Stegmayr, P. Stenvinkel, C. Tetta, C. Wanner, W. Zidek; European Uremic Toxin Work Group (EUTox). Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 2) T. Niwa. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 72: 1–11, 2010 [PMC free article] [PubMed] [Google Scholar]
- 3) T. Niwa. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 20(Suppl): S2–S6, 2010 [DOI] [PubMed] [Google Scholar]
- 4) T. Niwa, M. Ise. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 124: 96–104, 1994 [PubMed] [Google Scholar]
- 5) T. Niwa, M. Ise, T. Miyazaki. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 14: 207–212, 1994 [DOI] [PubMed] [Google Scholar]
- 6) T. Niwa. Mass spectrometry in the search for uremic toxins. Mass Spectrom. Rev. 16: 307–332, 1997 [DOI] [PubMed] [Google Scholar]
- 7) T. Niwa. Recent progress in the analysis of uremic toxins by mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2600–2606, 2009 [DOI] [PubMed] [Google Scholar]
- 8) T. Niwa. Update of uremic toxin research by mass spectrometry. Mass Spectrom. Rev. 30: 510–521, 2011 [DOI] [PubMed] [Google Scholar]
- 9) K. Kikuchi, Y. Itoh, R. Tateoka, A. Ezawa, K. Murakami, T. Niwa. Metabolomic analysis of uremic toxins by liquid chromatography/electrospray ionization-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 1662–1668, 2010 [DOI] [PubMed] [Google Scholar]
- 10) K. Kikuchi, Y. Itoh, R. Tateoka, A. Ezawa, K. Murakami, T. Niwa. Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST-120 by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 2997–3002, 2010 [DOI] [PubMed] [Google Scholar]
- 11) Y. Itoh, A. Ezawa, K. Kikuchi, Y. Tsuruta, T. Niwa. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 403: 1841–1850, 2012 [DOI] [PubMed] [Google Scholar]
- 12) P. A. Aronov, F. J. Luo, N. S. Plummer, Z. Quan, S. Holmes, T. H. Hostetter, T. W. Meyer. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13) W. R. Wikoff, A. T. Anfora, J. Liu, P. G. Schultz, S. A. Lesley, E. C. Peters, G. Siuzdak. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U.S.A. 106: 3698–3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14) T. Niwa, T. Miyazaki, S. Tsukushi, K. Maeda, Y. Tsubakihara, A. Owada, T. Shiigai. Accumulation of indoxyl-beta-d-glucuronide in uremic serum: Suppression of its production by oral sorbent and efficient removal by hemodialysis. Nephron 74: 72–78, 1996 [DOI] [PubMed] [Google Scholar]
- 15) T. Sakai, A. Takadate, M. Otagiri. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 18: 1755–1761, 1995 [DOI] [PubMed] [Google Scholar]
- 16) E. Schepers, G. Glorieux, R. Vanholder. The gut: The forgotten organ in uremia? Blood Purif. 29: 130–136, 2010 [DOI] [PubMed] [Google Scholar]
- 17) G. Lesaffer, R. De Smet, F. M. Belpaire, B. Van Vlem, M. Van Hulle, R. Cornelis, N. Lameire, R. Vanholder. Urinary excretion of the uraemic toxin p-cresol in the rat: Contribution of glucuronidation to its metabolization. Nephrol. Dial. Transplant. 18: 1299–1306, 2003 [DOI] [PubMed] [Google Scholar]
- 18) N. Meert, E. Schepers, G. Glorieux, M. Van Landschoot, J. L. Goeman, M. A. Waterloos, A. Dhondt, J. Van der Eycken, R. Vanholder. Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: Clinical data and pathophysiological implications. Nephrol. Dial. Transplant. 27: 2388–2396, 2012 [DOI] [PubMed] [Google Scholar]
- 19) B. K. Meijers, H. De Loor, B. Bammens, K. Verbeke, Y. Vanrenterghem, P. Evenepoel. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 4: 1932–1938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20) B. Meijers, N. D. Toussaint, T. Meyer, B. Bammens, K. Verbeke, Y. Vanrenterghem, P. G. Kerr, P. Evenepoel. Reduction in protein-bound solutes unacceptable as marker of dialysis efficacy during alternate-night nocturnal hemodialysis. Am. J. Nephrol. 34: 226–232, 2011 [DOI] [PubMed] [Google Scholar]
- 21) E. Bone, A. Tamm, M. Hill. The production of urinary phenols by gut bacteria and their possible role in the causation of large bowel cancer. Am. J. Clin. Nutr. 29: 1448–1454, 1976 [DOI] [PubMed] [Google Scholar]
- 22) K. Moldave, A. Meister. Synthesis of phenylacetylglutamine by human tissue. J. Biol. Chem. 229: 463–476, 1957 [PubMed] [Google Scholar]
- 23) J. W. Seakins. The determination of urinary phenylacetylglutamine as phenylacetic acid. Studies on its origin in normal subjects and children with cystic fibrosis. Clin. Chim. Acta 35: 121–131, 1971 [DOI] [PubMed] [Google Scholar]
- 24) D. Yang, M. Beylot, K. C. Agarwal, M. V. Soloviev, H. Brunengraber. Assay of the human liver citric acid cycle probe phenylacetylglutamine and of phenylacetate in plasma by gas chromatography-mass spectrometry. Anal. Biochem. 212: 277–282, 1993 [DOI] [PubMed] [Google Scholar]