Abstract
Cigarette smoke causes direct oxidative DNA damage as well as indirect damage through inflammation. Epidemiological studies show a strong relationship between secondhand smoke and cancer; however, the mechanisms of secondhand smoke-induced cancer are not well understood. Animal models with either i) deficient oxidative DNA damage repair, or ii) a decreased capacity to combat oxidative stress may help determine the pathways important in mitigating damage caused by smoke. In this study, we used mice lacking Ogg1 and Myh, both of which are involved in base excision repair by removing oxidatively damaged DNA bases. Gclm-deficient mice, which have decreased levels of glutathione (GSH), were used to look at the role of smoke-induced oxidative damage. Ex vivo experiments show significantly elevated levels of DNA single-strand breaks and chromosomal aberrations in peripheral blood lymphocytes from Ogg1−/−Myh−/− double knockout mice compared to wild type (WT) mice after 24 hours of exposure to cigarette smoke extract (CSE). The average γH2AX foci per cell was significantly elevated 3 hours after exposure to CSE in cells from Ogg1 −/−Myh −/− double knockout mice compared to wildtype mice. In vivo we found that all mice had increased markers of DNA damage after exposure to side-stream tobacco smoke (SSTS). Ogg1−/− Myh−/− and Gclm−/− mice had altered levels of peripheral blood glutathione after SSTS exposure whereas wild type mice did not. This may be due to differential regulation of glutathione synthesis in the lung. We also found that Ogg1−/−Myh−/− mice had a decreased lifespan after oral gavage with benzo[a]pyrene compared to wildtype mice and sham-exposed Ogg1−/−Myh−/− mice. Our results are important in investigating the roles of oxidative stress and oxidative DNA damage repair in cigarette smoke-induced cancers and characterizing the role of genetic polymorphisms in smoke-related disease susceptibility.
Keywords: DNA repair, Glutathione, Ogg1, Myh, DNA damage
INTRODUCTION
In 1964 The Surgeon General’s report classified tobacco smoke as a carcinogenic compound and in 1992, the EPA classified secondhand smoke as a Group A human carcinogen after evaluating human and animal data (Respiratory health effects of passive smoking: lung cancer and other disorders. 1992. EPA: Washington D.C.). Secondhand smoke contains over 4000 chemicals of which at least 250 are toxic or carcinogenic (Report on Carcinogens, Eleventh Edition, 2005, U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program). While the negative health effects of smoking are well known, 43.4 million adults continue to smoke, exposing approximately 126 million nonsmokers (Cancer Facts & Figures, 2009, American Cancer Society), making secondhand smoke a major public health concern.
Both in vitro and in vivo studies in laboratory animals as well as in non-smokers show genotoxic effects of second hand and side-stream tobacco smoke (SSTS) (Husgafvel-Pursiainen 2004). DNA adducts and oxidative DNA damage have been found in people exposed to SSTS (DeMarini 2004; Husgafvel-Pursiainen 2004). Compounds in smoke can react directly with DNA leading to DNA adducts as well as indirectly by producing reactive oxygen species (ROS). Benzo[a]pyrene (B[a]P) is one of the most well-known carcinogenic components of smoke and causes DNA adducts, often leading to GC→TA mutations (Pfeifer, Denissenko et al. 2002; Husgafvel-Pursiainen 2004). B[a]P has been shown to increase pulmonary DNA adducts in vivo, and causes altered mutational profiles in human lung tumors (Bartsch 1996; Denissenko, Pao et al. 1996; Hecht 2002). Although G to T transversions are most often attributed to DNA adducts caused by compounds in smoke such as polyaromatic hydrocarbons (PAHs), oxidative DNA damage also commonly leads to GC→TA mutations.
It is well established that second hand smoke increases the risk of diseases such as cancer (Sasco, Secretan et al. 2004) and heart disease (Ambrose and Barua 2004), however, mechanisms of smoke-induced injury are still not fully understood. The classic model is that smoke exposure causes DNA damage and mutations in tumor suppressor genes or oncogenes which lead to carcinogenesis. More recently, other factors have been suggested to increase susceptibility to smoke-induced lung cancer including inflammation, cell cycle signaling, and rates of activation and detoxification of smoke particles (Engels, Wu et al. 2007; Schwartz, Prysak et al. 2007). Understanding how smoke exposure causes lung cancer will help to clarify why some smokers develop lung cancer while others do not. Approximately 85% of lung cancers are attributed to smoking, however, only 10–15% of smokers develop lung cancer (Schwartz, Prysak et al. 2007), implying that a subset of smokers is more susceptible to smoke-induced injury. The association between secondhand smoke and lung cancer is weaker so that susceptibility may play a more prominent role.
Since oxidative stress is important in smoke-induced damage, we studied the roles of antioxidant and DNA damage repair capacity in combating the effects of smoke. We assessed the effects of cigarette smoke extract (CSE) and SSTS on DNA damage and oxidative stress in different mouse models. We used mice deficient in Ogg1 (8-oxodG glycosylase) and Myh (MutY homologue), as well as mice deficient in Gclm (Glutamate-cysteine ligase, modifier subunit). Ogg1 is considered to be the major enzyme for repair of 8-oxodG (Evans, Dizdaroglu et al. 2004). Myh primarily repairs 8-oxodG:A and 8- oxodG:G mismatches during DNA replication and its activity is directed towards the daughter strand (Evans, Dizdaroglu et al. 2004). Spontaneously, over 30% of double mutant Ogg1−/−Myh−/− mice develop lung tumors after 12 months (Xie, Yang et al. 2004). Further characterization of these lung tumors show that 75% of sequenced K-ras alleles from tumors had a G to T transversion compared to no transversions in adjacent normal tissue (Xie, Yang et al. 2004). This is consistent with mice deficient in 8-oxodG repair and the types of mutation which lead to tumorigenesis (Xie, Yang et al. 2004). Subsequently, it was found that the number of 8-oxodG lesions in the liver, lungs, and small intestines of Ogg1−/−Myh−/− mice were at least tripled compared to wildtype controls (Russo, De Luca et al. 2004).
We used Gclm−/− mice to study the role of anti-oxidant defense in smoke-induced disease and injury. Gclm is important for glutathione synthesis. Glutathione is one of the most abundant reducing agents in the cell and has an important role in protection against reactive oxygen species (ROS), metabolism of nutrients and xenobiotics, and regulation of intracellular redox status (Wu, Fang et al. 2004). Gclm−/− mice have 9–16% of the normal GSH levels in the liver, lung, pancreas, erythrocytes, and plasma compared to wildtype littermates (Yang, Dieter et al. 2002). Gclm−/− mice also exhibit reduced cysteine levels in the kidney, pancreas, and plasma (Yang, Dieter et al. 2002).
To study the roles of DNA damage and oxidative stress in environmental tobacco smoke-induced cancer, we exposed Ogg1−/−Myh−/− , Gclm+/−, and Gclm−/− and wildtype mice or cells to SSTS or CSE and measured markers of DNA damage and oxidative stress. We observed that CSE induced DNA double strand breaks in mononucleated white blood cells, single strand breaks in leukocytes, and micronucleus formation in Ogg1−/−Myh−/− mutant but not in wildtype cells. We also found that γH2AX foci were increased in mice after SSTS exposure; however single-strand breaks and hOGG-1-induced DNA strand breaks were not significantly increased. SSTS increased GSH levels in Ogg1−/−Myh−/− , Gclm+/−, and Gclm−/− mice but not wildtype mice. Gene expression of oxidative stress and GSH regulatory proteins were also altered in the lungs of SSTS-exposed animals. Finally, we show that DNA repair deficient animals had decreased survival proportions after oral gavage of benzo[a]pyrene compared to wildtype animals. These results show that mice deficient in base-excision repair have a mild but significantly increased susceptibility to smoke and smoke components and that GSH is an important molecule in mitigating these effects.
MATERIALS AND METHODS
Mice Breeding and Care
Myh- and Ogg1-deficient mice have been described previously (Xie, Yang et al. 2004) and were backcrossed with C57BL/6J mice at least 4 times. Additionally, they have been backcrossed at least twice with C57BL/6J pun/pun mice. Gclm+/− mice have also been described previously and have been backcrossed at least 4 times with C57BL/6J pun/pun mice (McConnachie, Mohar et al. 2007). To obtain Gclm−/− mice, heterozygous females were crossed with heterozygous males. Myh−/−Ogg1−/− mice were obtained by crossing Ogg1+/−Myh+/− males and females and by crossing Ogg1−/−Myh+/− mice with Ogg1+/−Myh−/− or Ogg1−/−Myh+/− and Ogg1+/−Myh−/− with Ogg1+/−Myh+/− mice. Genotyping was be done by PCR as described previously (Xie, Yang et al. 2004; McConnachie, Mohar et al. 2007). Wild type mice were obtained from Gclm+/− crosses, Ogg1+/−Myh+/− crosses, or from our wildtype C57BL/6J pun/pun colony, used in all backcrosses. 8–10 week old male and female mice were used for in vivo experiments and 12–13 month old male and female mice were used for ex vivo experiments. Mice were bred in an institutional specific pathogen free animal facility under standard conditions with a 12 hr light/dark cycle according to Animal Research Committee regulations. Mice were fed a standard diet and water ad libitum.
Blood Collection
For exposure to CSE, peripheral blood was collected from experimental mice via terminal right ventricle cardiac puncture using a heparin-coated syringe (American Pharmaceutical Partners, Inc. Schaumburg, IL). At least 1 mL of blood was collected from each animal, aliquoted into 2 tubes for exposure to CSE or PBS and incubated at 37°C. For in vivo experiments, blood was collected by facial vein puncture using 5 mm sterile lancets (Medipoint Inc. Mineola, Ny). Blood from each mouse was collected into EDTA-coated tubes (Sarstedt Aktiengesellschaft & Co., Numbrecht).
Whole blood CSE exposure
Frozen stocks of cigarette smoke extract were supplied by the lab of Andrew Dannenberg at Cornell University as described previously (Du, Leung et al. 2007). Concentrated 40.3 puffs/mL cigarette smoke extract was diluted to a working solution of 5 puffs/mL with PBS. 5 puffs/mL cigarette smoke extract (CSE) was administered directly into whole peripheral blood to a final concentration of 1 puff/mL CSE and allowed to incubate in a shaking 37°C incubator for 3,6, or 24 hours.
SSTS exposure
Mice were exposed to SSTS in a Teague Enterprises Model TE-10 cigarette-smoking machine (http://memebers.dcn.org/svteague/catalog.html). Smoke from a smoldering Kentucky reference cigarette 2R4F was collected in a mixing chamber, then flowed through a dilution chamber before entering the exposure chamber. Mice were exposed in open cages with access to food and water ad libitum. 8–10 week old mice were exposed to an average of 43 mg/m3 TPM for 6 hours/day for 14 days. Daily exposures were separated with a 1 hour break between 3-hour exposures. TPM was measured by a particulate counter (DusTrak, TSI Inc., St. Paul, MN) and calibrated for tobacco smoke.
γ-H2AX Immunofluorescence
After erythrocyte lysis, cells were laid over poly -D-lysine-coated coverslips and fixed with 4% paraformaldehyde (Electron Microscopy Sciences) at room temperature as described previously (Goldstine, Nahas et al. 2006). Subsequently, cells were permeabilized with 0.5% Triton X-100 (Sigma), followed by 5 rinses in PBS. For ex vivo experiments blocking was done in aluminum-covered plates overnight at 4°C in 10% FBS. For in vivo experiments blocking was done for 1 hour at room temperature in 10% FBS. Coverslips were then incubated for 1 hour at room temperature with mouse anti-phospho-Histone H2A.X (Upstate, Temecula, CA) at a dilution of 1:400, then rinsed with 0.1% Triton X-100. Following a second 10% FBS blocking, cells were stained with FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:200 for 1 hour at room temperature. Coverslips were mounted onto slides using VECTASHIELD with DAPI (Vector Laboratories, Burlingame, CA). Foci were analyzed on a Zeiss automated microscope. At least 100 cells were counted per sample and cells with more than four distinct foci in the nucleus were considered positive for γH2AX.(Westbrook, Wei et al. 2009). Apoptotic cells, which have an approximate 10-fold increased in nuclear foci in damaged cells, were not included in analyses (Muslimovic, Ismail et al. 2008; Westbrook, Wei et al. 2009). Statistical analysis was done using ANOVA and tukey’s post hoc test in ex vivo experiments and two-way repeated measures ANOVA followed by Bonferroni post-tests for in vivo experiments (PRISM).
Micronucleus assay
3µl of whole blood were spread on a microscope slide and stained with Wright-Giemsa solution (Sigma-Aldrich, St. Louis, MO). At least 4000 erythrocytes were counted according to published recommendations (Hayashi, Tice et al. 1994). MN were counted and scored with an Olympus Ax70 (Tokyo, Japan) at 100X magnification. Statistical analysis was done using repeated measures ANOVA followed by a Bonferroni or Kruskal-Wallis post-tests , or a t-test to compare 2 groups. (GraphPad Prism).
DNA single strand breaks
Oxidative DNA damage and DNA strand breaks were measured in peripheral blood cells using the alkaline comet assay. In ex vivo experiments peripheral blood was collected before CSE incubation, and immediately after 3, 6, or 24 hours of incubation with CSE. For in vivo experiments blood was collected before, and after 1 and 2 weeks of SSTS exposure. Blood was diluted 1:1 with RPMI + 20% DMSO, slowly frozen and stored at −80C until the assay was performed. The comet assay was done as described previously (Singh, McCoy et al. 1988). Briefly, cells were mixed with low melting-point agarose, and placed in triplicate onto normal agarose layed over gelbond (Lonza Inc. Rockland, ME). The gel was immerse in lysis buffer (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, 1% Triton, and 10% DMSO), then alkaline electrophoresis buffer (0.3 M NaOH, 1 mM EDTA). After 20 minutes in the electrophoresis buffer at 4°C, the gel was run for 45 minutes at 300 mA, allowed to dry and then stained with SYBR Gold (Molecular Probes). Comet tail-moments were analyzed using CASP (Comet Assay Software Project, http://casp.sourceforge.net/). To measure oxidative DNA damage, the comet assay was modified to include an incubation step with hOGG1 (New England Biolabs, Ipswich, MA). As described previously, embedded cells were incubated with hOGG1 (1:300 in NEBuffer1 and BSA) at 37°C for 30 minutes following the lysis step (Smith, O'Donovan et al. 2006). Tail-moments were normalized to a control to account for inter-experimental variability. Statistical analyses were done using ANOVA (GraphPad Prism).
GSH measurements
Glutathione levels were determined in blood samples taken before, and after 1 and 2 weeks of SSTS exposure and stored at −80°C until analysis. GSH was extracted using 5% metaphosphoric acid and analyzed using the OxisResearch Bioxytech GSH/GSSG-412 kit (Portland, OR) which is based on the Tietze method (Tietze 1969). The assay was modified to fit into a 96-well plate. Statistical analyses were done using ANOVA.
Gene expression analysis
Lungs from mice at the end of the 2-week SSTS exposure or unexposed control mice were perfused and lavaged before immersion into RNAlater (Qiagen). Lungs were kept at 4°C for 24 hours then transferred to −80°C until RNA was isolated using the RNeasy Mini kit according to manufacturer’s instructions (Qiagen). cDNA was synthesized using SuperscriptIII (Invitrogen) according to manufacturer’s recommendations. Quantitative real-time PCR was performed on an ABI Prism 7500 gene expression system (Applied Biosystems) using Taqman gene expression assays for Nrf2, Hmox, Gclc, Gclm, Gsr, Gstα4, Mcp-1, IL-1β, Tnfα, and Tgfβ. Gapdh was used as an internal control. Each reaction was done in triplicate and analyzed using the relative standard curve method.
B[a]P survival proportions
Wildtype and Ogg1−/−Myh−/− double knockout mice were dosed twice a week for 1 month by oral gavage with Benzo[a] pyrene (Sigma, lot no.37F-0555). B[a]P was suspended in corn oil and mice were dosed at 100mg/kg body weight. Control mice were gavaged with corn oil only.
RESULTS
CSE exposure increased DNA double-strand breaks in mononucleated white blood cells from base excision repair deficient mice
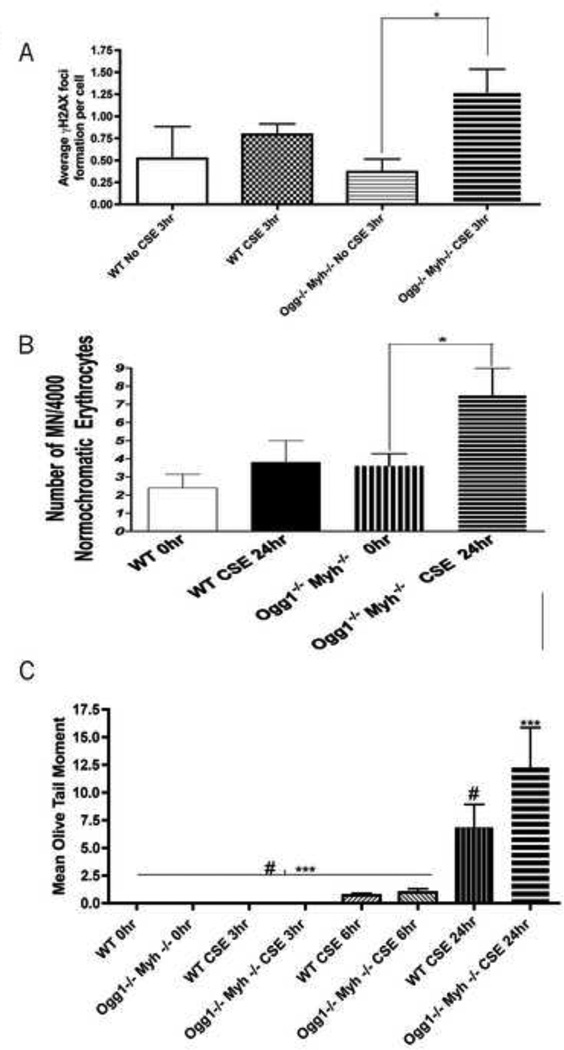
The frequency of DNA double stranded breaks in white blood cells (WBCs) from wildtype and Ogg1−/−Myh−/− mice was measured using the γH2AX assay. After 3 hours of exposure to 1 puff/mL CSE ex vivo, the average number of γH2AX foci per Ogg1−/−Myh−/− cell was four times higher than mononucleated WBCs in the non-exposed group (Figure 1A, p<0.05). No significant differences were found in wildtype WBCs exposed to 1 puff/mL CSE for 3 hours compared to non-exposed wildtype mononucleated WBCs. No differences were found between genotypes at any time point. (Figure 1A).
Figure 1. Ex-vivo DNA damage assays in peripheral leukocytes.
A.) Average number of γH2AX foci formed in peripheral leukocytes. Presence of double strand breaks was confirmed by immunofluorescence of γH2AX. At least 100 cells were analyzed per sample. Data represent mean ± SEM. Statistical analyses were done using ANOVA testing and Tukey’s post hoc analysis. n=5 in all groups. * indicates significant p<0.01 between no CSE exposed and CSE exposed lymphocytes at 3hr time point . B.) At least 4000 normochromatic erythrocytes were counted and scored for the presence of micronuclei. Data, statistical analyses, and error bars represent mean ± SEM of micronucleated normochromatic erythrocytes (MN-NCE) per 1000 NCEs. * indicate significant p<0.05 between no CSE exposed lymphocytes at 0hr and CSE exposed lymphocytes at 24hr. n=5 in all groups. Statistical analyses were done using ANOVA with Kruskal-Wallis Test and Dunn's Multiple Comparison Post hoc analysis C.) At least 100 “comets” were scored per mouse. n=5 in all groups. Data were log transformed before statistical analyses and error bars represent mean ± SEM. Statistical analyses were done using ANOVA testing and Tukey’s post hoc analysis. # indicates significant p< 0.05 between WT CSE exposed lymphocytes at 24hr and non-exposed and CSE exposed lymphocytes from previous time points . *** indicates significance p ≤ 0.001 between CSE exposed Ogg1−/−Myh−/− lymphocytes at 24hr and non-exposed and CSE exposed lymphocytes from previous time points.
CSE induced micronucleus formation in erythrocytes from base excision repair deficient mice after 24 hours
We found a significant induction of micronuclei in the peripheral blood from Ogg1−/− Myh−/− mice after incubation with CSE for 24 hours (Figure 1B, p<0.05). Micronuclei were slightly elevated in the peripheral blood from wildtype animals exposed to CSE for 24 hours , however, this difference was not significant. No differences were found between genotypes at any time point. These results suggest that cells from Ogg1−/− Myh−/− double knockout mice exhibit increased susceptibility to components of cigarette smoke extract.
CSE induced DNA strand breaks in peripheral leukocytes of wildtype and base excision repair deficient mice
Single- and double-strand breaks as well as alkalilabile sites were assessed by mean olive tail moment using the alkaline comet assay (Figure 1C). The mean olive tail moment from wildtype and Ogg1−/−Myh−/− double knockout mice remained low after 3 and 6-hour incubations with CSE. Mean olive tail moments were significantly higher after 24 hours of CSE incubation in both wildtype and Ogg1−/−Myh−/− double knockout mice compared to incubation with PBS only (p<0.05 and p<0.001, respectively) and compared to shorter incubation times. No differences were found between genotypes at any time point. (Figure 1C).
γH2AX foci and micronuclei are increased in mice after exposure to SSTS, however single-strand breaks, and hOGG1-induced DNA single-strand breaks were not
After 1 week of exposure to SSTS, γH2AX foci increased in all mice (Figure 2A, p<0.05). After 2 weeks of exposure, average γH2AX foci per cell were still above spontaneous levels, but did not increase above week 1 levels. No differences were found between genotypes at any time point. In Ogg1−/−Myh−/− mice, levels of γH2AX foci decreased significantly after 2 weeks of exposure compared to after 1 week. Since blood was taken within 15 minutes following exposure to SSTS, the γH2AX foci represent ongoing DNA damage and repair in these mice. Although γH2AX foci are increased after exposure to SSTS, micronuclei, which represent permanent damage following a double-strand break, are only significantly increased in wildtype and Gclm+/− mice after 2 weeks of exposure to SSTS (Figure 2B). Micronuclei are increased in Ogg1−/−Myh−/− and Gclm−/− mice, however the trend does not reach significance (Figure 2B). Importantly, however, spontaneous levels of micronuclei are lower in wildtype mice than Ogg1−/−Myh−/− mice (p<0.05 using the t test). DNA single-strand breaks as measured by the comet assay were not increased significantly after exposure to SSTS. Oxidized DNA damage, measured by the hOGG1-modified comet assay, which induces breaks at oxidized bases, was also increased but not significantly in response to SSTS (data not shown). A lack of significant DNA damage seen by the comet assay could be due to the sensitivity of the assays or number of mice since our data show slight but not significant increases after SSTS exposure.
Figure 2. In-vivo DNA damage assays in peripheral leukocytes.
A.) γH2AX foci in peripheral blood increase after 1 and 2 weeks of exposure to SSTS in all mice (p<0.05). n= 8 for wildtype mice and 6 for all other groups. B.) Micronuclei in normochromatic erythrocytes do not increase significantly after exposure to smoke. n= 5 for wildtype mice and 6 for all other groups. *indicates p<0.05, **indicates p<0.01, and ***indicates p<0.001. Error bars indicate the mean ± SEM. Significance is compared to before timepoints unless otherwise indicated.
Glutathione in the peripheral blood is differentially altered in response to SSTS depending on genotype
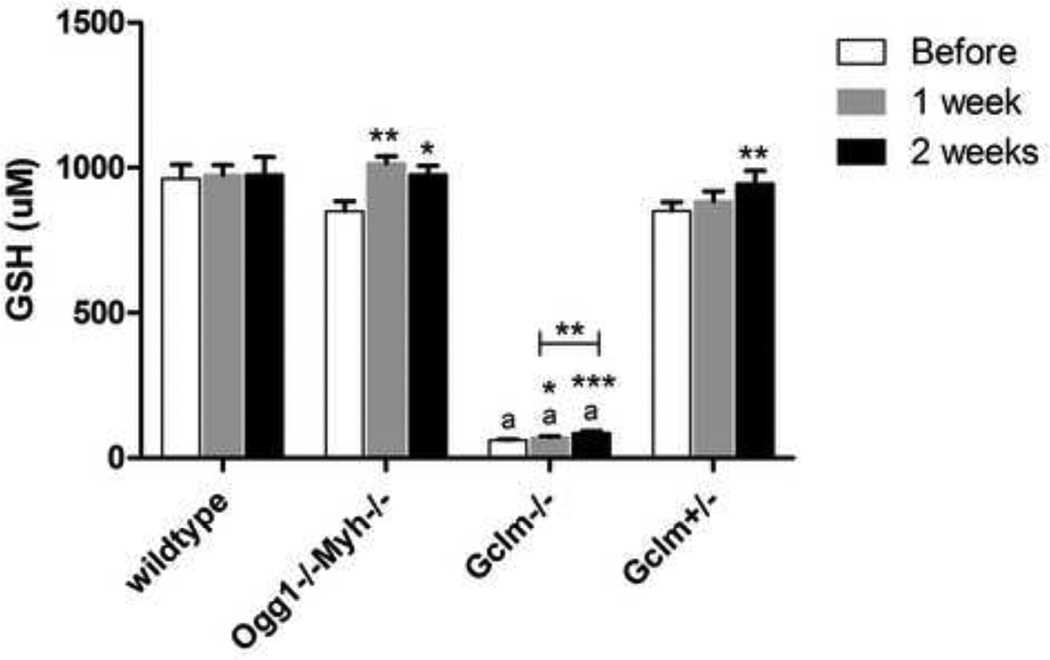
GSH is a major antioxidant in the cell. As expected, Gclm−/− mice have decreased levels of GSH at all time points (Figure 3A). Following exposure to SSTS, peripheral blood GSH levels were increased in all knockout mice over spontaneous levels (Ogg1−/−Myh−/−, Gclm−/−, and Gclm+/−) but not in wildtype mice (Figure 3A). GSH levels were significantly increased as early as 1 week in Ogg1−/−Myh−/− and Gclm−/− mice and after 2 weeks in Gclm+/− mice. Therefore, wildtype mice may be more efficient at regulating a stress response when exposed to sub-chronic doses of SSTS.
Figure 3. Peripheral blood levels of GSH are differentially altered after SSTS exposure.
A.) Peripheral blood GSH levels are differentially altered depending on genotype. GSH levels in Gclm−/− mice are significantly lower than all other mice at all timepoints. n= 8 for wildtype mice and 6 for all other groups. *indicates p<0.05, **indicates p<0.01, and ***indicates p<0.005. ‘a’ indicates p<0.005 comapred to all other genotypes. Error bars represent the mean ± SEM.
Gene expression of proteins involved in oxidative stress response and GSH regulation are altered in lungs after exposure to SSTS
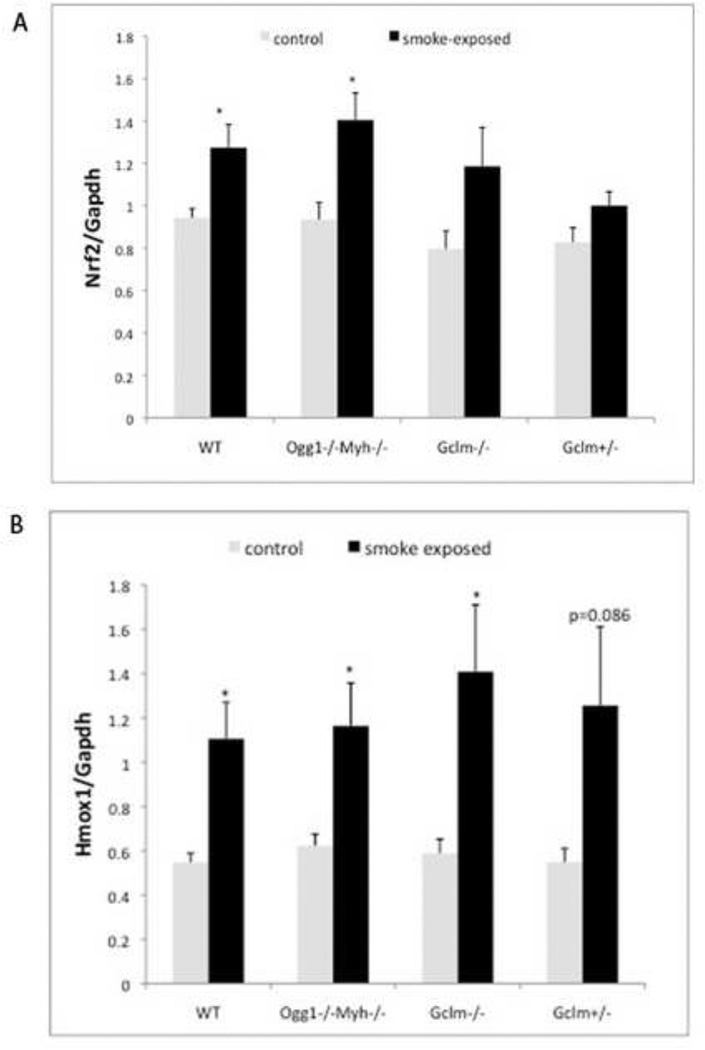
After 2 weeks of exposure to SSTS, Nrf2 expression is significantly increased in Ogg1−/−Myh−/− and wildtype mice (Figure 4A). Nrf2 is a transcription factor upregulated in response to oxidative stress and smoke (Adair-Kirk, Atkinson et al. 2008; Hubner, Schwartz et al. 2009). Hmox is regulated by Nrf2 and is increased in all mice (Figure 4B). This data is consistent with previous results showing increased Nrf2 and Hmox in response to smoke in wildtype mice (Adair-Kirk, Atkinson et al. 2008).
Figure 4. Gene expression of Nrf2 and Hmox proteins involved in oxidative stress response in the lung are upregulated in response to SSTS.
A.) Nrf2 expression changes, n=3 for each group. B.) Hmox expression changes, n=5 for each group. *indicates p<0.05. Error bars represent the mean ± SEM.
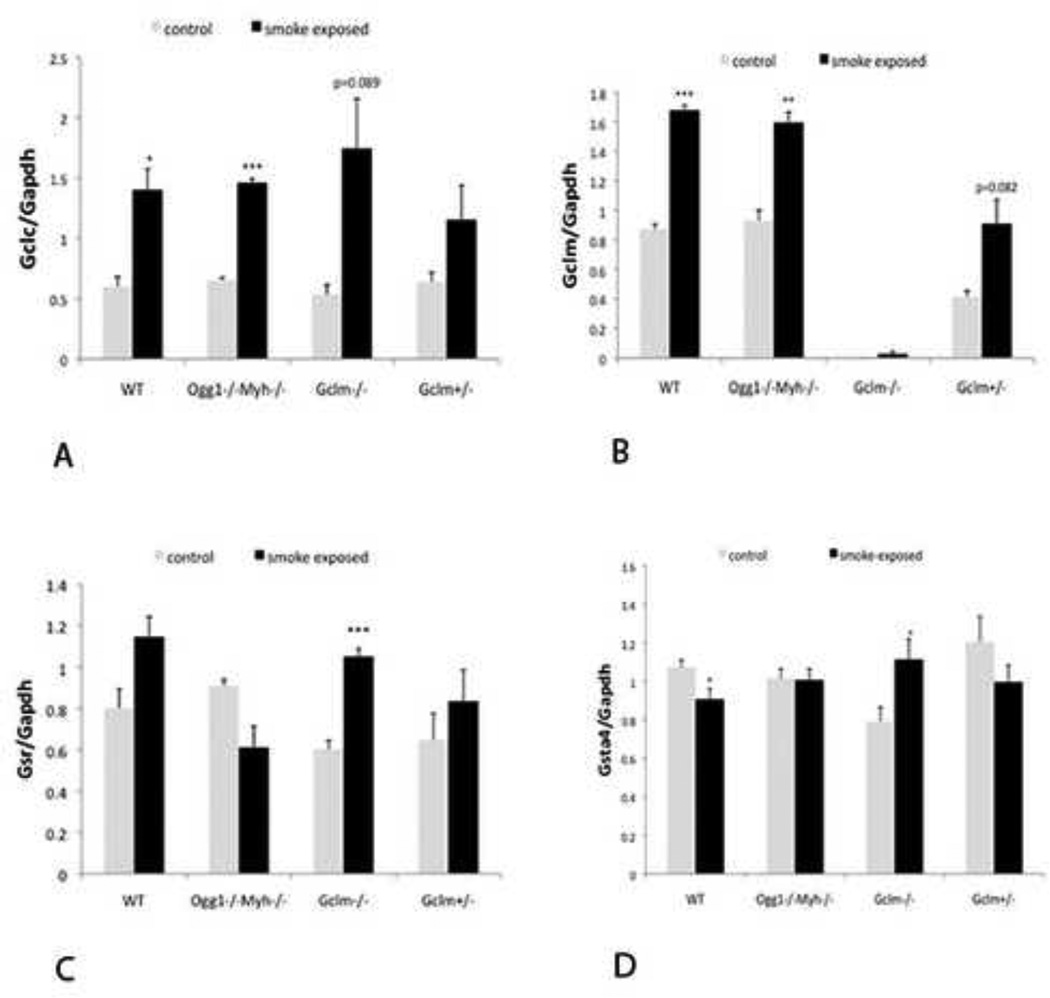
Since we found altered levels of GSH in the blood, we also looked at expression of genes involved in GSH homeostasis. Although McConnachie et al found a spontaneous increase of Gclc in the liver of Gclm−/− mice (McConnachie, Mohar et al. 2007), we did not see a similar increase in the lungs. Following exposure to smoke, however, Gclc expression was upregulated in Ogg1−/−Myh−/−, Gclm−/−, and wildtype mice (Figure 5A). In addition, Gclm expression was increased in response to SSTS in all mice except the Gclm-deficient mice (Figure 5B). Other genes involved in the GSH regulation include Glutathione reductase (Gsr), responsible for reducing GSSG to 2 GSH units and glutathione-S-transferases (Gstα4) which use GSH for detoxification. We found that Gsr is significantly upregulated in Gclm−/− mice (Figure 5C). We also found that Gstα4 is significantly upregulated in Gclm−/− mice (Figure 5D). Therefore, mice primarily responded to SSTS exposure by up-regulating genes involved in oxidative stress response and there are slight differences depending on genotype.
Figure 5. Expression of genes which affect GSH in the lung in response to SSTS.
A.) Gclc expression changes, n=3 for each group. B.) Gclm gene expression changes, n=3 for each group. C.) Gsr expression changes, n=3 for each group. D.) Gstα4 expression changes, n=5 for each group. *indicates p<0.05, ** indicates p<0.01, **indicates p<0.001. Error bars represent the mean ± SEM.
Ogg1 −/−Myh−/− double knockout mice exhibit decreased survival after exposure to B[a]P by oral gavage
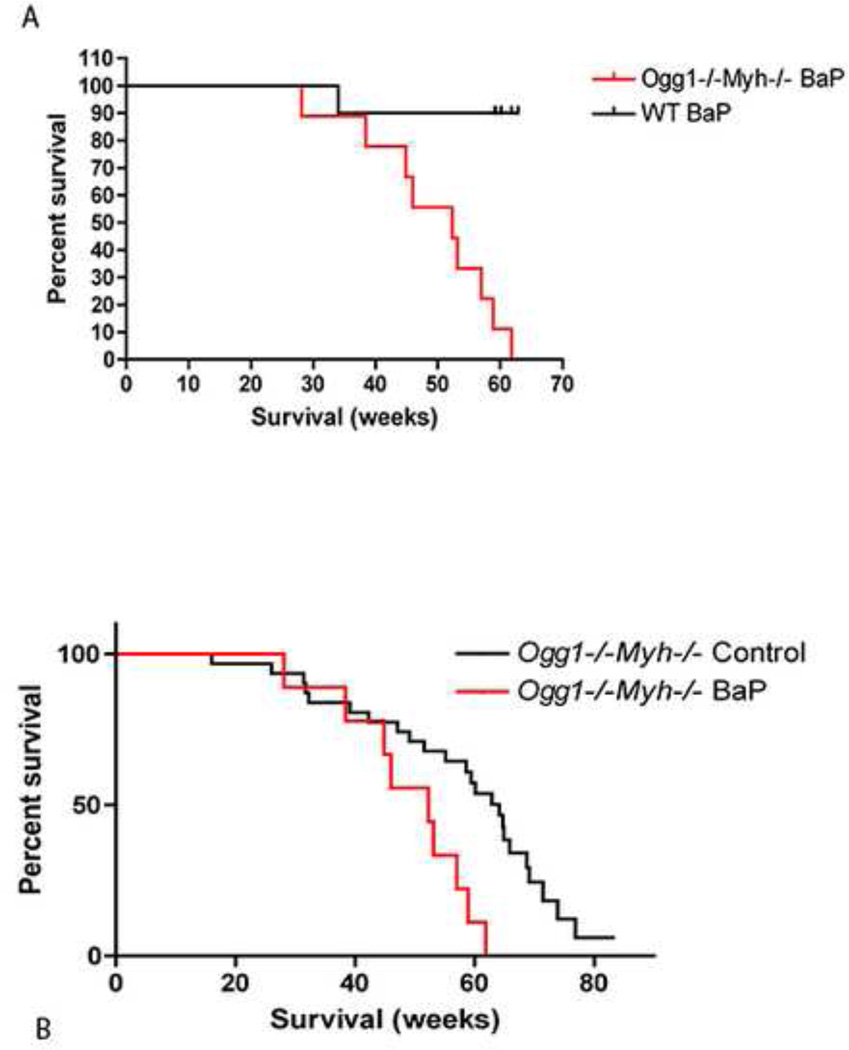
B[a]P and other polyaromatic hydrocarbons (PAHs) are thought to be the cause of the carcinogenic effects of exposure to coal tars, soots, and related materials (Hecht 2002). B[a]P has also been shown to induce tumor formation at the site of application in rodents (Hecht 2002). Metabolism of BaP to the major carcinogen BaP-diolepoxide , forms reactive oxygen species including superoxide, H2O2, •OH radicals and semiquinone. (Gao, Li et al.).These ROS induce DNA damage and can cause modifications to guanine residues to form the common 7,8-dihydro-8-oxoguanine (8oxo-dG) mutagenic legion .This specific legion is normally recognized by the Ogg1 enzyme. (Le Marchand, Donlon et al. 2002).Furthermore, (8oxo-dG) legions readily mispair with adenines to form transversion mutations, these mispaired adenines are removed by the MYH protein.(Miyaishi, Osawa et al. 2009) In this current work Ogg1−/−Myh−/− and wildtype mice were exposed to100 mg/kg B[a]P twice per week for 1 month by oral gavage. Ogg1−/−Myh−/− mice exposed to B[a]P have a significantly decreased median lifespan compared to B[a]P-exposed wildtype mice (Figure 6A, p<0.05). In addition, Ogg1−/−Myh−/− mice gavaged with B[a]P had a shorter median lifespan than Ogg1−/−Myh−/− mice gavaged with corn oil only (Figure 6B, p<0.005). These data show that the Ogg1−/−Myh−/− mice have increased susceptibility to a major component found in cigarette smoke.
Figure 6. Percent survival in Wildtype and Ogg1−/−Myh−/− mice after Benzo (a) pyrene gavaging.
A.) Percent survival in weeks in wildtype (black line) and Ogg1−/−Myh−/−(red line) mice after benzo (a) pyrene gavaging. B.) Percent survival in weeks in control Ogg1−/−Myh−/−(black line) and benzo (a) pyrene gavaged Ogg1−/−Myh−/− mice (red line). n=10 for wildtype mice and n=9 for Ogg1−/−Myh−/− mice. p< 0.005 for both curves.
DISCUSSION
We examined susceptibility to cigarette smoke extract and SSTS in mice deficient in the repair of oxidative DNA damage and in mice with decreased levels of GSH. In ex vivo CSE experiments we found that cells from Ogg1−/−Myh−/− mice had significantly higher amounts of chromosomal aberreations and single stranded breaks after 24-hour incubations assessed via micronucleus and comet assays, respectively. After a 3-hour CSE incubation we also observed a significant increase in DNA double-strand breaks assessed by γH2AX foci formation in peripheral blood lymphocytes. In vivo experiments showed that DNA double-strand breaks were increased in response to SSTS in all groups of mice. GSH homeostasis, however, was differentially regulated in Ogg1−/−Myh−/−, Gclm−/− and Gclm+/− mice compared to wildtype mice. Although in our in vivo experiments we did not observe a significant increase in DNA damage as measured by the comet assay after a two week SSTS exposure in all mice, a significant increase in ex vivo mouse lymphocytes suggests that the cells of the DNA repair deficient mice are more sensitive than that of wildtype mice exposed to CSE. There may be a dose or time-dependency which is important in differentiating these mice in vivo, as alluded to by the decreased γH2AX foci formation after 2 weeks of SSTS exposure in Ogg1−/−Myh−/− mice. In addition, Ogg1−/−Myh−/− mice were significantly more sensitive to B[a]P, a constituent of cigarette smoke.
In our experiments SSTS induced DNA double-strand breaks in peripheral blood cells in all mice after 1 week of exposure to SSTS, similar to what has been seen in cells exposed to smoke (Tanaka, Huang et al. 2007). Levels of DNA double-strand breaks decreased significantly in Ogg1−/−Myh−/− mice after 2 weeks of exposure and remained approximately the same or slightly decreased in all other mice indicating i) an increase in DNA repair capacity and/or ii) that SSTS-induced γH2AX foci had reached a saturation point so that there was an equilibrium between DNA damage and repair by 1 week. Although we found significant increases in DNA damage as measured by the micronucleus assay only in wildtype and Gclm+/− mice, in all groups of mice micronuclei levels seemed to increase over time. In support of this, slight but significant increases in peripheral blood micronuclei has been shown previously (Balansky, F et al. 1999). Since γH2AX foci form in response to DNA double-strand break recognition, and may signal repair, the lack of a significant increase in micronuclei could be indicative of proficient repair of double-strand breaks, or an indication that the measurement of micronuclei is not as sensitive after 1 and 2 weeks of exposure to SSTS. Micronuclei have been shown to increase 30 hours after a 1 day exposure to SSTS (Mohtashamipur, Norpoth et al. 1987). Micronuclei gradually accumulate in circulating blood until they reach a maximum level at 48–72 hours, then decrease (Mohtashamipur, Norpoth et al. 1987). Micronuclei gradually Chaubey, Bhilwade et al. 1993). Therefore, if repair capacity increases, circulating levels of micronuclei may decrease after 1–2 weeks of SSTS exposure. Single-strand breaks and hOGG1-induced single-strand breaks in peripheral blood lymphocytes also did not significantly increase in response to SSTS. It is possible that upregulation of protective enzymes such as DNA repair proteins prevented increases in DNA damage after 1 week of exposure since it was shown that HO-1 (Hmox1) and Ogg1 were upregulated as early as 4 days after exposure to diesel exhaust particles (Risom, Dybdahl et al. 2003). We also show an increase in Hmox1 after SSTS exposure. Although Ogg1 could not be upregulated in Ogg1−/−Myh−/− mice, there are several proteins which could help repair DNA damage (Evans, Dizdaroglu et al. 2004).
Since GSH is the most abundant small molecule antioxidant in the cell and its homeostasis is associated with several health effects (Wu, Fang et al. 2004; Ballatori, Krance et al. 2009), we measured circulating GSH levels. In response to SSTS exposure, we found that peripheral blood GSH levels significantly increased in Ogg1−/−Myh−/−, Gclm−/−, and Gclm+/− mice but not wild type mice. Since Ogg1−/−Myh−/− and wildtype mice in principle have equivalent abilities to regulate GSH, this was somewhat surprising. An increase in GSH in response to stress has been seen previously (Dickinson, Levonen et al. 2004; Yamamoto, Reliene et al. 2008), and specifically in a human lung cell line in response to cigarette smoke extract (Baglole, Sime et al. 2008). Since GSH regulation is important in many parameters of human health, changes in GSH levels may indicate disruption of normal processes (Wu, Fang et al. 2004; Ballatori, Krance et al. 2009). Different levels of peripheral blood GSH may be explained by differential regulation of enzymes involved in GSH metabolism as found in the lungs, in addition to Nrf2, which is a transcription factor shown to upregulate Gclc and Gclm (Nguyen, Sherratt et al. 2003; Dickinson, Levonen et al. 2004; Rangasamy, Cho et al. 2004). Although Gclc is upregulated in the lungs of wildtype, Ogg1−/−Myh−/− and Gclm−/− mice, Gclm is considered to be more involved in stress response (Dickinson, Levonen et al. 2004). Therefore, it is not surprising that Gclm is induced in all mice besides the knockout mouse. Gsr, important in reducing GSSG, is upregulated in Gclm−/− mice. Since these mice lack GSH sythesizing capacity, this may be a compensatory mechanism in Gclm−/−mice in response to SSTS. Gclm−/− mice were also the only group of mice to upregulate Gstα4. Gstα4 is a glutathione transferase which has been shown to detoxify 4-HNE (Engle, Singh et al. 2004), a product of lipid peroxidation. Therefore, an increase in Gstα4 in Gclm−/− mice may indicate the presence of oxidative stress.
The fact that mice respond to cigarette smoke with increased production of GSH and GSH metabolizing enzymes and possibly DNA repair functions might explain why it is difficult to induce cigarette smoke induced lung cancer in mice (Witschi, Joad et al. 1997; Coggins 2007). Of the small rodents, hamsters, rats, and mice have all been used to study effects of smoke. In many cases a small difference in smoke-exposed versus non-exposed animals was found, however the degree of carcinogenesis was relatively low (Coggins 2007). Newer models of smoke-induced lung cancer have been developed. One uses a high exposure concentration (100–250 TPM/m3) for up to 30 months which led to a significant induction of lung tumors and cancer in mice and rats (Mauderly, Gigliotti et al. 2004; Hutt, Vuillemenot et al. 2005; Hahn, Gigliotti et al. 2007). However, smoke exposed mice lived longer than control mice and had a significantly delayed onset of other types of cancer (Hutt, Vuillemenot et al. 2005) which is in agreement with our in vivo studies, showing an induction of defense enzymes and antioxidants which can reduce systemic genotoxicity.
In relation to smoking induced lung cancer the strongest and most widely studied carcinogens are the PAHs (Hecht 2002). B[a]P, one of the most extensively studied PAH present in cigarette smoke, has been shown to induce tumors at the site of application in rodents (Hecht 2002). Metabolism of PAHs causes genotoxic intermediates that can lead to ROS mediated G to T transversion mutations caused by elevated 8-OHdG adenine mispairing.(Gao, Li et al. ; Xie, Yang et al. 2004) These mutations are recognized and removed by the Ogg1, and MYH enzymes, respectively. Furthermore, this specific DNA alteration is elevated in peripheral leukocytes and lung tissue in smokers and lung cancer patients, making it advantageous to use base excision reapair deficient animals to study ROS mediated DNA damage caused by B[a]P exposure. In our experiments we gavaged wildtype and Ogg1−/−Myh−/− double knockout mice with 100 mg/kg B[a]P. Although extensive pathology was not conducted on these animals we observed a marked decrease in overall survival proportions in Ogg1−/−Myh−/− double knockout mice gavaged with B[a]P compared to B[a]P-gavaged wildtype and sham-exposed Ogg1−/−Myh−/− mice. In support of these observations, (Lan, Mumford et al. 2004) describes an increased risk of lung cancer in individuals with polymorphisms in DNA damage repair exposed to PAHs, including B[a]P, (Lan, Mumford et al. 2004). B[a]P DNA adducts are primarily repaired by nucleotide excision repair, but our results indicate that a certain proportion is also repaired by the base excision repair pathway or that B[a]P metabolites lead to damage which requires the base excision repair pathway.
Mechanistically the modes of cigarette smoke extract induced genotoxicity are not fully elucidated. Many studies suggest oxidative stress plays a major role in the genotoxic response (Asami, Manabe et al. 1997; Carnevali, Petruzzelli et al. 2003; Iimura and Iwahashi 2006; DeMarini, Gudi et al. 2008). However, these and many other aspects of cigarette smoke-induced carcinogenesis and tumorogenesis require further study. Our ex vivo results suggest an increased susceptibility to cigarette smoke induced genotoxicity in cells from base excision repair deficient mice compared to untreated wildtype mice. These data further identify the importance of proper DNA repair and removal of genotoxic insults from noxious environmental agents like cigarette smoke extract.
In conclusion, this study has observed a significant difference in ex vivo CSE-induced damage in base excision repair deficient mice compared to wildtype mice, differentially regulated GSH homeostasis in Ogg1−/−Myh−/−, Gclm−/−, and Gclm+/− mice compared to wildtype mice, and decreased survival proportions after exposure to B[a]P in Ogg1−/−Myh−/− mice compared to wildtype mice. These results are in agreement with epidemiological data showing an increased susceptibility to lung carcinogenesis in people with polymorphisms in OGG1 and MYH (Chang, Wrensch et al. 2009) (Le Marchand, Donlon et al. 2002). More studies using genetically modified animal models may expedite our understanding of cigarette smoke-induced cancer susceptibility.
-
!!
Increased CSE-induced DNA damage in Ogg1 −/−Myh−/− mice compared to WT mice
-
!!
GSH is differentially regulated in genetically modified mice compared to WT mice
-
!!
B[a]P treatment decreases survival in Ogg1−/−Myh−/− compared to WT mice
Abbreviations
- CSE
cigarette smoke extract
- OMM
Ogg1−/− Myh−/−
- WT
wild type
- ETS
environmental tobacco smoke
- SSTS
side stream tobacco smoke
- MN
micro nucleus
- DSBs
double stranded breaks
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair-Kirk TL, Atkinson JJ, et al. Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in Clara cells. Am J Respir Cell Mol Biol. 2008;39(4):400–411. doi: 10.1165/rcmb.2007-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Asami S, Manabe H, et al. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis. 1997;18(9):1763–1766. doi: 10.1093/carcin/18.9.1763. [DOI] [PubMed] [Google Scholar]
- Baglole CJ, Sime PJ, et al. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L624–L636. doi: 10.1152/ajplung.90215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balansky RM, F DA, et al. Induction, persistence and modulation of cytogenetic alterations in cells of smoke-exposed mice. Carcinogenesis. 1999;20(8):1491–1497. doi: 10.1093/carcin/20.8.1491. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Krance SM, et al. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch H. DNA adducts in human carcinogenesis: etiological relevance and structure-activity relationship. Mutat Res. 1996;340(2–3):67–79. doi: 10.1016/s0165-1110(96)90040-8. [DOI] [PubMed] [Google Scholar]
- Carnevali S, Petruzzelli S, et al. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L955–L963. doi: 10.1152/ajplung.00466.2001. [DOI] [PubMed] [Google Scholar]
- Chang JS, Wrensch MR, et al. Base excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African-Americans. Carcinogenesis. 2009;30(1):78–87. doi: 10.1093/carcin/bgn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey RC, Bhilwade HN, et al. Studies on the migration of micronucleated erythrocytes from bone marrow to the peripheral blood in irradiated Swiss mice. Int J Radiat Biol. 1993;63(2):239–245. doi: 10.1080/09553009314550311. [DOI] [PubMed] [Google Scholar]
- Coggins CR. An updated review of inhalation studies with cigarette smoke in laboratory animals. Int J Toxicol. 2007;26(4):331–338. doi: 10.1080/10915810701490190. [DOI] [PubMed] [Google Scholar]
- DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567(2–3):447–474. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- DeMarini DM, Gudi R, et al. Genotoxicity of 10 cigarette smoke condensates in four test systems: comparisons between assays and condensates. Mutat Res. 2008;650(1):15–29. doi: 10.1016/j.mrgentox.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Denissenko MF, Pao A, et al. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Levonen AL, et al. Human glutamate cysteine ligase gene regulation through the electrophile response element. Free Radic Biol Med. 2004;37(8):1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Du B, Leung H, et al. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: evidence for an epidermal growth factor receptor dependent mechanism. Cancer Res. 2007;67(18):8966–8972. doi: 10.1158/0008-5472.CAN-07-1388. [DOI] [PubMed] [Google Scholar]
- Engels EA, Wu X, et al. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67(13):6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- Engle MR, Singh SP, et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol Appl Pharmacol. 2004;194(3):296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, et al. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567(1):1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Gao M, Li Y, et al. A common carcinogen benzo[a]pyrene causes p53 overexpression in mouse cervix via DNA damage. Mutat Res. 724(1–2):69–75. doi: 10.1016/j.mrgentox.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Goldstine JV, Nahas S, et al. Constitutive phosphorylation of ATM in lymphoblastoid cell lines from patients with ICF syndrome without downstream kinase activity. DNA Repair (Amst) 2006;5(4):432–443. doi: 10.1016/j.dnarep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hahn FF, Gigliotti AP, et al. A review of the histopathology of cigarette smoke-induced lung cancer in rats and mice. Int J Toxicol. 2007;26(4):307–313. doi: 10.1080/10915810701483450. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Tice RR, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312(3):293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3(8):461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- Hubner RH, Schwartz JD, et al. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol Med. 2009;15(7–8):203–219. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husgafvel-Pursiainen K. Genotoxicity of environmental tobacco smoke: a review. Mutat Res. 2004;567(2–3):427–445. doi: 10.1016/j.mrrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hutt JA, Vuillemenot BR, et al. Life-span inhalation exposure to mainstream cigarette smoke induces lung cancer in B6C3F1 mice through genetic and epigenetic pathways. Carcinogenesis. 2005;26(11):1999–2009. doi: 10.1093/carcin/bgi150. [DOI] [PubMed] [Google Scholar]
- Iimura S, Iwahashi H. Enhancement by cigarette smoke extract of the radical formation in a reaction mixture of 13-hydroperoxide octadecadienoic acid and ferric ions. J Biochem. 2006;139(4):671–676. doi: 10.1093/jb/mvj082. [DOI] [PubMed] [Google Scholar]
- Lan Q, Mumford JL, et al. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25(11):2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Donlon T, et al. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(4):409–412. [PubMed] [Google Scholar]
- Mauderly JL, Gigliotti AP, et al. Chronic inhalation exposure to mainstream cigarette smoke increases lung and nasal tumor incidence in rats. Toxicol Sci. 2004;81(2):280–292. doi: 10.1093/toxsci/kfh203. [DOI] [PubMed] [Google Scholar]
- McConnachie LA, Mohar I, et al. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci. 2007;99(2):628–636. doi: 10.1093/toxsci/kfm165. [DOI] [PubMed] [Google Scholar]
- Miyaishi A, Osawa K, et al. MUTYH Gln324His gene polymorphism and genetic susceptibility for lung cancer in a Japanese population. J Exp Clin Cancer Res. 2009;28:10. doi: 10.1186/1756-9966-28-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtashamipur E, Norpoth K, et al. Clastogenic effect of passive smoking on bone marrow polychromatic erythrocytes of NMRI mice. Toxicol Lett. 1987;35(1):153–156. doi: 10.1016/0378-4274(87)90101-9. [DOI] [PubMed] [Google Scholar]
- Muslimovic A, Ismail IH, et al. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protoc. 2008;3(7):1187–1193. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, et al. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Denissenko MF, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risom L, Dybdahl M, et al. Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis. 2003;24(11):1847–1852. doi: 10.1093/carcin/bgg144. [DOI] [PubMed] [Google Scholar]
- Russo MT, De Luca G, et al. Accumulation of the oxidative base lesion 8-hydroxyguanine in DNA of tumor-prone mice defective in both the Myh and Ogg1 DNA glycosylases. Cancer Res. 2004;64(13):4411–4414. doi: 10.1158/0008-5472.CAN-04-0355. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Secretan MB, et al. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- Schwartz AG, Prysak GM, et al. The molecular epidemiology of lung cancer. Carcinogenesis. 2007;28(3):507–518. doi: 10.1093/carcin/bgl253. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Smith CC, O'Donovan MR, et al. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis. 2006;21(3):185–190. doi: 10.1093/mutage/gel019. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Huang X, et al. ATM activation accompanies histone H2AX phosphorylation in A549 cells upon exposure to tobacco smoke. BMC Cell Biol. 2007;8:26. doi: 10.1186/1471-2121-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Westbrook AM, Wei B, et al. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69(11):4827–4834. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witschi H, Joad JP, et al. The toxicology of environmental tobacco smoke. Annu Rev Pharmacol Toxicol. 1997;37:29–52. doi: 10.1146/annurev.pharmtox.37.1.29. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, et al. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Xie Y, Yang H, et al. Deficiencies in mouse Myh and Ogg1 result in tumor predisposition and G to T mutations in codon 12 of the K-ras oncogene in lung tumors. Cancer Res. 2004;64(9):3096–3102. doi: 10.1158/0008-5472.can-03-3834. [DOI] [PubMed] [Google Scholar]
- Yamamoto ML, Reliene R, et al. Effects of human Werner helicase on intrachromosomal homologous recombination mediated DNA deletions in mice. Mutat Res. 2008;644(1–2):11–16. doi: 10.1016/j.mrfmmm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dieter MZ, et al. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277(51):49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]