Abstract
Purpose: Recently, the authors proposed a free-breathing amplitude gating (FBAG) technique for PET/CT scanners. The implementation of this technique required specialized hardware and software components that were specifically designed to interface with commercial respiratory gating devices to generate the necessary triggers required for the FBAG technique. The objective of this technical note is to introduce an in-house device that integrates all the necessary hardware and software components as well as tracks the patient’s respiratory motion to realize amplitude gating on PET/CT scanners.
Methods: The in-house device is composed of a piezoelectric transducer coupled to a data-acquisition system in order to monitor the respiratory waveform. A LABVIEW® program was designed to control the data-acquisition device and inject triggers into the PET list stream whenever the detected respiratory amplitude crossed a predetermined amplitude range. A timer was also programmed to stop the scan when the accumulated time within the selected amplitude range reached a user-set interval. This device was tested using a volunteer and a phantom study.
Results: The results from the volunteer and phantom studies showed that the in-house device can detect similar respiratory signals as commercially available respiratory gating systems and is able to generate the necessary triggers to suppress respiratory motion artifacts.
Conclusions: The proposed in-house device can be used to implement the FBAG technique in current PET/CT scanners.
Keywords: amplitude gating, PET/CT, motion artifact
INTRODUCTION
Several respiratory gating techniques have recently been proposed in PET/CT imaging to suppress motion artifacts. These techniques can be divided into two categories: Phase gating and amplitude gating.1 In phase gating, the respiratory cycle is divided into multiple phase ranges (or bins) and the acquired data are sorted into each bin based on acquisition time within the respiratory cycle. This gating approach works well for patients with regular breathing cycles but results in strong artifacts with patients that have irregular respiration. As an alternative approach, amplitude gating has been proposed to divide the total respiration amplitude into different amplitude ranges (or gate) rather than phase ranges. It has been shown that amplitude gating techniques are better at suppressing respiratory motion artifacts when compared to phase gating.1
Current PET/CT scanners are only capable of phase gating. The use of amplitude gating in PET/CT imaging is currently the focus of many research groups.2, 3, 4, 5, 6 Some of the approaches that are proposed to realize amplitude gating include 4D PET/CT acquisition,2, 3 motion-incorporated reconstruction (MIR),4 and deep-inspiration breath hold (DIBH) technique.5 However, these methods are characterized by either a relatively high patient x-ray exposure and inaccuracy in deformable image registration (for 4D PET/CT and MIR) or patient noncompliance and extensive patient-technologist interaction (for DIBH). In order to overcome these drawbacks, we recently proposed a novel approach to implement and automate amplitude gating on current whole-body PET/CT scanners.6 The proposed approach is similar to the DIBH technique except that patients are allowed to breathe freely during the entire data-acquisition process (CT and PET) and therefore do not suffer from the limitations presented by the DIBH technique. In this approach, the respiratory amplitude captured during the CT scan is automatically matched with a corresponding amplitude range in the list-mode PET scan without the need for patient-technologist interaction. From here onward, this approach will be referred to as the free-breathing amplitude gating (FBAG) technique.
In order to implement FBAG, several criteria had to be satisfied: (1) A respiratory monitoring device had to record the patient’s breathing waveform; (2) triggers had to be sent to the PET list stream at the beginning and ending stages of the amplitude range corresponding to the amplitude captured during CT in each breathing cycle; and (3) a timer had to be designed to record the total accumulated time in the selected amplitude range in order to stop the scan when a predetermined accumulated time is reached. Currently, there are no commercial respiratory gating systems that can accomplish all of these requirements. In this regard, the implementation of FBAG had to rely on a multitude of hardware and software components that were designed and interfaced to one another. In this technical note, we introduce an in-house respiratory gating device that has the ability to integrate all the necessary hardware and software components into one system.
METHOD
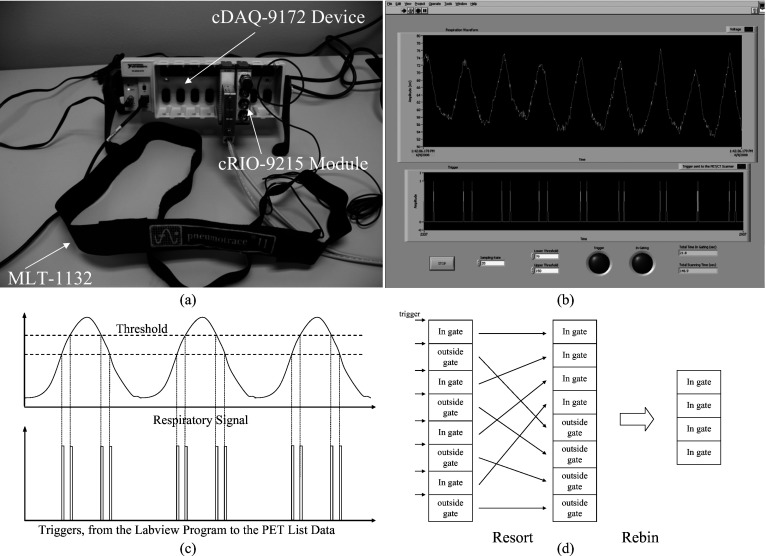
An MLT-1132 respiratory belt transducer (AD Instruments Inc., Colorado Springs, CO) was used to detect the patient’s respiratory signal [Fig. 1a]. This transducer consists of a piezoelectric element that transforms pressure into an electric signal. It measures the changes in thoracic or abdominal circumference during respiration to derive a real-time breathing signal when attached to the patient’s torso. The detailed description of this transducer can be found elsewhere.7 In order to detect the respiratory signal from this transducer, a NI-cDAQ-9172 data-acquisition device (National Instruments Corp., Austin, TX) was interfaced to the transducer through a CompactRIO™ cRIO-9215 module using a BNC connection [Fig. 1a]. The cRIO-9215 module has the capability to digitize the analog respiratory signal from the belt transducer. The detailed descriptions of the cDAQ-9172 device and cRIO-9215 module are available online.8, 9 Furthermore, a graphical user interface (GUI) software tool [Fig. 1b] was designed using LABVIEW® (National Instruments Corp., Austin, TX) to manipulate and display the detected respiratory signal as well as to store the real-time respiratory signal into a file. In order to enable the proposed device to acquire PET data in amplitude gating mode, the GUI software was also designed to inject a 5 V TTL trigger into the PET list stream whenever the measured respiratory amplitude crossed a preset amplitude range. The relationship between the respiratory signal detected from the belt transducer and the triggers generated by the LABVIEW® software is shown in Fig. 1c. The occurrence of each trigger was also recorded in the saved file, which allowed the automatic synchronization between the PET list stream and the recorded respiratory signal. A digital timer was also programmed in the LABVIEW® software to stop the acquisition whenever the accumulated scan duration, within the selected amplitude range, reached a user-set interval. The lower and upper thresholds of the selected amplitude range as well as the sampling rate of the device can all be selected manually in the front panel of the software. The total scan duration and the accumulated time within the preset amplitude range are also displayed in the front panel. Combining these components into a single system/device allows the implementation of FBAG without using any additional devices.
Figure 1.
(a) The MLT-1132 piezoelectric transducer along with the NI-cDAQ-9172 data-acquisition device and cRIO-9215 module. (b) The controlling and data manipulation software designed using LABVIEW®. (c) The relationship between the respiration signal and triggers from the LABVIEW® program (dashed lines represent the preset amplitude range). (d) The filtering process of the list-mode PET data.
Following data acquisition, the PET data corresponding to the selected amplitude range can then be reconstructed using the same method described in the FBAG approach.6 In brief, the PET list stream is first filtered to only select the recorded events that fall within the preset amplitude range. This filtering process is performed using a list-resorting program followed by the data-rebinning function of the PET/CT scanner [shown in Fig. 1d]. The filtering process is necessary since the rebinning function of the scanner cannot be configured to only select events that are acquired within the preset amplitude range. The result from such a filtering process is an amplitude-gated list-mode data which can be directly reconstructed to generate an amplitude-gated PET image after attenuation correction.
EVALUATION AND RESULTS
Volunteer study
In order to evaluate the performance of the in-house respiratory gating device, a volunteer study and a phantom study were conducted. The objective of the volunteer study focused on evaluating the ability of this device to accurately detect the respiratory waveform. For this objective, the performance of the in-house device was compared to the commercially available Anzai system (Anzai Medical Co. Ltd., Japan).
The breathing cycles of ten volunteers (six M, four F, ) were measured using both the Anzai respiratory gating system6 and our in-house device simultaneously. The Anzai strain gauge sensor was attached to an elastic belt which was placed around the volunteer’s torso while the belt transducer of our in-house device was secured right below the Anzai belt. Caution was exercised so that the two belts did not overlap or interfere with one another. All the volunteers were requested to breathe freely while lying down on the patient couch of the PET/CT scanner. For each volunteer, data acquisition lasted 6 min, and the two recorded waveforms were synchronized using a trigger generated from the Anzai system. After data acquisition, the two waveforms were then compared to one another by correlating the two waveform shapes as well as their respective Fourier transforms. Figure 2 shows the respiratory waveforms and their Fourier transforms for one volunteer. The similarity between the two waveforms in Fig. 2a and between their Fourier transforms in Fig. 2b suggests that our in-house respiratory gating device has the capability to generate a similar respiratory waveform as the commercially available Anzai respiratory gating system. The correlation coefficients between the two waveforms as well as between their Fourier transforms for all the ten volunteers are and , respectively.
Figure 2.

Results from the volunteer studies: (a) The respiratory waveform detected from the Anzai respiratory gating system and the in-house device. The Fourier transform of the two waveforms are shown in (b).
Phantom study
The objective of the phantom study was to test whether the in-house device can generate the necessary triggers to facilitate the implementation of the FBAG technique.
The phantom consisted of two moving spheres (33 and 22 mm) placed in a water tank. The two moving spheres were attached to a computer-controlled platform which was driven by a sinusoidal wave (2 cm amplitude, 5 s period). The spheres as well as the tank were filled with -FDG water with a sphere-to-background contrast of 5.1:1. The activity concentration in the background was 3.1 kBq/cc. The phantom was imaged in 3D mode while the elastic belt of our in-house system was wrapped around the platform. List-mode PET/CT with amplitude gating was then acquired. In this case, we chose to acquire the CT data when the moving spheres were at 90% of the maximum motion amplitude. In order to “freeze” the same motion amplitude captured during the CT scan, a preset amplitude range of 80%–100% of the maximum motion amplitude was selected for the list-mode PET scan. In this regard, the LABVIEW® software sent triggers to the PET list stream whenever the sphere motion crossed the edge of this preset amplitude range [Fig. 1c]. PET data acquisition was stopped when 3 min worth of data were accumulated within the preset amplitude range as determined by the programmed digital timer. This process resulted in a total of 10 min scan duration. The acquired list-mode data were then filtered and reconstructed using a 3D-IR algorithm (two iterations, 21 subsets) to generate an amplitude-gated PET image. For reference purposes, the original list-mode data were rebinned and reconstructed using the same algorithm to generate a static scan (ungated) of 10 min.
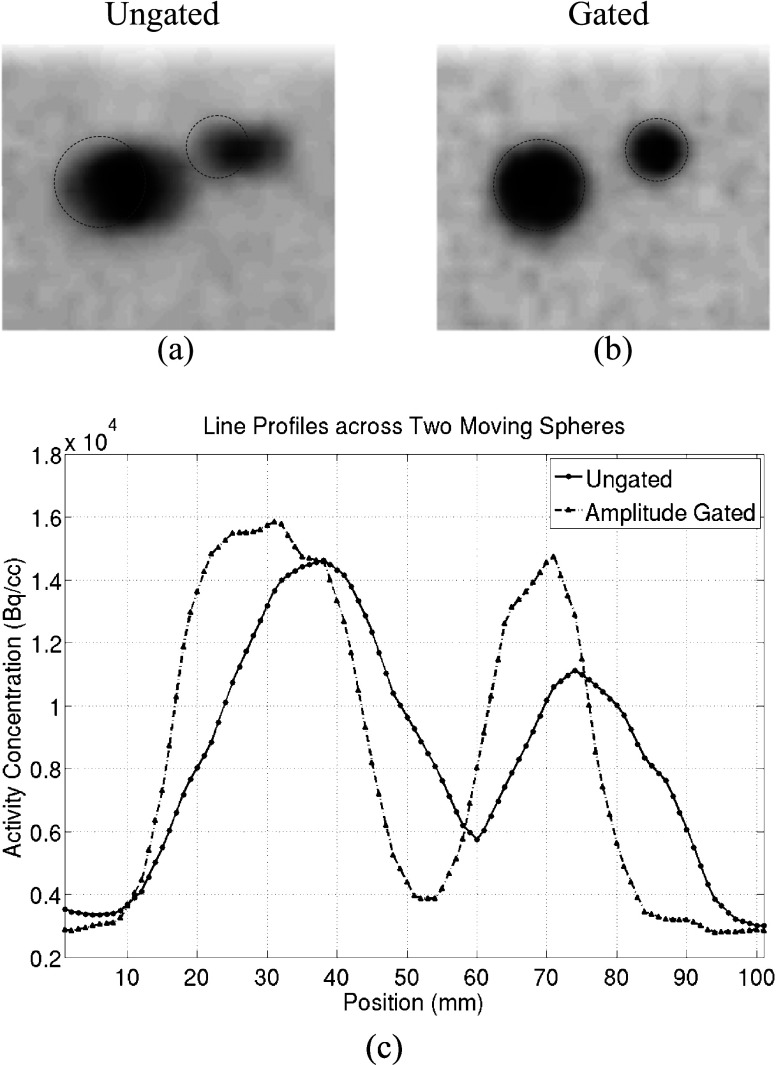
The two moving spheres in the ungated and gated PET images are shown in Figs. 3a, 3b, respectively. Dashed circles are superimposed on these images to represent the locations and sizes of the spheres in the CT images. Comparisons between the gated and ungated PET images indicate that the two moving spheres match well with their locations in the CT image in the gated PET image, while in the ungated image, the two spheres appear to extend beyond their positions in the CT image and appear blurred and elongated along their motion direction. Line profiles across the two spheres in both the gated and ungated images are shown in Fig. 3c. These line profiles suggest that the two moving spheres have higher contrast ratio (CR) in the gated image when compared to the ungated image. The quantitative results of AC and CR for both the ungated and gated PET images are summarized in Table 1. AC data in this table are normalized to the true sphere AC: 15.8 kBq/cc. This table shows that the max AC, mean AC, and CR of the two moving spheres in the gated images are improved by an average of 18.9%, 24.4%, and 40.4%, respectively, when compared to the ungated image.
Figure 3.
Results from the phantom study: The two moving spheres for the (a) ungated and (b) amplitude-gated PET image. The dashed circles represent the positions of the spheres in the corresponding CT images. The line profile across the two moving spheres is shown in (c).
Table 1.
AC and CR in the phantom study (AC data are normalized to the true sphere AC: 15.8 kBq/cc).
| Sphere | Image | ||
|---|---|---|---|
| Ungated | Amplitude-gated | ||
| Max AC | 33 mm | 0.93 | 1.01 |
| 22 mm | 0.72 | 0.93 | |
| Mean AC | 33 mm | 0.65 | 0.77 |
| 22 mm | 0.56 | 0.73 | |
| CR1 | 33 mm | 0.60 | 0.76 |
| 22 mm | 0.48 | 0.74 | |
.
DISCUSSION AND CONCLUSION
In this paper, we described an in-house respiratory gating device that can be used to implement and automate the FBAG approach that was previously described by our group.6 This in-house device consists of a commercially available piezoelectric transducer as well as a National Instruments (Austin, TX) data-acquisition device that are controlled by a LABVIEW® GUI software program. This device has the ability to detect the patient’s respiratory waveform and can send triggers to the PET list stream at predetermined amplitude settings in each breathing cycle while simultaneously monitoring the accumulated time within the gate. In this regard, this device has the advantage of integrating a multitude of features that otherwise would have required a commercially available system such as the real-time positioning management system (Varian, Palo Alto, CA) and Anzai system to be interfaced to a number of components to achieve the same objective. We tested the performance of this in-house device using volunteer and phantom studies. The results from these studies showed that our in-house device can detect similar respiratory signals as the commercial Anzai system and is able to generate the necessary triggers to suppress respiratory motion artifacts.
Our in-house respiratory gating system was designed specifically for amplitude gating. The reason for using this gating scheme is primarily due to its proposed benefit over phase gating techniques as has been shown by Dawood et al.1 However, our LABVIEW® software can also be configured to output triggers into the PET list stream whenever the detected respiratory signal crossed a user-set phase range rather than amplitude range, and hence allowing the device to function as a phase gating system. In the phantom study, only one amplitude range was selected to test the performance of this device which is a requirement for the FBAG approach6 as well as the DIBH technique. This device was not designed to generate multiple amplitude triggers as in 4D PET/CT acquisition since such gating methods are not recommended due to their disadvantages of increased x-ray exposure.
One potential advantage of our proposed gating device is its low cost when compared to other commercially available respiratory gating systems whose costs range in the tens of thousands of dollars. A breakdown of the total cost of the in-house system includes: (1) $235 for the MLT-1132 piezoelectric transducer from AD Instruments Inc. (Colorado Springs, CO), (2) $1049 for the NI-cDAQ-9172 data-acquisition device from National Instruments Corp. (Austin, TX), (3) $499 for the CompactRIO™ cRIO-9215 module from National Instruments Corp. (Austin, TX), and (4) $2599 for the LABVIEW® Full Development environment. The LABVIEW® software is installed in a PC or laptop with the following recommended system requirements: Pentium 4 or above, 1 GB memory, 40 GB hard disk, screen, and Windows® XP operating system. The cost of a typical laptop that satisfies these requirements is about $628 (http://www.dell.com), bringing the total price of the whole system to $5010 excluding the costs associated with product development and commercialization. This price constitutes less than 10% of the total cost of other commercially available systems.
One disadvantage of our in-house respiratory gating device is its relatively higher noise and hence lower SNR in detecting respiratory waveforms when compared to the Anzai system. The high noise from the MLT-1132 transducer can be easily identified from its frequency response [Fig. 2b] which had larger high-frequency components versus the Anzai waveform. In our volunteer study, the MLT-1132 transducer exhibited a noise level of less than 1 mV while the amplitudes of the detected respiratory waveforms were usually larger than 20 mV corresponding to a signal-to-noise ratio greater than 20. However, errors could be perceived to occur whenever the detected respiratory signal falls within the preset amplitude range due to the effects of the noise while in reality, the patient’s respiration has not reached that level. Similarly, errors could also exist whenever the detected respiratory signal falls outside the preset amplitude range while in reality the patient’s respiration level is still within that range. Such sources of error could potentially lead to additional blurring in the resultant gated PET images. To overcome these drawbacks, filters can be designed in the LABVIEW® software; however, such approaches, although they can improve the SNR of the detected respiratory signal, will result in a phase delay which, on the other hand, can also introduce similar errors into the gated images.
References
- Dawood M., Buther F., Lang N., Schober O., and Schafers K. P., “Respiratory gating in positron emission tomography: A quantitative comparison of different gating schemes,” Med. Phys. 34(7), 3067–3076 (2007). 10.1118/1.2748104 [DOI] [PubMed] [Google Scholar]
- Klein G. J., Reutter B. W., Ho M. H., Reed J. H., and Huesman R. H., “Real-time system for respiratory-cardiac gating in position tomography,” IEEE Trans. Nucl. Sci. 45, 2139–2143 (1998). 10.1109/23.708323 [DOI] [Google Scholar]
- Schäfers K. P. and Stegger L., “Combined imaging of molecular function and morphology with PET/CT and SPECT/CT: Image fusion and motion correction,” Basic Res. Cardiol. 103, 191–199 (2008). 10.1007/s00395-008-0717-0 [DOI] [PubMed] [Google Scholar]
- Qiao F., Pan T., Clark J. W., and Mawlawi O. R., “A motion-incorporated reconstruction method for gated PET studies,” Phys. Med. Biol. 51, 3769–3783 (2006). 10.1088/0031-9155/51/15/012 [DOI] [PubMed] [Google Scholar]
- Nemeh S. A. et al. , “Deep-inspiration breath-hold PET/CT of the thorax,” J. Nucl. Med. 48(1), 22–26 (2007). [PubMed] [Google Scholar]
- Chang G., Chang T., Pan T., Clark J. W., and Mawlawi O. R., “Implementation of an automated respiratory amplitude gating technique for PET/CT imaging: Clinical evaluation,” J. Nucl. Med. 51(1), 16–24 (2010). 10.2967/jnumed.109.068759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Available at http://www.adinstruments.com/products/product.php?id=MLT1132.
- Available at http://sine.ni.com/nips/cds/view/p/lang/en/nid/202545.
- Available at http://sine.ni.com/nips/cds/view/p/lang/en/nid/14166.