Abstract
Purpose: Four-dimensional computed tomography (4D-CT) is commonly used to account for respiratory motion of target volumes in radiotherapy to the thorax. From the 4D-CT acquisition, a maximum-intensity projection (MIP) image set can be created and used to help define the tumor motion envelope or the internal gross tumor volume (iGTV). The purpose of this study was to quantify the differences in automatically contoured target volumes for usage in the delivery of stereotactic body radiation therapy using MIP data sets generated from one of the four methods: (1) 4D-CT phase-binned (PB) based on retrospective phase calculations, (2) 4D-CT phase-corrected phase-binned (PC-PB) based on motion extrema, (3) 4D-CT amplitude-binned (AB), and (4) cine CT built from all available images.
Methods: MIP image data sets using each of the four methods were generated for a cohort of 28 patients who had prior thoracic 4D-CT scans that exhibited lung tumor motion of at least 1 cm. Each MIP image set was automatically contoured on commercial radiation treatment planning system. Margins were added to the iGTV to observe differences in the final simulated planning target volumes (PTVs).
Results: For all patients, the iGTV measured on the MIP generated from the entire cine CT data set was the largest. Expressed as a percentage of , 4D-CT iGTV (all sorting methods) ranged from 83.8% to 99.1%, representing differences in the absolute volume ranging from 0.02 to ; the largest average and range of 4D-CT iGTV measurements was from the PC-PB data set. Expressed as a percentage of (expansions applied to ), the 4D-CT PTV ranged from 87.6% to 99.6%, representing differences in the absolute volume ranging from 0.08 to . Regions of the measured respiratory waveform corresponding to a rapid change of phase or amplitude showed an increased susceptibility to the selection of identical images for adjacent bins. Duplicate image selection was most common in the AB implementation, followed by the PC-PB method. The authors also found that the image associated with the minimum amplitude measurement did not always correlate with the image that showed maximum tumor motion extent.
Conclusions: The authors identified cases in which the MIP generated from a 4D-CT sorting process under-represented the iGTV by more than 10% or up to when compared to the . They suggest utilization of a MIP generated from the full cine CT data set to ensure maximum inclusive tumor extent.
Keywords: four-dimensional computed tomography, 4D-CT, cine computed tomography, contouring, stereotactic body radiation therapy
INTRODUCTION
The motion of lung tumors during respiration has to be considered for radiation therapy treatment to the thorax.1, 2, 3, 4 Motion decreases the reproducibility of target position3, 5 and can ultimately lead to larger margins during radiation therapy treatment planning.6, 7, 8, 9 Instead of relying upon population-based data and generalized target volume expansions,9 it is desirable to individually assess tumor motion for all patients.10
The development of four-dimensional computed tomography (4D-CT) has enabled us to explicitly address the issue of target volume motion for individual patients.4 4D-CT relies upon a respiratory surrogate to track some physiological respiratory process, such as exterior chest wall motion or air flow during breathing.11 While the respiratory motion is tracked, CT data are acquired over the volume of interest. By combining information from the respiratory surrogate and the CT data, a collection of images can be created that represents different stages of the breathing process. In this collection of images, we can visualize how a particular lung tumor moves during respiration.
Current 4D-CT scanning techniques fall into one of the two general categories: (1) Cine CT acquisition with image sorting12 and (2) low-pitch helical acquisition with sinogram binning.13 In image sorting with cine CT, images are acquired with the table stationary for a specified time interval over a respiratory cycle. The table is then indexed, and additional images are acquired. Acquisition is followed by indexing until the desired volume has been scanned. The cine CT images and respiratory waveform are associated, and a subset of images that correspond to different snapshots of the tumor motion during respiration is selected.12 In sinogram binning with low-pitch helical acquisition, the selection of target phases or amplitudes is made prior to image reconstruction in the sinogram projection data. Images are reconstructed around the identified target phases from the stream of the sinogram data.13, 14 In both scanning techniques, the selected or reconstructed images are used to depict one respiratory cycle of the tumor motion. In practice, CT data (both cine and low-pitch helical) are collected for longer than one respiratory cycle to account for potential variation in respiration during the CT data acquisition.
Regarding image sorting with cine CT, two primary methods of image selection, or binning, have been previously implemented: Phase binning (PB) and amplitude binning (AB). In the PB method, images are selected to correspond to different phases of the respiratory waveform as calculated within the respiratory monitoring file.15, 16 The more recent AB implementation is based on the measured amplitude, and images are selected based on temporally associated amplitude and direction of respiration (i.e., inspiration or expiration).17, 18 The selected 4D-CT images are a subset of the cine CT image set. Both of these sorting processes result in the generation of multiple phases of 3D image sets, called the 4D-CT image set.
From the 4D-CT image set, a maximum-intensity projection (MIP) image, consisting of a pixel-by-pixel maximum CT number, can be generated.12 An identical process can be used to create a MIP from the entire unsorted cine CT image set. The MIP image set should ideally highlight the volume encompassing the location of the moving tumor at any point during respiration. Prior studies have investigated the level of utility and accuracy of target volume delineation with MIP images.19, 20 Rietzel et al.21 showed that the contours generated on 4D-CT phase images were very similar to those on 4D-CT MIP. At our institution, the MIP from the 4D-CT data subset (, phase-binning implementation) is used as a starting point to define the volume that encompasses the motion envelope of the moving gross tumor volume (GTV); this motion envelope has been named the internal gross tumor volume (iGTV). The final iGTV is defined using a combination of the and the ten phases of 4D-CT images.
It has been observed that changing the 4D-CT binning method leads to the selection of a different collection of images;17 however, the clinical implications of changing the constituent images on the resultant MIP data set are not clear. Prior studies have advocated the advantages of AB over PB in 4D-CT image selection either from a phantom study17 or limited patient studies.22 In the phantom study, the correlation between the external surrogate and the internal tumor motion was 1-to-1, and phase lag (departure from 1-to-1 correlation) was not simulated. The patient studies were limited in the number of patients and the magnitude of motion. To our knowledge, there has not been a comprehensive study to compare target volume delineation based on different 4D-CT sorting methodologies in a patient population. In a phantom study with irregular target motion, Park et al.20 noted that the MIP can underestimate target motion. We believe that clinical situations exist in which the currently utilized 4D-CT based MIP will under-represent the visible motion envelope of the moving lung tumor. The purpose of this study was to quantify the differences in automatically contoured target volumes for usage in the delivery of stereotactic body radiation therapy (SBRT) based on MIP data sets generated from one of the four methods: (1) Phase-binned (PB) based on retrospective phase calculations, (2) phase-corrected phase-binned (PC-PB) based on motion extrema, (3) amplitude-binned (AB), and (4) cine CT built from all available images. By doing so, we can determine the degree to which the 4D-CT selection process under-represents the extent of tumor motion in patient cases.
METHODS AND MATERIALS
Patient selection and image processing
This study was approved by our Institutional Review Board. Patients eligible for this study were those who had previously undergone thoracic 4D-CT scans at our institution between 2005 and 2010 for the treatment of nonsmall-cell lung cancer (NSCLC). Patients were candidates for SBRT and were chosen only if the lung tumor exhibited respiratory-induced motion of at least 1 cm and had a simple structure and clear boundaries to facilitate automatic contouring. The 4D-CT workflow used at our institution on our 8-slice PET/CT scanner (Discovery ST; GE Healthcare, Waukesha, WI) consists of a deep-inspiration breath-hold CT scout to determine maximum lung extent, followed by a free-breathing helical CT of the chest and abdomen, and then a free-breathing cine CT acquisition of the chest. The acquisition and usage of cine CT images in 4D-CT have been extensively described.12, 23 In short, a cine scan generates multiple images at a single bed position through the acquisition of projection data over a period of time equal to at least one respiratory cycle. The patient bed is then incremented a distance equal to the nominal radiation beam width so that image data is contiguous in space over the entire scan extent. For our patients, cine scan images were acquired at 120 kVp, 100 mA, detector configuration, 2.5 mm image thickness, and a 0.5 s/revolution gantry rotation time. The cine duration was set to approximately one breath cycle plus 1 s, and images were reconstructed at a cine reconstruction interval, typically 0.25 s.24
During the cine scan, respiratory motion was monitored and recorded by tracking the patient’s anterior abdominal surface with a commercial respiratory monitoring device [Real-time Position Management (RPM) system, Varian, Palo Alto, CA]. The monitoring system uses a reflective block placed on the patient’s abdomen in combination with a tracking infrared camera. Samples of the block amplitude are acquired at 30 Hz and stored in a data file. Within the data file, end-inspiration (EI) (or 0%) points are identified on the respiratory trace; the respiratory phase is then calculated between subsequent EI points, assuming a linear phase progression. Next, the cine images that have been created are temporally correlated with the respiratory data file, and the best-fit images are selected to meet a set of target parameters, thereby creating the 4D-CT image set.
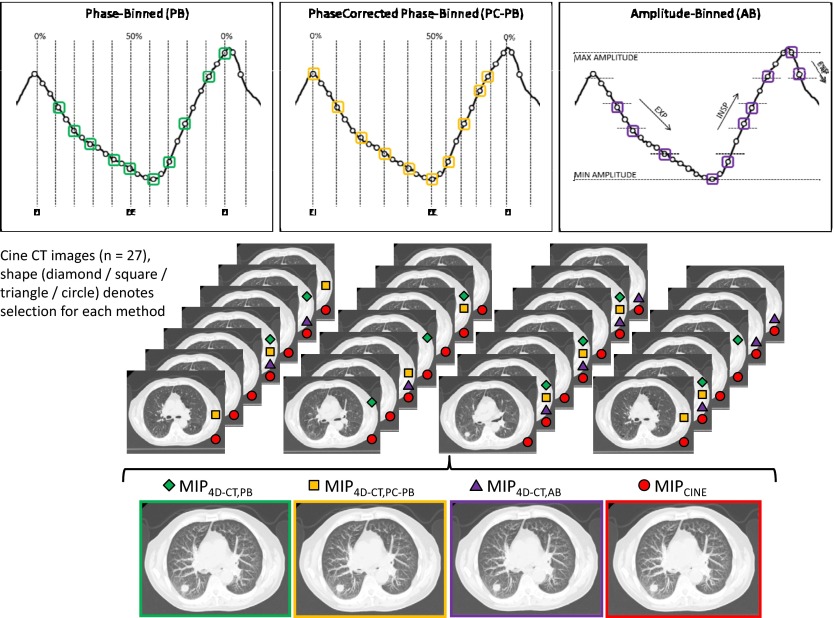
In this study, we examined four different MIP image sets: (1) PB based on retrospective phase calculations, (2) PC-PB based on motion extrema, (3) AB, and (4) cine CT built from all available images. We used previously developed sorting software to execute the different 4D-CT sorting methods (that is, the first three methods listed here) and to generate all four of the MIP image data sets.18 In the remainder of this paper, subscripts refer to the method employed to create a particular data set or to measure a particular value.
Method (1) utilizes the respiratory data file and associated phase calculations to perform the PB sorting. Within the respiratory data file, the 50% point is temporally centered between the identified EI points; no attempt is made to correlate the 50% point as the end-expiration (EE) or minimum measured RPM amplitude. Method (2) addresses this issue by applying a phase-based sorting method using a modified or phase-corrected (PC) calculation of numerical phase. A “PHASECORRECT” software was previously developed to retrospectively identify the EI and EE points as the respective maximum and minimum amplitudes, and then to recalculate the numerical phase between each identified points (0%–50% and 50%–100%), resulting in a modified or PC respiratory data file.14 Since the durations of expiration and inspiration are not typically equal when defining the EE as the minimum amplitude, we expected that images selected based on a PC respiratory file would be different from images selected by the unmodified respiratory file, as in the PB method. For both PB and PC-PB methods, images were directed into ten bins, with target phases of 0%–90% in steps of 10% phase angle.
The in method (3) is generated based on an AB methodology;17, 18 instead of sorting the images based on the calculated respiratory phase, images are sorted based on the measured amplitude of the respiratory signal. The implementation of AB identifies the images created at the maximum and minimum amplitudes for each bed position. From those extreme amplitudes, we define equally spaced target amplitudes for inspiration and for expiration. This method is meant to force the selection of the images correlated with the most extreme-amplitude positions and then to select images at equal amplitude increments during inspiration and expiration. There are ten bins defined for each bed position and ten images selected for each slice location. Figure 1 offers a graphical representation of the 4D-CT selection process for each of the three sorting methods applied to the same input data. Note that method (4), yielding , uses all available cine CT images.
Figure 1.
MIP images generated from one of the three 4D-CT sorting processes (PB, PC-PB, and AB) or from the use of all 27 cine CT images. Top: Respiratory signal at a single bed position on a representative patient case showing that the same input will lead to the selection of a different distribution of ten images for each different sorting method (bottom). Each of the three image sets is generated from the selected images.
We also developed programs to quantitatively analyze the PC respiratory data file. The average breath cycle was defined as the time between subsequent EI points occurring within the duration of the cine scan. Similarly, we defined the average expiration time between the marked EI and EE, and the average inspiration time between the marked EE and EI points. We calculated the average ratio of expiration time to inspiration time to understand the level of breathing asymmetry when identifying the motion extrema as the EI and EE points. We also calculated the estimated percentage of raw projection data not utilized when applying a 4D-CT sorting mechanism by comparing the average breath cycle and cine duration. Based on the data sufficiency condition (DSC) for a cine acquisition, we theoretically need projection data for the duration of only one breathing cycle plus one CT gantry rotation.14 We therefore defined the percentage of unused raw data as 1 minus the ratio of the average DSC duration and the clinically utilized cine duration (i.e., beam-on time per bed position).
Tumor geometric characterization
For each patient, we measured the tumor displacement on a standard three-plane view (anterior-posterior/superior-inferior/left-right) of the clinical PB 4D-CT and calculated the quadrature sum to find the total tumor excursion. To further characterize each tumor, we measured the approximate static tumor volume and largest dimension on the clinically acquired 4D-CT 50% phase images using an image workstation (Advantage Workstation 4.2; GE Healthcare, Waukesha, WI).
The four MIP image sets for each patient were sent to a commercial radiation treatment planning system (Pinnacle 8.1w; Philips Healthcare, Madison, WI). To minimize the effect of human interobserver variation, each data set was automatically contoured using a lower threshold of 600 (equivalent to −424 Hounsfield units) using the readily available tools within the software. The selected threshold value represented a value that maximized tumor volume, but limited volume growth into the surrounding lung parenchyma. The choice of tumors for this work made automatic contouring a reasonable estimate of the clinical (manual) contour, but also tended to emphasize smaller, less complex tumors with larger motions to amplify observed differences between the methods. The treatment planning software reported the total tumor volume, or iGTV in our case, based on the contoured tumors.
Since it is a standard practice to add clinical margins to the visible tumor iGTV, we next added a uniform margin expansion of 5 mm to each patient’s iGTV to arrive at a simulated planning target volume (PTV), as documented within Radiation Therapy Oncology Group 0915 for patients undergoing treatment with SBRT.25 The PTV represented the treatment volume while the patient is breathing normally during dose delivery. The process of adding simulated margins was meant to give a baseline understanding of how differences that we observed in our iGTV measurements would be affected by applied expansions. To compare the mean values of the contoured iGTV results, we performed a two-sided alternative paired t-test between the measurements performed on the and each of the three data sets, as well as between each of the measurements.
Image data set analysis
To understand how the distribution of selected images affects the generated MIP and resultant iGTV, we correlated the measured respiratory waveform and the selected images for each of the three 4D-CT methods. The time stamps in the respiratory data file and the cine CT image times were not properly aligned. Temporally matching the images with the respiratory data file was necessary.
The respiratory data file contains information on the x-ray beam status (beam-on or beam-off) with each data sample; each cine CT image is tagged with an image midscan time, the time at which half of the image data is collected for a full 360° acquisition. We developed programs to extract header information from the 4D-CT images for each 4D-CT sorting method, including the image midscan time, slice location, image bin, and image number. We also developed programs to calculate the time that the x-ray beam was on (i.e., cine duration) and the time that the x-ray beam was off during table translation using both the timing information from the respiratory data file and from the image midscan times. A timing inconsistency existed on the image midscan time stamps; the calculated x-ray beam-off time between subsequent bed positions varied by up to 1 s. We did not observe the same level of variance in the calculation of beam-off time using the respiratory data file. To make the data consistent, we added a correction factor to the images generated at each bed position to align them with the detected beam-on status indicator in the respiratory file. For the initial x-ray beam-on indicator at bed position n, a midscan time of the first produced image at bed position n, and a known gantry rotation time , the correction factor is
The addition of the correction factor forces the start of CT data collection (one-half gantry rotation before the midscan time of the first image) to the detected beam-on status indicator in the respiratory file.
RESULTS
Patient data and image processing
Twenty-eight patients (16 men and 12 women) who had previously undergone thoracic 4D-CT scans at our institution for the treatment of lung cancer were selected for this study. The average age was 76 yr, ranging from 55 to 87 yr. All of the tumors were classified as NSCLC. Twenty-six of the patients were treated with SBRT; two patients were treated with protons, but both had early stage NSCLC and were candidates for treatment with SBRT.
The clinical cine acquisition for our patients resulted in an average of 21.7 cine images available per slice location. The number of cine images available at each slice location was a function of patient-specific factors such as scan extent, cine duration, and cine interval, as well as hardware limitations (maximum of 3000 reconstructed images per series).
Analysis of the PC respiratory file showed an average patient respiratory cycle of 4.2 s, a value comparable to prior studies using similar respiratory monitoring systems.26 The average times for expiration and inspiration were 2.7 and 1.6 s, respectively, leading to an average ratio of expiration to inspiration time of 1.7. On average, an estimated 25% of the acquired CT data was unused when generating MIP from 4D-CT sorting methodologies.
Tumor geometric characterization
The average total tumor motion was measured to be 1.5 cm, ranging from 1.0 to 2.9 cm. The average static tumor volume was , with an average largest dimension of 2.3 cm. Table 1 summarizes the results of the automatically contoured iGTV on each of the four MIP data sets. In all cases, the had the largest magnitude. This makes theoretical sense in that approximately twice as many images are used to build the as any of the image sets. Expressed as a percentage of , our values ranged from 83.8% to 99.1%. The absolute volume differences between the and the three measurements ranged from 0.02 to . Figure 2 shows the histogram associated with the results of calculating as a percentage of ; it exhibits the fact that the PC-PB method led to the largest , but also had the largest range. Considering only the 4D-CT volumes, the largest iGTV was captured by the PC-PB method for 20 of the patients, by the PB method for 5 of the patients, and by the AB method for 3 of the patients.
Table 1.
Summary of tumor geometric characterization. iGTV measurements resulting from the automatic contouring process are shown. Volume differences are expressed as a percentage of the larger or as appropriate or as an absolute volume difference between the cine and each 4D-CT volume.
| Pt. No. | Tumor motion(cm) | Static volume | Volume differences, iGTV | Volume differences, PTV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| as % of (absolute difference, ) | as % of (absolute difference, ) | |||||||||||
| PB | PC-PB | AB | PB | PC-PB | AB | |||||||
| 1 | 1.1 | 2.01 | 10.89 | 10.31 | 10.42 | 10.26 | 94.7% (0.58) | 95.7% (0.46) | 94.3% (0.63) | 95.3% (1.65) | 97.3% (0.95) | 94.2% (2.05) |
| 2 | 2.5 | 1.06 | 2.92 | 2.55 | 2.65 | 2.48 | 87.4% (0.37) | 90.7% (0.30) | 84.9% (0.47) | 94.0% (0.98) | 89.9% (1.66) | 87.8% (2.00) |
| 3 | 1.2 | 2.76 | 5.58 | 5.39 | 5.53 | 5.34 | 96.6% (0.19) | 99.1% (0.05) | 95.7% (0.24) | 97.6% (0.49) | 99.6% (0.08) | 97.6% (0.50) |
| 4 | 1.0 | 0.82 | 1.81 | 1.70 | 1.72 | 1.54 | 93.8% (0.11) | 95.3% (0.09) | 84.9% (0.27) | 97.1% (0.31) | 98.2% (0.19) | 91.5% (0.91) |
| 5 | 2.8 | 3.72 | 10.71 | 9.48 | 10.02 | 9.43 | 88.4% (1.24) | 93.6% (0.69) | 88.0% (1.29) | 93.3% (2.29) | 97.6% (0.82) | 91.9% (2.76) |
| 6 | 1.0 | 2.22 | 5.22 | 4.70 | 4.77 | 4.50 | 90.1% (0.52) | 91.4% (0.45) | 86.2% (0.72) | 93.1% (1.52) | 94.1% (1.29) | 87.6% (2.72) |
| 7 | 1.5 | 7.57 | 11.76 | 11.05 | 11.40 | 11.02 | 94.0% (0.71) | 96.9% (0.36) | 93.7% (0.75) | 95.5% (1.61) | 98.5% (0.55) | 95.4% (1.63) |
| 8 | 1.5 | 0.94 | 2.88 | 2.62 | 2.66 | 2.53 | 90.9% (0.26) | 92.3% (0.22) | 87.8% (0.35) | 94.6% (0.87) | 96.1% (0.64) | 93.4% (1.06) |
| 9 | 1.6 | 10.78 | 16.79 | 15.80 | 16.06 | 15.36 | 94.1% (1.00) | 95.6% (0.74) | 91.4% (1.44) | 95.9% (1.91) | 95.9% (1.92) | 93.5% (3.01) |
| 10 | 1.8 | 7.01 | 12.22 | 11.47 | 11.53 | 11.47 | 93.8% (0.76) | 94.3% (0.70) | 93.8% (0.75) | 97.2% (1.21) | 96.7% (1.42) | 96.3% (1.60) |
| 11 | 1.0 | 3.37 | 4.27 | 4.06 | 4.01 | 4.01 | 95.0% (0.21) | 93.9% (0.26) | 93.8% (0.27) | 96.0% (0.72) | 97.2% (0.51) | 96.9% (0.57) |
| 12 | 1.9 | 3.84 | 9.82 | 9.18 | 9.21 | 8.86 | 93.5% (0.64) | 93.8% (0.61) | 90.3% (0.95) | 94.6% (1.85) | 95.6% (1.50) | 91.4% (2.95) |
| 13 | 1.0 | 5.38 | 7.31 | 6.95 | 7.07 | 7.14 | 95.1% (0.36) | 96.7% (0.24) | 97.7% (0.17) | 96.7% (0.84) | 97.7% (0.59) | 98.7% (0.32) |
| 14 | 1.6 | 8.76 | 10.61 | 9.70 | 10.00 | 9.12 | 91.4% (0.91) | 94.2% (0.61) | 86.0% (1.49) | 93.8% (2.05) | 96.4% (1.19) | 90.8% (3.02) |
| 15 | 1.7 | 0.89 | 3.47 | 3.06 | 3.09 | 2.94 | 88.2% (0.41) | 89.1% (0.38) | 84.7% (0.53) | 93.7% (1.11) | 96.5% (0.61) | 92.1% (1.38) |
| 16 | 1.0 | 6.14 | 8.01 | 7.21 | 7.56 | 7.52 | 90.0% (0.80) | 94.3% (0.45) | 93.8% (0.49) | 92.5% (2.00) | 96.6% (0.90) | 95.6% (1.18) |
| 17 | 1.1 | 5.39 | 6.88 | 6.40 | 6.50 | 6.24 | 93.0% (0.48) | 94.5% (0.38) | 90.7% (0.64) | 95.5% (1.11) | 96.9% (0.78) | 94.4% (1.39) |
| 18 | 1.6 | 3.72 | 8.09 | 6.84 | 6.98 | 7.17 | 84.6% (1.25) | 86.2% (1.12) | 88.6% (0.92) | 92.3% (2.15) | 93.1% (1.91) | 93.8% (1.71) |
| 19 | 1.0 | 0.51 | 0.92 | 0.89 | 0.90 | 0.88 | 96.9% (0.03) | 97.4% (0.02) | 95.3% (0.04) | 97.9% (0.15) | 98.5% (0.11) | 97.8% (0.15) |
| 20 | 2.9 | 1.21 | 3.28 | 2.75 | 2.93 | 2.95 | 83.8% (0.53) | 89.4% (0.35) | 90.0% (0.33) | 94.7% (1.00) | 96.4% (0.69) | 94.3% (1.08) |
| 21 | 1.0 | 6.75 | 11.12 | 10.35 | 9.92 | 10.24 | 93.1% (0.77) | 89.2% (0.88) | 92.1% (1.20) | 95.4% (1.81) | 97.6% (0.94) | 93.8% (2.42) |
| 22 | 2.2 | 17.20 | 39.33 | 35.80 | 36.90 | 35.13 | 91.0% (3.54) | 93.8% (2.43) | 89.3% (4.20) | 93.2% (6.14) | 96.0% (3.58) | 91.8% (7.42) |
| 231 | 1.0 | 22.86 | 25.48 | 24.32 | 24.68 | 24.34 | 95.5% (1.16) | 96.8% (0.81) | 95.5% (1.14) | 97.1% (2.15) | 97.8% (1.61) | 97.2% (2.04) |
| 24 | 1.5 | 1.94 | 4.84 | 4.63 | 4.53 | 4.60 | 95.7% (0.21) | 93.5% (0.31) | 95.0% (0.24) | 97.5% (0.50) | 96.6% (0.69) | 95.0% (1.02) |
| 251 | 1.2 | 27.63 | 53.73 | 53.01 | 52.69 | 52.14 | 98.7% (0.73) | 98.1% (1.04) | 97.0% (1.60) | 99.1% (1.23) | 98.5% (1.98) | 99.0% (1.28) |
| 26 | 1.0 | 5.32 | 5.97 | 5.82 | 5.82 | 5.82 | 97.5% (0.15) | 97.5% (0.15) | 97.5% (0.15) | 98.4% (0.34) | 98.1% (0.41) | 98.6% (0.31) |
| 27 | 1.0 | 12.50 | 19.79 | 19.36 | 19.47 | 18.76 | 97.8% (0.43) | 98.4% (0.32) | 94.8% (1.03) | 98.0% (1.07) | 98.7% (0.71) | 96.3% (1.97) |
| 28 | 1.1 | 10.77 | 16.35 | 15.17 | 15.29 | 15.03 | 92.8% (1.19) | 93.5% (1.07) | 91.9% (1.32) | 94.8% (2.32) | 95.1% (2.19) | 94.1% (2.66) |
| Max | 2.9 | 27.63 | 53.73 | 53.01 | 52.69 | 52.14 | 98.7% (3.53) | 99.1% (2.44) | 97.7% (4.20) | 99.1% (6.14) | 99.6% (3.58) | 99.0% (7.42) |
| Min | 1.0 | 0.51 | 0.92 | 0.89 | 0.90 | 0.88 | 83.8% (0.03) | 86.2% (0.02) | 84.7% (0.04) | 92.3% (0.15) | 89.9% (0.08) | 87.6% (0.15) |
| Mean | 1.5 | 6.54 | 11.43 | 10.73 | 10.87 | 10.60 | 92.8% (0.70) | 94.1% (0.55) | 91.6% (0.84) | 95.5% (1.48) | 96.7% (1.09) | 94.3% (1.83) |
| SD | 0.57 | 6.68 | 11.59 | 11.21 | 11.26 | 11.02 | 3.8% (0.66) | 3.1% (0.48) | 4.0% (0.80) | 1.9% (1.12) | 1.9% (0.76) | 3.0% (1.40) |
The patient was a candidate for SBRT, but was clinically treated with protons.
Figure 2.

Histogram of as a percent of for each of the 4D-CT sorting methods. The PC-PB method had the largest average result, but also exhibited the largest range of values. The AB method resulted in the smallest average as a percentage of for our patient population.
The results of the two-sided alternative paired t-test showed statistically significant differences between the and each of the three measurements (all cases, ). Similarly, the two-sided alternative paired t-test indicated that there were statistically significant differences when comparing the 4D-CT methods to one another (all cases, ).
The values that we observed between the and the measurements (i.e., as a percentage of ) decreased after adding the simulated uniform margin expansions. The smallest as a percentage of that we observed was 87.6%, as shown in Table 1. Although the as a percent of appeared to approach unity as we added expansions, it is important to note that the volume of affected tissue is larger per percentage point for the PTV than for the iGTV (i.e., 1% of the PTV is larger in magnitude than 1% of the iGTV). The absolute volume difference between the and each of the measurements ranged from 0.08 to .
Image data set analysis
By overlaying the respiratory waveform and the image midscan times of the selected 4D-CT images, it became apparent that situations existed in which an identical image was selected as the best fit for two adjacent bins. In other words, for a given bed position, less than ten unique images were being selected. This has important implications when developing a MIP from the 4D-CT images, as our data in Table 1 showed that the volume measured on the , built from all available cine CT images, was always larger than the volume measured on any , built from a subset of the cine CT images. Figure 3 offers clinical examples for which both a PC-PB and an AB sorting methodology led to the selection of an identical image for two adjacent bins.
Figure 3.

Clinical example of breathing cycles and their associated cine CT image midscan times. White circles on the waveform indicate an available cine CT image from a single bed position. A single box indicates that cine CT image was selected by the PC-PB method (left) or the AB method (right). Multiple boxes indicate that a particular cine CT image was selected multiple times for adjacent bins. A particularly short inhalation period or steep amplitude measurement (high time rate of change of amplitude either during inhalation or during exhalation) led to a higher likelihood of duplicate image selection with PC-PB and AB sorting.
Figure 4 illustrates the distribution of adjacent bin pairs that exhibited duplicate image selection for each of the 4D-CT image sorting processes over the entire scan extent. Image duplication occurred most frequently in the AB data set (which affected 25 of the patients), followed by PC-PB sorting (17 affected patients), and PB sorting (4 affected patients). We calculated the probability of duplicate image selection at a given bed position as 6%, 12%, and 51% for the PB, PC-PB, and AB methods, respectively. When considering only the bed positions that captured the tumor, image duplication affected 18 patients with the AB method, 6 patients with the PC-PB method, and 2 patients with the PB method. The PB method generally showed a uniformly distributed low frequency of image duplication occurrences, and duplication tended to be associated with irregular fast breathing. When PC-PB sorting was used, duplication tended to occur during the inspiration phase (between 50% and 100%) of the breath cycle. This phenomenon can be explained by our results from the analysis of the PC respiratory waveform data showing that the inspiration portion of the waveform (50%–100%) was temporally shorter than the expiration portion. In effect, the sorting process attempted to select more images than were available in the shorter inspiration time. The AB method exhibited two distinct regions of image duplication occurrences. The steep portions of the respiratory waveform were associated with a high time rate of change of amplitude. The sorting process attempted to select more images than were available in a short period of time during the steep portions of the inspiration and expiration.
Figure 4.

Distribution of adjacent bin pairs that included duplicate images for each of the three 4D-CT sorting processes. This distribution was used to identify what portion of the breathing cycle was most susceptible to duplicate image selection for each method. For PC-PB, duplicate image selection occurs predominantly during the temporally shorter inspiration. For AB, two distinct regions of duplication occur during the steep inhalation and exhalation portions of the respiratory waveform.
Selection of maximum motion extent image with 4D-CT sorting
During the automatic contouring process, we observed cases for which the superior portion of the indicated the presence of lung tumor that the did not capture. To investigate, we used our data that correlated the respiratory waveform and the selected 4D-CT images. We found that the image associated with the minimum amplitude measurement did not always correlate to the image that showed maximum tumor motion extent on the superior portion of the contoured iGTV.
Figure 5 provides a clinical example of a series of cine CT images generated during a 4D-CT scan. Images that were selected by an AB methodology are identified. Images (a)–(d) represent a chronological series of images near the EE point, all located in the same slice location at the superior portion of the tumor motion envelope. As we progress forward in time from image (a) to image (d), we see the tumor become more visible and then start to diminish again. Since image (d) was associated with the lowest amplitude, it was selected by the AB sorting methodology even though the appearance of the tumor was diminished. Furthermore, since the images immediately before image (d) that do show full motion extent have similar amplitudes, they were not selected; the nearest selected prior image was reconstructed a full 1.5 s before image (d) and showed no tumor tissue.
Figure 5.

Top: Clinical example of (left) a breathing cycle with boxed AB selections and (right) of the mismatch between the image at minimum amplitude (d) and visualization of maximum tumor motion [(b) or (c)]. Image (a) shows the tumor starting to move into the image plane as the patient exhales. Image (c), occurring 0.3 s prior to the minimum RPM amplitude image, has the highest tumor visibility and density. Image (d), associated with the extreme-amplitude measurement, exhibits suppressed density and blurred visualization.
DISCUSSION
This study quantified the differences in the iGTV when generating the MIP from three 4D-CT sorting methods and from the entire cine CT data set for tumors exhibiting motion of at least 1 cm. While in most cases the generated from the 4D-CT MIP closely approximated the generated from all the cine CT images, we did identify some situations for which the motion extent was under-represented in the by more than 10%. Since a is built from a subset of the available cine CT images (only 10 binned images as opposed to the 20–40 images available from cine CT), we mathematically expect that it will be smaller than , becoming more apparent in patients having higher amplitude tumor motions.
Of particular interest in this study were the clinical cases in which the was significantly smaller than the . This and other studies have identified reasons that the captured target volume with 4D-CT is smaller.27 It has been shown that PB methodologies do not explicitly select the images corresponding to the extreme amplitudes.28 When multiple EE or EI points are identified at a single bed position, only the best-fit phase image is selected without regard to maximum amplitude. The development of AB was meant to alleviate this issue. However, this study has shown that the image from an extreme-amplitude measurement does not always represent the maximum tumor motion extent; therefore, one cannot assume that selection of the extreme-amplitude images will capture the largest representative tumor volume from a given acquisition. This mismatch may occur for a variety of reasons, such as phase lag between the respiratory trace and the internal anatomy. Also, the image that is created at the minimum amplitude has a temporal window equal to the rotation time29 (0.5 s in our clinic). Figure 6 shows the temporal window of an image overlaid onto a respiratory waveform using the clinical example presented in Fig. 5. In the example, the patient inhaled sharply after the minimum amplitude was reached, indicating that the tumor likely moved out of the imaging plane, while the image data were being collected, resulting in a blurred lower-density tumor appearance.
Figure 6.

Left: Temporal window of the image at minimum amplitude overlaid on the respiratory waveform for the clinical case presented in Fig. 5. The temporal window captures a large portion of the inhalation phase, causing the blurry appearance of image (d). Right: Contouring shows that the (left) and (center) exhibit close agreement with the outermost contour from the . (right) does not exhibit full visualization of the superior portion of tumor motion envelope when compared to the outermost contour.
It should be noted that the selection of an incorrect maximum extent image can also exist with a PB methodology. In particular, in PC-PB we identify the EE or 50% phase as the minimum amplitude and the EI or 0% phase as the maximum amplitude. This definition leads to a strong likelihood that the image closest to the minimum amplitude will be selected as the 50% phase image. However, the PC-PB method may still capture the image of maximum motion extent. While the AB method ignored the neighboring prior images because they had similar associated amplitudes, the PC-PB method distributes the selected images approximately evenly across the time axis from 0% to 50% and may inadvertently select a more appropriate maximum extent image in a different image bin. Figure 6 shows that the and the closely approximated the tumor appearance in the , whereas the did not.
This study has also shown that retrospective 4D-CT sorting is susceptible to the selection of the same image for multiple bins. From the perspective of the MIP, fewer constitutive images will lead to a smaller resultant iGTV and should theoretically be more apparent for highly mobile tumors. Because of the potential for misses when generating a MIP from a 4D-CT image set, we believe that there is merit in using the to evaluate tumor motion extent. By using the , one can reliably expect that the visual representation of the iGTV will include all tumor positions captured by the CT acquisition. The trade-off for a more representative iGTV with is that the image set does exhibit higher anatomical noise—surrounding lung parenchyma appears denser and there is a slight loss of contrast between the tumor and surrounding tissue. However, this loss of contrast did not adversely affect our automatic contouring process. Further studies should be performed to assess whether the margin expansion process should be modified while incorporating the for iGTV identification—while our results indicate that the resultant iGTV will be larger with than with , we expect the uncertainty in motion will decrease, possibly necessitating smaller margins.4
It has been previously suggested that one could bypass the 4D-CT sorting process, instead relying on the MIP and respiratory-averaged CT generated from the entire cine CT data set.18, 28 By doing so, one could minimize or even avoid the extra expenses associated with commercial 4D-CT hardware and software. We should continue to be aware of the limitations of contouring on MIPs, such as overwrite of the target volume by higher density surrounding tissue.21, 30, 31 However, methods such as the generation of a weighted MIP address the overwrite and loss of contrast that occur in regions near the diaphragm.32 Breathing irregularities such as coughing or deep inhalation may cause the tumor motion envelope to be exaggerated with both and .33 It may be advisable to consider an alternative method of respiratory monitoring to ensure that irregular breathing does not occur during the scan period.
One strength of the current study lies in our identification of causes of MIP volume differences on clinical data. The findings may shed light on future improvements to methods of 4D-CT sorting. Our data show that the AB sorting method had the lowest average contoured iGTV, which suggests that modifications of the AB method should be considered. For example, the current AB method associates the nearest-neighbor amplitude value closest with the time-aligned image; alternatively, one could associate an average amplitude to each cine CT image by considering the amplitude values within the temporal window of each image. To test the feasibility, we applied this method to the example presented in Fig. 5 and found that the correct maximum extent image was selected.
While this study did not focus specifically on the low-pitch helical acquisition method of 4D-CT, there are some thematic similarities that may offer insight for further improvement. Information related to the identification of a maximum extent image may allow for a more appropriate sinogram binning mechanism. Users of helical 4D-CT should be aware of the potential for underestimation of in their iGTV delineation.
CONCLUSIONS
In most cases exhibiting tumor motion of at least 1 cm, a MIP generated from a 4D-CT sorting method does a clinically adequate job of capturing the full extent of tumor motion. However, we observed clinical situations for which the iGTV was under-represented in the by more than 10% or up to when compared to the iGTV from . To avoid potential geometric miss, we recommend use of the , generated from all cine CT images, for contouring the target volume. With regard to the 4D-CT volume measurements, the PC-PB method had the largest average iGTV, followed by the PB method, then the AB method. The AB method was the most susceptible to selection of identical images for two adjacent image bins.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of Valen Johnson (Department of Biostatistics) and Kathryn Carnes (Scientific Publications), both from the University of Texas M.D. Anderson Cancer Center.
References
- Mayo J. R., Muller N. L., and Henkelman R. M., “The double-fissure sign: A motion artifact on thin-section CT scans,” Radiology 165, 580–581 (1987). [DOI] [PubMed] [Google Scholar]
- Crawford C. R., King K. F., Ritchie C. J., and Godwin J. D., “Respiratory compensation in projection imaging using a magnification and displacement model,” IEEE Trans. Med. Imaging 15, 327–332 (1996). 10.1109/42.500141 [DOI] [PubMed] [Google Scholar]
- Balter J. M., Ten Haken R. K., Lawrence T. S., Lam K. L., and Robertson J. M., “Uncertainties in CT-based radiation therapy treatment planning associated with patient breathing,” Int. J. Radiat. Oncol., Biol., Phys. 36, 167–174 (1996). 10.1016/S0360-3016(96)00275-1 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Mageras G. S., Balter J. M., Emery R. S., Forster K. M., Jiang S. B., Kapatoes J. M., Low D. A., Murphy M. J., Murray B. R., Ramsey C. R., Van Herk M. B., Vedam S. S., Wong J. W., and Yorke E., “The management of respiratory motion in radiation oncology report of AAPM Task Group 76,” Med. Phys. 33, 3874–3900 (2006). 10.1118/1.2349696 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Kini V. R., Vedam S. S., and Mohan R., “Potential radiotherapy improvements with respiratory gating,” Australas. Phys. Eng. Sci. Med. 25, 1–6 (2002). 10.1007/BF03178368 [DOI] [PubMed] [Google Scholar]
- Giraud P., De Rycke Y., Dubray B., Helfre S., Voican D., Guo L., Rosenwald J. C., Keraudy K., Housset M., Touboul E., and Cosset J. M., “Conformal radiotherapy (CRT) planning for lung cancer: Analysis of intrathoracic organ motion during extreme phases of breathing,” Int. J. Radiat. Oncol., Biol., Phys. 51, 1081–1092 (2001). 10.1016/S0360-3016(01)01766-7 [DOI] [PubMed] [Google Scholar]
- Alasti H., Cho Y. B., Vandermeer A. D., Abbas A., Norrlinger B., Shubbar S., and Bezjak A., “A novel four-dimensional radiotherapy method for lung cancer: Imaging, treatment planning and delivery,” Phys. Med. Biol. 51, 3251–3267 (2006). 10.1088/0031-9155/51/12/017 [DOI] [PubMed] [Google Scholar]
- ICRU Report No. 50, “Prescribing, recording and reporting photon beam therapy” (ICRU, Bethesda, MD, 1993).
- ICRU Report No. 62, “Prescribing, recording and reporting photon beam therapy” (ICRU, Bethesda, MD, 1999).
- Stevens C. W., Munden R. F., Forster K. M., Kelly J. F., Liao Z., Starkschall G., Tucker S., and Komaki R., “Respiratory-driven lung tumor motion is independent of tumor size, tumor location, and pulmonary function,” Int. J. Radiat. Oncol., Biol., Phys. 51, 62–68 (2001). 10.1016/S0360-3016(01)01621-2 [DOI] [PubMed] [Google Scholar]
- Lu W., Parikh P. J., Hubenschmidt J. P., Bradley J. D., and Low D. A., “A comparison between amplitude sorting and phase-angle sorting using external respiratory measurement for 4D CT,” Med. Phys. 33, 2964–2974 (2006). 10.1118/1.2219772 [DOI] [PubMed] [Google Scholar]
- Pan T., Lee T. Y., Rietzel E., and Chen G. T., “4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT,” Med. Phys. 31, 333–340 (2004). 10.1118/1.1639993 [DOI] [PubMed] [Google Scholar]
- Keall P. J., Starkschall G., Shukla H., Forster K. M., Ortiz V., Stevens C. W., Vedam S. S., George R., Guerrero T., and Mohan R., “Acquiring 4D thoracic CT scans using a multislice helical method,” Phys. Med. Biol. 49, 2053–2067 (2004). 10.1088/0031-9155/49/10/015 [DOI] [PubMed] [Google Scholar]
- Pan T., “Comparison of helical and cine acquisitions for 4D-CT imaging with multislice CT,” Med. Phys. 32, 627–634 (2005). 10.1118/1.1855013 [DOI] [PubMed] [Google Scholar]
- Ford E. C., Mageras G. S., Yorke E., and Ling C. C., “Respiration-correlated spiral CT: A method of measuring respiratory-induced anatomic motion for radiation treatment planning,” Med. Phys. 30, 88–97 (2003). 10.1118/1.1531177 [DOI] [PubMed] [Google Scholar]
- Vedam S. S., Keall P. J., Kini V. R., Mostafavi H., Shukla H. P., and Mohan R., “Acquiring a four-dimensional computed tomography dataset using an external respiratory signal,” Phys. Med. Biol. 48, 45–62 (2003). 10.1088/0031-9155/48/1/304 [DOI] [PubMed] [Google Scholar]
- Wink N., Panknin C., and Solberg T. D., “Phase versus amplitude sorting of 4D-CT data,” J. Appl. Clin. Med. Phys. 7, 77–85 (2006). 10.1120/jacmp.2027.25373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Sun X., and Luo D., “Improvement of the cine-CT based 4D-CT imaging,” Med. Phys. 34, 4499–4503 (2007). 10.1118/1.2794225 [DOI] [PubMed] [Google Scholar]
- Rietzel E., Liu A. K., Chen G. T., and Choi N. C., “Maximum-intensity volumes for fast contouring of lung tumors including respiratory motion in 4DCT planning,” Int. J. Radiat. Oncol., Biol., Phys. 71, 1245–1252 (2008). 10.1016/j.ijrobp.2008.03.030 [DOI] [PubMed] [Google Scholar]
- Park K., Huang L., Gagne H., and Papiez L., “Do maximum intensity projection images truly capture tumor motion?,” Int. J. Radiat. Oncol., Biol., Phys. 73, 618–625 (2009). 10.1016/j.ijrobp.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Rietzel E., Chen G. T., Choi N. C., and Willet C. G., “Four-dimensional image-based treatment planning: Target volume segmentation and dose calculation in the presence of respiratory motion,” Int. J. Radiat. Oncol., Biol., Phys. 61, 1535–1550 (2005). 10.1016/j.ijrobp.2004.11.037 [DOI] [PubMed] [Google Scholar]
- Abdelnour A. F., Nehmeh S. A., Pan T., Humm J. L., Vernon P., Schoder H., Rosenzweig K. E., Mageras G. S., Yorke E., Larson S. M., and Erdi Y. E., “Phase and amplitude binning for 4D-CT imaging,” Phys. Med. Biol. 52, 3515–3529 (2007). 10.1088/0031-9155/52/12/012 [DOI] [PubMed] [Google Scholar]
- Rietzel E., Pan T., and Chen G. T., “Four-dimensional computed tomography: Image formation and clinical protocol,” Med. Phys. 32, 874–889 (2005). 10.1118/1.1869852 [DOI] [PubMed] [Google Scholar]
- Chi P. C., Balter P., Luo D., Mohan R., and Pan T., “Relation of external surface to internal tumor motion studied with cine CT,” Med. Phys. 33, 3116–3123 (2006). 10.1118/1.2241993 [DOI] [PubMed] [Google Scholar]
- Videtic G. M., Singh A. K., and Chang J. Y., “A randomized phase II study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer,” Radiation Therapy Oncology Group RTOG 0915 (NCCTG N0927) (2009).
- Pan T., Mawlawi O., Luo D., Liu H. H., Chi P. C., Mar M. V., Gladish G., Truong M., J.Erasmus, Jr., Liao Z., and Macapinlac H. A., “Attenuation correction of PET cardiac data with low-dose average CT in PET/CT,” Med. Phys. 33, 3931–3938 (2006). 10.1118/1.2349843 [DOI] [PubMed] [Google Scholar]
- Mutaf Y. D., Antolak J. A., and Brinkmann D. H., “The impact of temporal inaccuracies on 4DCT image quality,” Med. Phys. 34, 1615–1622 (2007). 10.1118/1.2717404 [DOI] [PubMed] [Google Scholar]
- Riegel A. C., Chang J. Y., Vedam S. S., Johnson V., Chi P. C., and Pan T., “Cine computed tomography without respiratory surrogate in planning stereotactic radiotherapy for non-small-cell lung cancer,” Int. J. Radiat. Oncol., Biol., Phys. 73, 433–441 (2009). 10.1016/j.ijrobp.2008.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Pan T., and Shen Y., “Multislice helical CT: Image temporal resolution,” IEEE Trans. Med. Imaging 19, 384–390 (2000). 10.1109/42.870249 [DOI] [PubMed] [Google Scholar]
- Underberg R. W., Lagerwaard F. J., Slotman B. J., Cuijpers J. P., and Senan S., “Use of maximum intensity projections (MIP) for target volume generation in 4DCT scans for lung cancer,” Int. J. Radiat. Oncol., Biol., Phys. 63, 253–260 (2005). 10.1016/j.ijrobp.2005.05.045 [DOI] [PubMed] [Google Scholar]
- Bradley J. D., Nofal A. N., El Naqa I. M., Lu W., Liu J., Hubenschmidt J., Low D. A., Drzymala R. E., and Khullar D., “Comparison of helical, maximum intensity projection (MIP), and averaged intensity (AI) 4D CT imaging for stereotactic body radiation therapy (SBRT) planning in lung cancer,” Radiother. Oncol. 81, 264–268 (2006). 10.1016/j.radonc.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Pan T., Chang J., Riegel A., Ahmad M., Sun X., and Luo D., “New weighted maximum-intensity-projection (MIP) images for assessing tumor motion in the thorax (abstract),” Med. Phys. 35(6), 2710–2711 (2008). 10.1118/1.2961687 [DOI] [Google Scholar]
- Starkschall G., Desai N., Balter P., Prado K., Luo D., Cody D., and Pan T., “Quantitative assessment of four-dimensional computed tomography image acquisition quality,” J. Appl. Clin. Med. Phys. 8, 2362–2381 (2007). 10.1120/jacmp.v8i3.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]