Abstract
Staphylococcus aureus is a successful human pathogen that has developed several approaches to evade the immune system, including resistance strategies to prevent oxidative killing by immune cells. One mechanism by which this evasion occurs is by production of superoxide dismutase enzymes, which require manganese as a cofactor. Manganese is acquired by the manganese transporter MntABC. One component of this operon, MntC, has been proposed as a potential vaccine candidate due to its early in vivo expression and its ability to provide protection in preclinical models of staphylococcal infection. In the current study, we interrogate the role of this protein in protecting S. aureus from oxidative stress. We demonstrate that mutation of mntC in a number of invasive S. aureus clinical isolates results in increased sensitivity to oxidative stress. In addition, we show that while downregulation of mntC transcription is triggered upon exposure to physiological concentrations of manganese, MntC protein is still present on the bacterial surface at these same concentrations. Taken together, these results provide insight into the role of this antigen for the pathogen.
Introduction
Staphylococcus aureus is the causative agent of diseases ranging from benign skin infections to life-threatening endocarditis, osteomyelitis, toxinoses, and bacteremia. Proliferation and virulence of this pathogen is dependent on its ability to scavenge essential metals which are normally sequestered by the host [1]. Historically, emphasis has been placed on the role of iron in S. aureus virulence (for recent reviews see [2]–[5]). However, recent reports suggest that manganese is also necessary for S. aureus virulence [6]–[8]. Resistance to oxidative stress by S. aureus can be attributed to the activity of superoxide dismutase enzymes, which use manganese as a cofactor and inactivate reactive oxygen species [9]–[11]. Corbin et al. demonstrated that calprotectin, a neutrophil-associated protein which chelates Mn2+, limits growth of S. aureus both in vitro and in abscesses [6]. Manganese chelation by calprotectin lowers S. aureus superoxide dismutase activity and renders the bacteria more sensitive to oxidative stress and killing by neutrophils [12].
ATP-binding cassette (ABC) transporters have been shown to play a central role in manganese transport in several species of bacteria [13]–[15]. However, the mechanism of manganese uptake by S. aureus is incompletely defined. S. aureus encodes an ABC transporter, MntABC, where mntA encodes the ATP-binding protein, mntB encodes the integral membrane protein, and mntC encodes the cation-binding lipoprotein. While S. aureus also encodes an additional manganese transporter, the Nramp homolog mntH, it appears that S. aureus imports manganese primarily by its ABC transporter [7]. Horsburgh et al showed that mutation of mntA in a laboratory isolate, 8325-4, resulted in a reduced growth rate and greater sensitivity to oxidative stress in vitro (although this strain background encodes a truncated copy of mntH [16]). In addition, it was shown that transcription of mntABC is down regulated by its cognate repressor, MntR, which, upon binding to Mn2+, blocks transcription of the mntABC operon. Deletion of mntA also resulted in slightly lowered virulence in a murine abscess model, but only when mntH was also insertionally inactivated [7].
MntC is a component of a tetravalent S. aureus vaccine currently under investigation in human clinical trials (U. S. National Institutes of Health Clinical Trial registry numbers NCT01364571 and NCT01643941) which, in addition to MntC, includes antigens designed to address immune evasion (capsular polysaccharide) and adhesion to host tissues (clumping factor A) [17]. It has recently been shown that MntC is a highly conserved protein among staphylococci; MntC homologs from 11 Staphylococcus species were found to share an average pairwise identity of 72% [18]. In addition, MntC was found to be surface expressed rapidly following infection of mice with a diverse group of S. aureus isolates [18]. Active vaccination with purified recombinant MntC significantly reduced both S. aureus and S. epidermidis bacterial burden in a mouse bacteremia model, suggesting that it may be an effective vaccine antigen for the prevention of S. aureus infection [18].
Here we present a brief report describing the precise regulation of mntABC expression by MntR and corresponding MntC protein levels. We also demonstrate that MntC is surface expressed at physiological concentrations of manganese and that it plays a key role in providing resistance to oxidative stress across diverse disease-causing clinical isolates.
Materials and Methods
Primers, strains, plasmids, and growth media
Primers, strains, and plasmids used in the current study are listed in Table 1. To make TSB-c medium, TSB (BD, Franklin Lakes, NJ) was stirred in the presence of 1% (w/v) Chelex 100 beads (Sigma-Aldrich, St. Louis, MO) for 4 hours. The medium was then filter-sterilized and supplemented with MgCl2 and Fe(II)SO4 to final concentrations of 50 µM and 1 µM, respectively. Inductively-coupled mass spectrometry analysis (performed by Robertson Microlit Laboratories, Ledgewood, NJ) of four independently prepared batches of the TSB medium that had been treated with Chelex 100 beads revealed that the medium contained 50 nM manganese ±17 nM.
Table 1. Primers, strains, and plasmids used in the study.
| Primers: | ||
| Primer name: | Sequencea: | |
| oLH431 | GCT GCT GAA TTC TAT TCT AAA TGC ATA ATA AAT ACT G | |
| oLH432 | TGT CAC TTT GCT TGA TAT ATG AG | |
| oLH398 | CTC ATA TAT CAA GCA AAG TGA CAT TTA CTT GAC ATG ATA AAT ATT CTC | |
| oLH433 | GTC ATA GAG AGT CCT CCT CTG AAT TAA AAG TTT AGG CTA ACC TAA TTA ATT G | |
| oLH434 | CAG AGG AGG ACT CTC TAT GAC | |
| oLH435 | GCT GCT GGA TCC TCA ATG TAT CTT ATC ATG TCT GC | |
| oLH442 | GTC ATA GAG AGT CCT CCT CTG TGT CAC TTT GCT TGA TAT ATG AG | |
| oLH443 | CTC ATA TAT CAA GCA AAG TGA CAC AGA GGA GGA CTC TCT ATG AC | |
| oLH202 | GCT GCT GGA TCC AAG AAA GCG GTA AAA CTA ATT CC | |
| oLH203 | GTA GCA TGT CTC ATT CAA TTT AAG GAT TGC CTT TAA ATA GTC C | |
| oLH204 | GGA CTA TTT AAA GGC AAT CCT TAA ATT GAA TGA GAC ATG CTA C | |
| oLH205 | GCA AAC ATG TTC ATT GCA TTT TCA AAA CTG GTT TAA GCC GAC | |
| oLH206 | GTC GGC TTA AAC CAG TTT TGA AAA TGC AAT GAA CAT GTT TGC | |
| oLH207 | GCT GCT GTC GAC AAT TCA CTA TTT CAG GGT TGG C | |
| oLH81 | GCT GCT GGA TCC ACA CTA CGA CAG ATT TGT ACC | |
| oLH82 | CCG TTC CCA ATT CCA CAT TGT TAC CAG CCT GTT CTA AGG C | |
| oLH83 | GCC TTA GAA CAG GCT GGT AAC AAT GTG GAA TTG GGA ACG G | |
| oLH84 | GGT TTA ACA TCT TTT GAT ACT GCT TAA CAA TTA TTA GAG GTC ATC G | |
| oLH85 | CGA TGA CCT CTA ATA ATT GTT AAG CAG TAT CAA AAG ATG TTA AAC C | |
| oLH86 | GCT GCT GGA TCC AAC TTC TAG CTT TTC TCT TTC G | |
| Strains: | ||
| Strain name: | Relevant characteristics: | Source or reference: |
| MRSA252 | Template for pI258 transcriptional terminator (TT) | [37] |
| Newman | Template for PmntABC promoter fragment | [38] |
| COL | Template for mntR-1, mntR-2, mntC-1, and mntC-2 fragment for gene knockout constructs | [39] |
| RN4220 | Restriction-deficient RN450 | [40] |
| RN4220 mntC::neo | mntC knockout strain of RN4220 | This study |
| RN4220 mntR::ermC | mntR knockout strain of RN4220 | This study |
| RN4220 geh::pLH75 | pLH75 integrated at the geh locus by L54a integrase encoded on pLH69, which was subsequently cured from the strain | This study |
| RN4220 geh::pLH76 | pLH76 integrated at the geh locus by L54a integrase encoded on pLH69, which was subsequently cured from the strain | This study |
| 8325-4 | NCTC8325 cured of three phophage | [41] |
| 8325-4 geh::pLH75 | geh::pLH75 allele from RN4220 geh::pLH75 transduced into 8325-4 | This study |
| 8325-4 geh::pLH76 | geh::pLH76 allele from RN4220 geh::pLH76 transduced into 8325-4 | This study |
| 8325-4 mntC::neo | mntC::neo allele moved from RN4220 mntC::neo by transduction | This study |
| 8325-4 mntR::ermC | mntR::ermC allele moved from RN4220 mntR::ermC by transduction | This study |
| 8325-4 mntR::ermC geh::pLH76 | mntR::ermC allele from RN4220 mntR::ermC introduced into 8325-4 geh::pLH76 by transduction | This study |
| PFESA0179 | S. aureus clinical isolate, CC5 | This study |
| PFESA0190 | S. aureus clinical isolate, CC5 | This study |
| PFESA0237 | S. aureus clinical isolate, CC8 | This study |
| PFESA0105 | S. aureus clinical isolate, CC22 | This study |
| PFESA0164 | S. aureus clinical isolate, CC30 | This study |
| PFESA0186 | S. aureus clinical isolate, CC30 | This study |
| PFESA0005 | S. aureus clinical isolate, CC45 | This study |
| PFESA0113 | S. aureus clinical isolate, CC45 | This study |
| PFESA0176 | S. aureus clinical isolate, CC45 | This study |
| PFESA0106 | S. aureus clinical isolate, CC45 | This study |
| PFESA0175 | S. aureus clinical isolate, CC45 | This study |
| PFESA0119 | S. aureus clinical isolate, CC8, USA300 | This study |
| Plasmids: | ||
| Plasmid name: | Relevant characteristics: | Source: |
| pRL-null | Template for Renilla luciferase, Rluc | Promega |
| pLH69 | pNL9164 with the L54a int gene cloned in at the SphI and KpnI sites | This study |
| pLH71 | attP-tet cassette cloned into the AatII site in pUC19 | This study |
| pLH75 | TT-Rluc cassette cloned into the integrative vector, pLH71, at the EcoRI/BamHI sites. | This study |
| pLH76 | TT-PmntABC-Rluc cassette cloned into the integrative vector, pLH71, at the EcoRI/BamHI sites. | This study |
| pLH26 | mntC::neo cassette cloned into pSPT181 at the BamHI site. | This study |
| pLH47 | mntR::ermC cassette cloned into pSPT181 at the BamHI/SalI sites. | This study |
| pE194 | Template for ermC | [42] |
| pSPT181 | Temperature-sensitive E. coli-S. aureus shuttle vector | [43] |
| pUB110 | Template for neo | ATCC |
Construction of a mntC knockout strain
A mntC::neo knockout cassette consisting of two 800 bp fragments (mntC-1 and mntC-2) flanking the neomycin resistance gene, neo, was constructed. The primer sets and DNA templates used to amplify each of the fragments were: (i) mntC-1: oLH81/oLH82, S. aureus COL genomic DNA; (ii) neo: oLH83/oLH84, pUB110; (iii) mntC-2: oLH85/oLH86, S. aureus COL genomic DNA. All PCRs were performed with iProof polymerase (Bio-Rad, Hercules, CA), and all cloning and molecular biology techniques were performed as described [19]. After purification, these three PCR products were spliced together by amplification with oLH81 and oLH86, and the knockout cassette was cloned into pSPT181 at the BamHI site. After electroporation and chromosomal integration into S. aureus RN4220, the final mntC::neo mutant strain was obtained after plasmid excision by secondary recombination. The mutation was introduced into other S. aureus isolates by transduction using protocols described previously [20]. In the resulting neomycin resistant transductants, insertional inactivation of mntC was confirmed by PCR. Mutants were shown to be of the expected strain lineage with a Qualicon RiboPrinter (DuPont, Wilmington, DE).
Construction of a mntR knockout strain
A mntR::ermC knockout cassette, consisting of two ∼1.2 kb fragments (mntR-1 and mntR-2) flanking the erythromycin resistance gene, ermC, was constructed. The primer sets and DNA templates used to amplify each of the fragments were: (i) mntR-1: oLH202/oLH203, S. aureus COL genomic DNA; (ii) ermC: oLH204/oLH205, pE194; (iii) mntR-2: oLH206/oLH207, S. aureus COL genomic DNA. After purification, these three products were spliced together by amplification with oLH202 and oLH207, and the knockout cassette was cloned into pSPT181 at the BamHI and SalI sites. After electroporation and chromosomal integration into S. aureus RN4220, the final mntR::ermC mutant strain was obtained after plasmid excision by secondary recombination. This mutation was transduced into S. aureus 8325-4 geh::pLH76 essentially as described elsewhere [20]. In the resulting erythromycin resistant transductants, insertional inactivation of mntR was confirmed by PCR. Mutants were shown to be of the expected strain lineage with a Qualicon RiboPrinter.
Construction of TT-PmntABC-Rluc and TT-Rluc reporter fusions and reporter assays
To more easily monitor the levels of mntABC promoter activity, a transcriptional fusion was constructed comprised of the Renilla luciferase (Rluc) gene placed under the control of the mntABC promoter (PmntABC). To prevent non-specific read-through transcription, a transcriptional terminator (TT) from pI258 (as described in [21]) was amplified with primers oLH431 and oLH432. The PmntABC promoter fragment, which included the MntR binding box sequences [7], [22], was amplified from S. aureus Newman genomic DNA with primers oLH398 and oLH433. The promoterless Rluc reporter gene was amplified from pRL-null (Promega, Madison, WI) with primers oLH434 and oLH435. Each of these products was purified (QIAquick PCR Purification Kit, Qiagen, Valencia, CA) and were spliced together by amplification with primers oLH431 and oLH435 to give TT-PmntABC-Rluc. A promoterless version of this cassette, TT-Rluc, was constructed with PCR primers oLH431 and oLH442 for TT and oLH443 and oLH435 for Rluc. These two PCR products were purified and spliced together as before to yield TT-Rluc. The TT-Rluc and TT-PmntABC-Rluc cassettes were cloned into the L54a attP-encoding integrative vector, pLH71 (see Table 1 for more detail), at EcoRI and BamHI sites yielding pLH75 and pLH76, respectively. These plasmids were transformed into S. aureus RN4220/pLH69 by electroporation, where pLH69 encodes the phage L54a integrase [23]–[26]. After integration of the plasmids into the chromosome at geh was confirmed, pLH69 was cured from the strain, and the cassettes were transduced into S. aureus strains 8325-4 and 8325-4 mntR::ermC essentially as described elsewhere [20].
S. aureus strains with chromosomally integrated reporters bearing either a promoterless Rluc (8325-4 geh::pLH75) or PmntABC-Rluc (8325-4 geh::pLH76) were cultured at 37°C in TSB-c medium to mid-exponential phase of growth, then subcultured into the same medium containing increasing concentrations of supplemental MnSO4 to a final optical density at 600 nm (OD600) of 0.01. After one hour incubation with shaking at 37°C, 40 µL of each culture was mixed with 10 µL of 5X Renilla luciferase lysis buffer (Promega) in an opaque white 96-well plate, and the plate was shaken for two minutes at 200 rpm at room temperature. Fifty µL of Renilla luciferase substrate (Promega) was added and the samples were shaken for an additional two minutes before measuring relative luminescence on a Spectramax M5e (Molecular Devices, Sunnyvale, CA).
Western blot hybridization for MntC
Cells were grown overnight in TSB-c at 37°C with shaking, and subcultured (1:200 dilution) the following day in fresh TSB-c supplemented with the indicated concentration of MnSO4. Cells were harvested in the mid-exponential phase of growth, lysed with lysostaphin, and proteins were denatured in SDS-PAGE loading buffer. Following electrophoretic fractionation and transfer, Western blots were probed with murine anti-MntC 78-7 monoclonal antibody (at 3 µg/mL) as the primary antibody, and goat anti-mouse IgG (H+L)-AP Conjugate (Bio-Rad; diluted 1:5,000) as the secondary antibody. Western blots were processed using standard protocols [27].
Assessment of MntC surface expression by whole cell ELISA
Selected clinical isolates from each clonal complex and their cognate mntC::neo mutant derivatives were grown in 5 mL TSB-c overnight at 37°C with shaking. The next day, cultures were diluted 1∶200 into TSB-c containing 400 nM MnSO4. Cultures were shaken at 37°C until they reached an OD600 = 0.6 – 0.9. An OD600 equivalent of 5 was collected, pelleted by centrifugation, and the supernatant was decanted. Cells were resuspended in 1 mL of 1X DPBS (Invitrogen, Carlsbad, CA) +20% glycerol and frozen at −70°C until use. Frozen cells were diluted 1∶10 in 1X DPBS, and 100 µL of each strain of S. aureus was added per well to a NUNC Maxisorp plate (Thermo Fisher Scientific, Waltham, MA). Plates were incubated at 4°C for 16 hours. The plate was blocked with 25 µL of porcine serum (J R Scientific, Woodland, CA) per well, and plates were incubated at 4°C for an additional 2 hours. Plates were washed with 300 µL/well three times with TBS containing 0.1% Brij-35 (CellGro/Mediatech, Manassas, VA). One hundred µL of 4 mg/mL IgG-purified polyclonal antibodies from either naïve or anti-MntC rabbit sera [18] diluted 1∶800 in 20% porcine serum in sterile 1X DPBS was added to each well and incubated at 4°C for 2 hours. Plates were washed three times as described above, then 100 µL of goat anti-rabbit-AP secondary antibody (Southern Biotech, Birmingham, AL) diluted 1∶1000 in 20% porcine serum was added to each well and plates were incubated at room temperature for 1 hr. Plates were then washed three times as described above, followed by the addition of 100 µL Alkaline Phosphatase Reagent for ELISA (Sigma-Aldrich). Plates were incubated for 15 min at room temperature and optical densities at 405 nm were determined. The experiment was performed four times, and each of these experiments was done with an independently grown panel of S. aureus cells.
Assessment of MICs to methyl viologen
The Minimal Inhibitory Concentration (MIC) of methyl viologen (Sigma-Aldrich) necessary to inhibit bacterial growth was determined by broth microdilution according to standard protocols [28] with either TSB-c or TSB-c supplemented with 10 µM MnSO4 as the growth medium. MIC determinations were made during at least two independent assays.
Results and Discussion
mntABC transcription and MntC expression are observed at physiological levels of Mn2+
The US Department of Health reports that the normal range for manganese levels in humans is ∼4 to 15 µg/L in blood, which is equivalent to 73 nM to 273 nM [29]. It has been reported that manganese-mediated repression of mntABC transcription occurs via MntR [7]. However, the manganese concentrations tested were higher than levels normally encountered in human blood (≥1 µM) and therefore higher than what S. aureus would normally encounter during bacteremia. We therefore sought to more clearly define the concentrations of Mn2+ that regulate mntABC at the level of transcript and protein expression. A transcriptional fusion of the mntABC promoter with Rluc was constructed to facilitate precise definition of the manganese concentration required for the regulation of mntABC transcription.
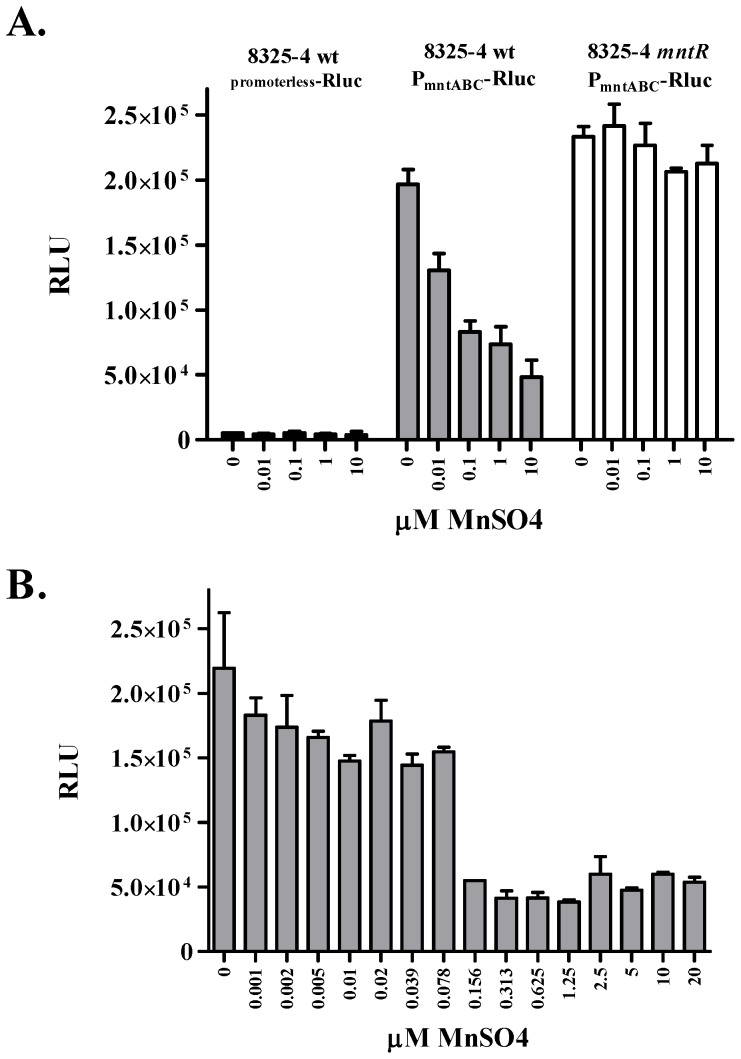
S. aureus strains with chromosomally integrated reporters bearing either a promoterless Rluc (S. aureus 8325-4 geh::pLH75) or PmntABC-Rluc (8325-4 geh::pLH76) were cultured in TSB-c medium containing various concentrations of supplemental MnSO4. The promoterless Rluc strain had minimal levels of reporter activity at all concentrations of MnSO4 tested (Figure 1A). In contrast, S. aureus 8325-4/ PmntABC-Rluc, which contained Rluc under the control of a 67 bp mntABC promoter fragment, exhibited high levels of reporter activity in the absence of supplemental MnSO4. Consistent with previously published results [7], reporter activity decreased as a function of increasing MnSO4 in the culture.
Figure 1. Promoter activity of S. aureus PmntABC in response to Mn2+.
Panel A: Luciferase reporter activity of S. aureus 8325-4 geh::pLH75 (Rluc without a promoter, black bars), S. aureus 8325-4 geh::pLH76 (PmntABC-Rluc, gray bars), and S. aureus 8325-4 mntR::ermC geh::pLH76, (PmntABC-Rluc with mntR deleted, white bars) in the presence of increasing Mn2+ as revealed by luminescence from the Rluc reporter. Panel B: mntABC promoter activity of 8325-4 geh::pLH76 in the presence of two-fold serial dilutions of Mn2+ as revealed by luminescence from the Rluc reporter. Error bars indicate one standard deviation from the mean of triplicate wells.
The observed repression of reporter activity in the presence of manganese is likely due to the metalloregulatory protein, MntR, responding to increasing Mn2+ as described previously [7]. To confirm this hypothesis, a mntR knockout mutant was constructed. As shown in Figure 1A, deletion of mntR relieved the manganese-dependent repression of the PmntABC-Rluc reporter. These results indicate that expression and regulation of this promoter-reporter system work in a similar fashion to what has been published previously [7]. Unlike the earlier study that utilized a mntA-lacZ fusion containing a 578 bp promoter fragment, these results illustrate that a 67 bp PmntABC fragment is sufficient to support both expression of mntABC and Mn2+-dependent regulation by MntR.
To determine the minimum concentration of manganese at which expression of mntABC was repressed by MntR, S. aureus 8325-4/ PmntABC-Rluc was cultured in the presence of two-fold serial dilutions of Mn2+ (Figure 1B). At concentrations of 78 nM MnSO4 and below, levels of reporter activity were high (between 1.5×105 and 2×105 relative light units, RLU). Higher concentrations triggered repression of reporter activity, and maximal repression was observed at concentrations of ≥156 nM MnSO4. Despite this repression, reporter activity was still observed, indicating that, under these growth conditions, MntR only down regulates expression without abolishing it. These results indicate that S. aureus responds to extracellular concentrations of manganese in the range found in human blood (73 nM to 273 nM). The observation that mntABC transcription is not completely repressed at these concentrations correlates well with in vivo expression of MntC [18]. The Mn2+ concentration at which promoter repression occurs also correlates with the experimentally determined binding affinity of purified MntC for Mn2+, which is in the nanomolar range [30].
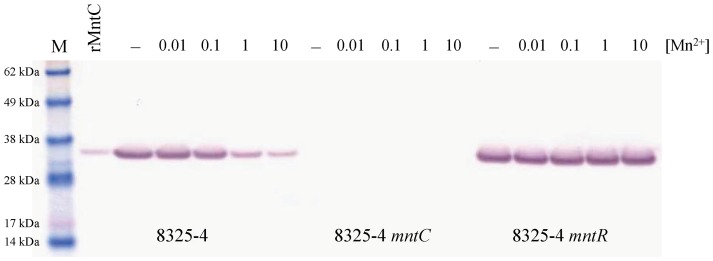
To determine the degree to which manganese-dependent repression of mntABC RNA expression impacts MntC protein production, S. aureus 8325-4 wild-type, 8325-4 mntC, and 8325-4 mntR strains were cultured in TSB-c supplemented with increasing concentrations of Mn2+. As shown in Figure 2, expression of MntC protein was highest in the absence of manganese. In addition, there was a clear down-regulation of MntC protein levels in the S. aureus 8325-4 wild-type strain as a function of increasing levels of Mn2+ in the culture medium. As expected, no MntC protein was detectable in S. aureus 8325-4 mntC at any concentration of Mn2+ tested. For S. aureus 8325-4 mntR, levels of MntC protein were high and not affected by the concentration of manganese in the growth medium. These results indicate that, although manganese-mediated repression of mntABC transcription by MntR lowers the concentration of MntC protein, it is still expressed at the manganese concentrations which were tested under these growth conditions.
Figure 2. Western blot expression of MntC in S. aureus cultures supplemented with manganese.
S. aureus strains 8325-4, 8325-4 mntC, and 8325-4 mntR were grown in TSB-c medium in the presence of increasing concentrations of manganese. Cells were grown in the absence of supplemental manganese (minus sign) or in the presence of 0.01 µM, 0.1 µM, 1 µM, or 10 µM manganese as indicated, and samples of crude lysates were resolved by SDS-PAGE. Recombinant expressed MntC (rMntC) and protein molecular weight standards (M) are included for reference.
MntC is present on the surface of diverse S. aureus clinical isolates
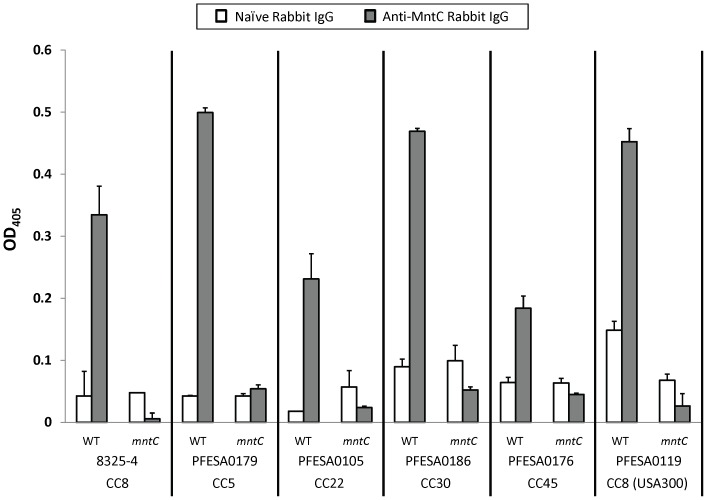
Although mntC has been shown to be highly conserved across S. aureus isolates, its role in bacterial fitness, virulence and resistance to neutrophil killing in clinically relevant disease-causing isolates has yet to be demonstrated. In order to show that, (1) the results described above with the laboratory strain 8325-4 could be extended to clinically relevant strains, and (2) the levels of MntC detected by Western Blot correlate with surface expression, we performed whole cell ELISAs using anti-MntC polyclonal antibodies on representative clinical isolates grown in vitro. The isolates were selected from the five S. aureus clonal complexes most often associated with global disease: CC5, CC8, CC22, CC30, and CC45 [31]. As shown in Figure 3, we found that MntC was detected on the surface of each of the clinical isolates tested and that the level of expression for three of the five strains was equivalent to that observed in the 8325-4 laboratory strain.
Figure 3. Relative surface expression of MntC across S. aureus clinical isolates as measured by whole cell ELISA.
S. aureus clinical isolates (WT) representing five different clonal complexes and their isogenic mntC mutants (mntC) were grown in TSB-c supplemented with 400 nM MnSO4, then tested by whole cell ELISA for relative expression of MntC using polyclonal antibodies purified either from naïve rabbits (unshaded bars) or rabbits immunized with purified MntC (shaded bars). Wild-type 8325-4 and its isogenic mntC mutant were evaluated as comparators. Results were plotted following background correction: average background signal (from assays performed without primary naïve rabbit IgG or primary anti-MntC antibodies) were subtracted from the average sample signals from assays performed with primary antibody. Error bars indicate the standard deviation from duplicate samples. Representative data from one of four experiments yielding similar results is shown (see Materials and Methods).
Inactivation of mntC results in sensitivity to oxidative stress across S. aureus clinical isolates
Polymorphonuclear neutrophils are important cellular mediators of the immune response to S. aureus. These immune cells are adept at killing engulfed bacteria through the production of a respiratory burst, i.e., a potent cocktail of superoxide radicals, hydrogen peroxide, and hydroxyl radicals that is secreted into the phagosome [32]. Methyl viologen (or paraquat), a quaternary ammonium compound which generates intracellular superoxide [33], [34], is frequently used in vitro to mimic the oxidative stress that the bacteria encounter during the respiratory burst. Increased sensitivity to methyl viologen was observed with a S. aureus mntA mutant made in the laboratory strain background, 8325-4 [7]. This mutation in the first gene in the operon (mntA) likely resulted in polar effects that disrupt expression of the downstream genes mntB and mntC. In addition, it has been shown that S. aureus 8325-4 encodes a truncated copy of the Nramp homolog mntH [16], so it cannot be concluded that the mutation in mntA was the sole cause of the observed sensitivity to methyl viologen (i.e., the observed phenotype may require mutation of both manganese transport systems). As a result, experiments were performed to look specifically at the contribution of the MntC vaccine antigen to S. aureus sensitivity to oxidative stress. Further, given that the previous observation was made with only a single laboratory strain of S. aureus, it was important to determine whether mntC-inactivation was similarly important for resistance to oxidative stress in diverse S. aureus strains that cause human disease. To test this possibility, a mntC mutation was transduced from S. aureus RN4220 into twelve clinical isolates from nosocomial infections representing different clonal complexes, including the five most often associated with invasive disease. Among the 12 isogenic pairs of clinical isolates were two MRSA strains, including one USA300, a lineage associated mainly with community-acquired infections. In order to quantitate the effect of mntC inactivation, minimum inhibitory concentrations (MICs) of methyl viologen were determined for wild-type and mntC isogenic strains in Mn2+-depleted media (Table 2). Without exception, all mntC strains were more sensitive to methyl viologen than their wild-type counterparts. Relative to the respective wild type strains, the methyl viologen MICs were at least 8-fold lower in the mntC mutants. When mntC was supplied in trans, this effect was reversed and resistance to methyl viologen was restored to wild-type levels (data not shown). Further, resistance to methyl viologen was restored when the mntC mutant strains were cultured in the presence of manganese (Table 2). These data demonstrate that increased sensitivity to oxidative stress is a general characteristic when mntC is disrupted in S. aureus. We conclude that the MntC plays a central role in resistance to oxidative stress across invasive S. aureus clinical isolates.
Table 2. Sensitivity of genetically diverse S. aureus mntC strains to oxidative stress.
| MV MIC (mM)a TSB-c | MV MIC (mM)a TSB-c + Mnb | ||||||
| Strain | Origin | Clonal Complex | wt | mntC | Fold Change in Sensitivity | wt | mntC |
| 8325-4 | Lab. isolate | CC8 | 25 | 0.2 | 125 | 25 | 25 |
| PFESA0179 | UK | CC5 | 25 | 1.56 | 16 | 25 | 25 |
| PFESA0190 | UK | CC5 | 25 | 1.56 | 16 | 25 | 25 |
| PFESA0237 | UK | CC8 | 50 | 6.25 | 8 | 25 | 25 |
| PFESA0105 | Netherlands | CC22 | 25 | 0.39 | 64 | 25 | 25 |
| PFESA0164 | UK | CC30 | 12.5 | 0.78 | 16 | 12.5 | 12.5 |
| PFESA0186 | UK | CC30 | 25 | 0.39 | 64 | 25 | 25 |
| PFESA0005 | USA | CC45 | 50 | 0.78 | 64 | 25 | 25 |
| PFESA0113 | UK | CC45 | 25 | 3.12 | 8 | 25 | 25 |
| PFESA0176 | UK | CC45 | 25 | 3.12 | 8 | 25 | 25 |
| PFESA0106 | Canada | CC45 | 12.5 | 0.78 | 16 | 12.5 | 12.5 |
| PFESA0175 | Finland (MRSA) | CC45 | 50 | 0.78 | 64 | 25 | 25 |
| PFESA0119 | USA (MRSA) | CC8 (USA300) | 25 | 0.39 | 64 | 25 | 25 |
MV MIC: minimum inhibitory concentration of methyl viologen.
TSB-c + Mn: TSB-c supplemented with 10 µM MnSO4.
MntC regulation and its role in resistance to oxidative stress
In the current study, we sought to further define the regulation of MntC and its role in resistance to oxidative stress in S. aureus. Using a chromosomally-integrated mntABC promoter-reporter fusion, we demonstrated that transcription of mntABC is repressed in the presence of Mn2+ in a MntR-dependent manner. Further, we showed that down-regulation of the promoter-reporter fusion occurred at physiologically relevant concentrations of manganese [29]. Under these growth conditions, expression from the mntABC promoter was down regulated at Mn2+ concentrations greater than 156 nM. However, complete repression was never observed, even at micromolar concentrations of manganese. In contrast, Horsburgh et al, reported complete repression of mntABC at very high concentrations of manganese by Northern blot analysis (≥10 µM) [7]. This discrepancy could be explained either by the relative sensitivity of the Rluc reporter construct as compared to a Northern blot, or by the potential contribution of additional negative regulators whose binding sites were not present in the 67 bp promoter fragment used in our construct. We also found that MntC was surface expressed at 400 nM MnSO4 in S. aureus clinical isolates representative of the most prevalent disease-causing strains. Finally, mntC mutation correlated with heightened sensitivity to oxidative stress in a wide spectrum of clinical isolates.
Several antigens have been proposed as potential vaccine components for the prevention of staphylococcal infections [35]. Because manganese plays an important role in S. aureus virulence, antibodies resulting from vaccination with MntC have the potential to prevent MntC-mediated uptake of Mn2+ by S. aureus, thereby rendering the organism more sensitive to oxidative killing by host immune cells. The results presented above, in combination with the high degree of protein sequence conservation and the demonstration of surface expression during murine models of infection [18] and human infection [36] make MntC an interesting candidate for assessment as a S. aureus vaccine antigen.
Acknowledgments
We thank Terri Mininni (Pfizer) for providing anti-MntC 78-7 monoclonal antibody, Yekaterina Timofeyeva (Pfizer) and Guy Singh (Pfizer) for providing the purified rabbit anti-MntC polyclonal antibodies, Paul Liberator (Pfizer), Robert Donald (Pfizer), and Timothy Foster (Trinity College Dublin) for critical review of the manuscript, and Alexey Gribenko (Pfizer) and Yury Matsuka (Pfizer) for helpful discussions.
Funding Statement
This work was supported by Pfizer Vaccine Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cassat JE, Skaar EP (2012) Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin Immunopathol 34: 215–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haley KP, Skaar EP (2012) A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes Infect 14: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammer ND, Skaar EP (2012) The impact of metal sequestration on Staphylococcus aureus metabolism. Curr Opin Microbiol 15: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maresso AW, Schneewind O (2006) Iron acquisition and transport in Staphylococcus aureus . Biometals 19: 193–203. [DOI] [PubMed] [Google Scholar]
- 5. Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev 73: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, et al. (2008) Metal Chelation and Inhibition of Bacterial Growth in Tissue Abscesses. Science 319: 962–965. [DOI] [PubMed] [Google Scholar]
- 7. Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, et al. (2002) MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Molecular Microbiology 44: 1269–1286. [DOI] [PubMed] [Google Scholar]
- 8. Kehl-Fie TE, Skaar EP (2010) Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clements MO, Watson SP, Foster SJ (1999) Characterization of the major superoxide dismutase of Staphylococcus aureus and its role in starvation survival, stress resistance, and pathogenicity. J Bacteriol 181: 3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ (2003) Role and regulation of the superoxide dismutases of Staphylococcus aureus . Microbiology 149: 2749–2758. [DOI] [PubMed] [Google Scholar]
- 11. Valderas MW, Hart ME (2001) Identification and characterization of a second superoxide dismutase gene (sodM) from Staphylococcus aureus . J Bacteriol 183: 3399–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, et al. (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus . Cell Host Microbe 10: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tseng H-J, McEwan AG, Paton JC, Jennings MP (2002) Virulence of Streptococcus pneumoniae: PsaA Mutants Are Hypersensitive to Oxidative Stress. Infect Immun 70: 1635–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng H-J, Srikhanta Y, McEwan AG, Jennings MP (2001) Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Molecular Microbiology 40: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 15.Wichgers Schreur PJ, Rebel JMJ, Smits MA, van Putten JPM, Smith HE (2011) TroA of Streptococcus suis is required for manganese acquisition and full virulence. J Bacteriol: JB.05305–05311. [DOI] [PMC free article] [PubMed]
- 16. O'Neill AJ (2010) Staphylococcus aureus SH1000 and 8325-4: comparative genome sequences of key laboratory strains in staphylococcal research. Lett Appl Microbiol 51: 358–361. [DOI] [PubMed] [Google Scholar]
- 17. Anderson AS, Miller AA, Donald RGK, Scully IL, Nanra JS, et al. (2012) Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Human Vaccines & Immunotherapeutics 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, et al. (2012) Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis . J Infect Dis 205: 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Russell DW, editors (2001) Molecular Cloning: A Laboratory Manual. .Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- 20.McNamara PJ (2008) Genetic Manipulation of Staphylococcus aureus In: Lindsay JA, Staphylococcus: Molecular Genetics. Norfolk, UK: Caister Academic Press. pp. 89–130.
- 21. Charpentier E, Anton AI, Barry P, Alfonso B, Fang Y, et al. (2004) Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol 70: 6076–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ando M, Manabe YC, Converse PJ, Miyazaki E, Harrison R, et al. (2003) Characterization of the Role of the Divalent Metal Ion-Dependent Transcriptional Repressor MntR in the Virulence of Staphylococcus aureus . Infect Immun 71: 2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee CY, Buranen SL (1989) Extent of the DNA sequence required in integration of staphylococcal bacteriophage L54a. J Bacteriol 171: 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee CY, Buranen SL, Ye ZH (1991) Construction of single-copy integration vectors for Staphylococcus aureus . Gene 103: 101–105. [DOI] [PubMed] [Google Scholar]
- 25. Ye ZH, Buranen SL, Lee CY (1990) Sequence analysis and comparison of int and xis genes from staphylococcal bacteriophages L54a and phi 11. J Bacteriol 172: 2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye ZH, Lee CY (1989) Nucleotide sequence and genetic characterization of staphylococcal bacteriophage L54a int and xis genes. J Bacteriol 171: 4146–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher S, Winston S, Fuller S, Hurrell J (2008) Immunoblotting and Immunodetection. Current Protocols in Molecular Biology: John Wiley & Sons, Inc. pp. 10.18.11–10.18.28. [DOI] [PubMed]
- 28.(2012) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard M07-A9. Villanova, PA: NCCLS.
- 29.Agency for Toxic Substances and Disease Registry website. Available: http://www.atsdr.cdc.gov/ToxProfiles/tp151-c1-b.pdf. Accessed 2013 Sep 24.
- 30.Gribenko A, Mosyak L, Ghosh S, Parris K, Svenson K, et al. Three-dimensional structure and biophysical characterization of Staphylococcus aureus cell surface antigen - manganese transporter MntC. J Mol Biol: In press. [DOI] [PubMed]
- 31. Deurenberg RH, Stobberingh EE (2008) The evolution of Staphylococcus aureus . Infect Genet Evol 8: 747–763. [DOI] [PubMed] [Google Scholar]
- 32. Malachowa N, DeLeo FR (2011) Staphylococcus aureus survival in human blood. Virulence 2: 567–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carr RJ, Bilton RF, Atkinson T (1986) Toxicity of paraquat to microorganisms. Appl Environ Microbiol 52: 1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hassan HM, Fridovich I (1978) Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli . J Biol Chem 253: 8143–8148. [PubMed] [Google Scholar]
- 35. Broughan J, Anderson R, Anderson AS (2011) Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines 10: 695–708. [DOI] [PubMed] [Google Scholar]
- 36. den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, et al. (2013) Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One 8: e53391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, et al. (2004) Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A 101: 9786–9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duthie ES, Lorenz LL (1952) Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6: 95–107. [DOI] [PubMed] [Google Scholar]
- 39. Niemeyer DM, Pucci MJ, Thanassi JA, Sharma VK, Archer GL (1996) Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus . J Bacteriol 178: 5464–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, et al. (1983) The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305: 709–712. [DOI] [PubMed] [Google Scholar]
- 41. Novick R (1967) Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus . Virology 33: 155–166. [DOI] [PubMed] [Google Scholar]
- 42. Iordanescu S (1976) Three distinct plasmids originating in the same Staphylococcus aureus strain. Arch Roum Pathol Exp Microbiol 35: 111–118. [PubMed] [Google Scholar]
- 43. Janzon L, Arvidson S (1990) The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus . EMBO J 9: 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]