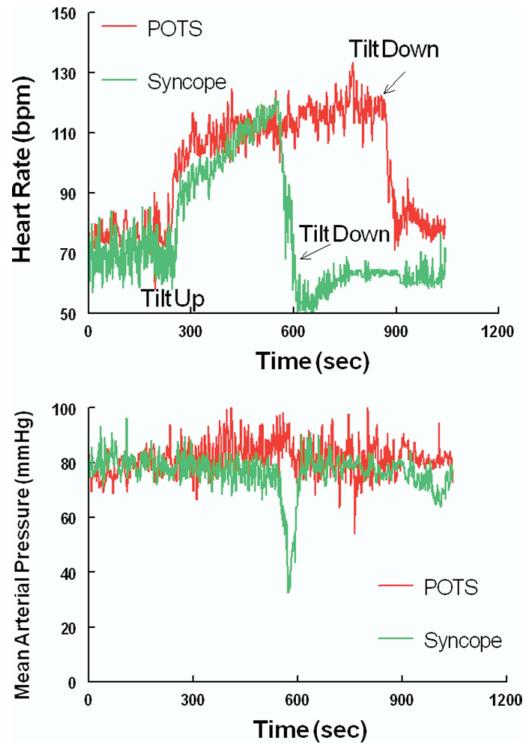
As awareness of postural tachycardia syndrome (POTS) has increased in recent years,1-3 our laboratory has received numerous patient referrals for “POTS going to faint.” These comprise patients who, during tilt table testing, have development of excessive tachycardia, sometimes gradually decreasing blood pressure and symptoms of orthostatic intolerance, followed by a simple postural faint (current designation “reflex neurocardiogenic syncope”4). Consider 2 representative teenage patients placed on a tilt table for a 20- to 30-minute supine equilibration period and then tilted upright to 70 degrees. Both have development of excessive increases in heart rate within 5 minutes of the onset of upright tilt (Figure 1). Both become lightheaded and nauseated and have development of progressive neurocognitive impairment. Respirations deepen, and end tidal carbon dioxide is significantly decreased. They are both pale and somewhat diaphoretic. Blood pressure may slowly decrease. Just before pronouncing the diagnosis of POTS, 1 patient abruptly loses consciousness in association with a rapid decrease in blood pressure and heart rate characteristic of the “vasovagal faint” variant of reflex syncope because of the decrease in blood pressure (the vaso or vascular part) followed immediately by a sudden drop in heart rate (the vagal part).5 This causes an equally abrupt change in the diagnosis from POTS to simple faint. The other patient continues to have an excessively increased heart rate and symptoms of orthostatic intolerance but has no decrease in blood pressure or heart rate, or loss of consciousness. Blood pressure and heart rate data for the representative tilt subjects are shown in Figure 1. These scenarios have fostered concerns about the clinical and physiological similarities and differences between simple faint and POTS. The answer appears to be a qualified no to clinical similarity and a qualified yes to physiological similarity. One could easily reverse this question and ask whether there are clinical and physiological differences between simple faint and POTS. The answer appears to be a qualified yes to clinical difference and a qualified no to physiological difference. The most notable differences between POTS and simple fainting are found in their clinical presentations, and therefore history taking is paramount in diagnoses. POTS is a chronic day-to-day form of orthostatic intolerance, and simple faint is most often episodic and associated with long periods of “wellness.”
Figure 1.
The figure shows heart rate in the top panel and mean arterial pressure in the bottom panel during upright tilt to 70 degrees. A representative patient with POTS is shown in red and a representative patient with syncope is shown in green. Excessive tachycardia and symptoms occur in both patients during tilt. Tilt is terminated in the patient with syncope at the time of faint, and the table is placed in the supine position.
ACUTE AND CHRONIC ORTHOSTATIC INTOLERANCE
Orthostatic intolerance (OI) is defined by the presence of symptoms and signs while upright, relieved by recumbency. Symptoms include dizziness, impending loss of consciousness, headache, fatigue, neurocognitive or sleep disturbance, exercise intolerance, nausea/abdominal pain, heat, and sweating.6 These roughly divide into symptoms of reduced cerebral and regional circulatory blood flow7,8 and symptoms of sympathetic activation.9 Acute orthostatic intolerance during adolescence usually presents as episodic simple postural faint (vasovagal syncope), and chronic orthostatic intolerance presents with POTS in which symptoms are associated with excessive upright tachycardia.10 Both vasovagal syncope and POTS are defined clinically; there is no other reference standard test for their diagnosis. Syncope (fainting) is defined by a sudden transient loss of consciousness and postural tone caused by cerebral hypoperfusion.11 Cerebral blood flow needs to decrease to approximately 50% of normal before consciousness is seriously impaired.12 Symptoms and signs of impending faint are the signs and symptoms of orthostatic intolerance: commonly pallor, nausea or stomach discomfort, headache, cold or warm feelings, and dizziness, which is often initially misinterpreted as a more ill-defined lightheadedness or cognitive loss. Post faint recovery may be protracted with residual pallor and weakness.
Generally, syncope can result from serious cardiovascular and cerebrovascular pathology.13 This is beyond the scope of the present review, which focuses exclusively on orthostatic intolerance. POTS is identified with chronic orthostatic intolerance14-16 and is defined operationally by symptoms of OI in association with excessive tachycardia. In adults excessive postural tachycardia comprises an increase of heart rate by 30 or more beats/min during upright tilt or to a heart rate exceeding 120 beats/min within 10 minutes of being placed upright.1 Hypotension has been until recently an exclusionary criterion during the 10 minute testing period but can occur later during tilt table testing in some patients with typical chronic OI (Phillip Low, personal communication, April 2008) in which it is associated with severe cerebral hypoperfusion.17,18 Some patients have waxing and waning symptoms, although usually not at regular intervals. In healthy young people, especially those with a low supine resting heart rate, the normal postural increase in heart rate is often much larger than in adults. The reason for this is straightforward: tonic vagal activity is maximum during the teenage years19 and low heart rates are common, particularly in young athletes.20 Baroreflex-mediated vagal withdrawal (maximum vagal withdrawal is typically associated with a heart rate in the range of 100 beats/min in orthotopic transplants21) occurs during the standing process22 and a relatively large increase in heart rate is common. A diagnosis of POTS in adolescent patients requires chronic symptoms of OI, as well as “excessive tachycardia” that appear with standing and are relieved by recumbency. During laboratory tilt testing symptoms of OI are typically duplicated and are associated with an excessive increase in heart rate that is usually much in excess of 30 beats/min. Symptoms may worsen with prolonged standing. Hypotension and fainting may occur during long stand times, and the patients are trained to sit or lie down once symptoms occur. However, other patients with POTS will not faint throughout 30 or more minutes of tilt. In addition, many healthy volunteers can have symptoms and signs of POTS during laboratory testing but not during daily life where they are generally untroubled by chronic OI.23
DEFINING ILLNESS IN OI
How then to define illness? The 2 general ways that patients are evaluated for medical ailments are by history/physical examination and by laboratory tests. There are no evidence-based studies indicating the clinical utility of brain imaging studies. For orthostatic intolerance, tilt table testing, a kind of orthostatic stress test, has been used as a primary laboratory tool. Tilt testing was first used in the 19th century,24 and later by NASA and aerospace scientists as a test for physiological changes with change of posture.25 The first research reports with tilt testing used to evaluate simple faint were published in the 1980s.26,27 Although tilt testing can be made reasonably specific and sensitive in the evaluation of fainting in adults, debate continues concerning excessive false-positive and false-negative results in children and adolescents.23,28 Thus on tilt table tests “fainters” do not always faint, and non-fainters sometimes do. Interestingly, evidence suggests that so-called false-positive results may indeed have a propensity to faint masked by movement-induced skeletal muscle pump activity.29 This may be consistent with the idea that fainting is a normal response in the pediatric age range, but that the threshold for fainting varies widely from person to person.30 Indeed, fainting is exceedingly common with onset typically in the pediatric age range.31 However, just because something is “normal” does not make it desirable. Thus it is reasonable to speak of illness when orthostatic intolerance occurs with frequency or severity sufficient to impair quality of life.
Under the laboratory conditions that use tilt table stress combined with lower body negative pressure (which further forces blood to fill lower body veins and produces interstitial transudation), virtually all healthy volunteers can be made to experience a form of faint, vasovagal or otherwise.32 So is tilt table testing useful? Perhaps it does not provide an accurate diagnostic tool for children. However, as shown below, it has considerable value in physiological assessment. Data from prior experiments indicate detectable differences of episodic fainters from healthy control subjects during the measurement of regional blood flows even in so-called “false-negative” results.
CLINICAL DIAGNOSES OF SIMPLE FAINT AND POTS
What does this mean for the diagnosis of fainting? Particularly for children, clinically important fainting is a real-world phenomenon but not necessarily a laboratory phenomenon. If fainting importantly interferes with the quality of life, it can be considered an illness that requires treatment. Recent work, including a position statement from the American Heart Association/American College of Cardiology,4 emphasizes the preeminence of the history and physical examination in the diagnosis of syncope, especially “neurally mediated reflex faints.”33
With greater awareness of the existence of POTS comes an increasing diagnosis of POTS with syncope, usually on the basis of orthostatic testing results. The problem with the tilt studies described in the first paragraph is that the most important diagnostic feature, clinical history, is omitted. On the one hand, typical young fainters have episodic illness. Thus fainting episodes usually occur sporadically, separated by fairly long intervals during which the patient is entirely well. On the other hand, almost all young patients with POTS are chronically ill and uncommonly have fainting as part of the real-world syndrome. Work, school, and physical activities are often highly compromised. Fainters lose consciousness in real life, but patients with POTS typically do not (although they can be made to lose consciousness in the tilt test laboratory). Thus we have on the one hand, real-world fainters, who are typically well for the most part, and on the other hand real-world patients with POTS who are typically ill for the most part, interspersed with occasional periods of wellness.
What do we expect of tilt testing in patients with POTS? As might be inferred from chronic symptoms, orthostatic intolerance and tachycardia is usually easily provoked in the laboratory. If they are well, tilt findings may be minimal, but this is seldom the case, and findings and symptoms tend to be quite consistent. Ten-minute upright tilt testing is a standard for POTS.15 In our experience protracted tilt tests or tilt combined with pharmacologic potentiation in patients with POTS may provoke a faint, but similar faints do not occur in real life where patients sit or lie down to relieve symptoms.
WHY ARE “POSITIVE” TILT TEST RESULTS SIMILAR (EARLY ON) IN FAINTERS AND POTS?
The common physiological underpinnings of simple postural faint and POTS explain the reflex tachycardia observed in both POTS and simple faint. Tachycardia can occur as a reflex response to pressure or volume stimuli. For example, on standing a significant fraction of thoracic blood is rapidly translocated to the dependent body parts. The shift in blood volume unloads the baroreflexes, which evoke compensatory sympathetic activation and vagal withdrawal and result in tachycardia and widespread vasoconstriction. Young and healthy subjects may have near-complete translocation of blood occurring well before autonomic compensation can be effective. This produces short-lived hypotension and dizziness that resolves within 1 minute and is normal.34,35 One way for excessive tachycardia to occur is for there to be excessive thoracic hypovolemia. This occurs for example, if there is concurrent hemorrhage or dehydration. The resulting “excessive” tachycardia is therefore part of an appropriate autonomic response.
Other ways to produce excessive tachycardia might include inappropriate vagal withdrawal or changes in numbers or function of cardiac muscarinic receptors. However, such changes would not account for very high heart rates.36 Similarly, sympathetic excitation increases tachycardia directly through sinus node beta receptors.37 Thus up-regulation of sympathetic nerve activity or beta receptor numbers or signal transduction, as occur in inappropriate sinus tachycardia,38 can increase heart rate. Heart rate is stimulated by circulating catecholamines, and modulated by other factors such as angiotensin-II and nitric oxide, which largely function within the framework provided by the autonomic nervous system.
In a recent discussion of POTS,39 Grubb divided patients with POTS into 2 physiological groups on the basis of the redistribution of blood volume40 or a hyperadrenergic state of uncertain origin.41,42 Contributions may also arise from changes in hematocrit and in blood viscosity.43 Of these variants the redistributive form is most common. This has been attributed to lower extremity blood pooling and vasodilation in “neurogenic POTS,”44,45 or to our category of “normal flow POTS” in which splanchnic vasodilation and blood pooling occur.8 The finding that splanchnic blood pooling is important in orthostatic intolerance is not surprising because the splanchnic organs, comprising liver, spleen, stomach, pancreas, and intestines, are the largest reservoir of venous blood. In man much of its reservoir function is attributable to the gastrointestinal tract which has active venoconstrictive and arterial vasoconstrictive capabilities.46 At rest, the splanchnic venous reservoir is highly compliant.47 There is rich splanchnic innervation and good evidence that intact innervation of arterial and venous vasculature is required for BP maintenance during postural changes.48
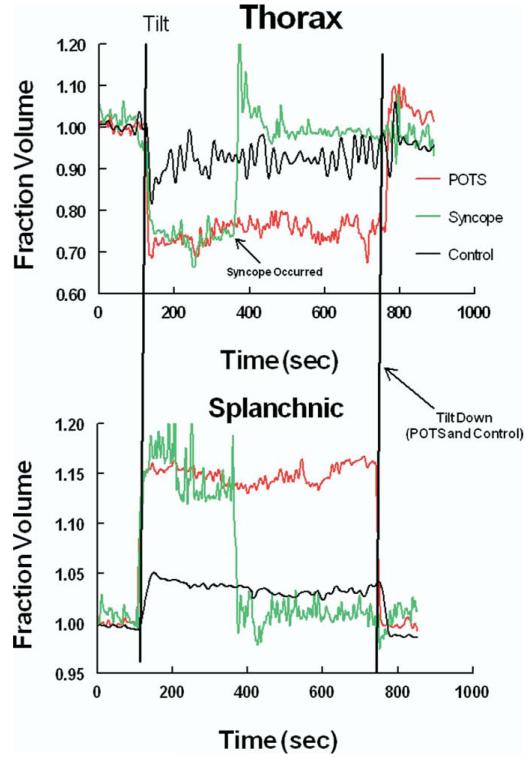
Most interesting, however, is that similar splanchnic pooling occurs in postural fainting and POTS; and splanchnic pooling is reciprocally related to thoracic hypovolemia, which can account for reflex tachycardia in both fainting and POTS (Figure 2).7,8,49 Differences among POTS, syncope, or healthy subjects in other vascular compartments, such as legs or pelvic vasculature, are not found during upright tilt. Further analysis demonstrates that splanchnic pooling is caused by selective splanchnic vasodilation in patients with POTS and fainting.8,49
Figure 2.
The figure shows fractional changes in regional blood volumes during 70-degree upright tilt. Changes in thoracic blood volumes are shown in the top panel. Changes in splanchnic blood volumes are shown in the bottom panel. Data from a representative patient with POTS are shown in red. Data from a representative patient with syncope are shown in green. Data from a healthy volunteer control subject are shown in black. There is a more marked decrease in thoracic blood volume and increase in splanchnic blood volume in the patient with POTS and the patient with syncope compared with the control subject.
UNANSWERED QUESTIONS
Selective splanchnic vasodilation is common during eating where it is related to the elaboration of cholecystokinin and serotonin.50 Splanchnic vasodilation also occurs in abnormal hemodynamic states such as sepsis where it is related to the production of nitric oxide by inducible nitric oxide synthase, and to inflammatory mediators51; and in portal hypertension where it may relate to excessive nitric oxide production by Enos.52 To date, however, there is no evidence-based explanation for selective splanchnic vasodilation in simple faint or POTS.
If the initiating physiological change in reflex syncope and in POTS is splanchnic hyperemia, why do fainters faint episodically, but patients with POTS do not; why are fainters well most of the time, but patients with POTS are not? The answer appears to be in the central mediation of the fainting response: its pathogenesis remains “elusive,” and its threshold varies from person to person.53
CONCLUSIONS
Clinical approaches serve best to separate reflex syncope from POTS. Orthostatic intolerance can be evaluated with orthostatic stress testing (standing, upright tilt table testing) and laboratory instruments can be used to evaluate and study pathophysiology. The use of laboratory tools has shown that the “front-end” pathophysiology of simple faint and POTS is similar, holding out a promise of potential treatment once that is understood. Precise pathophysiology and thus effective medical treatment of the central vasovagal response remains elusive.
Glossary
- OI
Orthostatic intolerance
- POTS
Postural tachycardia syndrome
Footnotes
The author declares no potential, perceived, or real conflicts of interest.
REFERENCES
- 1.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 2.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–7. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS) J Pediatr. 2004;145:725–30. doi: 10.1016/j.jpeds.2004.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickberger SA, Benson DW, Biaggioni I, Callans DJ, Cohen MI, Ellenbogen KA, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation. 2006;113:316–27. doi: 10.1161/CIRCULATIONAHA.105.170274. [DOI] [PubMed] [Google Scholar]
- 5.Lewis T. A lecture on vasovagal syncope and the carotid sinus mechanism: with comments on Gower’s and Nothnagel’s syndrome. Br Med J. 1932;1:873–6. doi: 10.1136/bmj.1.3723.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523–8. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation. 2004;110:2575–81. doi: 10.1161/01.CIR.0000145543.88293.21. [DOI] [PubMed] [Google Scholar]
- 8.Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2006;290:H665–H673. doi: 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JM, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–13. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, et al. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–9. doi: 10.1161/01.cir.98.20.2154. [DOI] [PubMed] [Google Scholar]
- 11.Folino AF, Russo G, Porta A, Buja G, Cerutti S, Iliceto S. Modulations of autonomic activity leading to tilt-mediated syncope. Int J Cardiol. 2007;120:102–7. doi: 10.1016/j.ijcard.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 12.Folino AF. Cerebral autoregulation in neurally mediated syncope: victim or executioner? Heart. 2006;92:724–6. doi: 10.1136/hrt.2005.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuriachan V, Sheldon RS. Current concepts in the evaluation and management of syncope. Curr Cardiol Rep. 2008;10:384–90. doi: 10.1007/s11886-008-0061-x. [DOI] [PubMed] [Google Scholar]
- 14.Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999;317:88–101. doi: 10.1097/00000441-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 16.Streeten DH. Orthostatic intolerance. A historical introduction to the pathophysiological mechanisms. Am J Med Sci. 1999;317:78–87. doi: 10.1097/00000441-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke. 1998;29:1876–81. doi: 10.1161/01.str.29.9.1876. [DOI] [PubMed] [Google Scholar]
- 18.Jacob G, Atkinson D, Jordan J, Shannon JR, Furlan R, Black BK, et al. Effects of standing on cerebrovascular resistance in patients with idiopathic orthostatic intolerance. Am J Med. 1999;106:59–64. doi: 10.1016/s0002-9343(98)00364-7. [DOI] [PubMed] [Google Scholar]
- 19.Lenard Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–12. doi: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Stork TL, Perron AD, Brady WJ. The athlete’s electrocardiogram. Am J Emerg Med. 2006;24:77–86. doi: 10.1016/j.ajem.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Arrowood JA, Minisi AJ, Goudreau E, Davis AB, King AL. Absence of parasympathetic control of heart rate after human orthotopic cardiac transplantation. Circulation. 1997;96:3492–8. doi: 10.1161/01.cir.96.10.3492. [DOI] [PubMed] [Google Scholar]
- 22.Moak JP, Bailey JJ, Makhlouf FT. Simultaneous heart rate and blood pressure variability analysis. Insight into mechanisms underlying neurally mediated cardiac syncope in children. J Am Coll Cardiol. 2002;40:1466–74. doi: 10.1016/s0735-1097(02)02273-8. [DOI] [PubMed] [Google Scholar]
- 23.de Jong-de Vos van Steenwijk CC, Wieling W, Johannes JM, Harms MP, Kuis W, et al. Incidence and hemodynamic characteristics of near-fainting in healthy 6- to 16-year old subjects. J Am Coll Cardiol. 1995;25:1615–21. doi: 10.1016/0735-1097(95)00056-a. [DOI] [PubMed] [Google Scholar]
- 24.Hill L. The influence of the force of gravity on the circulation of the blood. J Physiol. 1895;18:15–53. doi: 10.1113/jphysiol.1895.sp000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellebrandt FA, Franseen EB. Physiologic study of vertical stance in man. Physiol Rew. 1943;23:220–5. [Google Scholar]
- 26.Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet. 1986;1:1352–5. doi: 10.1016/s0140-6736(86)91665-x. [DOI] [PubMed] [Google Scholar]
- 27.Abi-Samra F, Maloney JD, Fouad-Tarazi FM, Castle LW. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. Pacing Clin Electrophysiol. 1988;11:1202–14. doi: 10.1111/j.1540-8159.1988.tb03973.x. [DOI] [PubMed] [Google Scholar]
- 28.Fouad FM, Sitthisook S, Vanerio G, Maloney J, III, Okabe M, Jaeger F, et al. Sensitivity and specificity of the tilt table test in young patients with unexplained syncope. Pacing Clin Electrophysiol. 1993;16:394–400. doi: 10.1111/j.1540-8159.1993.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 29.Claydon VE, Hainsworth R. Increased postural sway in control subjects with poor orthostatic tolerance. J Am Coll Cardiol. 2005;46:1309–13. doi: 10.1016/j.jacc.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Julu PO, Cooper VL, Hansen S, Hainsworth R. Cardiovascular regulation in the period preceding vasovagal syncope in conscious humans. J Physiol. 2003;549:299–311. doi: 10.1113/jphysiol.2002.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheldon RS, Sheldon AG, Connolly SJ, Morillo CA, Klingenheben T, Krahn AD, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49–54. doi: 10.1111/j.1540-8167.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 32.LeLorier P, Klein GC, Krahn A, Yee R, Skanes A, Shoemaker JK. Combined head-up tilt and lower body negative pressure as an experimental model of orthostatic syncope. J Cardiovasc Electrophysiol. 1993;14:920–4. doi: 10.1046/j.1540-8167.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- 33.Brignole M, Alboni P, Benditt DG, Bergfeldt L, Blanc JJ, Bloch Thomsen PE, et al. Guidelines on management (diagnosis and treatment) of syncope—update 2004. Europace. 2004;6:467–537. doi: 10.1016/j.eupc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Stewart JM. Transient orthostatic hypotension is common in adolescents. J Pediatr. 2002;140:418–24. doi: 10.1067/mpd.2002.122643. [DOI] [PubMed] [Google Scholar]
- 35.Sheriff DD, Nadland IH, Toska K. Hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol. 2007;103:452–8. doi: 10.1152/japplphysiol.01190.2006. [DOI] [PubMed] [Google Scholar]
- 36.Raczkowska M, Eckberg DL, Ebert TJ. Muscarinic cholinergic receptors modulate vagal cardiac responses in man. J Auton Nerv Syst. 1983;7:271–8. doi: 10.1016/0165-1838(83)90080-2. [DOI] [PubMed] [Google Scholar]
- 37.Dunlop D, Shanks RG. Selective blockade of adrenoceptive beta receptors in the heart. Br J Pharmacol Chemother. 1968;32:201–18. doi: 10.1111/j.1476-5381.1968.tb00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiale PA, Garro HA, Schmidberg J, Sanchez RA, Acunzo RS, Lago M, et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006;3:1182–6. doi: 10.1016/j.hrthm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Grubb BP, Karabin B. Cardiology patient page. Postural tachycardia syndrome: perspectives for patients. Circulation. 2008;118:e61–e62. doi: 10.1161/CIRCULATIONAHA.107.761650. [DOI] [PubMed] [Google Scholar]
- 40.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–13. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 41.Streeten DH. Pathogenesis of hyperadrenergic orthostatic hypotension. Evidence of disordered venous innervation exclusively in the lower limbs. J Clin Invest. 1990;86:1582–8. doi: 10.1172/JCI114878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan J, Shannon JR, Diedrich A, Black BK, Robertson D. Increased sympathetic activation in idiopathic orthostatic intolerance: role of systemic adrenoreceptor sensitivity. Hypertension. 2002;39:173–8. doi: 10.1161/hy1201.097202. [DOI] [PubMed] [Google Scholar]
- 43.Yamanouchi Y, Jaalouk S, Shehadeh AA, Fouad-Tarazi FM. Venous dysfunction and the change of blood viscosity during head-up tilt. Pacing Clin Electrophysiol. 1998;21:520–7. doi: 10.1111/j.1540-8159.1998.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 44.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–14. doi: 10.1056/NEJM200010053431404. [DOI] [PubMed] [Google Scholar]
- 45.Stewart JM. Microvascular filtration is increased in postural tachycardia syndrome. Circulation. 2003;107:2816–22. doi: 10.1161/01.CIR.0000070951.93566.FC. [DOI] [PubMed] [Google Scholar]
- 46.Rothe CF. Venous system: physiology of the capacitance vessels. In: Shepherd JT, Abboud FM, Geiger SR, editors. The cardiovascular system: peripheral circulation and organ blood flow. handbook of physiology. American Physiologic Society; Bethesda: 1983. pp. 397–452. sect 22 vol III, part 1. [Google Scholar]
- 47.Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhuri KR, Thomaides T, Mathias CJ. Abnormality of superior mesenteric artery blood flow responses in human sympathetic failure. J Physiol. 1992;457:477–89. doi: 10.1113/jphysiol.1992.sp019388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol. 2008;295:H372–81. doi: 10.1152/ajpheart.00101.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartor DM, Verberne AJ. Cholecystokinin selectively affects presympathetic vasomotor neurons and sympathetic vasomotor outflow. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1174–84. doi: 10.1152/ajpregu.00500.2001. [DOI] [PubMed] [Google Scholar]
- 51.Yorganci K, Sayek I, Ismailoglu UB, Sahin-Erdemli I. Detrimental effects of nitric oxide on mesenteric circulation during endotoxaemia and its reversal by aminoguanidine. Eur J Surg. 2000;166:888, 93. doi: 10.1080/110241500447290. [DOI] [PubMed] [Google Scholar]
- 52.Wiest R, Shah V, Sessa WC, Groszmann RJ. NO overproduction by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. Am J Physiol. 1999;276:G1043–51. doi: 10.1152/ajpgi.1999.276.4.G1043. [DOI] [PubMed] [Google Scholar]
- 53.Mosqueda-Garcia R, Furlan R, Md JT, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope [In Process Citation] Circulation. 2000;102:2898–906. doi: 10.1161/01.cir.102.23.2898. [DOI] [PubMed] [Google Scholar]