Abstract
A large number of studies have provided evidence for the efficacy of psychological and other non-pharmacological interventions in the treatment of chronic pain. While these methods are increasingly used to treat pain, remarkably few studies focused on the exploration of their neural correlates. The aim of this article was to review the findings from neuroimaging studies that evaluated the neural response to distraction-based techniques, cognitive behavioral therapy (CBT), clinical hypnosis, mental imagery, physical therapy/exercise, biofeedback, and mirror therapy. To date, the results from studies that used neuroimaging to evaluate these methods have not been conclusive and the experimental methods have been suboptimal for assessing clinical pain. Still, several different psychological and non-pharmacological treatment modalities were associated with increased painrelated activations of executive cognitive brain regions, such as the ventral- and dorsolateral prefrontal cortex. There was also evidence for decreased pain-related activations in afferent pain regions and limbic structures. If future studies will address the technical and methodological challenges of today’s experiments, neuroimaging might have the potential of segregating the neural mechanisms of different treatment interventions and elucidate predictive and mediating factors for successful treatment outcomes. Evaluations of treatment-related brain changes (functional and structural) might also allow for sub-grouping of patients and help to develop individualized treatments.
Keywords: Pain, neuroimaging, non-pharmacological, psychological modulation, analgesia
Introduction
With increased evidence for the efficacy of psychological interventions in the treatment of acute and chronic pain [74, 77, 108], these methods are more and more commonly used in pain clinics, either alone or in combination with pharmacological treatment. While these interventions have been investigated by a large number of behavioral studies, remarkably few studies have focused on the exploration of their neural correlates. As we will discuss, an understanding of the neurobiological mechanisms by which these techniques produce alterations in the experience of pain could have a number of significant implications, which might ultimately enhance treatment efficacy. The aim of this review is to highlight the findings from neuroimaging studies that evaluated the neural effects of commonly used psychological and other non-pharmacological treatments for pain. In brief, the present article summarizes the neuroimaging findings from seven different treatment modalities: distraction based techniques, cognitive behavioral therapy (CBT), clinical hypnosis, mental imagery, physical therapy/exercise, biofeedback, and mirror therapy.
The brain is a potent source of pain modulation, and since the advent of neuroimaging techniques, the neural underpinnings of endogenous modulation of pain have been extensively investigated in healthy volunteers. For example, functional Magnetic Resonance Imaging (fMRI) and Positron Emission Tomography (PET) have been used to assess changes in central pain processing in response to distraction techniques [9, 118], hypnotic states [125, 130] and altered anticipation of pain [26, 68, 83, 157]. Overall, findings suggest that psychological modulation of pain is accompanied by objectively measurable changes in neuronal activity (during administration of a noxious stimulus) [162] in regions such as the lateral Prefrontal Cortex (PFC) [119], the rostral Anterior Cingulate Cortex (ACC) [15, 40, 157], the dorsal part of the ACC [9, 94], or the Periaqueductal Gray (PAG) [151] (see Figure 1). Results from neuroimaging studies in healthy volunteers have been important in moving beyond self-report and assessing the neural underpinnings of “psychological” pain modulation in the central nervous system. Direct evidence of neural networks that support endogenous modulation of pain is likely to have improved the general acceptance of psychological treatment strategies and promoted their legitimacy as an effective component of multidisciplinary pain treatment.
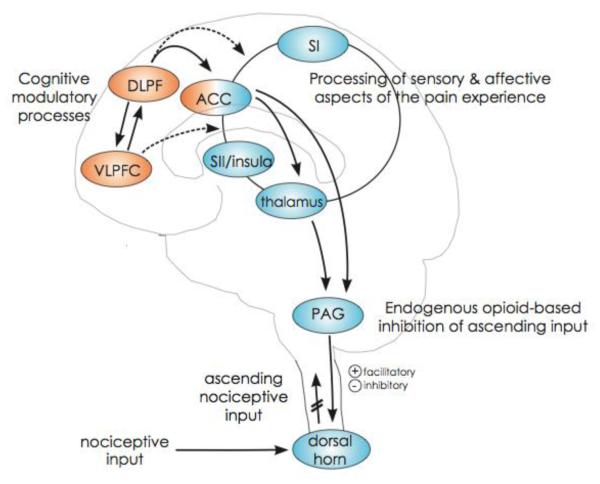
Figure 1. Possible neural pathways of cognitive pain modulation (adapted from Wiech, Ploner and Tracey, 2008).
Cognitive modulations of pain are related to activation of prefrontal brain areas such as the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), and to the anterior cingulate cortex (ACC); shown in orange. These regions may modulate activation in afferent pain regions in the cortex (ACC, primary- and secondary somatosensory cortex, insula and thalamus), as well as the periaqueductal gray (PAG) and dorsal horns of the spinal cord; shown in blue. The DLPFC and VLPFC are connected to the ACC, which, in turn, projects to thalamus and the PAG, a core component of the descending pain modulatory system. This system eventually facilitates and/or inhibits pain processing at the level of the spinal cord dorsal horn. Direct cortico–cortical modulations from VLPFC and DLPFC to pain-associated cortical areas are probable but have not been directly shown yet (broken lines). Areas most closely associated with afferent pain processing are densely interconnected, as indicated by the connecting circle.
Brain imaging studies have contributed to the understanding of cerebral changes associated with chronic pain [5, 140, 141] and evidence includes structural [21, 32, 45, 86, 138], functional [7, 56, 67, 70, 141] and neurochemical brain alterations [59, 75, 165] in patients, compared to carefully matched controls. There are indications of a linear relationship between specific brain changes and the duration of chronic pain [86], suggesting that long-term exposure to pain might cause central alterations such as decreased grey matter of the prefrontal cortex [7], and not vice versa [99, 136]. Furthermore, recent evidence points towards normalization of brain structure and function in response to successful treatment for chronic pain [58, 114, 116, 136, 144], indicating that brain plasticity can be bidirectional. In the presence of persistent pain, patients often suffer from cognitive deficits, and recent neuroimaging studies have linked pain patients’ cognitive dysfunction to specific changes in brain structure [95] and function [54], providing evidence for a pain-cognition interaction. Given the plasticity of the brain in response to analgesia, and the close interaction between pain modulation and executive cognitive function, it is likely that psychological interventions targeting clinical pain are associated with adaptive changes in brain function or structure. To date, few studies have evaluated the neural underpinnings of psychological treatments for pain in clinical populations. It is our hope that this review will inspire neuroscientists and pain clinicians to collaborate in future studies where neuroimaging is used as a tool to evaluate non-pharmacological pain treatments.
Neuroimaging of distraction-based techniques in pain patients
Both clinical and experimental studies have demonstrated that noxious stimuli can be perceived as significantly less painful when the attention is directed away from them [140, 153]. For instance, several authors have observed that measures of pain sensitivity are decreased when the attention of healthy volunteers is directed towards simultaneously presented visual [94, 106] auditory [24, 38], olfactory [155] or tactile [94] stimuli, and/or engaging activities [25].
Numerous imaging experiments in healthy volunteers have demonstrated that pain reductions during distraction are associated with decreased pain-evoked activations in structures belonging to the thalamo-cortical ascending pain network [6], such as the thalamus, primary and secondary somatosensory cortices, insula and ACC [10, 17, 24, 51, 66, 94, 143, 152, 154, 163]. Moreover, neurophysiological studies in monkeys have shown that nociceptive neurons in the medullary dorsal horn and the medial thalamus are less responsive to noxious stimuli when the monkey is attending to a distracter, compared to attending to the pain [22, 23]. Distraction from pain is not only accompanied by reduced activity in some pain processing structures, but also by increased activity in brain regions such as the PAG, the rostral parts of the ACC and the orbitofrontal cortex [51, 151, 152]. Taken together, these studies suggest that distraction-related reductions in reported pain are associated with objective neurophysiological changes, some of which occur at early stages of sensory processing, and can therefore not be explained in terms of report bias. It is important to note that some brain structures, such as the ACC, may be divided in sub-regions that serve different aspects of pain processing [156], and therefore a region can sometimes be associated with increases or decreases in response to pain modulation, depending on the exact anatomical location. For example, activation of the rostral ACC has been demonstrated during inhibition of pain [15, 120], whereas activation of the more posterior parts of the ACC has been associated with increased pain affect [46, 47, 139].
In clinical settings, distraction has been employed to reduce pain during uncomfortable procedures, such as venipuncture [4, 166], cataract surgery [146], endoscopy [90] and wound care [65, 110]. Among the most well studied clinical applications of a distraction-based technique is the use of immersive virtual reality (VR) in acute burn patients. When distracted with VR, patients often report significant reductions in pain and discomfort during the painful procedures of wound cleaning and debridement [65, 110]. Although no fMRI study has, to our knowledge, investigated the neural underpinning of VR-analgesia in pain patients, the use of VR in healthy volunteers undergoing heat pain stimulation [66] was shown to be associated with reduced pain-related fMRI signals in the ACC, insula, thalamus, primary and secondary somatosensory cortices, suggesting that an analogous mechanism might mediate the analgesic effect of VR in patient care. While distraction techniques have proved to be successful means of reducing acute pain, their application as strategies to control chronic pain might present some difficulties. In fact, studies have suggested that patients with chronic pain have an attentional bias towards pain, or a failure to disengage from pain [122, 145]. Thus, such patients might benefits from the application of techniques focused at modifying the attentional bias [19, 20].
In summary, results from the literature suggest that distraction could be successfully used as a pain control strategy and neural correlates to distraction based interventions include reduced activation in afferent pain regions, as well as increased activation in structures of the descending pain inhibitory circuitry.
Functional imaging of CBT in pain patients
Cognitive Behavioral Therapy (CBT) is an evidence-based psychotherapeutic method, rooted in behaviorism and cognitive psychological theory. Since the introduction of CBT-based treatments for chronic pain more than 35 years ago [48, 78], there have been many published reports of symptom improvements in patients with various forms of chronic pain [14, 64, 93, 97, 160]. CBT is often conducted through weekly therapy sessions, in combination with home assignments. Central to treatments based on CBT is the identification of maladaptive cognitions and behavior patterns, such as catastrophizing thoughts [39] or avoidance behaviors [100], which can be targeted by exposure-oriented interventions.
Despite the common use of CBT for chronic pain, only two studies have investigated the neural correlates of CBT in pain patients [71, 89]. The two studies used similar treatment protocols, including weekly CBT sessions in small groups of 3-5 patients, combined with home assignments. Lackner and colleagues [89], used PET to evaluate the effect of a 10-week CBT trial in 8 female patients with Irritable Bowel Syndrome (IBS) and Jensen et al [71] used fMRI to assess the response to a randomized, 12-week, waiting-list controlled trial in 43 female patients with Fibromyalgia syndrome (FM). Results from both studies found that the clinical effect of CBT was paired with significant changes in brain activations post-treatment. More specifically, patients with IBS displayed decreased PET O-15 resting-state activity in the limbic regions post-treatment, including the parahippocampal gyrus and cingulate cortex, bilaterally. It is possible that the decreased limbic activity in response to CBT reflected attenuated vigilance and attention to pain, as both these measures improved post-treatment. fMRI measures in patients with FM revealed that CBT led to increased pain-evoked activation of the lateral PFC after treatment, compared to controls (see Figure 2). Moreover, patients treated with CBT displayed increased connectivity between the PFC and the thalamus. The thalamus is a critical relay site for afferent signals, including pain [150], and decreased thalamic activity has previously been implicated in pain pathology [28, 69, 88, 112]. Therefore, increased coherence between the PFC and the thalamus might be an indication of attenuated central pain pathology.
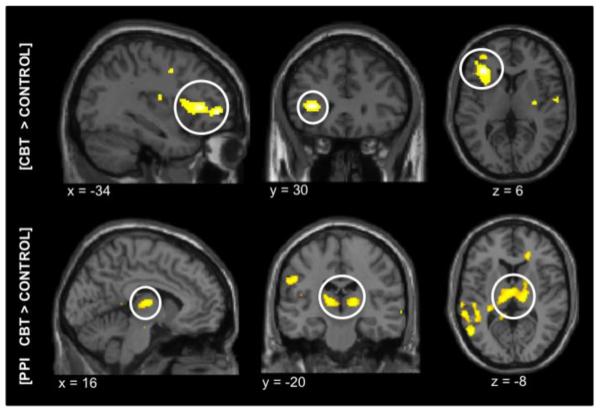
Figure 2. Results from an fMRI evaluation of CBT in FM patients (Jensen et al. 2011).
Statistical maps of the fMRI activations during subjectively calibrated pressure pain (n=43). All maps represent brain activations after treatment minus before treatment, for the contrast [CBT > control group]. The top panel: significantly increased pain-evoked brain activity in the left lateral prefrontal cortex in the CBT group after treatment, compared to controls. The lower panel: significantly increased connectivity between the left lateral prefrontal cortex and the thalamus in the CBT group, compared to controls, measured by Psychophysiological Interaction effects. All anatomical locations are given in Montreal Neurological Institute coordinates (MNI).
As an interesting comparison, neuroimaging evaluations of CBT in psychiatric conditions have often reported decreased activation of limbic regions and increased function of the prefrontal cortex (PFC) in response to treatment (for a review see Ribeiro et al.) [133], suggesting that CBT can lead to decrements in maladaptive cognitive and emotional processes (e.g., fear, negative affect, obsessive thoughts, etc.) through increased prefrontal control. Moreover, pre-treatment activity in the ventromedial PFC predicted a positive response to CBT in depression, suggesting that patients with less pre-treatment impairments might be more likely to benefit from cognitive behavioral interventions [134].
In summary, neuroimaging has contributed to the understanding of the neural changes that can be attributed to CBT in chronic pain. However, there are a number of limitations to the present findings. Firstly, the study in IBS assessed the post-treatment effects of CBT without including a control group in the analysis. This means that the natural history or test-retest effects may have confounded the neuroimaging results. Secondly, only six patients were included in the evaluation of post-treatment effects of CBT, suggesting that the study was underpowered and should be interpreted with caution. The FM study included a waiting-list control group but there was no active control for CBT, e.g. weekly social sessions or lectures about pain. Future studies should validate these preliminary findings by randomizing patients to either a CBT arm, pharmacological arm, CBT + pharmacological arm, active control or natural history. Contrasting information about the functional brain changes in response to CBT and other treatments will increase the chances of finding specific treatment mechanisms for CBT, if they exist. CBT is a relatively expensive treatment and future studies should break down the different components of CBT and see if any specific intervention contributes more to the changes in central processing seen after treatment. In fact, most CBT programs, beyond addressing the maladaptive cognitive and behavioral patterns, also teach elements of physical therapy, mental imagery, or distraction techniques, and therefore studying these modalities separately is of related interest.
Functional imaging of clinical hypnosis in pain patients
Hypnosis is a procedure in which suggestions for imaginative experiences are presented after an hypnotic induction, often including suggestions of relaxation, deep breathing and/or focused attention.[57]. The subject is guided by the hypnotherapist to respond to suggestions for changes in subjective experience, perception, sensation, emotion, thought, or behavior [57]. In the context of pain relief, an initial suggestion of relaxation is often followed by different suggestions which can enhance distraction, positive affect, feelings of self efficacy and control over pain or alter the way pain is experienced [117]. The psychological mechanisms involved in hypnotic analgesia have not yet been fully explained [73, 126], but it is likely that several factors contribute to the desired effect, e.g. altered expectancy, reappraisal, sense of control, mood, and attentional focus.
Studies in healthy volunteers have demonstrated a variety of neural underpinnings for hypnotic analgesia. Work by Rainville, Hofbauer, and collaborators has established that suggestions aimed at modifying pain unpleasantness are linearly associated with pain-related activation in the ACC [128], while those altering pain intensity correlate with activity in primary sensory cortex (SI) [63]; suggesting that these discrete aspects of the painful experience could be modulated separately [127]. Collectively, the analgesic effect of hypnotic modulation has repeatedly been associated with altered activity in the ACC [42, 129]. Furthermore, hypnotic analgesia has been proposed to be mediated by an increase in the functional connectivity between the mid-cingulate cortex and a large cortico-subcortical network including the brainstem, thalamus, insulae, ACC, the supplementary premotor cortex and the right PFC, suggesting an alteration in the integration of sensory, affective, cognitive and behavioral aspects of the pain experience [43].
Therapist-delivered hypnosis was shown to benefit patients suffering from chronic pain [73] but self-hypnosis can also lead to analgesia in patients, similarly to other therapies involving relaxation or imagery [72, 73]. To date, four neuroimaging studies evaluated the effects of hypnotic analgesia in chronic pain patients: two studies in FM [35, 164], one in temporo-mandibular joint disorder [1], and one in patients with chronic low back pain [115]. In contrast to assessments of hypnotic analgesia in healthy volunteers, none of these studies found activity changes in the ACC when comparing hypnotic analgesia to a control condition, and each showed a unique pattern of changed brain activations [1, 35, 115, 164]. Some key methodological differences could explain the absence of a consensual finding from these studies. For example, the study by Derbyshire et al. collapsed hyperalgesic and hypoalgesic suggestions and compared the hypnotic state to the non-hypnotic state, instead of comparing the hypnotic analgesia versus the same non-hypnotic suggestions [35], while Nusbaum et al. did not test the statistical difference between activations in the hypnotic state and the control state [115]. These results could therefore justify further studies asking a more specific question, such as: is hypno-analgesia in patients mediated by different central circuits compared to healthy volunteers? Brain imaging techniques might also help to better understand hypnotic susceptibility, as this personal quality seems to be a determining factor in the response to hypno-analgesia [33, 107]. Finally, these technologies could also participate in identifying other predictors of response to hypnotherapy for pain, since hypnotic susceptibility appears to explain only a part of the difference in pain ratings in an hypnotic analgesia condition [1].
Neuroimaging of mental imagery in pain patients
Mental images are sensory experiences in the absence of a direct percept, resulting from a cognitive process by which perceptual information is retrieved from memory to create a new experience, allowing to ‘see with the mind’s eye’ or ‘smell with the mind’s nose’ [84]. Mental imagery-based therapy is frequently used in clinical practice for pain relief and consists of suggesting specific (positive) mental images, which the patient tries to experience in his/her mind [121]. Clinical trials of mental imagery have shown benefits for patients with chronic pain [e.g. 3, 8, 18, 49, 50, 87, 91, 92, 96, 102, 124]. Nonetheless, there are methodological issues with many of the studies conducted in this field, as discussed in recent literature reviews [109, 123].
Mental imagery techniques are carried out specifically without the ‘induction’ phase characteristic of hypnosis. The hypnotic induction might in fact be less important to obtain responsiveness to suggestions than once thought, and most of the effects of hypnosis could be due to the underlying suggestions [82]. In fact, well-controlled hypnotic inductions with no specific suggestions seem to have no analgesic effect [63, 127] thus providing a rationale for studying the neurophysiological underpinnings of suggestions.
However, all brain imaging studies of mental imagery-based pain modulation published to date are control conditions in hypnosis experiments [35, 42, 43, 115]. These control conditions could be more or less valid, as the suggestions in hypnosis and mental-imagery are sometimes different [43] or given in a different way (e.g. tone of voice, prosody) [115]. This, as well as participant’s expectations towards hypnosis could significantly impact the effectiveness of the control [82], and therefore the conclusions held about this technique. Furthermore, all of these studies selected participants with high hypnotic suggestibility [35, 42, 43, 115], leaving many questions open about the excluded patients with low hypnotic suggestibility [105].
In summary, while brain activity during mental imagery for pain relief has been assessed using functional neuroimaging [115], no study has compared a mental imagery task to a control condition with similar cognitive load to investigate the specific neural underpinnings of this technique. The studies that compared mental imagery with hypnosis prevented the assessment of specific mental imagery mechanisms by collapsing the mental imagery condition with a rest condition [42, 43]. Future studies could also compare different suggestions, potentially demonstrating the recruitment of different brain networks, and hence the ability of mental imagery to rely on different cognitive mechanisms. This could be useful, as patients with different psychological profiles could benefit more from one or the other cognitive modulation of pain. This viewpoint is supported by studies demonstrating that the meaning of a visual image likely plays an important role in shaping brain responses (e.g., viewing a specific religious image has different effects on pain perception in individuals with differing belief systems) [161], highlighting the potential benefits of tailoring images and suggestions to individual patients.
Neuroimaging of physiotherapy/exercise in pain patients
Physiotherapy and exercise-based interventions for the management of chronic pain are widely recommended and have proven effective in reducing pain and improving physical function in a variety of pain syndromes, including FM [80], back pain [61], arthritis [79], and many others. These treatments take a variety of forms, from one-on-one sessions with a licensed physiotherapist, to group aquatherapy sessions, to prescription of stretching and muscle-strengthening exercises at home. In general, the available data suggests that any type of physiotherapy intervention that increases physical activity (e.g., stretching, cardiovascular conditioning, strength training, etc.) may have pain-relieving benefits, with few differences between various schools of physiotherapy or types of exercise [29]. A recent systematic review concluded that clinical improvements in back pain following exercise and physiotherapy treatment were largely uncorrelated with objective improvements in musculoskeletal performance (i.e., range of motion, skeletal muscle strength, muscular endurance), suggesting that other changes, potentially in the central processing of pain or mood, play an important role in these treatments [149].
Recent findings, both from animal studies and clinical trials, provide evidence for central nervous system mechanisms in exercise-induced analgesia [13, 148]. Unfortunately, to date there are few functional neuroimaging studies of the specific pathways by which physiotherapy and exercise may reduce pain perception. One recent report in healthy volunteers used transcranial magnetic stimulation to demonstrate that administration of a noxious mechanical stimulus enhanced the amplitude of motor-evoked potentials [62]. The performance of isometric exercise reduced pain intensity and attenuated the pain-induced increase in motor-evoked potentials, suggesting that exercise may lead to modulation of corticomotor excitability [62]. Somewhat surprisingly, no studies have directly examined brain responses to pain before and after a standardized exercise protocol. Such a study might pose some interpretive difficulties, as recent exercise would likely alter respiration, cardiopulmonary function, and cerebral blood flow, complicating the characterization of the blood oxygenation level dependent fMRI signal following a bout of exercise. However, the PAG and subthalamic nucleus are intimately involved in initiating and integrating cardiovascular responses to exercise [11], and in particular the PAG plays an important role in opioid-dependent pain modulation, highlighting a possible mechanistic link between exercise and pain reduction.
Rather than directly study an exercise intervention, a recent fMRI study of heat pain responses in FM patients and controls separated participants as a function of recent physical activity [101]. Patients and controls who were more physically active over the preceding week demonstrated reduced heat pain ratings and increased brain responses to painful heat in pain-modulatory areas such as the dorsolateral PFC, though PAG responses were unrelated to physical activity measures [101]. The dorsolateral PFC and related areas have been linked to cognitively driven pain inhibition in the context of, for example, placebo analgesia [85]. It is possible that there is substantial mechanistic overlap between physiotherapy/exercise-based interventions and psychological treatments. Outcomes studies suggest that treatments targeted at increasing physical activity among chronic pain patients produce strong reductions in symptoms of distress and catastrophizing thoughts, and that these cognitive and emotional changes mediate the observed reductions in pain intensity and physical disability that the treatment produces [52, 147]. Future trials of exercise and physiotherapy interventions will have to evaluate weather changes in cerebral pain processing are attributable specifically to increases in physical activity, or to ancillary changes in the psychosocial domain.
Neuroimaging of biofeedback in pain patients
Biofeedback is comprised of a set of methodologies whereby individuals are provided with real-time feedback of different physiological parameters, with the goal of developing awareness of and control over these bodily processes, some of which may traditionally be considered “involuntary” [55]. In the context of pain management, the physiological targets of biofeedback are typically processes that are directly associated with pain exacerbations, or stress responses that are presumed to exacerbate pain. Recent systematic reviews and meta-analyses provide empirical support for a variety of biofeedback methods for managing chronic headache, back pain, orofacial pain, etc [2, 81, 113]. Physiological targets include, among others, heart rate and heart rate variability, surface electromyography of skeletal muscle, skin temperature, galvanic skin response.
Most recently, Electroencephalography (EEG) biofeedback has been studied in several trials in patients with FM. In EEG biofeedback, the amplitudes of resting EEG frequency bands (i.e., Theta 4–8 Hz: associated with early-stage sleep; Alpha 8–12 Hz: associated with a relaxed and defocused attentional state; Beta 13 Hz or greater: associated with higher-order cognition) are represented visually and participants attempt to learn to increase or decrease the power or amplitude of particular frequency bands. Prior work in healthy adults had demonstrated that subjects were able to alter the relative power of particular EEG frequency bands; for example, increasing the Theta/Alpha ratio [12], or reducing relative Alpha power [137]. In studies of patients with FM, participants were able to learn to modulate EEG signals over the course of ten to forty sessions [27, 76]. In general, patients treated with EEG biofeedback showed improvements in a variety of symptoms, including pain, fatigue, anxiety etc. Putative mechanisms that might underlie such effects include the promotion of functional neuroplasticity and enhancement of motor cortex excitability [137], as well as the facilitation of thalamocortical inhibitory pathways [76].
To date, no functional neuroimaging studies have scanned participants before and after the application of a pain-reducing biofeedback technique, so data on the neural mechanisms underlying the analgesic benefits of biofeedback are not available. Some researchers have used real-time fMRI to perform “neurofeedback” during a scan, providing immediate visual feedback to subjects about brain activation in areas involved in pain processing and regulation such as the ACC. This emerging technique has been discussed in detail by deCharms et al [34] and Weiskopf et al [159].
One significant challenge in this field will be the selection of the physiological parameter to provide feedback on. While EEG is an attractive target, other processes have been effectively targeted as well. For example, a recent study of heart rate variability and biofeedback (intended to dampen autonomic reactivity) in patients with FM reported increased heart rate variability over the course of 10 weeks of training, which was associated with significant decreases in pain and depression, and improvement in physical function at 3-month follow-up [60]. In this field, additional research is needed to clarify which physiological parameters subjects are most able to modulate, and which parameters are most closely related to pain. In addition, further studies will be required to evaluate the specificity of any brain responses to pain, since biofeedback treatments may produce a host of non-specific effects [81].
Neuroimaging of mirror therapy in pain patients
Mirror therapy is an exposure-based therapy for painful limbs, using a 24 × 24 inches mirror box, propped in between the patient’s unaffected and affected limb [131]. When looking at the box, the patient sees the mirror image of the unaffected limb taking the place of the affected limb in their visual field. By moving the normal limb, the affected side is perceived to move as well. Mirror boxes are available for as little as 40 U.S. dollars and treatment can be effectively self-delivered at home [31].
The discovery of mirror neurons in the brain [36], and reports of perceptual correlates of cortical reorganization following limb amputation [132], has provided a rationale for mirror therapy in patients with hemiparesis or chronic pain. As mirror neurons are activated both during the performance of a motor action and during observation of another individual performing a similar action [44, 98] [135], it has been suggested that the treatment mechanisms in mirror therapy are related to brain neuroplasticity induced by the vicarious (“mirror”) activation of the somatotopic representation of the affected limb [53].
A few randomized controlled trials have assessed the effect of mirror therapy for pain. One study was a 4-week crossover trial in 18 patients with phantom limb pain (PLP) [30], and one was a 6-week crossover trial performed in 13 patients with complex regional pain syndrome Type I [111]. In both studies the majority of subjects who received mirror therapy had more than 50% improvement of pain when assessed at 8 and 24 weeks, respectively.
The neural correlates to the treatment effect of mirror therapy have been investigated by means of fMRI. In patients with stroke, the mirror illusion was associated with increased activations in the precuneus and the posterior cingulate cortex; areas associated with awareness of the self and spatial attention [104]. Interestingly, the authors did not observe mirror-therapy related brain activations in areas of the motor cortex or other regions of mirror neuron system. The findings from this stroke trial question whether mirror neurons underlie the efficacy of mirror therapy. In a follow up randomized clinical trial by the same group, 16 subjects with stroke underwent 6 weeks of mirror therapy, or a control condition, and repeated fMRI scans. While mirror therapy was only weakly effective in improving function, it was associated with a shift in activation balance of the primary motor cortex toward the affected brain hemisphere, suggesting cortical reorganization [103]. In 14 patients with upper extremity amputation (7 with and 7 without PLP), Diers and colleagues demonstrated that patients without PLP had greater mirror-related activation in primary somatosensory and primary motor cortices during fMRI scanning [37]. However, in a recent fMRI treatment study by Seidel and colleagues [142], 12 sessions of mirror therapy in amputees with PLP were not associated with changes in mirror-related activation in the primary somatosensory and motor cortices, even though patients reported more than 50% mean improvement of pain. These results question whether sensorimotor reorganization underlies mirror therapy success or if it is a relevant precondition for pain relief.
While mirror therapy is a promising intervention for painful conditions such as PLP and complex regional pain syndrome, there is a need for larger clinical trials with better chances of detecting possible responders and non-responders. Neuroimaging techniques are well suited to study potential reorganization of cortical function and future studies might provide better characterization of the neural changes attributable to mirror therapy. A large randomized clinical trial that combines continuous measures of treatment outcomes and neuroimaging assessments, would be a most favorable approach to addressing these issues.
Discussion
To date, only few studies used functional neuroimaging to evaluate psychological and other non-pharmacological treatments for pain, however, the advances of modern neuroimaging techniques holds the promise for more such studies in the future. Hitherto, most neuroimaging methods were optimized for experimental pain paradigms, and the ability to assess clinical improvements of naturally occurring pain have been limited. Nevertheless, new neuroimaging techniques are being developed and Arterial Spin Labeling, for example, could be a useful tool for measuring slow fluctuations of chronic pain [158]. Also, the development of spinal fMRI tools [16, 41] might lead to better characterization of differences in pain processing after treatment with psychological and other non-pharmacological interventions. If future studies will address the technical and methodological challenges of today’s experiments, neuroimaging might have the potential of segregating the neural mechanisms of different treatment interventions and elucidate predictive and mediating factors for successful treatment outcomes. Evaluations of treatment-related brain changes (functional and structural) might also allow for sub-grouping of patients and help to develop individualized treatments. Compared to pharmacological treatment, psychological and non-pharmacological treatments for pain often require more personal and economic resources. Therefore, it would be valuable to develop standardized neuroimaging assessments for predicting responders to certain treatment modalities. Moreover, it is possible that neuroimaging will become a standardized method for acquiring biomarkers/objective correlates to pain ratings in clinical trials. Finally, while the translation from the neuroimaging-lab to the clinic could lead to some clear benefits, it is also likely that the understanding of how psychological treatments change the brain will increase our general understanding of the role of the brain in chronic pain.
Highlights.
Summary of neuroimaging studies of psychological treatments for clinical pain
Advantages/limitations of using neuroimaging to evaluate these interventions
Future implications for using neuroimaging to develop effective treatments for pain
Acknowledgements
KBJ is supported by a Marie Curie Fellowship (COFAS) from the Swedish Council for Working Life and Social Welfare. RLG receives partial support from several grants: NIH #1P01AT006663-01; NIH/NCCAM #R01AT005280, #R01AT006146-01; NIH/NIDA #P01AT00663, #R03 DA030512-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abrahamsen R, Dietz M, Lodahl S, Roepstorff A, Zachariae R, Ostergaard L, Svensson P. Effect of hypnotic pain modulation on brain activity in patients with temporomandibular disorder pain. Pain. 2010;151(3):825–833. doi: 10.1016/j.pain.2010.09.020. [DOI] [PubMed] [Google Scholar]
- [2].Aggarwal VR, Lovell K, Peters S, Javidi H, Joughin A, Goldthorpe J. Psychosocial interventions for the management of chronic orofacial pain. Cochrane Database Syst Rev. 2011;11:8456. doi: 10.1002/14651858.CD008456.pub2. [DOI] [PubMed] [Google Scholar]
- [3].Albright GL, Fischer AA. Effects of warming imagery aimed at trigger-point sites on tissue compliance, skin temperature, and pain sensitivity in biofeedback-trained patients with chronic pain: a preliminary study. Percept Mot Skills. 1990;71(3):1163–1170. doi: 10.2466/pms.1990.71.3f.1163. [DOI] [PubMed] [Google Scholar]
- [4].Alhani F. The effect of programmed distraction on the pain caused by venipuncture among adolescents on hemodialysis. Pain Manag Nurs. 2010;11(2):85–91. doi: 10.1016/j.pmn.2009.03.005. [DOI] [PubMed] [Google Scholar]
- [5].Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Progress in Neurobiology. 2009;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [7].Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic Back Pain Is Associated with Decreased Prefrontal and Thalamic Gray Matter Density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baird CL, Murawski MM, Wu J. Efficacy of guided imagery with relaxation for osteoarthritis symptoms and medication intake. Pain Manag Nurs. 2010;11(1):56–65. doi: 10.1016/j.pmn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith S, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125(2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- [10].Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. 2002;125(2):310–319. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- [11].Basnayake SD, Green AL, Paterson DJ. Mapping the Central Neurocircuitry that Integrates the Cardiovascular Response to Exercise in Humans. Exp Physiol. 2011;7 doi: 10.1113/expphysiol.2011.060848. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [12].Batty MJ, Bonnington S, Tang BK, Hawken MB, Gruzelier JH. Relaxation strategies and enhancement of hypnotic susceptibility: EEG neurofeedback, progressive muscle relaxation and self-hypnosis. Brain research bulletin. 2006;71(1-3):83–90. doi: 10.1016/j.brainresbull.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [13].Bender T, Nagy G, Barna I, Tefner I, Kadas E, Geher P. The effect of physical therapy on beta-endorphin levels. Eur J Appl Physiol. 2007;100(4):371–382. doi: 10.1007/s00421-007-0469-9. [DOI] [PubMed] [Google Scholar]
- [14].Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37(10):1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- [15].Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- [16].Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathway. Journal of Anatomy. 2005;207(1):19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15(2):293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- [18].Brown JM. Imagery coping strategies in the treatment of migraine. Pain. 1984;18(2):157–167. doi: 10.1016/0304-3959(84)90883-2. [DOI] [PubMed] [Google Scholar]
- [19].Browning M, Holmes EA, Harmer CJ. The modification of attentional bias to emotional information: A review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci. 2010;10(1):8–20. doi: 10.3758/CABN.10.1.8. [DOI] [PubMed] [Google Scholar]
- [20].Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased Gray Matter Volumes in the Cingulo-Frontal Cortex and the Amygdala in Patients With Fibromyalgia. Psychosom Med. 2009;71(5):566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- [22].Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Experimental brain research. 1989;78(2):415–418. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- [23].Bushnell MC, Duncan GH, Dubner R, He LF. Activity of trigeminothalamic neurons in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. Journal of neurophysiology. 1984;52(1):170–187. doi: 10.1152/jn.1984.52.1.170. [DOI] [PubMed] [Google Scholar]
- [24].Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Campbell CM, Witmer K, Simango M, Carteret A, Loggia ML, Campbell JN, Haythornthwaite JA, Edwards RR. Catastrophizing delays the analgesic effect of distraction. Pain. 2010;149(2):202–207. doi: 10.1016/j.pain.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage. 2006;32(4):1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- [27].Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl Psychophysiol Biofeedback. 2011;36(3):193–200. doi: 10.1007/s10484-011-9159-9. [DOI] [PubMed] [Google Scholar]
- [28].Cauda F, Sacco K, D’Agata F, Duca S, Cocito D, Geminiani G, Migliorati F, Isoardo G. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci. 2009;26(10):138. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cazzola M, Atzeni F, Salaffi F, Stisi S, Cassisi G, Sarzi-Puttini P. Which kind of exercise is best in fibromyalgia therapeutic programmes? A practical review. Clinical and experimental rheumatology. 2010;6(63):117–124. [PubMed] [Google Scholar]
- [30].Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, Heilman KM, Tsao JW. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357(21):2206–2207. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- [31].Darnall BD. Self-delivered home-based mirror therapy for lower limb phantom pain. Am J Phys Med Rehabil. 2009;88(1):78–81. doi: 10.1097/PHM.0b013e318191105b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- [33].De Pascalis V, Magurano MR, Bellusci A, Chen AC. Somatosensory event-related potential and autonomic activity to varying pain reduction cognitive strategies in hypnosis. Clin Neurophysiol. 2001;112(8):1475–1485. doi: 10.1016/s1388-2457(01)00586-7. [DOI] [PubMed] [Google Scholar]
- [34].deCharms RC. Applications of real-time fMRI. Nature reviews Neuroscience. 2008;9(9):720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- [35].Derbyshire SW, Whalley MG, Oakley DA. Fibromyalgia pain and its modulation by hypnotic and non-hypnotic suggestion: An fMRI analysis. Eur J Pain. 2009;13(5):542–550. doi: 10.1016/j.ejpain.2008.06.010. [DOI] [PubMed] [Google Scholar]
- [36].di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- [37].Diers M, Christmann C, Koeppe C, Ruf M, Flor H. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010;149(2):296–304. doi: 10.1016/j.pain.2010.02.020. [DOI] [PubMed] [Google Scholar]
- [38].Dunckley P, Aziz Q, Wise RG, Brooks J, Tracey I, Chang L. Attentional modulation of visceral and somatic pain. Neurogastroenterol Motil. 2007;19(7):569–577. doi: 10.1111/j.1365-2982.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- [39].Edwards RR, Clifton OB, Bathon J, Haythornthwaite JA. Catastrophizing and Pain in Arthritis, Fibromyalgia, and Other Rheumatic Diseases. Arthritis & Rheumatism. 2006;55(2):325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- [40].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the Opioidergic Descending Pain Control System Underlies Placebo Analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- [41].Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct Evidence for Spinal Cord Involvement in Placebo Analgesia. Science. 2009;326(5951):404–405. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- [42].Faymonville ME, Laureys S, Degueldre C, DelFiore G, Luxen A, Franck G, Lamy M, Maquet P. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology. 2000;92(5):1257–1267. doi: 10.1097/00000542-200005000-00013. [DOI] [PubMed] [Google Scholar]
- [43].Faymonville ME, Roediger L, Del Fiore G, Delgueldre C, Phillips C, Lamy M, Luxen A, Maquet P, Laureys S. Increased cerebral functional connectivity underlying the antinociceptive effects of hypnosis. Brain Res Cogn Brain Res. 2003;17(2):255–262. doi: 10.1016/s0926-6410(03)00113-7. [DOI] [PubMed] [Google Scholar]
- [44].Filimon F, Nelson JD, Hagler DJ, Sereno MI. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. Neuroimage. 2007;37(4):1315–1328. doi: 10.1016/j.neuroimage.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41:66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- [46].Foltz EL, White LE. The role of rostral cingulumotomy in “pain” relief. Int J Neurol. 1968;6(3-4):353–373. [PubMed] [Google Scholar]
- [47].Foltz EL, White LE., Jr. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- [48].Fordyce WE. Behavioral methods for chronic pain and illness. C.V. Mosby; St. Louis, MO: 1976. [Google Scholar]
- [49].Fors EA, Gotestam KG. Patient education, guided imagery and pain related talk in fibromyalgia coping. European Journal of Psychiatry. 2000;14(4):233–240. [Google Scholar]
- [50].Fors EA, Sexton H, Gotestam KG. The effect of guided imagery and amitriptyline on daily fibromyalgia pain: a prospective, randomized, controlled trial. J Psychiatr Res. 2002;36(3):179–187. doi: 10.1016/s0022-3956(02)00003-1. [DOI] [PubMed] [Google Scholar]
- [51].Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14(4):827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- [52].George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40(11):694–704. doi: 10.2519/jospt.2010.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage. 2003;20(1):107–111. doi: 10.1016/j.neuroimage.2003.09.024. [DOI] [PubMed] [Google Scholar]
- [54].Glass JM, Williams DA, Fernandez-Sanchez ML, Kairys A, Barjola P, Heitzeg MM, Clauw DJ, Schmidt-Wilcke T. Executive Function in Chronic Pain Patients and Healthy Controls: Different Cortical Activation During Response Inhibition in Fibromyalgia. The journal of pain. 2011;12(12):1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Glick RM, Greco CM. Biofeedback and primary care. Prim Care. 2010;37(1):91–103. doi: 10.1016/j.pop.2009.09.005. [DOI] [PubMed] [Google Scholar]
- [56].Gracely RH, McGrath P, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5(1):5–18. doi: 10.1016/0304-3959(78)90020-9. [DOI] [PubMed] [Google Scholar]
- [57].Green JP, Barabasz AF, Barrett D, Montgomery GH. Forging ahead: The 2003 APA Division 30 definition of hypnosis. Int J Clin Exp Hypn. 2005;53:259–264. doi: 10.1080/00207140590961321. [DOI] [PubMed] [Google Scholar]
- [58].Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis and rheumatism. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- [59].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased Central μ-Opioid Receptor Availability in Fibromyalgia. J Neurosci. 2007;27(37):10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, Buyske S, Lehrer PM. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl Psychophysiol Biofeedback. 2007;32(1):1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- [61].Henschke N, Kuijpers T, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, Koes BW, van Tulder MW. Trends over time in the size and quality of randomised controlled trials of interventions for chronic low-back pain. European spine journal. 2011;12(12):1219–1229. doi: 10.1007/s00586-011-2023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hoeger Bement MK, Weyer A, Hartley S, Yoon T, Hunter SK. Fatiguing exercise attenuates pain-induced corticomotor excitability. Neuroscience letters. 2009;452(2):209–213. doi: 10.1016/j.neulet.2009.01.038. [DOI] [PubMed] [Google Scholar]
- [63].Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86(1):402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- [64].Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychology. 2007;26:1–9. doi: 10.1037/0278-6133.26.1.1. [DOI] [PubMed] [Google Scholar]
- [65].Hoffman HG, Chambers GT, Meyer WJ, 3rd, Arceneaux LL, Russell WJ, Seibel EJ, Richards TL, Sharar SR, Patterson DR. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Ann Behav Med. 2011;41(2):183–191. doi: 10.1007/s12160-010-9248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hoffman HG, Richards TL, Coda B, Bills AR, Blough D, Richards AL, Sharar SR. Modulation of thermal pain-related brain activity with virtual reality: evidence from fMRI. Neuroreport. 2004;15(8):1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- [67].Hsieh J-C, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63(2):225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- [68].Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of pain expressed in the human anterior cingulate cortex: a positron emission tomography study. Neuroscience letters. 1999;262(1):61–64. doi: 10.1016/s0304-3940(99)00060-9. [DOI] [PubMed] [Google Scholar]
- [69].Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63(1):55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- [70].Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered Functional Connectivity Between the Insula and the Cingulate Cortex in Patients With Temporomandibular Disorder: A Pilot Study. Headache. 2011 doi: 10.1111/j.1526-4610.2011.01998.x. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jensen KB, Kosek E, Wicksell RK, Kemani M, Olsson GL, Merle JV, Kadetoff D, Ingvar M. Treatment with Cognitive Behavioral Therapy increases the activity of the prefrontal cortex in patients suffering from chronic pain. 2012. In Press.
- [72].Jensen MP. Hypnosis for chronic pain management: A new hope. Pain. 2009;146(3):235–237. doi: 10.1016/j.pain.2009.06.027. [DOI] [PubMed] [Google Scholar]
- [73].Jensen MP, Patterson DR. Hypnotic treatment of chronic pain. J Behav Med. 2006 Feb 01;29(1):95–124. doi: 10.1007/s10865-005-9031-6. [DOI] [PubMed] [Google Scholar]
- [74].Johansson C, Dahl J, Jannert M, Melin L, Andersson G. Effects of cognitive-behavioral pain-management programs. Beh Res Ther. 1998;36:915–930. doi: 10.1016/s0005-7967(98)00079-5. [DOI] [PubMed] [Google Scholar]
- [75].Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, Derbyshire S, Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33(10):909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- [76].Kayiran S, Dursun E, Dursun N, Ermutlu N, Karamursel S. Neurofeedback intervention in fibromyalgia syndrome; a randomized, controlled, rater blind clinical trial. Appl Psychophysiol Biofeedback. 2010;35(4):293–302. doi: 10.1007/s10484-010-9135-9. [DOI] [PubMed] [Google Scholar]
- [77].Keefe FJ. Cognitive behavioral therapy for managing pain. The Clinical Psychologist. 1996;49(3):4–5. [Google Scholar]
- [78].Keefe FJ, Gil KM. Behavioral concepts in the analysis of chronic pain syndromes. Journal of Consulting and Clinical Psychology. 1986;54:776–783. doi: 10.1037//0022-006x.54.6.776. [DOI] [PubMed] [Google Scholar]
- [79].Keefe FJ, Somers TJ. Psychological approaches to understanding and treating arthritis pain. Nat Rev Rheumatol. 2010 Apr;6(4):210–216. doi: 10.1038/nrrheum.2010.22. [DOI] [PubMed] [Google Scholar]
- [80].Kelley GA, Kelley KS, Jones DL. Efficacy and effectiveness of exercise on tender points in adults with fibromyalgia: a meta-analysis of randomized controlled trials. Arthritis. 2011 doi: 10.1155/2011/125485. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol. 2011;7:411–434. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]
- [82].Kirsch I, Mazzoni G, Montgomery GH. Remembrance of hypnosis past. Am J Clin Hypn. 2007;49(3):171–178. doi: 10.1080/00029157.2007.10401574. discussion 179-180, 183-174. [DOI] [PubMed] [Google Scholar]
- [83].Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain Activity Associated with Expectancy-Enhanced Placebo Analgesia as Measured by Functional Magnetic Resonance Imaging. J Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2(9):635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- [85].Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- [86].Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated Brain Gray Matter Loss in Fibromyalgia Patients: Premature Aging of the Brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kwekkeboom KL, Wanta B, Bumpus M. Individual difference variables and the effects of progressive muscle relaxation and analgesic imagery interventions on cancer pain. J Pain Symptom Manage. 2008;36(6):604–615. doi: 10.1016/j.jpainsymman.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kwiatek R, Barnden L, Tedman R, Jarrett R, Chew L, Rowe C, Pile K. Regional cerebral blood flow in fibromyalgia: Single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis & Rheumatism. 2000;43(12):2823–2833. doi: 10.1002/1529-0131(200012)43:12<2823::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [89].Lackner JM, Lou Coad M, Mertz HR, Wack DS, Katz LA, Krasner SS, Firth R, Mahl TC, Lockwood AH. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther. 2006;44(5):621–638. doi: 10.1016/j.brat.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lembo T, Fitzgerald L, Matin K, Woo K, Mayer EA, Naliboff BD. Audio and visual stimulation reduces patient discomfort during screening flexible sigmoidoscopy. Am J Gastroenterol. 1998;93(7):1113–1116. doi: 10.1111/j.1572-0241.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- [91].Lewandowski WA. Patterning of pain and power with guided imagery. Nurs Sci Q. 2004;17(3):233–241. doi: 10.1177/0894318404266322. [DOI] [PubMed] [Google Scholar]
- [92].Lewandowski WA, Jacobson A, Palmieri PA, Alexander T, Zeller R. Biological Mechanisms Related to the Effectiveness of Guided Imagery for Chronic Pain. Biol Res Nurs. 2011;13(4):364–375. doi: 10.1177/1099800410386475. [DOI] [PubMed] [Google Scholar]
- [93].Ljotsson B, Falk L, Vesterlund AW, Hedman E, Lindfors P, Ruck C, Hursti T, Andreewitch S, Jansson L, Lindefors N, Andersson G. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome--a randomized controlled trial. Behav Res Ther. 2010;48(6):531–539. doi: 10.1016/j.brat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [94].Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, Tracey I. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12(9):2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- [95].Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131(12):3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- [96].Mannix LK, Chandurkar RS, Rybicki LA, Tusek DL, Solomon GD. Effect of Guided Imagery on Quality of Life for Patients With Chronic Tension-Type Headache. Headache: The Journal of Head and Face Pain. 1999;39(5):326–334. doi: 10.1046/j.1526-4610.1999.3905326.x. [DOI] [PubMed] [Google Scholar]
- [97].Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: Cognitive-behavioral therapy vs. supportive psychotherapy. Pain. 2009;141(1-2):31–40. doi: 10.1016/j.pain.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Matthys K, Smits M, Van der Geest JN, Van der Lugt A, Seurinck R, Stam HJ, Selles RW. Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch Phys Med Rehabil. 2009;90(4):675–681. doi: 10.1016/j.apmr.2008.09.571. [DOI] [PubMed] [Google Scholar]
- [99].May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [100].McCracken LM, Samuel VM. The role of avoidance, pacing, and other activity patterns in chronic pain. Pain. 2007;130(1-2):119–125. doi: 10.1016/j.pain.2006.11.016. [DOI] [PubMed] [Google Scholar]
- [101].McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. The journal of pain. 2011;12(6):640–651. doi: 10.1016/j.jpain.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Menzies V, Taylor AG, Bourguignon C. Effects of guided imagery on outcomes of pain, functional status, and self-efficacy in persons diagnosed with fibromyalgia. J Altern Complement Med. 2006;12(1):23–30. doi: 10.1089/acm.2006.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Michielsen ME, Selles RW, van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, Smits M, Ribbers GM, Bussmann JB. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25(3):223–233. doi: 10.1177/1545968310385127. [DOI] [PubMed] [Google Scholar]
- [104].Michielsen ME, Smits M, Ribbers GM, Stam HJ, van der Geest JN, Bussmann JB, Selles RW. The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J Neurol Neurosurg Psychiatry. 2011;82(4):393–398. doi: 10.1136/jnnp.2009.194134. [DOI] [PubMed] [Google Scholar]
- [105].Milling LS, Coursen EL, Shores JS, Waszkiewicz JA. The predictive utility of hypnotizability: the change in suggestibility produced by hypnosis. Journal of consulting and clinical psychology. 2010;78(1):126–130. doi: 10.1037/a0017388. [DOI] [PubMed] [Google Scholar]
- [106].Miron D, Duncan GH, Bushnell MC. Effects of attention on the intensity and unpleasantness of thermal pain. Pain. 1989;39(3):345–352. doi: 10.1016/0304-3959(89)90048-1. [DOI] [PubMed] [Google Scholar]
- [107].Montgomery GH, Duhamel KN, Redd WH. A meta-analysis of hypnotically induced analgesia: How effective is hypnosis? Int J Clin Exp Hypn. 2000;48(2):138–153. doi: 10.1080/00207140008410045. [DOI] [PubMed] [Google Scholar]
- [108].Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1-2):1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- [109].Morone NE, Greco CM. Mind-body interventions for chronic pain in older adults: a structured review. Pain medicine. 2007;8(4):359–375. doi: 10.1111/j.1526-4637.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- [110].Morris LD, Louw QA, Grimmer-Somers K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. The Clinical journal of pain. 2009;25(9):815–826. doi: 10.1097/AJP.0b013e3181aaa909. [DOI] [PubMed] [Google Scholar]
- [111].Moseley GL. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain. 2004;108(1-2):192–198. doi: 10.1016/j.pain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [112].Mountz J, Bradley L, Modell J, Alexander R, Triana-Alexander M, Aaron L, Stewart K, Alarcón G, Mountz J. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum. 1995;38(7):926–938. doi: 10.1002/art.1780380708. [DOI] [PubMed] [Google Scholar]
- [113].Nestoriuc Y, Martin A, Rief W, Andrasik F. Biofeedback treatment for headache disorders: a comprehensive efficacy review. Appl Psychophysiol Biofeedback. 2008;33(3):125–140. doi: 10.1007/s10484-008-9060-3. [DOI] [PubMed] [Google Scholar]
- [114].Niddam D, Chan R-C, Lee S-H, Yeh T-C, Hsieh J-C. Central modulation of pain evoked from myofascial trigger point. Clin J Pain. 2007;23:440–448. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- [115].Nusbaum F, Redoute J, Le Bars D, Volckmann P, Simon F, Hannoun S, Ribes G, Gaucher J, Laurent B, Sappey-Marinier D. Chronic low-back pain modulation is enhanced by hypnotic analgesic suggestion by recruiting an emotional network: a PET imaging study. Int J Clin Exp Hypn. 2011;59(1):27–44. doi: 10.1080/00207144.2011.522874. [DOI] [PubMed] [Google Scholar]
- [116].Obermann M, Nebel K, Schumann C, Holle D, Gizewski ER, Maschke M, Goadsby PJ, Diener HC, Katsarava Z. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73(12):978–983. doi: 10.1212/WNL.0b013e3181b8791a. [DOI] [PubMed] [Google Scholar]
- [117].Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull. 2003;129(4):495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- [118].Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95(1-2):1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- [119].Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150(1):59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- [120].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- [121].Pincus D, Sheikh AA. Imagery for Pain Relief: A Scientifically Grounded Guidebook for Clinicians. Routledge; New York: 2009. [Google Scholar]
- [122].Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychological bulletin. 2001;127(5):599–617. doi: 10.1037/0033-2909.127.5.599. [DOI] [PubMed] [Google Scholar]
- [123].Posadzki P, Ernst E. Guided Imagery for Musculoskeletal Pain: A Systematic Review. Clin J Pain. 2011 doi: 10.1097/AJP.0b013e31821124a5. Epub ahead. [DOI] [PubMed] [Google Scholar]
- [124].Raft D, Smith RH, Warren N. Selection of imagery in the relief of chronic and acute clinical pain. J Psychosom Res. 1986;30(4):481–488. doi: 10.1016/0022-3999(86)90087-5. [DOI] [PubMed] [Google Scholar]
- [125].Raij TT, Numminen J, Närvänen S, Hiltunen J, Hari R. Brain correlates of subjective reality of physically and psychologically induced pain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2147–2151. doi: 10.1073/pnas.0409542102. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rainville P. Hypnosis and the analgesic effect of suggestions. Pain. 2008;134(1-2):1–2. doi: 10.1016/j.pain.2007.10.030. [DOI] [PubMed] [Google Scholar]
- [127].Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82(2):159–171. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- [128].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain Affect Encoded in Human Anterior Cingulate But Not Somatosensory Cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- [129].Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci. 2002;14(6):887–901. doi: 10.1162/089892902760191117. [DOI] [PubMed] [Google Scholar]
- [130].Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral Mechanisms of Hypnotic Induction and Suggestion. J Cogn Neurosci. 1999;11(1):110–125. doi: 10.1162/089892999563175. [DOI] [PubMed] [Google Scholar]
- [131].Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132(7):1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- [132].Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258(5085):1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- [133].Ribeiro Porto P, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does Cognitive Behavioral Therapy Change the Brain? A Systematic Review of Neuroimaging in Anxiety Disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:114–125. doi: 10.1176/jnp.2009.21.2.114. [DOI] [PubMed] [Google Scholar]
- [134].Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. Journal of Psychiatric Research. 2012 doi: 10.1016/j.jpsychires.2010.09.007. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- [136].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ros T, Munneke MA, Ruge D, Gruzelier JH, Rothwell JC. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur J Neurosci. 2010;31(4):770–778. doi: 10.1111/j.1460-9568.2010.07100.x. [DOI] [PubMed] [Google Scholar]
- [138].Ruscheweyh R, Deppe M, Lohmann H, Stehling C, Floel A, Ringelstein EB, Knecht S. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152(4):904–911. doi: 10.1016/j.pain.2011.01.013. [DOI] [PubMed] [Google Scholar]
- [139].Schnitzler A, Ploner M. Neurophysiology and functional neuroanatomy of pain perception. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2000;17(6):592–603. doi: 10.1097/00004691-200011000-00005. [DOI] [PubMed] [Google Scholar]
- [140].Schweinhardt P, Bushnell MC. Pain imaging in health and disease--how far have we come? J Clin Invest. 2010;120(11):3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. NeuroImage. 2008;40(2):759–766. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- [142].Seidel S, Kasprian G, Furtner J, Schopf V, Essmeister M, Sycha T, Auff E, Prayer D. Mirror Therapy in Lower Limb Amputees - A Look Beyond Primary Motor Cortex Reorganization. Rofo. 2011;183(11):1051–1057. doi: 10.1055/s-0031-1281768. [DOI] [PubMed] [Google Scholar]
- [143].Seminowicz DA, Mikulis DJ, Davis KD. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112(1-2):48–58. doi: 10.1016/j.pain.2004.07.027. [DOI] [PubMed] [Google Scholar]
- [144].Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Sharpe L, Dear BF, Schrieber L. Attentional biases in chronic pain associated with rheumatoid arthritis: hypervigilance or difficulties disengaging? The journal of pain : official journal of the American Pain Society. 2009;10(3):329–335. doi: 10.1016/j.jpain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- [146].Simmons D, Chabal C, Griffith J, Rausch M, Steele B. A clinical trial of distraction techniques for pain and anxiety control during cataract surgery. Insight. 2004;29(4):13–16. [PubMed] [Google Scholar]
- [147].Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The journal of pain. 2006;7(4):261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- [148].Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Steiger F, Wirth B, de Bruin ED, Mannion AF. Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. European spine. 2011 doi: 10.1007/s00586-011-2045-6. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Tracey I, Mantyh PW. The Cerebral Signature for Pain Perception and Its Modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [151].Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22(7):2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain. 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033. [DOI] [PubMed] [Google Scholar]
- [153].Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95(3):195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- [154].Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29(3):705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106(1-2):101–108. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- [156].Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature reviews Neuroscience. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- [158].Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115(2):364–374. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Weiskopf N. Real-time fMRI and its application to neurofeedback. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.10.009. Epub ahead. [DOI] [PubMed] [Google Scholar]
- [160].Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141(3):248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- [161].Wiech K, Farias M, Kahane G, Shackel N, Tiede W, Tracey I. An fMRI study measuring analgesia enhanced by religion as a belief system. Pain. 2008;139(2):467–476. doi: 10.1016/j.pain.2008.07.030. [DOI] [PubMed] [Google Scholar]
- [162].Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in cognitive sciences. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- [163].Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27(1):59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- [164].Wik G, Fischer H, Bragee B, Finer B, Fredrikson M. Functional anatomy of hypnotic analgesia: a PET study of patients with fibromyalgia. Eur J Pain. 1999;3(1):7–12. doi: 10.1053/eujp.1998.0093. [DOI] [PubMed] [Google Scholar]
- [165].Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25(12):3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- [166].Yoo H, Kim S, Hur HK, Kim HS. The effects of an animation distraction intervention on pain response of preschool children during venipuncture. Appl Nurs Res. 2011;24(2):94–100. doi: 10.1016/j.apnr.2009.03.005. [DOI] [PubMed] [Google Scholar]