Abstract
Pneumococcal polysaccharide vaccines have been used to elicit a protective anti-pneumococcal polysaccharide antibody response against S. pneumoniae in healthy individuals. Identifying human B cells which respond to T-cell independent type-2 antigens, such as pneumococcal polysaccharides, has been challenging. We employed pneumococcal polysaccharides directly conjugated to fluorophores in conjunction with flow cytometry to identify the phenotype of B cells that respond to pneumococcal polysaccharide vaccination. We have previously identified that the majority of pneumococcal polysaccharide-selected cells responding to vaccination are CD27+IgM+ (IgM+ memory) cells. In this study, we further characterized pneumococcal polysaccharide-selected cells in the peripheral blood to better identify how the various B cell phenotypes responded 7 and 30 days post-immunization. We show that 7 days post-immunization the majority of pneumococcal polysaccharide-selected IgM+ memory cells (PPS14+ 56.5%, PPS23F+ 63.8%) were CD19+CD20+CD27+IgM+CD43+CD5+/−CD70−, which was significantly increased compared to pre-immunization levels. This phenotype is in alignment with recent publications describing human B-1 cells. PPS-responsive B cells receded to pre-immunization levels by day-30. These findings suggest that this B-1 like cell population plays an important role in early responses to S. pneumoniae infection and possibly other T-cell independent type-2 antigens in humans.
1. Introduction
Increased antibiotic resistance among many Streptococcus pneumoniae serotypes associated with disease, including pneumococcal polysaccharides 14 and 23F (PPS14, PPS23F), emphasizes the need for improved vaccine strategies, especially for those at highest risk for invasive disease including elderly and immunocompromised individuals [1–5]. Vaccination results in PPS-specific IgM and IgG opsonophagocytic antibodies (Ab) which are critical for bacterial clearance [6–10].
The nature of the immune cells involved in the production of Ab against these T-independent Type II (TI-2) polysaccharide antigens is controversial. Splenic marginal zone B cells (MZB) produce recirculating plasmacytes and memory B cells, capable of rapidly producing opsonizing IgM and IgG Abs against TI-2 antigens [7, 11–16]. The role of MZB in response to TI-2 antigens is also supported by the finding that individuals who respond poorly to pneumococcal vaccinations tend to lack IgM+ memory B cells. This includes patients with congenital neutropenia, common variable immunodeficiency, HIV infection, have been splenectomized, infants <2 years old with an underdeveloped marginal zone, and elderly populations [11,14,15,17–20]. Alternatively, B-1 cells have also been implicated in the production of plasmacytes and memory B cells capable of rapidly producing IgM and IgG Abs against TI-2 antigens [7,12,21–25].
Previous studies demonstrate mouse B-1 cells transferred into RAG−/− mice produce PPS-specific Abs and provide protection against lethal challenge [21,22]. While it is thought that B-1 cells contribute to the immune response against pathogens expressing TI-2 antigens in humans, the direct relevance of B-1 cells has been unclear due to the difficulty in identifying human B-1 cell equivalents. In mice, B-1 cells can be divided into two subtypes, B-1a and B-1b cells. B-1b cells have the ability to produce Abs that can provide a long-term adaptive immune response to TI-2 antigens like polysaccharides [21–23,26,27]. Human B-1 cells on the other hand are controversial themselves. It is unclear if the same division of B-1 cells that exists in mice is recapitulated in humans. Previous studies demonstrate that CD5 expression on human B cells is insufficient to characterize B-1 cells as it is used in mice [27–30]. Recent publications have described a mouse B-1a like subset in humans [27,30]. It is currently unclear if there is a mouse B-1b like equivalent in humans capable of responding to TI-2 antigens such as those used for pneumococcal polysaccharide vaccination (PPV).
We have previously shown, using fluorescently labeled PPS14 and PPS23F, the majority of PPS-specific B cells responding to vaccination are IgM+ memory cells (CD27+IgM+) [31]. The goal of the present study was to further characterize PPS-specific PPV responding cells with respect to expression of CD43 and CD5 used to characterize this putative B-1 cell population. Our results identify PPS14- and PPS23F-reactive B cell populations that circulate in the peripheral blood 7- and 30-days post-immunization in response to PPV. We show the majority of PPS-specific B cells on day-7 are phenotypically characterized as CD19+CD20+CD3−CD70−CD27+IgM+CD43+CD5+/−. This population is in alignment with recent reports of human B-1 cells [30,32–34]. We also show that 30 days post-immunization, this population recedes towards pre-immunization levels.
2. Materials and Methods
2.1 Human Volunteers
Seventeen healthy volunteers participated in the University of Toledo IRB committee approved study (IRB #105137). Volunteers were 24–30 years old (mean=26.6) and pneumococcal polysaccharide vaccine naïve. Volunteers were questioned about medications, previous illness, and present health before immunization with PPV, Merck (23-valent pneumococcal polysaccharide vaccine).
2.2 Labeling of polysaccharide 14 and 23F with fluorescent dye
Conjugation of PPS14 to cascade blue (CB) ethylenediamine (Invitrogen catalog C-621) or PPS23F to 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF; Sigma-Aldrich Fluka catalog 36565) was carried out as previously described [31].
2.3 Flow Cytometry
Peripheral blood was collected from volunteers pre- and post-immunization at days 0, 7, and 30. After Ficoll-gradient centrifugation and washing, cells were resuspended in FACS buffer (PBS, 0.1% FCS, 2mM EDTA). Before staining, cells were absorbed with 10µg/ml cell wall polysaccharide (Statens Serum Institut; MiraVista Diagnostics, Indianapolis, IN) and PPS22F (American Type Culture Collection) to reduce nonspecific binding [35]. B cells were labeled with 10µg/ml, either PPS14-CB or PPS23F-DTAF (BioCentra, Sugar Land, TX). Fluorochrome-conjugated mAbs (BD Bioscience or eBioscience) to the following anti-human Ags were used: CD19 (APC-Cy7), CD27 (PerCP-Cy5.5), IgM (allophycocyanin), IgD (Alexa Fluor 700), CD5 (PE-Cy7), CD43 (PE), CD20 (Alexa Fluor 700), CD70 (CB and FITC). Cells were washed and suspended in FACS buffer and analyzed with FACSAria using FACSDiva software (BD Biosciences). FCS files were further analyzed using FlowJo software (Tree Star, Ashland, OR).
2.4 Pneumococcal Polysaccharide ELISA
ELISA was performed to examine anti-PPS-specific human Abs in all volunteers. The PPS-ELISA is modification from the World Health Organization assay [36,37]. All steps were performed as reported previously [31].
2.5 Opsonophagocytic Assay
Opsonophagocytic assay was performed as previously described [38,39]. Briefly, S. pneumoniae, serotypes 14 and 23F, were incubated with serial diluted heat-inactivated sera. Newborn rabbit serum (Pel-Freez, Brown Deer, WI.) was added as source of complement. Differentiated HL-60 cells were added at an E:T ratio of 400:1. Sera were tested in duplicate. Results were obtained Opsotiter1 software program (University of Alabama at Birmingham).
2.6 Statistical analysis
Geometric mean concentration of IgG, IgM, and IgA, and flow cell numbers, specific to PPS14 and PPS23F, were calculated for each group. Correlation between groups was examined using Pearson’s correlation coefficient. Comparison between two group values was performed using unpaired t test. The p-values <0.05 were considered to be significant.
3. Results
3.1 Ab titers increase significantly post-immunization with PPV
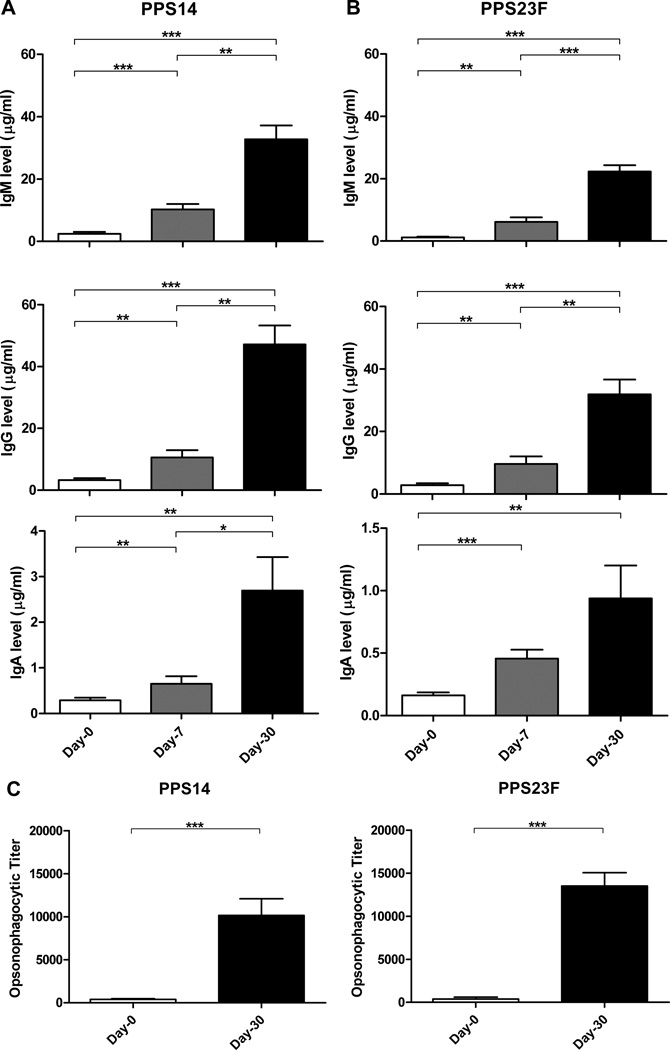
To show that our young healthy donor population (n=17) responded normally to immunization with PPV, we tested sera collected on day-0, day-7, and day-30 for PPS14 and PPS23F Ab responsiveness. Following the World Health Organization recommendations, sera were first absorbed with PPS22F and cell wall polysaccharide to prevent overestimation of PPS-specific Ab concentrations [35,39,40]. Day-30 post-immunization, donors showed a significant increase in PPS14-specific IgM from 2.4±2.4µg/ml to 32.7 ±18.0 µg/ml (p<0.001), IgG from 3.3 ±2.5µg/ml to 47.1 ±24.0 µg/ml (p=0.0023), and IgA from 0.3 ±0.2µg/ml to 2.7 ±2.9µg/ml (p=0.0056) (Fig.1A). Similarly, post-immunization PPS23F-specific Ab levels were significantly increased for IgM from 1.2 ±0.8µg/ml to 22.3 ±7.9µg/ml (p<0.0001), IgG from 2.8 ±2.3µg/ml to 31.9 ±18.9µg/ml (p<0.0001), and IgA from 0.2 ±0.1µg/ml to 0.9 ±1.1µg/ml (Fig.1B). IgG concentrations showed a greater increase post-immunization compared to IgM concentrations for both PPS. These ELISA data confirm that our donor population responded to PPV immunization resulting in a minimal two-fold increase in serotype-specific Ab.
Figure 1. Polysaccharide specific Ig and opsonophagocytic titers increase post-immunization with PPV.
Healthy young volunteers were immunized with PPV. Serum samples were obtained on days 0, 7, and 30. Serum samples were tested for PPS14-specific (A) and PPS23F-specific (B) IgM, IgG, IgA concentrations by ELISA expressed as µg/ml. Serum samples from day-0 and day-30 were tested for S. pneumoniae PPS-specific antibody opsonophagocytic activity (C) expressed as opsonophagocytic titer. Mean values with SEM shown. *p < 0.05, **p < 0.01, ***p<0.001.
3.2 OPT increases significantly 30 days post-immunization with PPV
Donor sera collected on day 0 and day 30 were tested for functional opsonophagocytic response using both PPS14 and PPS23F expressing S. pneumoniae. The reciprocal of the Ab dilution required to obtain 50% opsonophagocytic killing by differentiated HL-60 cells (opsonophagocytic titer-OPT) was calculated. There was a significant increase in the OPT post-immunization compared to pre-immunization for both PPS14 (p=0.0001) and PPS23F (p<0.0001) (Fig.1C). These results confirmed that vaccination of our sample population with PPV elicited a functional immune response against serotype-specific PPS [41].
3.3 PPV induces transient increase in PPS-specific B cells in peripheral blood
To characterize the phenotype of B cells that responded to vaccination with PPV, donor peripheral blood samples were analyzed pre- and post-immunization. The percentage of PPS14-selected B cells increased significantly (p=0.001) from an average of 1.5% on day-0 to 5.0% by day-7 and decreased to 3.5% by day-30. Similarly, PPS23F-selected B cells increased significantly (p=0.001) from an average of 1.1% on day 0 to 4.2% by day 7 and decreased significantly (p=0.034) to 2.0% by day-30 (Table I, Supplemental). The percentage of PPS14+ B cells on day-30 remained statistically higher (p=0.002) compared to pre-immunization levels. In contrast, the percentage of PPS23F+ B cells returned to pre-immunization levels.
Table I.
CD19+PPS+ B cells.
| PPS+ B cells |
%PPS14+ day 0 |
%PPS14+ day 7 |
%PPS14+ day 30 |
%PPS23F+ day 0 |
%PPS23F+ day 7 |
%PPS23F+ day 30 |
|---|---|---|---|---|---|---|
| Mean | 1.5 | 5.0 | 3.5 | 1.1 | 4.2 | 2.0 |
| SEM | 1.1 | 2.8 | 1.5 | 0.6 | 2.9 | 1.4 |
Mean percentage ± SEM of CD19+ B cells stained with fluorescently labeled PPS14 or PPS23F from peripheral blood samples obtained pre-immunization (PPS14 n=10, PPS23F n=11) and 7 (PPS14 n=16, PPS23F n=17) and 30 (PPS14 n=10, PPS23F n=10)
3.4 Majority of PPS-specific B cells express CD27 and IgM post-immunization with PPV
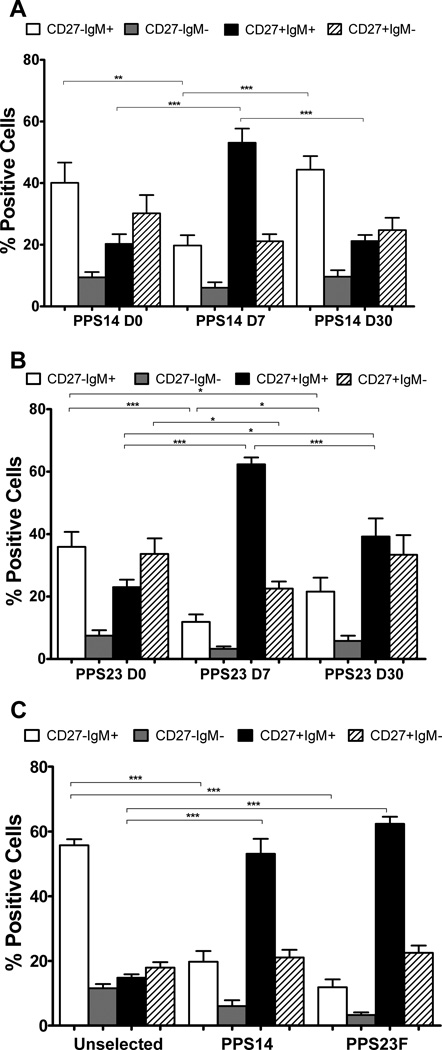
Pre-immunization, the phenotypic analysis of CD27 and IgM for PPS-selected and unselected cells were both similar to our previously published results demonstrating B cells pre-immunization primarily consisted of naïve B cells (38–59%) [31]. PPS-selection day-7 post-immunization revealed a highly significant decrease in representation of naïve B cells (PPS14 p=0.0056, PPS23F p<0.0001). These changes were coupled with highly significant increases in IgM+ memory cells (PPS14/23F p<0.0001) compared to pre-immunization (Fig.2A,B). The majority of PPS-selected B cells (PPS14 53.1%, PPS23F 62.4%) day-7 post-immunization were IgM+ memory cells (Fig.2,3A). This population was significantly higher (PPS14/23F p<0.0001) than the unselected population post-immunization. In contrast, day-7 post-immunization class-switched memory cells represented a smaller portion of the PPS-selected B cells (PPS14 21.1%, PPS23F 22.5%) (Fig.2C).
Figure 2. Majority of PPS-specific B cells express CD27 and IgM post-immunization with PPV.
Lymphocyte enriched peripheral blood samples were stained with immunofluorescent PPS and antibodies to be evaluated by flow cytometry. The phenotype distribution of CD27 and IgM on CD19+ B cells was compared for day-0, day-7, and day-30 for PPS14-(A) and PPS23F- (B) selected cells. The phenotypic distribution of CD27 and IgM was compared on day-7 for unselected, PPS14- and PPS23F- selected cells (C). Mean values with SEM shown. In each sample, 100,000 events were recorded. *p < 0.05, **p < 0.01, ***p<0.001.
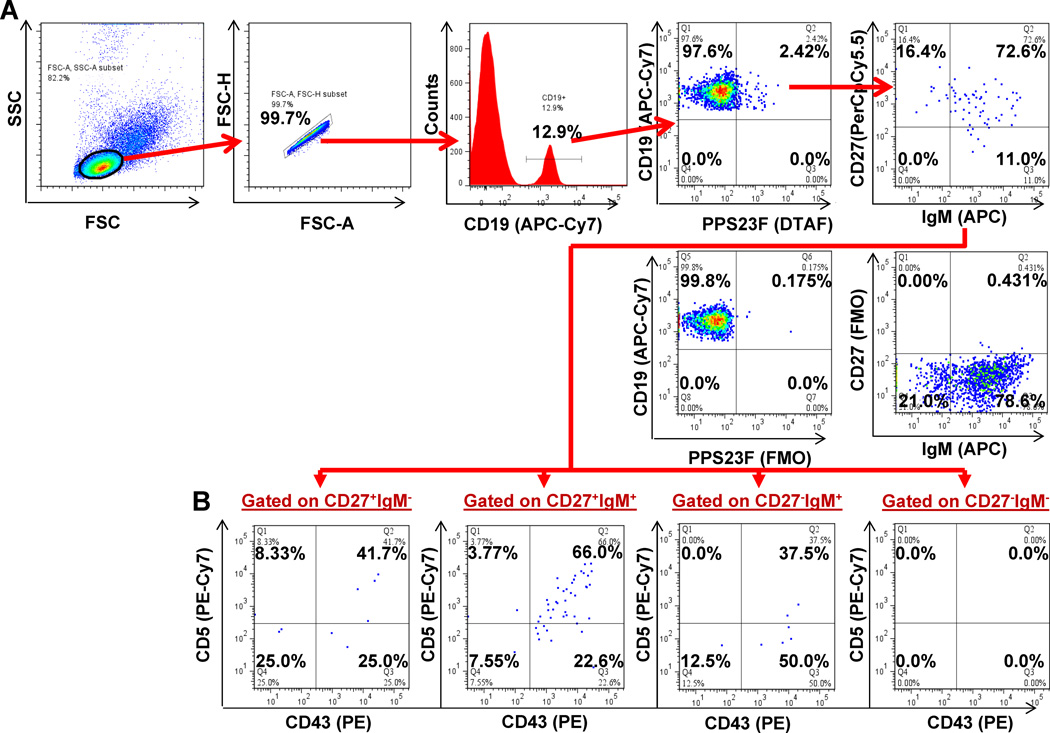
Figure 3. Phenotypic analysis of human peripheral blood B cells by flow cytometry.
Lymphocyte-enriched peripheral blood samples were stained with immunofluorescent PPS and antibodies to be evaluated by flow cytometry. Lymphocytes were plotted (FSC-A, FSC-H) for doublet discrimination. Singlet lymphocytes were plotted on a histogram to gate on B cells (APC-Cy7:CD19+). B cells were plotted using either Cascade Blue:PPS14 or 5-DTAF:PPS23F to identify PPS-selected verses unselected cells. Each of these two populations were subdivided into four sub-populations: naïve (CD27−IgM+), class-switched (CD27−IgM−), IgM+ memory (CD27+IgM+), and class-switched memory (CD27+IgM−) B cells. We further subgated each of these populations based on CD43 and CD5 expression. All flow cytometry results were analyzed and plotted using Fluorescence Minus One controls (FMO). Representative CD27 vs. IgM plot (A) and CD43 vs. CD5 plots (B) for each CD27 vs. IgM quadrant shown. 100,000 events were recorded.
Day-30 post-immunization the phenotypic distributions of PPS-selected cells returned towards pre-immunization levels (Fig.2A,B). PPS14-selected naïve cells on day-30 showed a significant increase compared to day-7 (p=0.0001), returning to pre-immunization levels. In contrast, IgM+ memory cells decreased significantly (p<0.0001) to pre-immunization levels by day-30. PPS14-selected cells on day-30 showed no significant difference for naive and IgM+ memory cells compared to day-0 (Fig.2A). PPS23F-selected cells showed similar trends. PPS23F-selected naive cells increased significantly (p=0.0461) toward pre-immunization levels by day-30, but remained significantly (p=0.0413) lower compared to day-0. In contrast, PPS23F-selected IgM+ memory cells decreased significantly (p=0.0002) toward pre-immunization levels by day-30, but remained significantly higher compared to day-0 (p=0.0151) (Fig.2B). Therefore, by day-30, the distribution of PPS-selected cells returned towards pre-immunization phenotype distributions.
Strong positive correlations were found between the percentage of PPS-selected IgM+ memory cells on day-7 and IgM Ab concentrations on day-30 for both PPS14 (r2=0.87, p<0.0001) and PPS23F (r2 =0.98, p<0.0001) (data not shown). In contrast, correlations were not found between the percentage of PPS-selected class-switched memory cells on day-7 and IgG Ab concentrations on day-30 for either PPS14 (r2=−0.3451, p=0.2268) and PPS23F (r2=−0.3934, p=0.1317) (data not shown).
3.5 Majority of PPS-specific IgM+ Memory B cells express CD43 and CD5 post-immunization with PPV
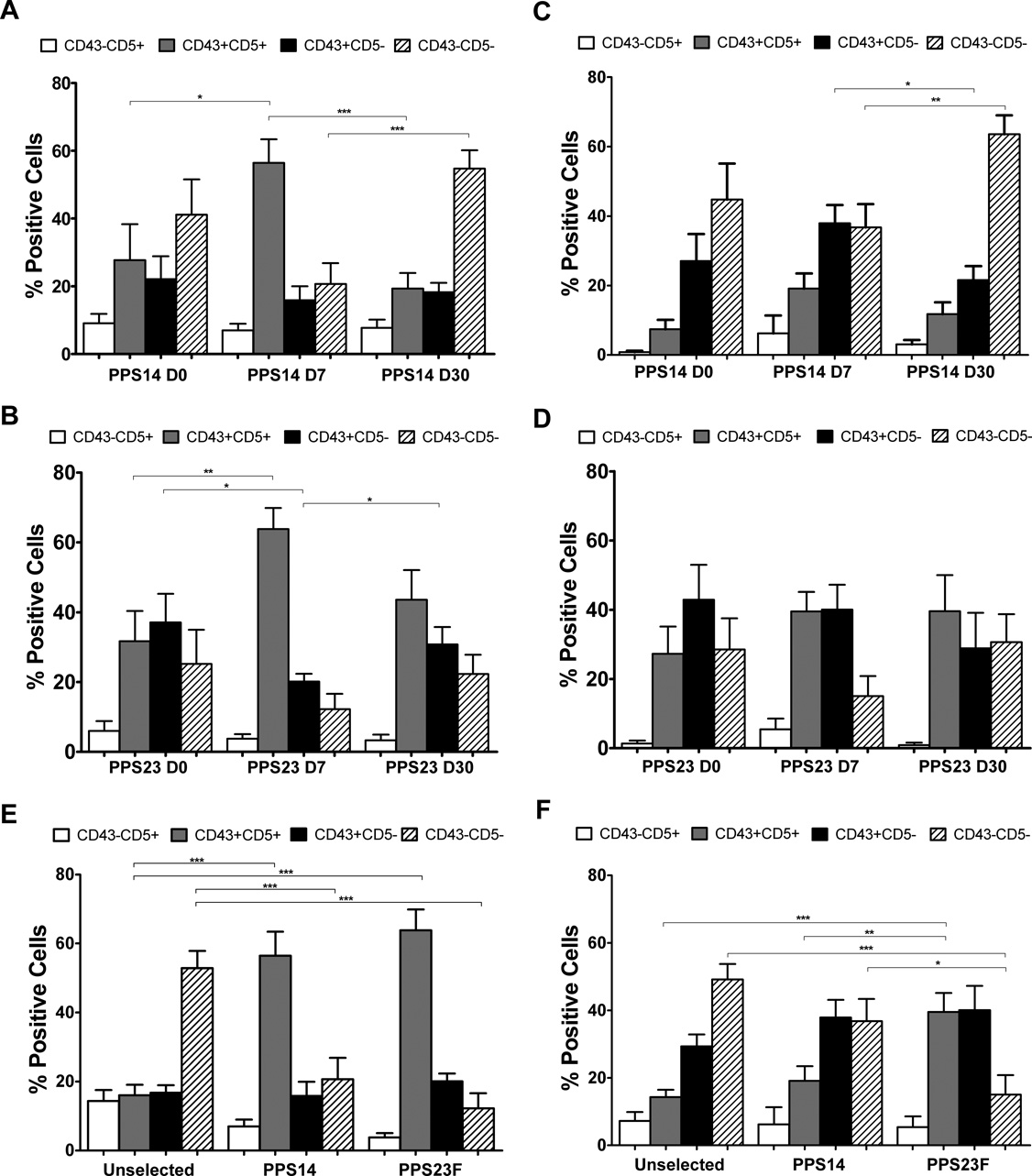
We further characterized peripheral blood for CD43 and CD5 expression within each of the previously identified CD27 and IgM quadrants (Fig.3B). Pre-immunization, within the CD27+ populations, CD43 and CD5 analysis showed no significant difference between selected and unselected cells (data not shown). Within the PPS-selected IgM+ memory populations, there were significant increases in CD43+CD5+ expression on PPS-specific cells from day-0 to day-7 (PPS14: 27.7%–56.5% p=0.027, PPS23F: 31.7%–63.8%, p=0.005) (Fig.4A,B). There were no significant changes within the PPS-selected class-switched memory populations from day-0 to day-7 (Fig.4C,D). On day 7 post-immunization, the majority of PPS-selected IgM+ memory cells were CD43+CD5+ (PPS14 56.5%, PPS23F 63.8%). This was in contrast to the unselected IgM+ memory cell population which showed the majority of cells were CD43−CD5− (52.9%), while the CD43+CD5+ population represented only 16% of these cells. The percentage of PPS-selected CD27+IgM+CD43+CD5+ cells were significantly higher (PPS14 p<0.0001, PPS23F p<0.0001) compared to analysis using unselected cells (Fig.4E). Within the class-switched memory PPS-selected population, significant changes between unselected and PPS-selected cells were only found in response to PPS23F, revealing a significant (p<0.0001) increase in CD5+CD43+ cells paired with a significant (p<0.0001) decrease in CD5−CD43− cells (Fig.4F). Thus, 7 days post-immunization the majority of PPS+ peripheral blood B cells responding to PPV were CD27+IgM+CD43+CD5+.
Figure 4. Majority of PPS-specific IgM+ Memory B cells express CD43 and CD5 post-immunization with PPV.
Lymphocyte enriched peripheral blood samples were stained with immunofluorescent PPS and antibodies to be evaluated by flow cytometry. The phenotypic distribution of CD43 and CD5 on B cells within the IgM+ memory and class-switched memory populations was compared for day-0, day-7, and day-30 for PPS14- (A,C) and PPS23F (B,D) selected cells. The phenotypic distribution of CD43 and CD5 on B cells within the IgM+ memory (E) and class-switched memory (F) populations was compared on day-7 for unselected, PPS14- and PPS23F- selected cells. Mean values with SEM shown. In each sample, 100,000 events were recorded. *p < 0.05, **p < 0.01, ***p<0.001.
Day-30 post-immunization, within the PPS-selected IgM+ memory populations, the phenotypic distributions of CD43 and CD5 returned toward pre-immunization levels. For PPS14-selected IgM+ memory cells, the predominant (56.5%) CD43+CD5+ population on day-7 decreased significantly (19.3%, p=0.0005) to day-0 levels. At the same time within this population, the CD43−CD5− population which was reduced (20.7%) on day-7 significantly increased (54.7%, p=0.0007) to day-0 levels (Fig.4A). For IgM+ memory PPS23F-selected cells the predominant (63.8%) CD43+CD5+ population on day-7 regressed to 43.6%, but remained higher than day-0 levels (31.7%) (Fig.4B). With in the class-switched memory population significant changes in PPS-selected cells between day-7 and day-30 were only seen within the PPS14-selected CD5− populations. However, the distribution of CD43 and CD5 did not show any statistical significant differences between day-0 and day-30 (Fig.4C,D). Therefore, by day-30, the distribution of PPS-selected cells resembled pre-immunization phenotype distributions with no significant differences.
3.6 PPS-selected B cells phenotypically resemble human B-1 cells
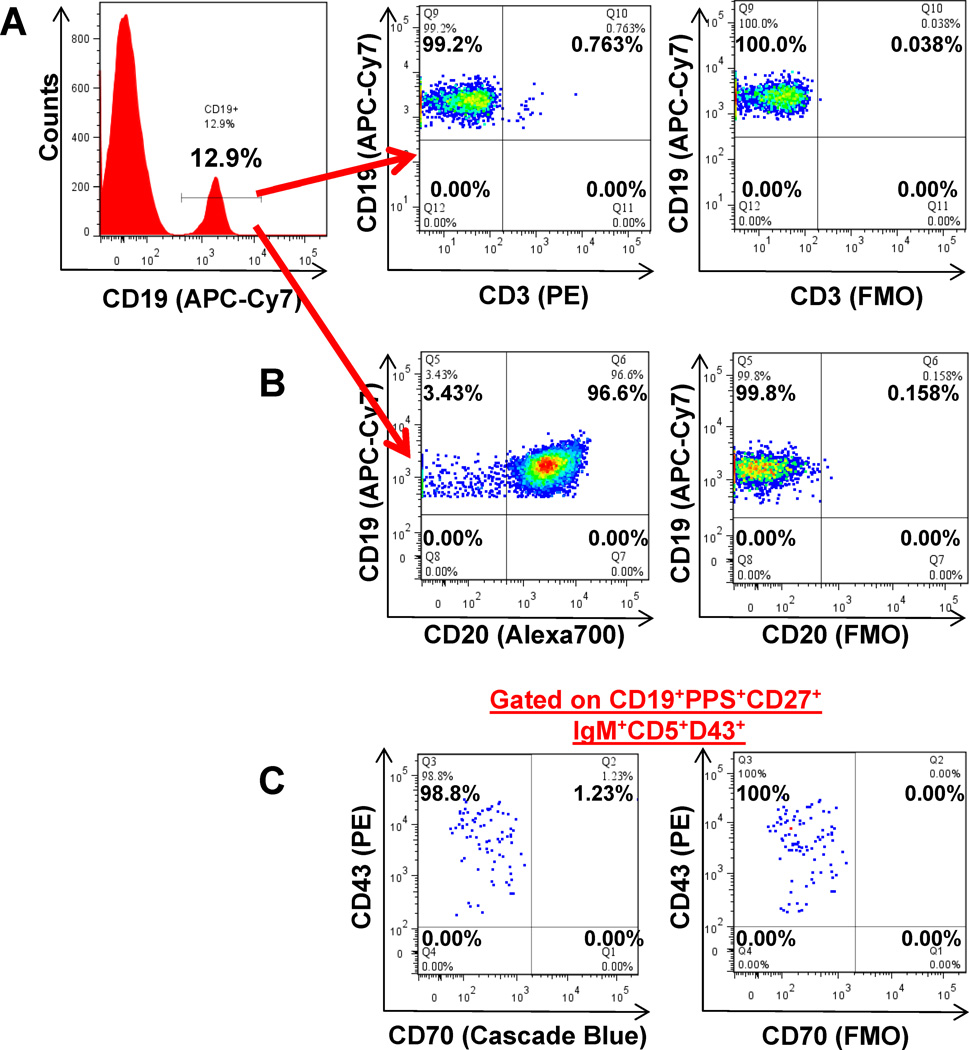
Recent reports have expressed interest in distinctly identifying B cells from T cells or B/T-cell doublets due to the heterogeneic distribution of CD43 and CD5 on T-cells [33]. CD3 analysis showed that 96.93 ±2.88% of our day-7 CD19+ cells did not express CD3 and therefore did not represent T-cells or B/T-cell doublets (Fig.5A). To clarify the proportion of day-7 CD19+ cells that could represent CD19+CD20− plasmablasts, we analyzed CD20 expression. Within the CD19+ cells, the vast majority of cells (95.0 ±1.87%) were also CD20+ (Fig.5B). To demonstrate that our PPS-selected cells do not represent IgM+ memory cells expressing CD43 and CD5 as inducible activation markers, and to allow direct comparison to recent studies, we analyzed CD70 expression [30,32]. Within the CD19+ cells, 99.22 ±1.32% of PPS-selected, and 98.98 ±1.07% of our PPS-selected CD27+IgM+CD43+CD5+ populations 7 days post-immunization were also found to be CD70− (Fig.5C).
Figure 5. PPS-selected B cells phenotypically resemble human B-1 cells.
Four healthy human donors were immunized with PPV. Lymphocyte enriched peripheral blood samples were stained with immunofluorescent PPS and antibodies to be evaluated by flow cytometry on day-7. Representative plot of CD3 expression on CD19+ B cells (A). Representative plot of CD20 expression on CD19+ B cells (B). Representative plot of PPS23F-selected IgM+ memory cells gated for CD43+ and CD5+ expression and plotted for CD43 and CD70 expression (C). All flow cytometry results were analyzed and plotted using Fluorescence Minus One controls (FMO). 100,000 events were recorded.
4. Discussion
Polysaccharide-specific ELISA and opsonophagocytic assays demonstrated that our volunteers were immunocompetent and responded normally to PPV. Samples showed variability in Ab concentrations and OPT between individuals and between serotypes. Others have also shown that a strong Ab response to one serotype does not necessarily correlate with a strong response to other serotypes [31,41,42].
The B cells responsible for eliciting protective Abs in response to PPV vaccination remains an active and controversial area of research. The goal of our study was to further characterize the phenotype of PPS-specific B cells that respond to PPV vaccination in healthy individuals using fluorescently conjugated PPS which we have previously described [31]. Day-7 post-immunization, the percentage of PPS-selected cells increased significantly compared to day-0 background levels. By day-30, the percentage of PPS-selected cells declined toward pre-immunization levels. Thus, at its peak, a mere 4–5% of B cells were PPS-specific emphasizing the importance of PPS-selection to analyze changes in cell populations responding to PPV as shown by day-7 post-immunization unselected versus PPS-selected results. PPS-selection on day-7 demonstrated a predominant IgM+ memory population in response to PPV clearly distinguished from the unselected phenotype analysis as in our previous study [31]. These changes reverted toward pre-immunization levels by day-30 post-immunization. Interestingly, PPS23F-selected IgM+ memory cells showed a significant decrease by day-30, but remained significantly higher than day-0 levels. In contrast, PPS14-selected IgM+ memory cells on day-30 showed no significant difference compared to day-0 levels. Others have also reported a heterogeneous response to various PPS [43]. Some differences may be due to PPS structural differences, previous donor exposure to specific serotypes, and/or pre-immunization Ab concentrations.
The CD27+IgM+ B cell population can further be subdivided into IgM memory B cells (CD27+IgM+CD43−) and B1 cells (CD27+IgM+CD43+). In mice, B1 cells have been extensively characterized, are subdivided into B1a and B1b cells, and are vitally important in the protective immune response against S. pneumoniae. Griffin et al recently characterized a human equivalent of B1 cells. The role of these cells in the human immune response to PPV remains to be defined [7,22,23,30]. We analyzed the presence of CD43 on the surface of PPS-labeled CD27+IgM+ B cells as percentage of PPS-positive B cells classified as B1 cells (CD43+) or those that are IgM memory cells (CD43−). The CD43 and CD5 sub-gated populations of unselected cells showed no significant differences in phenotype distribution between day-0, 7, and 30 analysis (data not shown). This is expected since the PPS+ B cell population is a small fraction of total B cells. However, within the PPS-specific IgM+ memory population, the percentage of CD43+CD5+ B cells increased significantly by day-7 post-immunization, and reverted toward pre-immunization levels by day-30. We also demonstrated strong correlations between the proportion of day-7 PPS-selected IgM+ memory B cells and day-30 post-immunization PPS-specific IgM concentrations. This suggests that the percentage of PPS-specific IgM+ memory cells on day-7 can serve as a surrogate marker for PPV responsiveness.
The majority population responding to PPV is in alignment with recent descriptions of putative human B-1 cells and includes a population capable of producing anti-polysaccharide Ab [30,32]. To determine to what degree this PPS-selected majority population resembled the putative B-1 population, we performed additional phenotypic analysis for CD3, CD20, and CD70. The vast majority of these cells didn’t represent CD3+ T cells, CD19+CD20− plasmablasts, or CD70+ activated memory and naïve cells expressing CD43 and CD5.
This analysis reveals additional populations of PPS-specific B cells, demonstrating that the immune response to PPV is in fact a heterogeneous and complex orchestration of many cell types and does not solely consist of the majority B-1 like population. Verbinnen et al have shown that the putative B1 cell population described by Griffin et al were capable of producing PPS-specific IgM and IgG detected by ELISPOT for PPS1 and PPS4 [30,32]. It is therefore possible that our CD27+IgM- B cells may have also represent class switched B1 cells which have been described by Hass et al in mice [24]. Additional future studies like the ELISPOT performed by Verbinnen et al and the spontaneous Ig secretion performed by Griffin et al can determine if this putative PPS-specific B-1 like population has the same functional characteristics as B-1b cells. Moreover, other populations of B cells may contribute to the immune response within different time frames and in other compartments such as the spleen or bone marrow. However, the significant increase in this B-1 like population, regardless of its classification, over a relatively short time in the peripheral blood suggests that this population may have great importance in controlling the early stages of infection just as B-1b cells have been shown to function [12]. The relatively quick regression of this majority population from the blood suggests likely sequestration in other compartments.
The majority of PPS14- and PPS23F-selected B cells responding to PPV in the peripheral blood was characterized as CD19+CD20+CD3−IgM+CD27+CD43+CD5+/−CD70−. This analysis in young adults lays a foundation against which comparisons can be made for high risk populations, including the elderly and HIV-infected individuals, which we are currently pursuing [44]. We hypothesize that high risk populations may show a very different distribution of phenotypes compared to the healthy individuals. Identifying changes or deficiencies in the PPS-specific responding B cell populations of elderly and HIV-infected individuals may be used to improve vaccination schedules and develop new therapies leading to improved patient protection.
Supplementary Material
Highlights.
We studied the B cell response of healthy individuals vaccinated with PPV-23.
PPS-specific B cells were identified by flow cytometry pre- and post-vaccination.
PPS-specific B cells were CD19+CD20+CD3−CD27+IgM+CD43+CD5+/−CD70−.
B cells share same phenotype as recently described putative human B1 cells.
Acknowledgements
We thank all of the volunteers who participated in this study.
Abbreviations
- Ab
Antibodies
- PPS
Pneumococcal polysaccharide
- TI-2
T-independent type II
- MZB
Splenic marginal zone B cell
- PPV
Pneumococcal polysaccharide vaccination
- OPT
Opsonophagocytic Titer
- FMO
Fluorescence Minus One
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors do not have any commercial or other association that might pose a conflict of interest.
References
- 1.Song JH, Dagan R, Klugman KP, Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. 2012;30:2728–2737. doi: 10.1016/j.vaccine.2012.01.091. [DOI] [PubMed] [Google Scholar]
- 2.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 3.Westerink MA, Schroeder HW, Jr, Nahm MH. Immune Responses to pneumococcal vaccines in children and adults: Rationale for age-specific vaccination. Aging and disease. 2012;3:51–67. [PMC free article] [PubMed] [Google Scholar]
- 4.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis. 2005;191:2038–2045. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 5.Breiman RF, Spika JS, Navarro VJ, Darden PM, Darby CP. Pneumococcal bacteremia in Charleston County, South Carolina. A decade later. Arch Intern Med. 1990;150:1401–1405. [PubMed] [Google Scholar]
- 6.Garcia-Suarez Mdel M, Vazquez F, Mendez FJ. Streptococcus pneumoniae virulence factors and their clinical impact: An update. Enferm Infecc Microbiol Clin. 2006;24:512–517. doi: 10.1157/13092469. [DOI] [PubMed] [Google Scholar]
- 7.Taillardet M, Haffar G, Mondiere P, Asensio MJ, Gheit H, Burdin N, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114:4432–4440. doi: 10.1182/blood-2009-01-200014. [DOI] [PubMed] [Google Scholar]
- 8.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 9.Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, et al. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Human vaccines. 2011;7:919–928. doi: 10.4161/hv.7.9.15996. [DOI] [PubMed] [Google Scholar]
- 10.Ortqvist A. Pneumococcal vaccination: current and future issues. Eur Respir J. 2001;18:184–195. doi: 10.1183/09031936.01.00084401. [DOI] [PubMed] [Google Scholar]
- 11.Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, et al. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 12.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 13.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- 14.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 17.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- 19.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 21.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–336. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 28.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunological reviews. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 30.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaskhely N, Mosakowski J, Thompson RS, Khuder S, Smithson SL, Westerink MA. Phenotypic analysis of pneumococcal polysaccharide-specific B cells. J Immunol. 2012;188:2455–2463. doi: 10.4049/jimmunol.1102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbinnen B, Covens K, Moens L, Meyts I, Bossuyt X. Human CD20+CD43+CD27+CD5- B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae. J Allergy Clin Immunol. 2012;130:272–275. doi: 10.1016/j.jaci.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells are CD3-: A reply to "A human equivalent of mouse B-1 cells?" and "The nature of circulating CD27+CD43+ B cells". J Exp Med. 2011;208:2566–2569. doi: 10.1084/jem.20111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Griffin DO, Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol. 2012;3:122. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–272. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon Nahm DG. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn Ps ELISA). (89SF Version) 2006:1–29. [Google Scholar]
- 37.Moon Nahm DG. Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn Ps ELISA). (007 Version) 2011:1–29. [Google Scholar]
- 38.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolibab K, Smithson SL, Shriner AK, Khuder S, Romero-Steiner S, Carlone GM, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2:10. doi: 10.1186/1742-4933-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nahm MGD. Training manual for enzyme linked immunosobent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (PnPg ELISA) 2002 [Google Scholar]
- 41.Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011;79:314–320. doi: 10.1128/IAI.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol. 1996;98:205–215. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 43.Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol. 2008;181:5306–5312. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- 44.Leggat DJ, Thompson RS, Khaskhely NM, Iyer AS, Westerink MA. The Immune Response to Pneumococcal Polysaccharides 14 and 23F Among Elderly Individuals Consists Predominantly of Switched Memory B Cells. J Infect Dis. 2013 doi: 10.1093/infdis/jit139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.