Abstract
Telomeric DNA at eukaryotic chromosome ends terminates with single stranded 3′ G-rich overhangs. The overhang is generated by the interplay of several dynamic processes including semiconservative DNA replication, 3′ end elongation by telomerase, C-strand fill-in synthesis and nucleolytic processing. The mammalian CST (CTC1-STN1-TEN1) complex is directly involved at several stages of telomere end formation. Elucidation of its structural organization and identification of interaction partners support the notion that mammalian CST is, as its yeast counterpart, a RPA-like complex. CST binding at mammalian telomere 3′ overhangs increases upon their elongation by telomerase. Formation of a trimeric CST complex at telomeric 3′overhangs leads to telomerase inhibition and at the same time mediates a physical interaction with DNA polymerase-α. Thus CST seems to play critical roles in coordinating telomerase elongation and fill-in synthesis to complete telomere replication.
Keywords: CST, DNA polymerase-α, replication, telomerase, telomere
Telomeres and Telomere Replication Problems
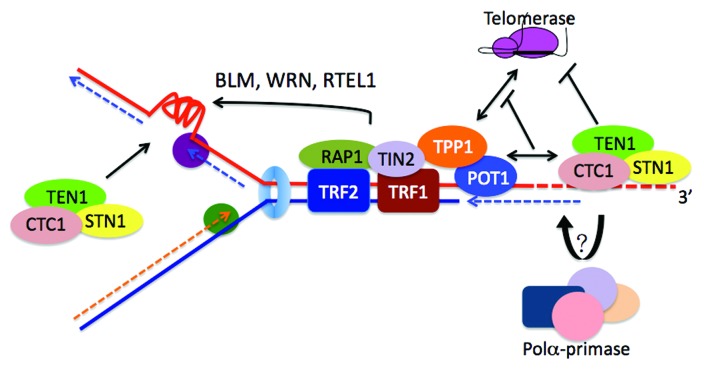
In eukaryotes, the natural chromosome termini dodge the surveillance by the DNA repair machinery through forming a specialized structure.1 Loss of telomere integrity triggers a DNA damage response and repair activities that cause genomic instability and proliferative defects. In most eukaryotes, telomeres are comprised of long segments of DNA duplexes consisting of short tandem repetitive sequences, and terminate with 3′-protruding G-rich overhangs. In mammals, telomeres are associated with the shelterin protein complex comprising TRF1, TRF2, RAP1, TIN2, TPP1 and POT1.1 TRF1 and TRF2 bind double-stranded telomeric DNA directly. Heterodimers of POT1-TPP1, which bind telomeric 3′ overhangs are delivered to telomere termini via TIN2, which interacts with the duplex binding proteins TRF1 and TRF2. In addition, a conserved trimeric protein complex termed CST (CTC1-STN1-TEN1) also associates with telomeres.
The end-replication problem invokes that in the absence of telomerase telomeres shorten upon semiconservative DNA replication. Telomeric DNA strands replicated by lagging-strand synthesis may shorten because of the removal of the ultimate RNA primers. Telomeric strands replicated by leading strand synthesis must shorten because the blunt-ended leading strand products that may be produced transiently become processed by nucleases in order to generate 3′ overhangs.2,3 Telomerase extends the telomeric G strands by reverse transcription of the telomerase RNA template.4 The subsequent fill-in synthesis of the complementary C-strand may complete end replication. In addition, the repetitive telomere sequences are replication barriers that interfere with replication fork progression and can cause telomere instability.5
Mammalian CST is a RPA-Like Complex Binding to Telomeres
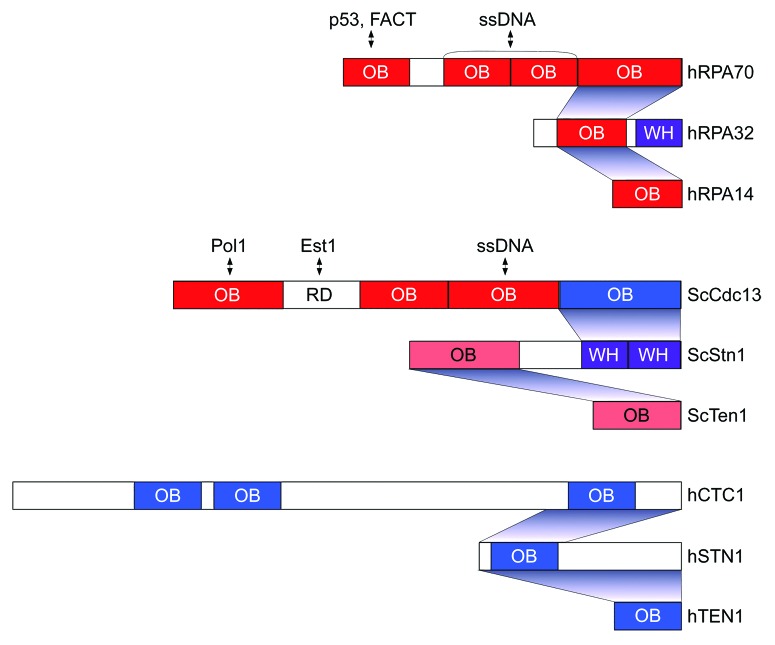
CST was first identified in Saccharomyces cerevisiae (S. cerevisiae) as a trimeric complex consisting of Cdc13, Stn1 and Ten1.6 Later CST was also found in multicellular organisms, including plants and vertebrates.7,8 Stn1 and Ten1 are well conserved throughout evolution whereas S. cerevisiae Cdc13 bears only little sequence similarity with the putative vertebrate ortholog which is referred to as CTC1 (conserved telomere maintenance component 1). S. cerevisiae CST is structurally related to the heterotrimeric replication protein A (RPA)-complex.6 Structural studies revealed that the components of S. cerevisiae CST contain OB folds with a similar domain organization and structural identity as RPA (Fig. 1).9 In addition, the C-terminus of ScStn1 comprises winged helix-turn-helix (wHTH) motifs analogous to that of RPA32.10 ScStn1 interacts with the C-terminus of ScCdc13 via its wHTH motifs, and with ScTen1 via its N-terminal putative OB fold. Like the RPA complex, yeast CST binds ssDNA. However, S. cerevisiae CST specifically binds the telomeric G-rich sequence in order to exert specific roles in coordinating replication events at telomeric termini, including telomerase regulation and C-strand fill-in synthesis. Specific recognition of telomeric ssDNA by CST in S. cerevisiae is mediated by one of the four OB folds of Cdc13 (Fig. 1).
Figure 1. Domain topology of the hRPA, ScCST, and hCST complexes. The OB folds (red: structures solved, pink: structures available from other yeasts, blue: predicted) and winged helix-turn-helix (WH) motifs (purple) are presented. The putative OB folds in hCST are assigned according to reference 13. Interaction domains between subunits of each complex are indicated by shaded areas. The domains in hRPA70 and ScCdc13 mediating other protein interactions and ssDNA binding are highlighted with the double arrow. The telomerase-recruitment domain (RD) domain in ScCdc13 mediates EST1 interaction.
CST subunits and in particular CTC1 in vertebrates and plants show significant sequence divergence from their yeast orthologs.7,8 Although no 3D structures are available, secondary structure predictions suggest that the components of CST in multicellular organisms also contain OB folds (Fig. 1). Human STN1 interacts with both CTC1 and TEN1 conferring the formation of a heterotrimeric CST complex.8,11 Particularly, the interaction with CTC1 is meditated by STN1 N-terminal OB-fold (Fig. 2). In this regard human STN1 resembles hRPA32 which uses its OB-fold to bind hRPA70 (Fig. 1). ScStn1 on the other hand uses separate domains to interact with Cdc13 and Ten1 (Fig. 1). Human CST (hCST) specifically binds to telomeric G-rich ssDNA in a size dependent manner,14 and Xenopus laevis CST complex also shows binding preference for G-rich sequences.12 Nevertheless, hCST is also able to bind to long (> 50 nt) ssDNA in a sequence-independent manner.8,11 The ssDNA binding of CST requires an intact trimeric complex and hCTC1 and the hSTN1/hTEN1 heterodimers do not have significant ssDNA binding activity on their own. This is in stark contrast to S. cerevisiae in which Cdc13 can bind single-stranded telomeric DNA in the absence of its partners. The following evidence supports the notion that mammalian CST binds directly the single-stranded telomeric DNA overhangs in vivo.11 First, deletion of the C-terminus of CTC1 abolishes its interaction with STN1 and telomere association. Second, depletion of the primary telomeric overhang associating protein POT-TPP1 increases hCST telomere association, suggesting that the two complexes compete for telomere overhang binding.11 In addition to CST-DNA interactions, protein-protein interactions between POT1-TPP1 and the CST complex may enhance telomere association.11,13,14
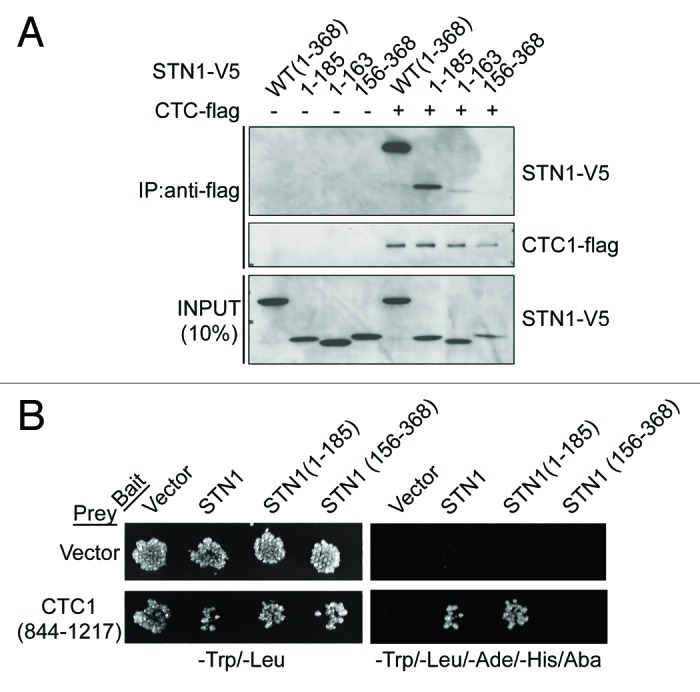
Figure 2. The C-terminus of CTC1 (844–1217) interacts with the N-terminus of STN1 (1–185). (A) Wild-type and mutant STN1-V5 were ectopically co-expressed with or without CTC1-flag in 293T cells for co-immunoprecipitation (co-IP) with anti-flag antibody. Western blots were performed to detect STN1-V5 and CTC1-flag proteins in cellular extracts (input) and immunoprecipitates (IP). (B) Indicated bait (STN1) and prey (CTC1) proteins were expressed as a fusion proteins with the Gal4-DNA binding domain and the Gal4-transcriptional activation domain, respectively, in diploid yeast cells grown on SD (synthetic dextrose-containing minimal medium) -Trp/-Leu agar plates for yeast two-hybrid assay. Protein interactions were detected by replica-plating and scoring for growth on SD/-Trp/-Leu/-Ade/-His/Aba (aureobasidin A) agar plates.
CST as a Telomerase Terminator
Recently we discovered that hCST plays crucial roles in telomerase inhibition and telomere length homeostasis,11 a function that is also seen with yeast CST.15,16 Studies from the Wright and Shay-lab suggested that in cancer cells telomere elongation by telomerase in S phase involves single rounds of processive elongation.17,18 This temporally limited telomerase reaction is partly mediated by TPP1 which binds and recruits telomerase to telomeres, and stimulates telomerase processivity in vitro when associating with POT1.19,20 Subsequently, the newly telomerase-elongated telomere overhangs may serve as a platform for hCST binding that confers telomerase inhibition to prevent telomere over-elongation.11 Indeed, perturbation of cellular hCST function by depleting individual components or expressing dominant mutant hCTC1 unleashes telomerase control resulting in telomere lengthening. In addition, a transient increase of hCST association with telomeres occurs during S/G2 phase in dependency of telomerase activity coinciding with telomerase inhibition. This model is also supported by in vitro experiments in which hCST binds as a unit to telomeric G-rich ssDNA leading to substrate sequestration of telomerase. In addition, the association of hCST with the telomeric 3′ overhang should also bring it in close proximity to POT1-TPP1 promoting their physical interaction which interferes with telomerase stimulation by POT1-TPP1.
Genetic studies revealed that ScCdc13, ScStn1 and ScTen1 negatively regulate telomerase when co-assembled in a trimeric protein complex at telomere overhangs at late S/G2.16,21 Nevertheless, ScCdc13 also has an essential role for telomerase recruitment through its interaction with the Est1 subunit of the telomerase holoenzyme.22 The interaction with ScStn1 may exclude ScCdc13 from further associating with Est1.21 Therefore, the cell cycle dependent telomere association of yeast CST may allow the temporal coupling of telomerase action to the following termination events, which is reminiscent to the situation at human telomeres.
CST in Telomere Fill-In Synthesis
Recent studies also suggest that the mammalian CST complex is involved in fill-in synthesis of the telomeric C-strand.13,23,24 Conventional DNA replication generates telomere leading strand daughters that are initially blunt after replication, whereas lagging strand daughters containing protruding 3′ ends as the final lagging RNA primer is positioned away from the ends. In addition, telomeric C-strands are resected by cellular nucleases and the G-strands are elongated by telomerase. These DNA replication and telomere processing events give rise to transient overhang extensions that are shortened during late S/G2 phase by the fill-in synthesis which involves CST.13,23,24 The precise role of CST in the C-strand fill-in synthesis of telomeres remains to be determined. Interestingly, it has been shown that DNA polymerase- α (polα)−primase complex associates with and is activated by CST.25 Thus, it is conceivable that CST mediates C-strand fill-in synthesis by telomeric recruitment and activation of DNA polα-primase. It is worth noting that in mouse cells the function of CST in fill-in synthesis requires its interaction with POT1b,13 which is reminiscent of telomerase inhibition by the interaction of CST with POT1-TPP1 as described above.
CST Coordinates Telomerase Inhibition and Fill-In Synthesis
Budding yeast CST presents a role for C-strand fill-in synthesis of telomerase elongated G-tails. The prevailing model suggests that CST recruits DNA polα-primase to telomere overhangs through direct physical interactions of Cdc13 and Stn1 with the Pol1 and Pol12 subunits of DNA polα, respectively.26,27 Notably, this CST-mediated C-strand fill-in synthesis is coordinated with telomerase regulation. Indeed, mutations in either CST (Cdc13) or DNA polα that affect their interactions lead to telomere lengthening.26,27 Therefore, it has been proposed that completion of the C-strand synthesis may contribute to the blockage of further telomerase action. In mammals, however, it has yet to be tested whether a similar inhibitory effect occurs on telomerase upon C-strand fill-in synthesis. Consistent with this notion, however, hypomorphic defects of DNA polα in mouse cells manifest a telomerase-meditated telomere lengthening phenotype.28 In addition, we found that DNA polα interacts with wild type hCTC1 but not a mutant hCTC1 lacking the C-terminal hSTN1 interaction domain (Fig. 3). Since an intact CST complex is also required for telomerase inhibition, this result would be consistent with hCST coordinating telomerase regulation via its interaction and stimulation of DNA polα-primase. Thus, C-strand fill-in synthesis coupled telomerase repression may be conserved in mammals.
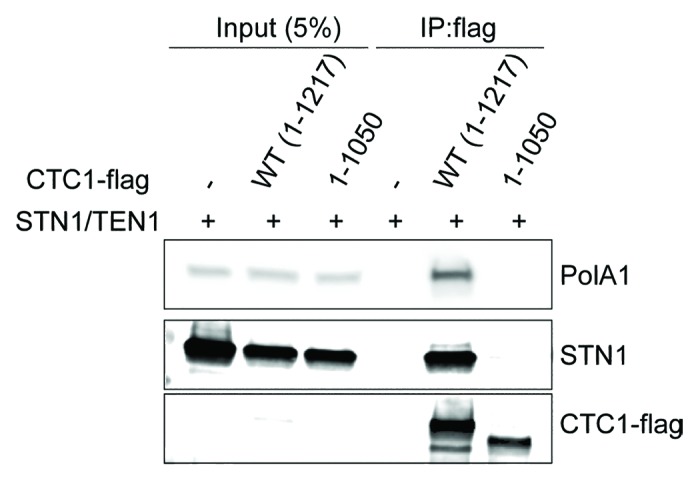
Figure 3. hCST interacts with DNA polα. STN1 and TEN1were ectopically co-expressed in the presence or absence of wild-type (WT) or mutant (1–1050) CTC1 in 293T cells for Co-IP using anti-flag antibody. CTC1-flag, STN1, and endogenous DNA polα (PolA1, catalytic subunit) were detected by western blot. CTC1-flag (1–1050) is defective in STN1 interaction as previously demonstrated.11
Perspectives
Although CST has significant structural and functional similarities between mammals, plants, and fungi, kingdom-specific roles at telomeres have evolved. Mammalian CST functions as a telomerase regulator and is involved in C-strand fill-in synthesis. However, unlike yeast CST, it seems not required for telomerase recruitment and telomere capping, as these essential functions are fulfilled by the other G-overhang binding complex TPP1-POT1. Furthermore, a novel function of CST in telomere duplex replication has been uncovered in mammals whereas in S. cerevisiae such a function remains elusive.23,24,29,30 Thus, it appears that mammalian CST is involved in multiple tasks of telomere replication crucial for telomere length homeostasis and structure integrity (Fig. 4). With the lessons from budding yeast, it is conceivable that the various telomeric functions are interconnected and coordinated during dynamic processes of telomere replication. Thus, dissecting the complex roles of CST function in mammalian cells will be an exciting and important topic of telomere biology research. Among others, it will rely on the identification of separation-of-function mutant CST proteins.
Figure 4. CST functions for telomere replication in mammals. Replication of telomere duplexes can be hindered by ssDNA secondary structures, such as G-quadruplexes. CST as well as shelterin proteins accompanied with cellular helicases are required for efficient telomere replication by the conventional DNA replication apparatus. At telomere ends, telomerase is recruited by TPP1 interaction adding fresh telomere repeats to the 3′ends (red dashed line). The telomerase reaction is terminated when CST binds to the newly telomerase-synthesized overhangs by substrate sequestration and interaction between POT1-TPP1 and CST. Through interaction with CST, DNA polα-primase may be recruited for the C-strand fill-in synthesis (blue dashed line).
Compound heterozygous mutations in CTC1 were recently found to cause short telomere syndromes, such as dyskeratosis congenita (DC) and Coats plus, but the disease pathology is unknown.31-34 Clinically, DC and Coats plus have overlapping clinical manifestations, such as bone marrow failure, but they also show diverse disease characteristics. DC is characterized with mucocutaneous abnormalities, including oral leukoplakia, nail dystrophy, and skin hyperpigmentation, while Coats plus is defined by exudative retinopathy and intracranial calcifications. In addition, in contrast to telomerase deficiency that is the cause of typical short telomere syndromes, CTC1 mutations cause telomere shortening in some but not all patients. Thus, these diverse cellular and clinical phenotypes may reflect the fact that CST has complex cellular functions both at telomeres and probably also at non-telomeric regions of human chromosomes. Future work is necessary to characterize the molecular consequences of the mutations in CTC1, and how they are attributed to cellular malfunctions. This analysis may help to delineate the complex roles of CST in mammals and uncover the molecular basis of the disease.
Acknowledgments
Research in JL’s laboratory was supported by the Swiss National Science Foundation, a European Research Council advanced investigator grant (grant agreement number 232812), an Initial Training Network (ITN) grant (CodeAge) from the European Commission’s Seventh Framework Programme (grant agreement number 316354), the Swiss Cancer League and EPFL.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/25701
References
- 1.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 2.Lingner J, Cooper JP, Cech TR. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269:1533–4. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- 3.Wellinger RJ, Ethier K, Labrecque P, Zakian VA. Evidence for a new step in telomere maintenance. Cell. 1996;85:423–33. doi: 10.1016/S0092-8674(00)81120-4. [DOI] [PubMed] [Google Scholar]
- 4.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 5.Jain D, Cooper JP. Telomeric strategies: means to an end. Annu Rev Genet. 2010;44:243–69. doi: 10.1146/annurev-genet-102108-134841. [DOI] [PubMed] [Google Scholar]
- 6.Gao H, Cervantes RB, Mandell EK, Otero JH, Lundblad V. RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol. 2007;14:208–14. doi: 10.1038/nsmb1205. [DOI] [PubMed] [Google Scholar]
- 7.Surovtseva YV, Churikov D, Boltz KA, Song X, Lamb JC, Warrington R, et al. Conserved telomere maintenance component 1 interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol Cell. 2009;36:207–18. doi: 10.1016/j.molcel.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake Y, Nakamura M, Nabetani A, Shimamura S, Tamura M, Yonehara S, et al. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Lewis KA, Wuttke DS. Telomerase and telomere-associated proteins: structural insights into mechanism and evolution. Structure. 2012;20:28–39. doi: 10.1016/j.str.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Yu EY, Yang Y, Confer LA, Sun SH, Wan K, et al. Stn1-Ten1 is an Rpa2-Rpa3-like complex at telomeres. Genes Dev. 2009;23:2900–14. doi: 10.1101/gad.1851909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LY, Redon S, Lingner J. The human CST complex is a terminator of telomerase activity. Nature. 2012;488:540–4. doi: 10.1038/nature11269. [DOI] [PubMed] [Google Scholar]
- 12.Nakaoka H, Nishiyama A, Saito M, Ishikawa F. Xenopus laevis Ctc1-Stn1-Ten1 (xCST) protein complex is involved in priming DNA synthesis on single-stranded DNA template in Xenopus egg extract. J Biol Chem. 2012;287:619–27. doi: 10.1074/jbc.M111.263723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P, Takai H, de Lange T. Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell. 2012;150:39–52. doi: 10.1016/j.cell.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan M, Qin J, Songyang Z, Liu D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J Biol Chem. 2009;284:26725–31. doi: 10.1074/jbc.M109.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellinger RJ, Zakian VA. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics. 2012;191:1073–105. doi: 10.1534/genetics.111.137851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009;28:2309–22. doi: 10.1038/emboj.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Abreu E, Kim J, Stadler G, Eskiocak U, Terns MP, et al. Processive and distributive extension of human telomeres by telomerase under homeostatic and nonequilibrium conditions. Mol Cell. 2011;42:297–307. doi: 10.1016/j.molcel.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell. 2009;138:463–75. doi: 10.1016/j.cell.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abreu E, Aritonovska E, Reichenbach P, Cristofari G, Culp B, Terns RM, et al. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol. 2010;30:2971–82. doi: 10.1128/MCB.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol. 2013;14:69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Hughes TR, Nugent CI, Lundblad V. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 2001;15:404–14. doi: 10.1101/gad.861001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001;104:387–96. doi: 10.1016/S0092-8674(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Stewart JA, Kasbek C, Zhao Y, Wright WE, Price CM. Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep. 2012;2:1096–103. doi: 10.1016/j.celrep.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Dai X, Chai W. Human Stn1 protects telomere integrity by promoting efficient lagging-strand synthesis at telomeres and mediating C-strand fill-in. Cell Res. 2012;22:1681–95. doi: 10.1038/cr.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casteel DE, Zhuang S, Zeng Y, Perrino FW, Boss GR, Goulian M, et al. A DNA polymerase-alphamiddle dotprimase cofactor with homology to replication protein A-32 regulates DNA replication in mammalian cells. J Biol Chem. 2009;284:5807–18. doi: 10.1074/jbc.M807593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossi S, Puglisi A, Dmitriev PV, Lopes M, Shore D. Pol12, the B subunit of DNA polymerase alpha, functions in both telomere capping and length regulation. Genes Dev. 2004;18:992–1006. doi: 10.1101/gad.300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi H, Zakian VA. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev. 2000;14:1777–88. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura M, Nabetani A, Mizuno T, Hanaoka F, Ishikawa F. Alterations of DNA and chromatin structures at telomeres and genetic instability in mouse cells defective in DNA polymerase alpha. Mol Cell Biol. 2005;25:11073–88. doi: 10.1128/MCB.25.24.11073-11088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JA, Wang F, Chaiken MF, Kasbek C, Chastain PD, 2nd, Wright WE, et al. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 2012;31:3537–49. doi: 10.1038/emboj.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu P, Min JN, Wang Y, Huang C, Peng T, Chai W, et al. CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J. 2012;31:2309–21. doi: 10.1038/emboj.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walne AJ, Bhagat T, Kirwan M, Gitiaux C, Desguerre I, Leonard N, et al. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica. 2013;98:334–8. doi: 10.3324/haematol.2012.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polvi A, Linnankivi T, Kivelä T, Herva R, Keating JP, Mäkitie O, et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am J Hum Genet. 2012;90:540–9. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller RB, Gagne KE, Usmani GN, Asdourian GK, Williams DA, Hofmann I, et al. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr Blood Cancer. 2012;59:311–4. doi: 10.1002/pbc.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet. 2012;44:338–42. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]