Abstract
The nuclear lamina underlies the inner nuclear membrane and consists of a proteinaceous meshwork of intermediate filaments: the A- and B-type lamins. Mutations in LMNA (encoding lamin A and C) give rise to a variety of human diseases including muscular dystrophies, cardiomyopathies and the premature aging syndrome progeria (HGPS). Duplication of the LMNB1 locus, leading to elevated levels of lamin B1, causes adult-onset autosomal dominant leukodystrophy (ADLD), a rare genetic disease that leads to demyelination in the central nervous system (CNS). Conversely, reduced levels of lamin B1 have been observed in HGPS patient derived fibroblasts, as well as fibroblasts and keratinocytes undergoing replicative senescence, suggesting that the regulation of lamin B1 is important for cellular physiology and disease. However, the causal relationship between low levels of lamin B1 and cellular senescence and its relevance in vivo remain unclear. How do elevated levels of lamin B1 cause disease and why is the CNS particularly susceptible to lamin B1 fluctuations? Here we summarize recent findings as to how perturbations of lamin B1 affect cellular physiology and discuss the implications this has on senescence, HGPS and ADLD.
Keywords: lamin B1, ADLD, senescence, telomeres, telomerase, p53
Introduction
In metazoans, the nuclear envelope consists of an inner and outer nuclear membrane, separated by a 40−50 nm perinuclear space. The nuclear envelope is spanned by nuclear pore complexes which facilitate transport between the nucleus and the cytoplasm. Underneath the inner nuclear membrane lies a 15–20 nm thick proteinaceous meshwork: the nuclear lamina. The lamina consists of intermediate filament proteins: lamin A, lamin C and lamin B1 and B2. B-type lamins are ubiquitously expressed in all cell types, including embryonic stem cells, whereas expression of A-type lamins is restricted to somatic lineages. Although initially thought to mainly support the structural integrity of the nucleus, it is now becoming clear that the lamins play an important role in various cellular processes, including DNA replication, cell cycle progression, chromatin organization and remodeling.1-3 Reflecting these diverse functions, mutations in lamina components give rise to a wide variety of diseases, collectively termed laminopathies.4,5 Mutations in LMNA can cause muscular dystrophies, lipodystrophies, cardiomyopathies and, most prominently, the premature aging syndrome Hutchinson-Gilford progeria (HGPS). Most cases of HGPS are caused by a single heterozygous base pair substitution in the LMNA gene (G608G, GGC > GGT), that gives rise to a truncated form of lamin A, called progerin.6,7
Although no dominant-acting missense or loss of function mutations have been identified in B-type lamins, duplication of the LMNB1 locus, leading to elevated lamin B1 protein levels, is associated with adult-onset autosomal dominant leukodystrophy (ADLD). ADLD is a rare genetic disease, similar to chronic progressive multiple sclerosis that affects myelination in the central nervous system (CNS).8 Akin to the molecular phenotype of ADLD, increased levels of lamin B1 have also been observed in lymphoblasts and fibroblasts from patients with ataxia telangiectasia (AT), whose clinical traits include neurological defects.9 AT is caused by mutations in ATM (ataxia telangiectasia mutated), a protein kinase that controls early steps during DNA damage response signaling. As a result, AT patients are sensitive to radiation and predisposed to cancers. The complex relationship between elevated lamin B1 levels and impaired DNA damage response with neurological defects and cancer predisposition remains elusive.
Interestingly, phenotypes associated with perturbations of lamin B1, such as in ADLD and AT, but also in mouse models lacking lamin B1, result in defects in the CNS.10,11 How do elevated levels of lamin B1 affect cellular physiology and what makes the CNS more susceptible to aberrant levels of lamin B1?
In contrast to the elevated levels of lamin B1 in ADLD and AT, low levels of lamin B1 have been observed in fibroblasts derived from patients with the accelerated aging syndrome HGPS,12,13 senescent human primary,14 WI-38, HCA2 and BJ fibroblasts.15,16 In contrast to quiescence, cellular senescence is an irreversible growth arrest. Several pathways have been implicated in triggering senescence. Of particular relevance are persistent DNA damage foci associated with critically shortened or deprotected telomeres.17,18 Telomeres cap the physical ends of linear chromosomes, consist of hexameric TTAGGG repeats and are bound by the shelterin protein complex.19 Due to the semi-conservative nature of DNA replication (the “end replication problem”), telomeres shorten throughout each replication cycle until critically shortened telomeres elicit a persistent DNA damage response that initiates cellular senescence.17 The “end replication problem” can be circumvented by the activation of telomerase, a ribonucleoprotein consisting of a reverse transcriptase (TERT) and its RNA moiety (TR).20 Telomerase is expressed in ~90% of cancers and embryonic stem cells and its expression immortalizes cells.21
Two rare diseases affecting the CNS have been associated with elevated levels of lamin B1, whereas reduced levels of lamin B1 have been observed in HGPS patient-derived fibroblasts and senescent normal fibroblasts in vitro. These findings raise several important questions: (1) Are the low levels of lamin B1 in senescent cells a cause or a consequence of senescence? (2) Does the loss of lamin B1 correlate with telomere shortening? (3) Is loss of lamin B1 a common feature of different somatic lineages undergoing senescence and are these findings relevant in vivo; for example during normal aging of human skin? (4) How do elevated levels of lamin B1 affect cellular physiology and (5) why are the brain and CNS particularly susceptible to lamin B1 fluctuations?
Lamin B1 Loss and Cellular Senescence: Cause or Consequence?
The correlation between low levels of lamin B1 and senescence raised the question whether lamin B1 depletion is a cause or a consequence of senescence. Previous studies investigated the consequences of lamin B1 depletion and led to conflicting results: in one report, silencing of lamin B1 in HeLa cells led to apoptosis,22 whereas recent work suggested that inhibition of lamin B1 in WI-38 cells causes senescence.15 Conversely, overexpression of lamin B1 appeared to enhance proliferation and delay the onset of senescence,15 whereas a second study showed that elevated levels of lamin B1 triggered senescence.9 To investigate the consequences of lamin B1 depletion or overexpression on cellular proliferation and senescence, we used constitutive or doxycyclin-inducible constructs to deplete (by shRNA) or overexpress lamin B1 in primary fibroblast lines and their telomerase-immortalized counterparts.14
In our hands, lamin B1-depleted cells have impaired proliferation, irrespective of whether they are primary cells or cells immortalized by telomerase. However, under normal cell culture conditions, we were unable to detect a significant increase in the numbers of cells staining positive for senescence-associated β-galactosidase (SA-β-gal) activity.14,23 To test whether lamin B1-depleted cells were more susceptible to senesce upon exposure to additional stressors, we grew lamin B1-depleted cells at low density (sparse) vs. sub-confluence. Interestingly, sparsely plated lamin B1-depleted cells were more prone to senesce (as judged by SA-β-gal staining), than cells grown at high density. These results demonstrate that although reduced levels of lamin B1 impair cell proliferation, they do not trigger premature senescence in either primary or telomerase-positive fibroblasts, unless subjected to additional stress, such as growth at low cell density.14
Do these in vitro results bear any physiological relevance in vivo? In the mouse, keratinocyte-specific depletion of Lmnb1, or hepatocyte-specific depletion of Lmnb1 and Lmnb2, affected neither epidermal keratinocyte proliferation, the development or maintenance of skin or hair,24 nor liver development and function, respectively.25 In contrast, in vitro mouse embryonic fibroblasts derived from Lmnb1-null mice, exhibited nuclear architecture defects, karyotypic abnormalities and premature senescence.26 Therefore, lamin B1 depletion alone does not trigger cellular senescence, unless the cells are exposed to additional stress: in the case of human lamin B1-depleted cells, growth under sparse conditions or in Lmnb1−/− mouse embryonic fibroblasts, growth under in vitro cell culture conditions.
To investigate what may lead to the impaired proliferation of lamin B1-depleted cells, we analyzed their cell cycle profile and consistently observed a slight increase in the percentage of cells in G2/M, as well as additional peaks to the right of the G2/M peak, suggesting a delay in mitosis and chromosomal instability.14 Consistent with these results, depletion of lamin B1 in mouse embryonic stem11 or HeLa cells27 led to mitotic defects, such as delayed pro-metaphase as well as abnormal mitotic spindle assembly. We speculate that these mitotic defects may ultimately lead to impaired chromosome segregation and karyotypic abnormalities. In conclusion, downregulation of lamin B1 is insufficient in triggering cellular senescence, unless it is accompanied by additional stress.
Does the Loss of Lamin B1 Correlate with Telomere Shortening?
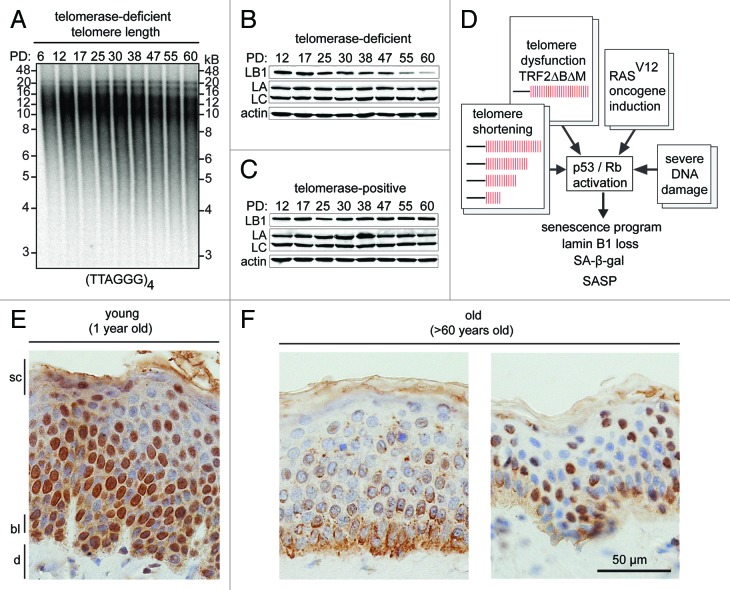
Gradual erosion of telomeric DNA due to the end replication problem occurs during each replication cycle, ultimately culminating in replicative senescence.17 To investigate whether lamin B1 reduction correlates with telomere shortening, we serially passaged human primary dermal fibroblasts until they reached replicative senescence (after ~60 PD) and monitored telomere length and lamin B1 levels throughout this time course. As shown in Figure 1A, telomere length gradually declined throughout the time course and correlated with a reduction in lamin B1 levels (Fig. 1B). In contrast, in cells immortalized by ectopic expression of telomerase, lamin B1 levels remained stable (Fig. 1C).14 Furthermore, induction of telomere-specific persistent DNA damage by removal of the shelterin component TRF2 (by expression of a dominant-negative allele of TRF2: TRF2∆B∆M), also triggered senescence and a concomitant reduction in lamin B1.14 Lastly, senescence induced by persistent DNA damage (by ionizing radiation) or by expression of an oncogenic form of H-RAS (RASV12) triggered senescence together with a loss of lamin B1.16
Figure 1. (A) In gel hybridization showing telomere shortening in primary dermal fibroblasts during continuous in vitro cell culture. A radiolabeled (TTAGGG)4 probe was used to visualize telomere repeats. Population doublings (PD) and molecular weight markers (kB) are indicated. (B) western blot shows lamin B1 (LB1), lamin A/C (LA and LC) and actin levels in primary dermal fibroblasts during a 60 PD time course (PDs are indicated on top). (C) western blot shows lamin B1, lamin A/C and actin levels in telomerase-positive dermal fibroblasts during extended in vitro culture (60 PD). (D) Schematic representation of signals (telomere shortening, telomere dysfunction, oncogene induction and severe DNA damage) that activate p53/Rb signaling pathways and initiate cellular senescence. (E) Loss of lamin B1 during normal skin aging in vivo. Immunohistochemistry of paraffin sections of normal human skin from (E) young (1 y) vs. (F) aged (over 60 y) individuals. Different skin layers are indicated: stratum corneum layer (sc); basal layer (bl); dermis (d), scale bar: 50 µm.
Taken together, these results suggest that DNA damage foci, caused by expression of oncogenic H-RAS and critically shortened or deprotected telomeres, lead to persistent activation of p53 and Rb, senescence and lamin B1 downregulation (Fig. 1D). If this sequence of events is correct, constitutive activation of p53 or Rb, in normal cells, should result in senescence and lamin B1 reduction. Freund et al. demonstrated this by activating p53—using the MDM2-antagonist Nutlin-3a, or by overexpressing p16INK4a to induce pRb—in the absence of additional DNA damage. Both procedures triggered cellular senescence and led to a reduction in lamin B1.16 Interestingly, while formation of senescence-associated heterochromatin foci and SA-β-gal activity only became apparent 7–10 d after inducing senescence, loss of lamin B1 levels was an early event during senescence and was apparent within 2 to 4 d.16 These results establish that loss of lamin B1 is a marker for senescence in human primary fibroblasts and prompted us to investigate whether these findings are relevant to other cell types of the human body.
Is Loss of Lamin B1 a Common Feature of Different Somatic Lineages Undergoing Senescence and Are These Findings Relevant In Vivo?
While fibroblasts reside in the dermis, keratinocytes populate the epidermal layer of the skin, form a protective barrier against pathogens and UV radiation and are important in the pathogenesis of various diseases affecting cutaneous and mucosal epithelia.28 To investigate lamin B1 dynamics during replicative aging of keratinocytes, we passaged two primary keratinocyte lines in parallel with a telomerase-immortalized keratinocyte cell line (N/TERT-1)29 until the primary lines reached replicative senescence. Senescent keratinocytes exhibited flattened morphology, stained positive for SA-β-gal activity and showed reduced levels of lamin B1 by immunofluorescence microscopy and western blotting.14 Thus, loss of lamin B1 characterizes both senescent fibroblasts and keratinocytes in vitro.
To test whether senescence-associated lamin B1 reduction occurs in vivo, Freund et al. induced senescence by irradiating mice and quantifying lamin B1 levels in liver sections.16 Irradiated mice exhibited lower mean lamin B1 (but not lamin C) staining intensities than non-irradiated controls 12 weeks after irradiation. These findings were extended by RT-PCR analysis showing increased expression of senescence-associated p16INK4a as well as lower levels of lamin B1 in liver, kidney, lung and skin of the irradiated mice. These results suggest that lamin B1 levels decline upon irradiation-induced senescence in murine cells in vivo.
To test whether lamin B1 levels decline in aged human tissues in vivo, we analyzed skin sections from one young (age 1) and several old individuals (age > 60) and compared lamin B1 levels. Consistent with our in vitro results, skin sections from the young donor exhibited robust lamin B1 staining (Fig. 1E), whereas sections from aged individuals showed a marked reduction of lamin B1 (Fig. 1F;14). Although the relationship may not be causal, the loss of lamin B1 correlates with increased numbers of senescent cells (by β-gal staining)23 and shortened telomeres30,31 in skin from aged individuals. Based on these results, we concluded that lamin B1 reduction is a hallmark of senescent keratinocytes in vitro and in vivo and may be a useful marker for skin aging.
ADLD and the Consequences of Lamin B1 Overexpression
While lamin B1 loss is a characteristic of senescent fibroblasts and keratinocytes, elevated levels of lamin B1 have been associated with ADLD, a progressive and fatal neurological disorder. Patients with ADLD exhibit autonomic symptoms, such as bowel/bladder dysfunction, male impotence, orthostatic hypotension and loss of fine motor skills starting at a mean age of ~40 y. On a pathological level, ADLD is characterized by widespread myelin loss in the CNS.8,32 Although similar to chronic progressive multiple sclerosis (MS), several features distinguish ADLD from MS: ADLD patients exhibit symmetrical myelin loss and show no indication of brain inflammation,33 whereas in multiple sclerosis, demyelination is asymmetrical and caused by an autoimmune response against myelin, leading to inflammation in the CNS.
To study the consequences of lamin B1 overexpression, we used doxycyclin-inducible (lentivirus-based) or constitutive (retrovirus based) vectors to express physiologically relevant levels of lamin B1. We used human fibroblast lines to perform these experiments, as they are more amenable to genetic manipulation and provide a more tractable system (than, for instance, neuronal lineages) to accurately assess the consequences of elevated lamin B1 levels on cell proliferation. Overexpression of lamin B1 led to a very subtle proliferation defect in primary fibroblasts that was rescued by expression of telomerase or by inactivation of the p53 signaling pathway.14 However, this subtle proliferation defect in fibroblasts may not be physiologically relevant as ADLD patients do not show any overt skin problems. Thus, in contrast to the CNS, which is strongly affected by elevated levels of lamin B1, fibroblasts appear to be resistant. This situation is also mirrored in mice with tissue specific Lmnb1 deficiency: depletion of Lmnb1 in liver or skin did not result in any abnormalities,24,25 whereas conditional forebrain specific deletion of Lmnb1 and Lmnb2 led to defects in neuronal migration and nuclear abnormalities.10
What makes the CNS so much more susceptible to fluctuations of the B-type lamins? Previous reports revealed significantly lower levels of lamin A in cells from chicken and mouse brains, as well as neuronal lineages derived from human induced pluripotent stem cells.34-36 More recently, it was shown that miR-9, a neural specific microRNA effectively suppresses lamin A expression in mouse and human neuronal lineages.37,38 Based on these observations, we speculated that reduced levels of lamin A may render these cells more susceptible to fluctuations of lamin B1.
Reduced Levels of Lamin A/C Exacerbate the Consequences of Lamin B1 Overexpression
To investigate whether reduced levels of lamin A exacerbates the consequences of elevated lamin B1 levels, we generated cell lines expressing ~50% of their original lamin A/C levels (by expression of a LMNA/C shRNA) and overexpressed lamin B1 (or a vector control) in these cells.14 Proliferation assays and growth analysis revealed that lamin A/C↓ cells were significantly more susceptible to lamin B1 overexpression than control cells: lamin B1↑lamin A/C↓ cells were severely impaired in their proliferation, arrested at G0/G1 and stained positive for SA-β-gal activity. They also showed a marked increase of DNA damage foci that co-localized with telomeres.
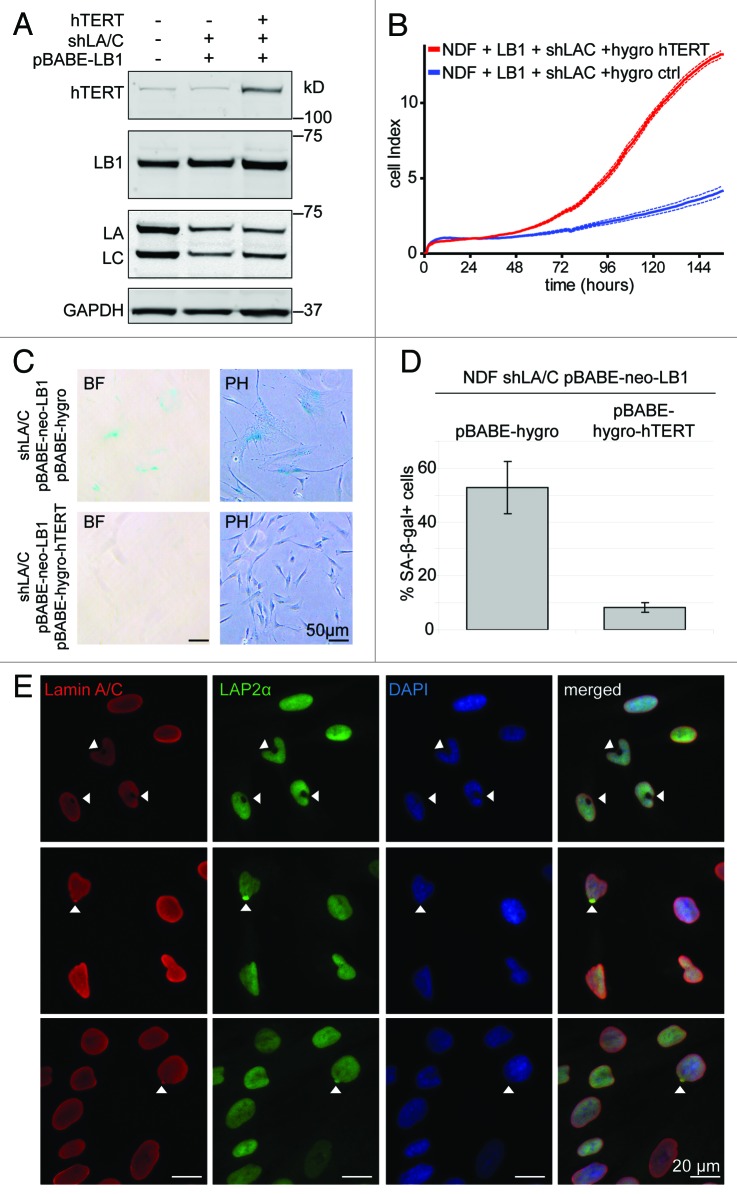
Telomere-associated DNA damage is particularly detrimental to cells, as it cannot be repaired by conventional DNA repair machinery and may explain the severe growth defects of these cells. As described before, the subtle proliferation defect caused by lamin B1 overexpression in normal fibroblasts was rescued by expression of telomerase. To test whether the severe lamin B1↑lamin A/C↓-induced proliferation defect could be rescued by telomerase, we introduced hTERT into lamin B1↑lamin A/C↓ cells (Fig. 2A). Lamin B1↑lamin A/C↓ cells expressing telomerase proliferated normally, despite retaining reduced levels of lamin A/C and overexpressing lamin B1. The restored proliferation of telomerase-positive lamin B1↑lamin A/C↓ cells was accompanied by a marked decrease of cells that stained positive for SA-β-gal activity (Fig. 2D). Taken together, these results demonstrate that the proliferation defect and premature senescence of lamin B1↑lamin A/C↓ cells is due to telomere-associated DNA damage and can be prevented by telomerase expression. A small percentage (~2−4%) of telomerase-immortalized lamin B1↑lamin A/C↓ cells had doughnut shaped nuclei. This nuclear architecture defect is similar to the doughnut-shaped nuclei observed in cells treated with farnesyl-transferase inhibitors or cells overexpressing a nonfarnesylated version of lamin B1.39 We also observed a few cells in which the lamina-associated polypeptide 2 α (LAP2α) aggregated at one pole of the nucleus (Fig. 2E), similar to a redistribution of LAP2α previously reported in cells from a patient with fetal lethal akinesis, due a LMNA mutation, which resulted in depletion of lamin A/C.40,41 Nevertheless, the physiological significance of this LAP2α re-localization remains unclear.
Figure 2. Ectopic expression of telomerase rescues severe proliferation defect of lamin B1↑lamin A/C↓ cells. (A) western blot showing telomerase, lamin B1 and lamin A/C levels in cells expressing pBABE-neo control or pBABE-neo-lamin B1 in the presence (shLA/C) or absence of reduced lamin A/C levels. Antibodies: telomerase (hTERT), lamin B1 (LB1); lamin A/C (LA/C) and GAPDH loading control. (B) Growth curve of lamin B1↑lamin A/C↓ cells expressing either pBABE-hygro-hTERT or pBABE-hygro control. (C) SA-β-gal staining of lamin B1↑lamin A/C↓ expressing pBABE-hygro-hTERT or pBABE-hygro (PH; phase contrast, BF; brightfield). Scale bar = 50 µm. (D) Quantification of SA-β-gal positive cells described in (C). (E) Immunofluorescence microscopy reveals cells with doughnut shaped nuclei (top row, arrowheads) and LAP2α aggregates (bottom two rows, arrowheads) in telomerase-positive lamin B1↑lamin A/C↓ cells. Antibodies: lamin A/C (red); LAP2α (green), DAPI (blue) and merged images. Scale bar: 20 µm
Conclusions and Perspectives: Loss of Lamin B1 is a Marker for Cellular Senescence In Vitro and In Vivo
Senescent cells accumulate with age in many tissues, in particular at sites of pre-neoplastic42 or age related pathologies, such as impaired heart regeneration43 and in aging skin.23 However, many senescence associated biomarkers are non-specific or hard to detect in vivo. For example, SA-β-gal activity has been widely used to identify senescent cells, but is notoriously difficult to detect in vivo. We and others have shown that loss of lamin B1 can serve as a marker for cellular senescence in vitro and in vivo.14-16 The reduction of lamin B1 levels that we observed in old skin14 is consistent with the reported increase in SA-β-gal positive cells in skin section of old individuals.23 Based on these results, loss of lamin B1 protein and mRNA levels may be useful in studying the contribution of senescence to skin aging or skin related pathologies. In this context, lamin B1 may also serve as a marker to assess the biological, rather than chronological age of human skin and possibly other tissues as lamin B1 levels remain stable in quiescent cells.14-16 Thus, lamin B1 levels in conjunction with other proliferation markers, such as Ki-67 may enable us to efficiently distinguish between quiescent and senescent cells.
It remains unclear what triggers senescence in aged human skin. In vitro, loss of telomeric DNA during extensive proliferation of primary fibroblasts correlates with loss of lamin B1. In vivo, telomere length declines in aged skin30,31 and thus, correlates with lamin B1 loss and senescence, but the causal relationship of these events remains to be established.
However, use of lamin B1 as a marker for senescence may not be restricted to skin: Freund et al. observed reduced levels of lamin B1 in mouse liver sections and other tissues, including skin, kidney and lungs, after irradiation, suggesting that “loss of lamin B1” faithfully detects irradiation-induced senescence in different tissues in vivo.16 It remains to be seen whether these findings are applicable to human tissues other than skin.
Lamin B1 Overexpression, Cellular Senescence and ADLD
In contrast to reduced lamin B1 levels being associated with aging and senescence, increased levels of lamin B1 are associated with ADLD and AT, two diseases with severe neurological defects associated with CNS demyelination. In fibroblasts, we and others have shown that elevated levels of lamin B1 trigger senescence.9,14 In cells from AT patients, increased oxidative stress and activation of the p38 MAPK pathway is thought to lead to overexpression of lamin B1 and senescence,9 while in ADLD patients, a duplication of the LMNB1 locus results in elevated lamin B1 levels.8 So how do elevated levels of lamin B1 trigger senescence? In fibroblasts, increased lamin B1 caused a very subtle proliferation defect that was rescued by inactivation of p53 or by expression of telomerase. This phenotype was exacerbated by reducing lamin A/C. Overexpression of lamin B1 with reduced lamin A/C led to severe proliferation defects, an increase in SA-β-gal positive cells, G1 arrest and telomere-associated DNA damage. This more severe lamin B1-dependent proliferation defect was also rescued by telomerase expression.
How relevant are these results to the pathology of ADLD and what is the mechanism that leads to telomere-associated DNA damage in the presence of elevated lamin B1 levels?
Previous reports suggested that elevated levels of lamin B1 in oligodendrocytes lead to a premature differentiation arrest and suppression of myelin biosynthesis (myelin-basic protein, proteolipid protein and myelin oligodendrocyte glycoprotein).44 A mouse model of ADLD that expresses additional copies of Lmnb145exhibited age-dependent histopathological and behavioral defects, such as axon demyelination, seizures and motor defects. Proteomic and RT-PCR data revealed decreased transcript and protein levels of myelin proteolipid protein. Based on these results, the authors concluded that lamin B1 is an important modulator of genes involved in myelin biosynthesis.45
Are these in vivo results compatible with our in vitro studies? Our in vitro data suggests that increased levels of lamin B1 trigger cellular senescence by damaging telomeres. So what is the link between telomeric DNA damage, senescence, myelination and cognitive defects? First, demyelination of neurons has been observed during human aging and in telomerase-deficient aged mice with critically shortened telomeres.46,47 Second, an age-dependent, DNA damage-response induced senescence-like state was recently described in several neuronal lineages in mouse.48 Third, and most strikingly, cognitive function and myelination of the CNS was improved upon reactivation of telomerase in aged telomerase-deficient mice.49
Based on these results, we speculate that demyelination, due to increased neuronal senescence during normal aging, may be accelerated by increased lamin B1 expression. Lamin B1 overexpression may not directly regulate expression of genes involved in myelin biosynthesis but rather lead to accelerated senescence, which in turn downregulates these genes. Although speculative, this model would complement our in vitro findings with recent results from the ADLD mouse model in vivo.
Although it remains unclear how elevated levels of lamin B1 (in the context of lower lamin A levels) lead to telomere-associated DNA damage, this telomere damage, as well as those induced by the expression of progerin, are prevented by ectopic expression of telomerase (or inactivation of p53 signaling).14,50 These results provide insights into how different lamin mutations can cause such a wide spectrum of human diseases. HGPS patients die of cardiovascular disease.51,52 Our iPSC based model of HGPS revealed that lineages of mesenchymal origin, such as vascular smooth muscle cells (VSMC) express the highest levels of lamin A (and mutant progerin), and thus, may be more prone to progerin-dependent defects. In contrast, neural lineages express very little lamin A / progerin and remain unaffected in HGPS patients.36 However, in ADLD the low levels of lamin A/C in neuronal lineages renders them more susceptible to elevated levels of lamin B1.14
Together, these studies underscore the importance of the nuclear lamina in regulating cellular proliferation and provide a framework as to how mutations in the nuclear lamina, or stochiomeric changes in lamina composition, lead to various human diseases. Understanding how progerin expression or lamin B1 overexpression independently cause telomeric DNA damage and cellular senescence will provide novel insights into the biology of telomeres and the nuclear lamina and may provide novel ways to treat laminopathies.
Acknowledgments
We are grateful to Colin Stewart for insightful comments on this manuscript, Declan Lunny and John Common for preparation and staining of skin sections. Shu Jin Lee (Division of Plastic, Reconstructive and Aesthetic Surgery, National University of Singapore) is thanked for providing us with skin samples. This work was supported by the Singapore Biomedical Research Council of the Singapore Agency for Science, Technology and Research (A*STAR), Singapore.
Glossary
Abbreviations:
- ADLD
adult-onset autosomal leukodystrophy
- HGPS
Hutchinson-Gilford progeria syndrome
- SA-β-gal
senescence-associated beta-galactosidase
- LMNB1
lamin B1
- LAP
lamina-associated polypeptide
- LMNA/C
lamin A/C
- hTERT
human telomerase reverse transcriptase
- TRF2
telomeric repeat-binding factor 2
Submitted
06/07/2013
Revised
07/16/2013
Accepted
07/16/2013
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/25808
References
- 1.Moir RD, Montag-Lowy M, Goldman RD. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol. 1994;125:1201–12. doi: 10.1083/jcb.125.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 3.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–98. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Burke B, Stewart CL. The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu Rev Genomics Hum Genet. 2006;7:369–405. doi: 10.1146/annurev.genom.7.080505.115732. [DOI] [PubMed] [Google Scholar]
- 5.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–8. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 8.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–23. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 9.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, et al. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–94. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011;22:4683–93. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, et al. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–10. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med. 2005;11:440–5. doi: 10.1038/nm1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taimen P, Pfleghaar K, Shimi T, Möller D, Ben-Harush K, Erdos MR, et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A. 2009;106:20788–93. doi: 10.1073/pnas.0911895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200:605–17. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011;25:2579–93. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell. 2012;23:2066–75. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–8. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 18.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–56. doi: 10.1016/S0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 19.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–52. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 21.Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Semin Cancer Biol. 2011;21:349–53. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 23.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, et al. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20:3537–44. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. Are B-type lamins essential in all mammalian cells? Nucleus. 2011;2:562–9. doi: 10.4161/nucl.2.6.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergnes L, Péterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A. 2004;101:10428–33. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, et al. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–93. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- 28.Albanesi C. Keratinocytes in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010;10:452–6. doi: 10.1097/ACI.0b013e32833e08ae. [DOI] [PubMed] [Google Scholar]
- 29.Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–47. doi: 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto M, Yamashita R, Ueda M. Telomere length of the skin in association with chronological aging and photoaging. J Dermatol Sci. 2006;43:43–7. doi: 10.1016/j.jdermsci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Izumiyama-Shimomura N, Sawabe M, Arai T, Aoyagi Y, Fujiwara M, et al. Comparative analysis of telomere lengths and erosion with age in human epidermis and lingual epithelium. J Invest Dermatol. 2002;119:1014–9. doi: 10.1046/j.1523-1747.2002.19523.x. [DOI] [PubMed] [Google Scholar]
- 32.Brussino A, Vaula G, Cagnoli C, Panza E, Seri M, Di Gregorio E, et al. A family with autosomal dominant leukodystrophy linked to 5q23.2-q23.3 without lamin B1 mutations. Eur J Neurol. 2010;17:541–9. doi: 10.1111/j.1468-1331.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 33.Padiath QS, Fu YH. Autosomal dominant leukodystrophy caused by lamin B1 duplications a clinical and molecular case study of altered nuclear function and disease. Methods Cell Biol. 2010;98:337–57. doi: 10.1016/S0091-679X(10)98014-X. [DOI] [PubMed] [Google Scholar]
- 34.Lehner CF, Stick R, Eppenberger HM, Nigg EA. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol. 1987;105:577–87. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Röber RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–78. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8:31–45. doi: 10.1016/j.stem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BS, Yang SH, et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci U S A. 2012;109:E423–31. doi: 10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nissan X, Blondel S, Navarro C, Maury Y, Denis C, Girard M, et al. Unique preservation of neural cells in Hutchinson- Gilford progeria syndrome is due to the expression of the neural-specific miR-9 microRNA. Cell Rep. 2012;2:1–9. doi: 10.1016/j.celrep.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Verstraeten VL, Peckham LA, Olive M, Capell BC, Collins FS, Nabel EG, et al. Protein farnesylation inhibitors cause donut-shaped cell nuclei attributable to a centrosome separation defect. Proc Natl Acad Sci U S A. 2011;108:4997–5002. doi: 10.1073/pnas.1019532108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M, et al. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol. 2007;176:163–72. doi: 10.1083/jcb.200606139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muchir A, van Engelen BG, Lammens M, Mislow JM, McNally E, Schwartz K, et al. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp Cell Res. 2003;291:352–62. doi: 10.1016/j.yexcr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 43.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–13. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 44.Lin ST, Fu YH. miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech. 2009;2:178–88. doi: 10.1242/dmm.001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heng MY, Lin ST, Verret L, Huang Y, Kamiya S, Padiath QS, et al. Lamin B1 mediates cell-autonomous neuropathology in a leukodystrophy mouse model. J Clin Invest. 2013;123:2719–29. doi: 10.1172/JCI66737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Dilley C, Mattson MP. DNA damage responses in neural cells: Focus on the telomere. Neuroscience. 2007;145:1439–48. doi: 10.1016/j.neuroscience.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Jo YS, Sung YH, Hwang IK, Kim H, Kim SY, et al. Telomerase deficiency affects normal brain functions in mice. Neurochem Res. 2010;35:211–8. doi: 10.1007/s11064-009-0044-3. [DOI] [PubMed] [Google Scholar]
- 48.Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, et al. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell. 2012;11:996–1004. doi: 10.1111/j.1474-9726.2012.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci. 2010;123:2605–12. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stehbens WE, Wakefield SJ, Gilbert-Barness E, Olson RE, Ackerman J. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol. 1999;8:29–39. doi: 10.1016/S1054-8807(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 52.Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–9. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]