Abstract
Objective
To evaluate the safety and efficacy of ketoprofen in Transfersome® gel (IDEA-033) in comparison with a ketoprofen-free vehicle (TDT 064) for the treatment of osteoarthritis (OA) of the knee.
Methods
Patients with knee OA (N = 866) were randomly assigned to receive topical IDEA-033 containing 100, 50, or 25 mg ketoprofen, or TDT 064 twice daily for 12 weeks, in a double-blind trial. The primary efficacy endpoint was the change in the Western Ontario and McMaster Universities (WOMAC®) Osteoarthritis Index pain subscale score. The coprimary efficacy endpoints were the WOMAC function subscale score and the patient global assessment of response to therapy. The secondary endpoints included the numeric pain rating for the first 14 days of treatment and the Outcome Measures in Rheumatology (OMERACT)-Osteoarthritis Research Society International (OARSI) responder rates.
Results
The WOMAC pain scores were reduced by approximately 50% or more in all four groups. The 100 and 50 mg ketoprofen groups, but not the 25 mg group, showed a superior reduction in the WOMAC pain score versus the TDT 064 group (100 mg: −57.4% [P = 0.0383]; 50 mg: −57.1% [P = 0.0204]; and 25 mg: −53.4% [P = 0.3616] versus TDT 064: −49.5%). The superiority of the ketoprofen-containing formulations was not demonstrated for the WOMAC function subscale score, whereas the patient global assessment of 50 mg ketoprofen group, but not the 100 or 25 mg group, was superior to that of the TDT 064 group (P = 0.0283). Responder rates were significantly higher for all the IDEA-033 groups versus the TDT 064 group, but were high in all groups (100 mg: 88.6%; 50 mg: 86.8%; 25 mg: 88.6%; and TDT 064: 77.5%). Dermal reactions were the only relevant drug-related adverse events in all four groups.
Conclusion
The 50 and 100 mg ketoprofen doses of IDEA-033 were only marginally superior to TDT 064 for reducing pain associated with knee OA. The study indicates a high treatment response to the topical ketoprofen-free vehicle TDT 064.
Keyword: Sequessome™, pain, non-steroidal anti-inflammatory drugs, drug-free, medical device
Introduction
Osteoarthritis (OA) is a major public health problem1,2 and one of the leading causes of disability in developed countries, particularly among the elderly.3 The American College of Rheumatology (ACR) 2012 guidelines4 recommend acetaminophen and oral nonsteroidal anti-inflammatory drugs (NSAIDs) as initial therapy for the systemic treatment of symptomatic OA. Oral NSAIDs have become a mainstay of treatment for symptomatic OA.5 However, the use of oral NSAIDs is associated with serious adverse events (AEs),6 with the typical OA population (where age and comorbidities increase the risks of long-term oral NSAID use) being particularly vulnerable.7 Nonselective cyclooxygenase (COX) inhibitors have the potential to cause gastrointestinal side effects, such as bleeding, in a dose-related manner.8 Gastrointestinal bleeds increase in frequency in patients aged >65 years and may be fatal.8 COX-II inhibitors are also associated with gastrointestinal side effects, albeit at a lower rate than nonspecific NSAIDs,9 and may increase the risk of cardiovascular events, such as myocardial infarction.10 Consequently, the use of NSAIDs is contraindicated or cautioned in a substantial number of patients due to comorbidities or concomitant medications.4 The guidelines state that even in those patients for whom oral NSAID treatment is considered appropriate, careful monitoring and use of the additional therapies to reduce the risk of complications is required.11 This presents a challenge, given the reliance on oral NSAIDs to manage the chronic pain associated with OA and also the availability of oral NSAIDs without prescription.
Topical formulations may present a treatment option, in particular for long-term use. However, the rate and depth of absorption of NSAIDs from topical formulations is variable,12 and there is some systemic absorption, albeit at a lower level compared with an equivalent oral dose.12 Systemic AEs are less common with topical formulations12 but cannot be excluded after long-term use of high doses of topical NSAIDs. Unfortunately, very few controlled studies have been performed to evaluate the long-term efficacy and safety of topical NSAIDs.
A topical formulation of ultradeformable phospholipid vesicles containing ketoprofen, a well established NSAID,13 (IDEA-033; IDEA AG, Munich, Germany) has been investigated previously in OA.17 Ultradeformable phospholipid vesicles (called Transfersome vesicles [trademark IDEA AG] when loaded with a pharmaceutical substance, and Sequessome vesicles [trademark Pro Bono Bio Entrepreneur Ltd, London, UK] without drug) are applied epicutaneously in an aqueous suspension. Once the vesicles are on the skin, the water starts to evaporate, and their movement through the intercellular spaces in the skin into subdermal tissue is driven by the transcutaneous water gradient.14,15 The vesicles do not enter the cutaneous microcirculation because of their size.16
A previous study17 demonstrated that IDEA-033 (110 mg dose of ketoprofen per knee twice daily [bid]) provided a symptomatic improvement compared with the oral celecoxib (200 mg daily) control group, within the 6-week treatment period.
The current study was conducted to confirm and extend the results of the earlier 6-week study. The highest dose of ketoprofen in the study reported in this paper (ie, 100 mg bid per knee) closely matches the dose that was demonstrated to be effective and well tolerated over a period of 6 weeks.17 The objective of this study was to evaluate three different doses of ketoprofen in Transfersome gel, to determine the dose(s) that provides clinically meaningful effects for treating the signs and symptoms associated with OA, as well as an acceptable safety profile.
Patients and methods
Study design and patients
This randomized, double-blind, placebo-controlled, parallel-group study (clinical trial registration number: NCT00316784) of patients with knee OA was conducted at 17 centers in Germany, five centers in Poland, five centers in Serbia, and four centers in Croatia. The study was approved by 18 ethics committees associated to the 31 centers, and the procedures followed were in accordance with the ethical standards of the responsible institutional and national committees and with the Helsinki Declaration of 1975, as revised in 2000.
All patients provided written informed consent before the start of the study. Eligible patients were aged 18–75 years and had a clinical diagnosis of OA in at least one knee for a minimum of 6 months, meeting at least two of the following inclusion criteria: morning stiffness lasting <30 minutes, crepitus on motion, or age ≥40 years. Patients had to meet the ACR clinical criteria for OA18 and have an ACR functional class rating of I, II, or III.19 The radiographic criteria for the index knee (defined as the knee with the dominant pain, where both knees were symptomatic) were a Kellgren–Lawrence score of grade 2 or 3,20 and radiographs had to have been taken within 6 months before baseline. In addition, patients had to be able to walk at least 100 feet without an assistive device. The patients had to have used a daily dose of an oral or rectal NSAID on at least 3 days per week during the 3 months before screening or on at least 25 of the 30 days before screening and had to be dissatisfied with their current NSAID treatment.
The exclusion criteria included: the presence of any causes of secondary OA;21 diseases of the spine or lower extremity joints potentially affecting the assessment of the index knee; severe coexisting diseases, such as peptic ulcers; severe renal, cardiovascular, or neurologic diseases; use of intra-articular medications or arthroscopy within the preceding 3 months; and skin lesions or dermatologic diseases in the treatment area.
Patients were required to stop treatment with their current NSAID and to return for a baseline visit at the end of a washout period. The length of the washout period was determined by the half-life of the patient’s NSAID and lasted for five half-lives plus 2 additional days.
At the baseline (randomization) visit, the OA flare criteria for the index knee were evaluated: patients with a Western Ontario and McMaster Universities (WOMAC®) Osteoarthritis Index 22 (version 3.1) visual analog scale (VAS) pain subscale score of >40 mm and an increase of ≥15 mm as compared with the value at the screening visit before stopping NSAID treatment were eligible for randomization. Patients were stratified in equal numbers into group 1 consisting of patients with only one symptomatic knee and group 2 consisting of patients with both knees symptomatic. If both knees were symptomatic (group 2) at the baseline visit, then the patients used the index knee to assess their symptoms, using the WOMAC scale, at each subsequent visit.22
After randomization to the treatment groups, the patients returned to the study center for visits at the end of weeks 2, 6, and 12.
Interventions
The patients in groups 1 and 2 were randomly assigned to receive either 25, 50, or 100 mg ketoprofen in Transfersome gel (IDEA-033; IDEA AG, Munich, Germany) or a matching amount of a ketoprofen-free vehicle gel (TDT 064; Pro Bono Bio Entrepreneur Ltd) that was identical to IDEA-033 in terms of appearance and constituents (with the exception of ketoprofen). Both products were applied twice daily for 12 weeks. The dosages of 25, 50, and 100 mg ketoprofen were selected because 25 mg was the lowest dose that covered the treatment area completely and 100 mg was the maximum dose that could be applied with a reasonable drying time.
The dosing was controlled by a dispensing device. Each dose was equivalent to one stroke of the respective gel dispenser. In cases where both knees were affected, one stroke had to be applied to each knee. The gel was spread gently and homogeneously over the knee(s) and around the knee, including the popliteal fossa, but sparing the patella. The upper boundary extended around the leg, from approximately 5 cm above the superior edge of the patella. The lower boundary extended around the leg from the inferior edge of the tibial tuberosity (approximately 5 cm below the inferior edge of the patella). In order to prevent any confounding effects from excessive massage, the patients were instructed that rubbing, kneading, and massaging had to be avoided. The gel had to dry for ≥15 minutes before putting on clothes.
Acetaminophen, up to a maximum daily dose of 2 g/day for up to 5 days during any 7-day period, was permitted for breakthrough pain or non-OA pain. Rescue medication use was documented and was not allowed within 48 hours before the study visits.
Assessments
The VAS version of the WOMAC Osteoarthritis Index (version 3.1), which includes subscales for pain, stiffness, and physical function, was used. At screening, the pain subscale served to define the eligibility for the study. At visit 2 (baseline visit), visit 3 (week 2), visit 4 (week 6), and visit 5 (end-of-study visit), all three of the subscales were rated by the patients, taking into account OA symptoms in the previous 24 hours. All ratings were performed before applying the study drug.
Every evening from day 1 to day 14, ie, the evening before visit 3, patients completed the numerical rating scale (NRS) version of the WOMAC pain subscale (version 3.1) in their diary.
At visits 3, 4, and 5, the patients provided a “patient global assessment” (PGA) of their response to therapy by answering the question “Considering the overall effects of the study drug on your OA symptoms, how would you rate your response to therapy today?” using the following five-point Likert scale: 0 = none (no good at all, ineffective); 1 = poor (some effect, but unsatisfactory); 2 = fair (reasonable effect, but could be better); 3 = good (satisfactory effect, with occasional episodes of pain and/or stiffness); and 4 = excellent (ideal response, virtually free of pain).
Safety assessments included a physical examination and routine laboratory tests, at screening and the end-of-study visit, as well as the following procedures at all visits: vital signs (sitting blood pressure and pulse rate, after a 15-minute rest), body weight, body temperature, and AE recording. The relationship of AEs to study drug treatment was assessed by the investigators, under blinded conditions. Serious AEs were defined as those requiring hospitalization.
Efficacy endpoints
The primary efficacy endpoint was the change from baseline to week 12 (or end of study) in the WOMAC VAS version pain subscale score (arithmetic mean of all individual scores of the subscale). The change from baseline to week 12 (or the end of study) in the WOMAC VAS version function subscale score (arithmetic mean of all individual scores of the subscale) and the PGA of the response to therapy were coprimary endpoints at week 12 (or end of study). Additional endpoints included the evaluation of the effects on pain during the first 14 days of treatment, using the WOMAC NRS version of the pain subscale and the evaluation of responder rates, according to the modified Outcome Measures in Rheumatology (OMERACT)-Osteoarthritis Research Society International (OARSI) criteria23 (≥50% improvement in pain or function together with an absolute improvement of 50 mm on the VAS, or two or more of the following: ≥20% improvement in pain together with an absolute improvement of ≥10 mm; ≥20% improvement in function together with an absolute change of 10 mm; and response of “good” or “excellent” on the PGA).
Statistical analysis
The sample size calculation was based on the Wilcoxon–Mann–Whitney procedure. The stipulations were: relevant superiority, ie, probability that a randomly selected patient of the test group would respond better than a randomly selected patient of the reference group, with a Mann–Whitney estimator of 0.60,24 one-sided probability of a type I error α = 0.025, and probability of a type II error β = 0.1. This resulted in a sample size of N = 180 per group. The planned number of patients per center was 12–48.
The statistical analyses were performed using SAS® software Version 9.1 (SAS Institute Inc, Cary, North Carolina, USA).
Mann–Whitney statistics and corresponding 90% confidence intervals (CIs) were calculated for the demographic and baseline characteristics.
The analysis of safety included all randomized patients who received at least one dose of study medication and had at least one follow-up visit. The randomized patients who received at least one dose of study medication were included in the intent-to-treat (ITT) population used for the evaluation of efficacy. The confirmatory analysis of the primary efficacy endpoint was based on the ITT population, using the last observation carried forward method.
Minimizing the required assumptions is a recommended approach for confirmatory efficacy analyses.25 Thus, a nonparametric assessment of the treatment effects across centers, regardless of the center-by-treatment interaction, was chosen as the primary analysis method, corresponding to the extended Mantel–Haenszel procedure, which is a stratified analysis and includes the number of patients per center as a weighting factor. Instead of integer scores, Wilcoxon scores were used because the values might have had skewed distributions and included some atypical outlier values (so-called Cochran–Mantel–Haenszel pooling).
Each ketoprofen dose group was tested versus the TDT 064 group, with factor treatment and stratification of the centers. The pooled P-value and the associated 95% CI provided the basis for the test decisions.
It was shown in the blind data review, before opening the treatment code, that decreases in pain were not shifted but were proportional. Therefore as the primary analysis, percent changes were evaluated instead of change from baseline values. The same applied to the other continuous variables. Absolute changes were computed in addition to percent changes, for exploratory purposes, if applicable.
The primary objective was evaluated for the primary efficacy variable, WOMAC pain score, using the ITT population. Confirmatory tests were performed within the framework of hierarchical stepwise testing, using a fixed sequence of tests (descending ketoprofen doses versus TDT 064) because all comparisons can be made with full α, starting with the highest dose. The coprimary and secondary objectives were evaluated in an exploratory/supportive manner without α adjustment due to multiple testing and was carried out independently by two different statisticians.
The AEs were categorized by the primary system organ class (SOC) and preferred term, as coded using the Medical Dictionary for Regulatory Activities (MedDRA).26 The AEs were tabulated by intensity/severity and relationship to the study drug. If, within an SOC, the number of patients who experienced a certain AE differed by more than 5% between treatment groups (absolute difference), the difference was further analyzed using Fisher’s exact test, with 95% CIs.
Results
Patients
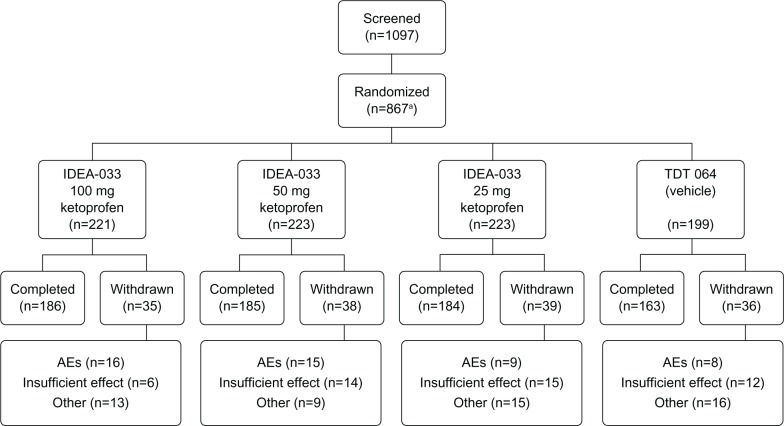
Overall, 1097 patients were screened, and 866 patients were randomized and received at least one dose of study medication between July 11, 2005 and February 6, 2006 (Figure 1). During the study, 148 (17%) patients discontinued treatment prematurely. The main reasons for early termination were withdrawal due to AEs (48 patients) and insufficient therapeutic effect (47 patients). In the IDEA-033 100 and 50 mg ketoprofen groups, more patients discontinued due to AEs than in the groups treated with 25 mg ketoprofen in IDEA-033 or TDT 064 (16 and 15 patients versus nine and eight patients, respectively). Discontinuation due to insufficient therapeutic effect was more frequent in the IDEA-033 50 and 25 mg ketoprofen groups and in the TDT 064 group than in the IDEA-033 100 mg ketoprofen group (14, 15, and 12 patients versus six patients, respectively).
Figure 1.
Flow of patients enrolled in the study.
Notes: aOne patient withdrew consent prior to treatment. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviation: AEs, adverse events.
The baseline characteristics for the ITT population reflect a typical OA population with respect to age and sex distribution (Table 1). The mean WOMAC VAS version pain subscale scores were 40.3–41.4 mm (standard deviation [SD]: 14.0–15.8) at screening and were 64.1–65.7 mm (SD: 13.2–14.4) after the NSAID washout, at visit 2 (baseline). The numbers of patients with one knee affected represent the 1:1 stratification. There were no statistically significant differences in the baseline characteristics of the four treatment groups.
Table 1.
Baseline characteristics of the patients (ITT population)
| Characteristic | IDEA-033
|
TDT 064
|
||
|---|---|---|---|---|
| 100 mg ketoprofen | 50 mg ketoprofen | 25 mg ketoprofen | ||
| (n = 211) | (n = 213) | (n = 214) | (n = 190) | |
| Women, n (%) | 147 (69.7) | 150 (70.4) | 152 (71.0) | 144 (75.8) |
| Age, years | ||||
| Mean ± SD | 61.8 ± 9.2 | 61.9 ± 9.7 | 61.6 ± 9.0 | 61.3 ± 9.3 |
| Median (range) | 64 (20–78) | 64 (19–75) | 63 (29–75) | 63 (34–77) |
| Weight, kg | ||||
| Mean ± SD | 84.2 ± 17.5 | 83.3 ± 16.4 | 82.9 ± 15.1 | 84.3 ± 15.2 |
| Median (range) | 83 (49–172) | 82 (49–135) | 80 (52–133) | 84 (48–129) |
| One knee affected, n (%) | 100 (47.4) | 99 (46.5) | 101 (47.2) | 87 (45.8) |
| Index knee right, n (%) | 116 (55.0) | 110 (51.6) | 116 (54.2) | 109 (57.4) |
| WOMAC score (VAS), mm | ||||
| Visit 1/screening | ||||
| Pain, mean ± SD | 40.67 ± 15.3 | 41.41 ± 15.2 | 40.30 ± 15.8 | 40.66 ± 14.0 |
| Visit 2/baseline | ||||
| Pain, mean ± SD | 65.7 ± 13.4 | 65.4 ± 14.4 | 64.1 ± 13.9 | 65.6 ± 13.2 |
| Function, mean ± SD | 52.9 ± 17.4 | 53.1 ± 18.3 | 51.2 ± 18.5 | 52.5 ± 16.8 |
| Stiffness, mean ± SD | 50.1 ± 22.1 | 54.6 ± 20.9 | 49.6 ± 21.5 | 50.9 ± 21.2 |
Notes: All but one patient (Asian) were Caucasian. There were no statistically significant differences between the study groups. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: ITT, intent-to-treat; mm, millimeter (on VAS); SD, standard deviation; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Efficacy
The WOMAC pain subscale scores were reduced, from baseline to the end-of-study visit, by 57.4% ± 29.3% for the IDEA-033 100 mg ketoprofen group (P = 0.0383), by 57.1% ± 31.7% for the IDEA-033 50 mg ketoprofen group (P = 0.0204), and by 53.4% ± 31.1% for the IDEA-033 25 mg group (P = 0.3616). This compared with a reduction in the WOMAC pain score for the TDT 064, of 49.5% ± 34.1% (Table 2). A statistically significant superiority of the IDEA-033 groups versus TDT 064 was not detected for the change in the WOMAC function subscale score (Table 2) but showed an average improvement of about 40% across all treatment groups, with numerically slightly better improvement for the IDEA-033 100 and 50 mg ketoprofen groups as compared with the 25 mg ketoprofen and TDT 064 groups. With respect to the PGA scores, only the IDEA-033 50 mg ketoprofen treatment group was statistically significantly superior to the TDT 064 group (P = 0.0283) (Table 2).
Table 2.
WOMAC (VAS version) pain and function subscale scores and patient global assessment of response to therapy
| Treatment | Baseline visit 2 | Week 12 (end-of-study visit) | Percent change from baseline | P-value |
|---|---|---|---|---|
| WOMAC pain subscale score | ||||
| IDEA-033 ketoprofen dosage | ||||
| 100 mg | 65.67 ± 13.38 | 28.39 ± 21.00 | −57.35 ± 29.30 | 0.0383 |
| 50 mg | 65.35 ± 14.35 | 27.92 ± 21.28 | −57.09 ± 31.70 | 0.0204 |
| 25 mg | 64.10 ± 13.90 | 29.88 ± 21.16 | −53.40 ± 31.10 | NS |
| TDT 064 | ||||
| – | 65.6 ± 13.2 | 32.57 ± 32.33 | −49.53 ± 34.05 | – |
| WOMAC function subscale score | ||||
| IDEA-033 ketoprofen dosage | ||||
| 100 mg | 52.85 ± 17.38 | 30.56 ± 21.44 | −42.01 ± 35.69 | NS |
| 50 mg | 53.05 ± 18.25 | 29.07 ± 21.20 | −44.70 ± 39.31 | NS |
| 25 mg | 51.21 ± 18.49 | 32.12 ± 19.62 | −37.08 ± 35.20 | NS |
| TDT 064 | ||||
| – | 52.54 ± 16.79 | 33.16 ± 21.75 | –36.10 ± 39.02 | – |
|
| ||||
| Patient global assessment of response to therapy (5-point Likert scale) | ||||
| Visit 3 (week 2) | Visit 5 (week 12) end-of-study visit | |||
|
| ||||
| IDEA-033 ketoprofen dosage | ||||
| 100 mg | 1.84 ± 1.10 | 2.23 ± 1.12 | NS | |
| 50 mg | 1.79 ± 1.10 | 2.36 ± 1.13 | 0.0283 | |
| 25 mg | 1.76 ± 1.07 | 2.23 ± 1.10 | NS | |
| TDT 064 | ||||
| – | 1.61 ± 1.03 | 2.11 ± 1.21 | – | |
Notes: Values are expressed as mean ± SD. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: ITT, intent-to-treat; NS, not statistically significantly different versus placebo; SD, standard deviation; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
The OMERACT–OARSI responder rate analysis indicated high response rates across all four treatment groups, with response rates between 88.6% for the IDEA-033 100 and 25 mg ketoprofen groups and 77.5% for the TDT 064 group (Figure 2). However, the IDEA-033 groups showed significantly higher response rates than did the TDT 064 group (Figure 2).
Figure 2.
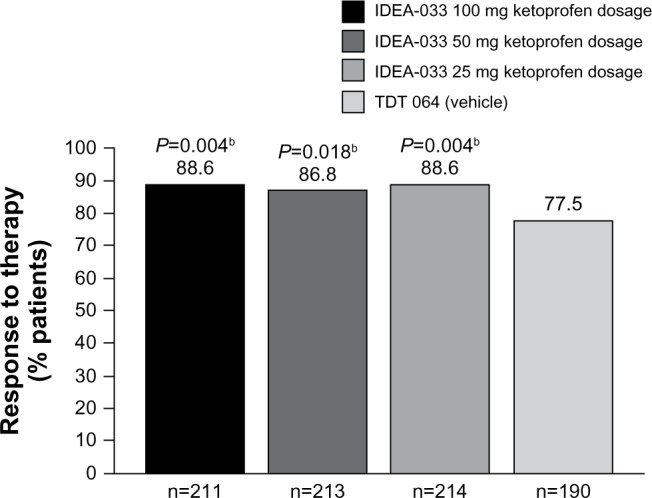
Responder rate,a according to the modified OMERACT-OARSI-criteria (ITT population).
Notes: aAnalyzed by visit, using a multiple regression model, with treatment group and baseline OA pain as the explanatory variables; bstatistically significant difference compared with the TDT 064 vehicle. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: ITT, intent-to-treat; OA, osteoarthritis; OMERACT, Outcome Measures in Rheumatology; OARSI, Osteoarthritis Research Society International.
The onset of a treatment effect, evaluated by the NRS version of the WOMAC pain scale, is shown in Table 3. The instrument was applied by diary for the first 14 days of the trial, always evaluating the effect for the previous 24 hours. Statistically significant effects in favor of the IDEA-033 100 mg and 50 mg ketoprofen groups were found, starting from the first time of evaluation (Day 2 - ie, within 1 day after the start of treatment) and maintained over the observation period of 14 days (except for day 3, for the 50 mg dose) (P = 0.0915).
Table 3.
Daily change from baseline in WOMAC NRS pain scale values (ITT population)
| Treatment
|
IDEA-033
|
TDT 064
|
|||||
|---|---|---|---|---|---|---|---|
| Day | 100 mg ketoprofen
|
50 mg ketoprofen
|
25 mg ketoprofen
|
||||
| % change | P-valuea | % change | P-valuea | % change | P-valuea | % change | |
| 2 | −1.76 | 0.0033 | −1.96 | 0.0023 | 0.6 | 0.0364 | 4.7 |
| 3 | −3.14 | 0.0269 | −1.74 | 0.0915 | 1.67 | 0.4478 | 2.89 |
| 4 | −5.63 | 0.0066 | −4.84 | 0.014 | −0.42 | 0.2097 | 2.44 |
| 5 | −8.3 | 0.0123 | −7.74 | 0.0167 | −0.91 | 0.5439 | 0.16 |
| 6 | −13.33 | 0.0005 | −8.44 | 0.0394 | −3.85 | 0.3155 | −1.08 |
| 7 | −14.3 | 0.0009 | −11.19 | 0.014 | −3.5 | 0.5758 | −2.22 |
| 8 | −12.98 | 0.0077 | −14.16 | 0.0023 | −4.06 | 0.5715 | −2.77 |
| 9 | −13.37 | 0.0267 | −14.82 | 0.007 | −4.22 | 0.9183 | −4.58 |
| 10 | −14.92 | 0.0123 | −15.22 | 0.0074 | −2.39 | 0.6819 | −4.65 |
| 11 | −16.77 | 0.0054 | −16.59 | 0.0045 | −4.98 | 0.9131 | −5.16 |
| 12 | −16.91 | 0.0014 | −17.81 | 0.0005 | −8.57 | 0.1365 | −3.27 |
| 13 | −19.93 | 0.0019 | −16.57 | 0.0216 | −10.21 | 0.3638 | −7.57 |
| 14 | −21.1 | 0.0021 | −20.95 | 0.0022 | −9.07 | 0.7212 | −8.21 |
Notes:
P-value versus TDT 064. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: ITT, intent-to-treat; NRS, numerical rating scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Safety
The numbers of patients with treatment-emergent AEs (occurring in ≥1% patients) are shown in Table 4.
Table 4.
Treatment-emergent AEs (≥1% of patients; based on safety population)
| MedDRA SOC | IDEA-033
|
TDT 064 (vehicle)
|
||
|---|---|---|---|---|
| Ketoprofen dosage
|
||||
| 100 mg | 50 mg | 25 mg | ||
| (n = 221) | (n = 223) | (n = 223) | (n = 199) | |
| Any | 112 (50.7) | 115 (51.6) | 105 (47.1) | 93 (46.7) |
| Skin and subcutaneous tissue disorders | 53 (24.0) | 43 (19.3) | 31 (13.9) | 39 (19.6) |
| Erythema | 19 (9.6) | 18 (8.1) | 9 (4.0) | 9 (4.5) |
| Skin irritation | 8 (3.6) | 11 (4.9) | 8 (3.6) | 9 (4.5) |
| Dry skin | 7 (3.2) | 5 (2.2) | 2 (0.9) | 9 (4.5) |
| Eczema | 5 (2.3) | 3 (1.3) | 5 (2.2) | 1 (0.5) |
| Contact dermatitis | 3 (1.4) | 3 (1.3) | 1 (0.4) | 4 (2.0) |
| Pruritus | 5 (2.3) | 2 (0.9) | 1 (0.4) | 2 (1.0) |
| Rash | 2 (0.9) | 4 (1.8) | 0 (0) | 3 (1.5) |
| Dermatosis | 3 (1.4) | 2 (0.9) | 0 (0) | 2 (1.0) |
| Allergic dermatitis | 3 (1.4) | 0 (0) | 1 (0.4) | 0 (0) |
| Exanthema | 0 (0) | 0 (0) | 3 (1.3) | 0 (0) |
| Musculoskeletal and connective tissue disorders | 26 (11.8) | 36 (16.1) | 25 (11.2) | 24 (12.1) |
| Respiratory, thoracic, and mediastinal disorders | 26 (11.8) | 24 (10.8) | 25 (11.2) | 25 (12.6) |
| Nervous system disorders | 19 (8.6) | 17 (7.6) | 14 (6.3) | 23 (11.6) |
| Gastrointestinal disorders | 10 (4.5) | 6 (2.7) | 6 (2.7) | 9 (4.5) |
| General disorders and administration site conditions | 5 (2.3) | 5 (2.2) | 11 (4.9) | 5 (2.5) |
| Vascular disorders | 5 (2.3) | 7 (3.1) | 5 (2.2) | 4 (2.0) |
| Investigations | 10 (4.5) | 2 (0.9) | 4 (1.8) | 2 (1.0) |
| Infections and infestations | 4 (1.8) | 4 (1.8) | 1 (0.4) | 6 (3.0) |
| Renal and urinary disorders | 4 (1.8) | 4 (1.8) | 5 (2.2) | 1 (0.5) |
| Blood and lymphatic system disorders | 4 (1.8) | 3 (1.3) | 2 (0.9) | 0 (0) |
| Surgical and medical procedures | 1 (0.5) | 2 (0.9) | 4 (1.8) | 2 (1.0) |
| Cardiac disorders | 3 (1.4) | 2 (0.9) | 3 (1.3) | 1 (0.5) |
Notes: Values are expressed as number (%) of patients. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: AEs, adverse events; MedDRA, Medical Dictionary for Regulatory Activities; SOC, system organ class.
Skin and subcutaneous tissue disorders were the most frequent drug-related AEs in all four treatment groups (Table 5), affecting 21.7%, 17.9%, 12.6%, and 17.6% of patients in the IDEA-033 100, 50, 25 mg ketoprofen, and TDT 064 groups, respectively. The statistical analysis did not show any significant differences between the treatments and TDT 064 in terms of the frequency of treatment-related skin and subcutaneous disorders. Erythema was the most frequent individual drug-related dermal AE, affecting 8.6%, 8.1%, 3.6%, and 4.5% of the patients in the IDEA-033 100, 50, 25 mg ketoprofen, and TDT 064 groups, respectively. Other drug-related dermal AEs, which occurred in 1%–5% of the patients in any of the treatment groups, were skin irritation, dry skin, eczema, contact dermatitis, pruritus, and rash. The majority of dermal reactions were of mild or moderate intensity and resolved without action. Those cases requiring intervention were resolved with the use of ointment, cool packs, or cream. Skin type, according to the Fitzpatrick classification,27 did not significantly influence the occurrence of drug-related dermal AEs.
Table 5.
Treatment-related AEs (based on safety population)
| MedDRA SOC | IDEA-033
|
TDT 064 (vehicle)
|
||
|---|---|---|---|---|
| Ketoprofen dosage
|
||||
| 100 mg | 50 mg | 25 mg | ||
| (n = 221) | (n = 223) | (n = 223) | (n = 199) | |
| Any | 50 (22.6)a | 43 (19.3)a | 31 (13.9)a | 35 (17.6) |
| Skin and subcutaneous tissue disorders | 48 (21.7)a | 40 (17.9)a | 28 (12.6)a | 35 (17.6)a |
| Nervous system disorders | 1 (0.5) | 0 | 0 | 0 |
| General disorders and administration site conditions | 0 | 1 (0.4) | 1 (0.4) | 0 |
| Investigations | 1 (0.5) | 1 (0.4) | 2 (0.9) | 0 |
| Infections and infestations | 1 (0.5) | 0 | 0 | 0 |
| Blood and lymphatic disorders | 2 (0.9) | 3 (1.3) | 0 | 0 |
| Immune system disorders | 0 | 1 (0.4) | 3 (1.3) | 0 |
Notes: Values are expressed as number (%) of patients.
Difference versus TDT 064 not statistically significant by Fisher’s exact test. IDEA-033 (IDEA AG, Munich, Germany); TDT 064 (Pro Bono Bio Entrepreneur Ltd, London, UK).
Abbreviations: AEs, adverse events; MedDRA, Medical Dictionary for Regulatory Activities; SOC, system organ class.
No clinically relevant changes in routine laboratory tests were detected in any of the treatment groups, with the exception of isolated cases of eosinophilia for all groups. A subanalysis of groups of special interest (ie, patients with cardiovascular risk factors and patients with use of low-dose aspirin) did not indicate significant changes in the safety laboratory parameters for any of the subgroups.
Discussion
In this randomized, double-blind, placebo-controlled study, all treatment groups, including the drug-free vehicle gel TDT 064 treatment group, showed improved WOMAC pain subscale scores, by approximately 50% or more, in patients with knee OA over a 12-week treatment period. Both IDEA-033 100 and 50 mg ketoprofen were superior to TDT 064 in relieving pain. However, the differences were small and might not be clinically relevant. The same applies to the statistically significant therapeutic effects on pain observed during the daily evaluations and first seen after 24 hours of treatment with IDEA-033 100 and 50 mg ketoprofen. Based on the results of the PGA, only IDEA-033 50 mg ketoprofen demonstrated statistically significant superiority to TDT 064 gel. None of the IDEA-033 groups were statistically significantly superior to TDT 064 with respect to the WOMAC function subscale score, and all treatment groups improved function by approximately 40% compared with baseline.
Simon et al28 reported a significant improvement in WOMAC pain score, using the five-point Likert scale version, after 12 weeks of therapy with topical diclofenac in dimethyl sulfoxide (DMSO) compared with a topical placebo and DMSO vehicle. Moreover, the effects were comparable with oral diclofenac. In a further 12-week study by Tugwell et al,29 topical diclofenac solution demonstrated equivalence to oral diclofenac. However, very few placebo-controlled, 12-week studies investigating the effect of topical NSAIDs have been published, and the variability between patients in the rates of absorption through the skin and in clinical effects complicates the interpretation of the available data.12
Another approach used in OA studies for evaluating the change from baseline within a treatment group and for describing the overall benefit to a patient is the determination of the “minimal clinical important improvement” (MCII) in OA.30 Absolute changes of −19.9 mm and relative changes of −40.8% are considered to be the MCII for pain related to knee OA.30 In this study, we observed average improvements of −34.2 mm (−53.4%) to −37.3 mm (−57.4%) for the IDEA-033 groups and −33.0 mm (−49.5%) for the TDT 064 group, indicating that the criteria of MCII were more than fulfilled for all treatment arms.
For all three primary and coprimary efficacy endpoints in this study and in the OMERACT–OARSI responder rate analysis, major responses were observed with the ketoprofen-free vehicle gel, TDT 064. This effect (77.5% responders) was substantial. Nonetheless, all three IDEA-033 groups were significantly superior to TDT 064 in the OMERACT–OARSI responder rate analysis (86.8%–88.6% responders). The possible reasons for such a pronounced vehicle response might include the intensive use of rescue medication or other (prohibited) concomitant analgesic medications within this group or that the vehicle itself exerts a treatment effect. Moreover, a high number of patients in the IDEA-033 groups withdrew from the study at an early stage, ie, before the full effect size of IDEA-033 had developed, and this may also have contributed to the lower than expected effect size in the IDEA-033 treatment groups.
A placebo effect has been observed in many OA trials and was reviewed recently by Doherty and Dieppe.31 This highlights various factors that can contribute to an improvement, independent of a direct pharmacologic effect, eg, the physical attributes of the test product, the method of delivery, the response expectancy and concealment, a provider effect, behavioral conditioning, and others. However, the response to the ketoprofen-free vehicle (TDT 064) seen in this study was even larger than that reported in a meta-analysis of knee OA studies.32 Zhang et al32 reported a mean effect size of 0.54 (95% CI: 0.49–0.60) for the placebo arms in all the knee OA studies analyzed and an effect size of 0.63 (95% CI: 0.47–0.80) for the placebo arms in all the topical NSAID studies analyzed. The corresponding effect size for TDT 064 in the study was 1.33 (95% CI: 1.18–1.47). This leaves unanswered the question of whether factors other than the usual placebo effects might have contributed to the large treatment response of TDT 064 observed in this study. Interestingly, two subsequent vehicle-controlled studies of IDEA-033, comparing 50 and 100 mg ketoprofen doses, respectively, with TDT 064 have also demonstrated a substantial treatment response to the vehicle over 12 weeks, as measured by the WOMAC pain, function, and joint stiffness subscales.33,34 In one of these studies, oral celecoxib was included as the active control.34 The pain reduction observed in the TDT 064 group was statistically noninferior to that of celecoxib and was also statistically significantly superior to that observed with an oral placebo.34
Overall, the epicutaneous administration of IDEA-033 in any of the three ketoprofen strengths (100, 50, or 25 mg) or of TDT 064 was well tolerated over the treatment period of 12 weeks. There were no significant differences between the groups in terms of the frequency of AEs. The skin and subcutaneous tissue disorders were the most common drug-related AEs, and all groups showed evidence of dermal irritation, which had a tendency to be dose related in the IDEA-033 groups. Dermal events were mainly mild or moderate and resolved without intervention in most cases. Photoallergic contact dermatitis, induced by ketoprofen and in particular, topical ketoprofen formulations, is a rare but well-established source of concern.35 However, no cases were observed in this study. No other types of treatment-related AEs occurred at a frequency of >1.5%. The subanalysis of patients with comorbidities relevant to NSAID treatment (eg, cardiovascular risk factors or concomitant use of low-dose aspirin) did not indicate an increase in treatment-related AEs among these patients. The range of safety laboratory values observed in this study remained within the range of values to be expected from the study population. The changes in these parameters were not dose related and included the ketoprofen-free vehicle gel group. In the majority of cases, they were not considered clinically relevant.
Whereas both the IDEA-033 100 and 50 mg ketoprofen doses were slightly more efficacious than the IDEA-033 25 mg ketoprofen dose and TDT-064, the IDEA-033 50 mg ketoprofen dose was slightly better tolerated than the 100 mg dose, particularly in terms of the occurrence of skin and subcutaneous tissue disorders. A subsequent controlled study in knee OA failed, however, to demonstrate a significant treatment effect for either the IDEA-033 100 or the 50 mg ketoprofen dose versus the ketoprofen-free vehicle TDT 064.33 Further investigation of TDT 064 in patients with knee OA will lead to a better understanding of its effect.
Supplementary table
Table S1.
Study investigators
| Investigator | Site location* |
|---|---|
| Croatia | |
| Dr Tatjana Kehler | Opatija |
| Dr Simeon Grazio (coordinator) | Zagreb |
| Dr Olga Badovinac | Zagreb |
| Dr Natalija Topolnjak | Varaždin |
| Germany | |
| Prof Gerold Stucki, MD, MS (coordinator) | München |
| Prof G. Stucki (Dr Borchers) | München |
| Dr med G. Lindner | Strausberg |
| Dr med Reiner Lehmann | Berlin |
| Dr med Klaus Lehnhardt | Bad Dürrheim |
| Dr med Werner Kneer | Stockach |
| Dr med Heike Dorow | Jena |
| Dr Roy Heller | Schwerin |
| Stefan Schäfer | Schwerin |
| Dr med Patrick Finkbeiner | Landau |
| Dr med Dieter Veith | Emmendingen |
| Dr med Annette Herzner | Hamburg |
| Dr med Matthias Groβann | Mannheim |
| Dr med Michael Schreinert | Berlin |
| Dr med Ralf Knels | Chemnitz |
| Dipl Med Janna Stöβel | Berlin |
| Dipl-Med DetlefWilcke | Berlin |
| Dr med Christina Mondorf | Frankfurt aM |
| Dr med Jutta Harten | Münster |
| Dr med Hans-Detlev Stahl | Leipzig |
| Poland | |
| Prof Leszek Szczepanski | Lublin |
| Dr Wieslawa Porawska | Poznan |
| Dr Maria Misterska-Skora | Wroclaw |
| Dr Jolanta Zarebska | Katowice |
| Prof Piotr Gluszko (coordinator) | Krakow |
| Serbia and Montenegro | |
| Prof Nemanja Damjanov (coordinator) | Belgrade |
| Prof Dr Gordana Devečerski | Novi Sad |
| Ass Prof Koviljka Čobeljić | Belgrade |
| Dr Miljanka Lazarević | Novi Sad |
| Prof Dr Stevan Jovic | Belgrade |
Note:
Site location of the investigator at the time of the study.
Acknowledgments
We are indebted to the patients and their physicians for participation in this study. The study investigators are listed in Table S1.
The sponsor, IDEA AG, provided study medication and financial support. Data analysis was provided by two independent statisticians representing two clinical research organizations (CRS Mannheim, idv Gauting, Germany). Bollin Strategies (funded by Pro Bono Bio Entrepreneur Ltd) provided editorial assistance with the preparation of the manuscript.
Footnotes
Author contributions
All study authors contributed to the trial design, interpretation of the data, and manuscript development. Werner Kneer and Egbert J Seidel served as principal investigators.
Disclosure
Werner Kneer and Egbert J Seidel received investigator grants from the sponsor, IDEA AG. Matthias Rother and Stefan Mazgareanu were employees of IDEA AG during the conduct of the study. Matthias Rother is a paid consultant of Pro Bono Bio Entrepreneur Ltd. The authors report no other conflicts of interest.
References
- 1.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350(9076):503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 2.Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clin Geriatr Med. 1998;14(3):435–454. [PubMed] [Google Scholar]
- 3.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Roddy E, Doherty M. Guidelines for management of osteoarthritis published by the American College of Rheumatology and the European League Against Rheumatism: why are they so different? Rheum Dis Clin North Am. 2003;29(4):717–731. doi: 10.1016/s0889-857x(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 6.Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int. 2012;32(6):1491–1502. doi: 10.1007/s00296-011-2263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth SH, Anderson S. The NSAID dilemma: managing osteoarthritis in high-risk patients. Phys Sportsmed. 2011;39(3):62–74. doi: 10.3810/psm.2011.09.1922. [DOI] [PubMed] [Google Scholar]
- 8.Tarone RE, Blot WJ, McLaughlin JK. Nonselective nonaspirin non-steroidal anti-inflammatory drugs and gastrointestinal bleeding: relative and absolute risk estimates from recent epidemiologic studies. Am J Ther. 2004;11(1):17–25. doi: 10.1097/00045391-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr Cartil. 2007;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Fosbøl EL, Gislason GH, Jacobsen S, et al. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85(2):190–197. doi: 10.1038/clpt.2008.204. [DOI] [PubMed] [Google Scholar]
- 11.Chan FK, Abraham NS, Scheiman JM, Laine L, First International Working Party on Gastrointestinal and Cardiovascular Effects of Nonsteroidal Anti-inflammatory Drugs and Anti-platelet Agents Management of patients on nonsteroidal anti-inflammatory drugs: a clinical practice recommendation from the First International Working Party on Gastrointestinal and Cardiovascular Effects of Nonsteroidal Anti-inflammatory Drugs and Anti-platelet Agents. Am J Gastroenterol. 2008;103(11):2908–2918. doi: 10.1111/j.1572-0241.2008.02200.x. [DOI] [PubMed] [Google Scholar]
- 12.Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Veys EM. 20 years’ experience with ketoprofen. Scand J Rheumatol Suppl. 1991;90(Suppl):S1–S44. [PubMed] [Google Scholar]
- 14.Cevc G, Schätzlein A, Richardsen H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim Biophys Acta. 2002;1564(1):21–30. doi: 10.1016/s0005-2736(02)00401-7. [DOI] [PubMed] [Google Scholar]
- 15.Cevc G, Gebauer D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophys J. 2003;84(2 Pt 1):1010–1024. doi: 10.1016/S0006-3495(03)74917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cevc G, Vierl U. Spatial distribution of cutaneous microvasculature and local drug clearance after drug application on the skin. J Control Release. 2007;118(1):18–26. doi: 10.1016/j.jconrel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Rother M, Lavins BJ, Kneer W, Lehnhardt K, Seidel EJ, Mazgareanu S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis. 2007;66(9):1178–1183. doi: 10.1136/ard.2006.065128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35(5):498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman R, Brandt K, Hochberg M, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthr Cartil. 1996;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 23.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr Cartil. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Miller JN, Mosteller F. Measuring gain in the evaluation of medical technology. The probability of a better outcome. Int J Technol Assess Health Care. 1988;4(4):637–642. doi: 10.1017/s0266462300007728. [DOI] [PubMed] [Google Scholar]
- 25.LaVange LM, Durham TA, Koch GG. Randomization-based nonparametric methods for the analysis of multicentre trials. Stat Methods Med Res. 2005;14(3):281–301. doi: 10.1191/0962280205sm397oa. [DOI] [PubMed] [Google Scholar]
- 26.Medical Dictionary for Regulatory Activities [homepage on the Inter-net] McLean: MedDRA MSSO; Available from: http://www.meddra.org/Accessed August 1, 2013 [Google Scholar]
- 27.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143(3):238–245. doi: 10.1016/j.pain.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol. 2004;31(10):2002–2012. [PubMed] [Google Scholar]
- 30.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doherty M, Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthr Cartil. 2009;17(10):1255–1262. doi: 10.1016/j.joca.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 33.Rother M, Conaghan PG. A randomized, double-blind, phase III trial in moderate osteoarthritis knee pain comparing topical ketoprofen in Trans-fersome gel with ketoprofen-free gel. J Rheumatol. 2013 doi: 10.3899/jrheum.130192. In press. [DOI] [PubMed] [Google Scholar]
- 34.Conaghan PG, Dickson J, Bolten W, Cevc G, Rother M. A multicentre, randomized, placebo- and active-controlled trial comparing the efficacy and safety of topical ketoprofen in Transfersome gel (IDEA-033) with keto-profen-free vehicle (TDT 064) and oral celecoxib for knee pain associated with osteoarthritis. Rheumatology (Oxford) 2013;52(7):1303–1312. doi: 10.1093/rheumatology/ket133. [DOI] [PubMed] [Google Scholar]
- 35.Devleeschouwer V, Roelandts R, Garmyn M, Goossens A. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Derm. 2008;58(3):159–166. doi: 10.1111/j.1600-0536.2007.01296.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Study investigators
| Investigator | Site location* |
|---|---|
| Croatia | |
| Dr Tatjana Kehler | Opatija |
| Dr Simeon Grazio (coordinator) | Zagreb |
| Dr Olga Badovinac | Zagreb |
| Dr Natalija Topolnjak | Varaždin |
| Germany | |
| Prof Gerold Stucki, MD, MS (coordinator) | München |
| Prof G. Stucki (Dr Borchers) | München |
| Dr med G. Lindner | Strausberg |
| Dr med Reiner Lehmann | Berlin |
| Dr med Klaus Lehnhardt | Bad Dürrheim |
| Dr med Werner Kneer | Stockach |
| Dr med Heike Dorow | Jena |
| Dr Roy Heller | Schwerin |
| Stefan Schäfer | Schwerin |
| Dr med Patrick Finkbeiner | Landau |
| Dr med Dieter Veith | Emmendingen |
| Dr med Annette Herzner | Hamburg |
| Dr med Matthias Groβann | Mannheim |
| Dr med Michael Schreinert | Berlin |
| Dr med Ralf Knels | Chemnitz |
| Dipl Med Janna Stöβel | Berlin |
| Dipl-Med DetlefWilcke | Berlin |
| Dr med Christina Mondorf | Frankfurt aM |
| Dr med Jutta Harten | Münster |
| Dr med Hans-Detlev Stahl | Leipzig |
| Poland | |
| Prof Leszek Szczepanski | Lublin |
| Dr Wieslawa Porawska | Poznan |
| Dr Maria Misterska-Skora | Wroclaw |
| Dr Jolanta Zarebska | Katowice |
| Prof Piotr Gluszko (coordinator) | Krakow |
| Serbia and Montenegro | |
| Prof Nemanja Damjanov (coordinator) | Belgrade |
| Prof Dr Gordana Devečerski | Novi Sad |
| Ass Prof Koviljka Čobeljić | Belgrade |
| Dr Miljanka Lazarević | Novi Sad |
| Prof Dr Stevan Jovic | Belgrade |
Note:
Site location of the investigator at the time of the study.