Abstract
Alginate microbeads have been investigated clinically for a number of therapeutic interventions, including drug delivery for treatment of ischemic tissues, cell delivery for tissue regeneration, and islet encapsulation as a therapy for type I diabetes. The physical properties of the microbeads play an important role in regulating cell behavior, protein release, and biological response following implantation. In this research alginate microbeads were synthesized, varying composition (mannuronic acid to guluronic acid ratio), concentration of alginate and needle gauge size. Following synthesis, the size, volume fraction, and morphometry of the beads were quantified. In addition, these properties were monitored over time in vitro in the presence of varying calcium levels in the microenvironment. The initial volume available for solute diffusion increased with alginate concentration and mannuronic (M) acid content, and bead diameter decreased with M content but increased with needle diameter. Interestingly, microbeads eroded completely in saline in less than 3 weeks regardless of synthesis conditions much faster than what has been observed in vivo. However, microbead stability was increased by the addition of calcium in the culture medium. Beads synthesized with low alginate concentration and high G content exhibited a more rapid change in physical properties even in the presence of calcium. These data suggest that temporal variations in the physical characteristics of alginate microbeads can occur in vitro depending on synthesis conditions and microbead environment. The results presented here will assist in optimizing the design of the materials for clinical application in drug delivery and cell therapy.
1 Introduction
Alginate hydrogels have received considerable attention for biomedical applications, including cell encapsulation [1–3], growth factor delivery [4–6], stem cell culture [7], and tissue engineering [8, 9] due to their mild gelling conditions, biocompatibility, and physical architecture [10, 11]. The success of these microbeads in biomedical applications depends, in part, on their physical properties. In fact, drug delivery and cell encapsulation systems depend on the ability to control the transport of soluble molecules through the hydrogel scaffold. The rate and the mechanism of solute diffusion is determined primarily by the physical properties of the material [12], therefore, the ability to design successful alginate systems is tantamount to being able to control the physical properties.
Alginate is a natural, acidic polysaccharide extracted from algae and is composed of monomeric units of 1,4′-β-D-mannuronic acid (M) and α-l-guluronic acid (G) (Fig. 1) [13]. Gelation occurs when divalent cations, such as Ca2+, Sr2+, or Ba2+ interact with G-monomers forming ionic bridges between adjacent alginate chains [14, 15]. Previous work has shown that simple physical parameters of alginate materials, including volume fraction, diameter, and shape, can be used to accurately predict solute diffusions from alginate disks [12]. Based on this information, a number of studies have been performed to determine how these properties can be influenced by alginate synthesis conditions. Variations in these conditions could alter transport properties and potentially influence biological performance. To the best of our knowledge, other researchers have not sought to address temporal variations in alginate microbead properties post-synthesis and whether these changes depend on synthesis conditions.
Fig. 1.
Structure of M and G chains in alginate alginate
Clinically, alginate microbeads have been used as drug and cell delivery systems for a number of applications. Alginate containing fibroblast growth factor-2 (FGF-2) and heparin have shown promise as angiogenic therapies for the treatment of coronary artery disease [16, 17]. However, this system requires that the transport of soluble factors from alginate microbeads is well understood [18]. Alginate microbeads have also been used to deliver cells in a clinical application of bone tissues engineering [19] and is in clinical trials for islet immunoisolation for the treatment of type I diabetes [1]. The viability of cells in these micro-beads is highly dependent on the ability to precisely control temporal variations in oxygen and nutrient levels within the alginate system.
In the present study, we investigated the effects of synthesis conditions on physical properties of alginate microbeads that can be used to model solute transport. Both initial properties and variations in these properties during 80 days of incubation were examined. Furthermore, the influence of calcium levels in the incubation medium on the stability of the microbeads is also presented.
2 Materials and methods
2.1 Supplies
Low viscosity (20–200 mPa·s) ultra pure alginate of high mannuronic acid (LVM) and high guluronic acid (LVG) content was purchased from NovaMatrix (Oslo, Norway). LVM and LVG alginates were reported by the manufacturer to have molecular weights 75–200 kDa and G/M ratios of ≤1 and ≥1.5, respectively. Solutions for alginate microbead fabrication were made using the following chemicals: HEPES, NaCl, and MgCl2 (Fisher Scientific); CaCl2 (Acros).
2.2 Fabrication of alginate beads
Microbeads were synthesized using a modification of a technique developed previously for islet encapsulation [2]. Two types of low viscosity alginate, LVM and LVG, were studied. Alginate solutions were loaded into a 2-channel air droplet micro-encapsulator (at air jacket pressure of 10 psi and alginate jacket pressure of 15 psi) and droplets were expelled through a syringe needle (various gauge sizes, 20–27 g) into a 1.1% CaCl2 cross-linking solution. The beads were incubated in cross-linking solution for 20 min, and then washed three times with a 1.1% CaCl2 solution in normal saline.
2.3 Alginate parameters
Various concentrations (1–3%) of LVM and LVG alginate were used to fabricate the microbeads. Alginate concentration, alginate type (LVG or LVM) and the needle gauge size of the encapsulating apparatus were varied and the resulting bead properties quantified. Volume fraction of alginate provides information about the amount of free volume available for diffusion. To determine volume fraction, empty alginate microbeads were fabricated, weighed in a swollen state and then allowed to dry in an incubator at 37°C for at least 24 h. Following incubation, the mass of the dried beads was measured. The volume fraction of alginate microbeads was calculated as follows:
| (1) |
where mdry is the weight of the dried alginate beads, mwet the weight of the water swollen alginate beads, ρwater the density of water (1 g/cm3) and ρalginate is the density of alginate or the inverse of the partial specific volume of (0.60 cm3/g) [20]. Microbead diameters were determined by sampling beads from each run and imaging beads (n = 27 for each run) using an Axiovert 200 inverted microscope (Carl Zeiss MicroImaging, Inc) at 10× (1.08 μm/pixel) and quantification with AxioVision AC 4.6 (Carl Zeiss).
2.4 Mathematical model of release from alginate microbeads
A transport model was developed and experimental tests performed in order to test whether the alginate parameters quantified in this study could be used to accurately predict solute diffusion. Since solute diffusion is hindered by the structure of the alginate beads, an obstruction model developed by Amsden and Turner [21] that takes into account the presence of impenetrable polymer chains as hindrances to protein diffusion was used to estimate the effective diffusion coefficient, Deff, of a solute in the alginate matrix:
| (2) |
where rs is the Stokes radius of the protein, rf the radius of the alginate fibers, and r̄ the average radius of the openings between the polymer chains. Do is the diffusion coefficient for the solute in the solvent under dilute conditions and in the absence of any alginate and was estimated using Stokes-Einstein equation:
| (3) |
where T is temperature, kb Boltzman constant and μ the viscosity of the solvent. The value for r̄ is determined from Eq. (3), where ks is a scaling constant for alginate in water [21] and ϕ is the alginate volume fraction.
| (4) |
The solute was assumed to be fibroblast growth factor-1 (FGF-1) and the model parameters used are provided in Table 1. This model has been shown to accurately approximate diffusion from cylindrical alginate gels [12, 21]. Assuming no convection, no chemical reaction (ri = 0) and only radial unsteady diffusion, the convection–diffusion equation:
| (5) |
can be simplified to Eq. (5), also known as Fick’s second law:
| (6) |
where C is the protein concentration, and r is radius.
Table 1.
Parameters used in predicting release from alginate microbeads
The following initial and boundary conditions were used to solve Eq. (5):
Initially (t = 0) concentration inside the bead is uniform at Co
Diffusion from the bead is spherically symmetric and uniform, at r = 0.
The surrounding fluid acts as an infinite sink, C = 0 at the surface (r = R) of the bead.
Using these conditions, Eq. (4) can be solved explicitly to give:
| (7) |
The concentration over the entire volume of the bead and the total amount of protein remaining within the bead was determined at various time points using MATLAB 6.1 (Natick, MA). The percent release (amount remaining/total encapsulated×100%) was plotted vs. time and compared to the experimental results described below.
2.5 Protein release studies
Experimental release studies were performed to compare to the model predictions. Microbeads were incubated with I125 labeled FGF-1 for 24 h prior to placing the radiolabeled FGF-1 loaded alginate microbeads in 2 mM CaCl2·2H2O at 37°C. Two hundred fifty beads were suspended in 3 mL of solution and the solution was replaced every 15 min in order to approximate infinite sink conditions. The solutions were analyzed for labeled protein content using a gamma counter (Perkin-Elmer Packard Cobra II Auto Gamma).
2.6 Microbead stability
Microbeads were incubated in three different solutions in a humidified atmosphere at 37°C: (1) normal saline (0.9% w/v), (2) saline with 22 mM (w/v) CaCl2·2H2O and (3) saline supplemented with a physiological concentration of calcium (2 mM) in order to explore the influence of calcium concentration on microbead stability. The media was replaced every 2 days. Images of the microbeads were taken every 3 days to assess bead diameter size and perimeter. Perimeter measurements were used to calculate the shape factor for the microbeads as follows: . A shape factor of one signifies a circle and less than one denotes a more irregular shape. The shape of the bead influences uniformity of diffusion within the microbead and also imperfectly shaped microbeads can elicit a biological response leading to the eventual failure of the microbead system. Additionally, volume fraction measurements were quantified every 2–5 days during the incubation period.
2.7 Statistical analysis
Alginate microbead properties are expressed as means ± standard deviations. To determine significant differences between groups of data, analysis of variance (ANOVA) was performed with a Tukey–Kramer post-test. In all cases, P < 0.05 was considered statistically significant. ANOVA sample size analysis of preliminary data was used to determine group sizes for comparison.
3 Results
3.1 Microbead size
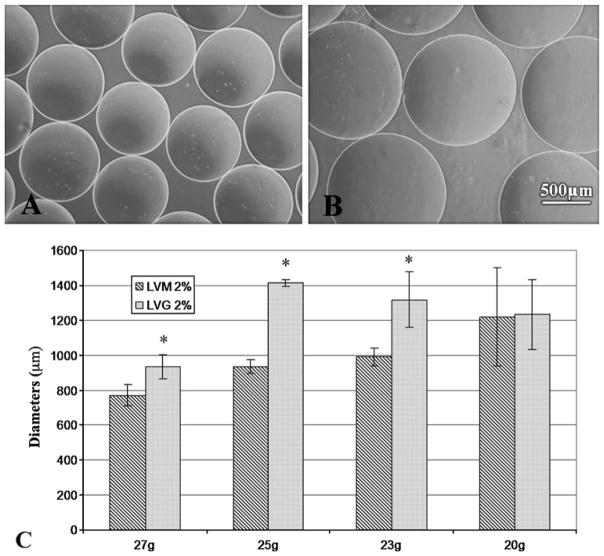
The size of microbeads plays an important role in determining solute diffusion distance to encapsulated cells and the maximum amount and duration of therapeutic solute release from delivery systems. Our data demonstrate that at equal alginate concentrations, microbeads made using LVM (Fig. 2a) are smaller than LVG beads (Fig. 2b). The diameter of the beads also varied with the needle size of the encapsulating apparatus (Fig. 2c). As needle gauge decreased (corresponding to an increase in needle diameter), the diameter of both LVM and LVG microbeads increased. The data show that the diameters of the micro-beads can be varied between 700–1400 μm, by a combination of varying needle sizes and alginate composition. Understanding how to control microbead parameters that affect solute release, such as bead size, will allow for guidance in fabrication and design of a versatile alginate system for various applications.
Fig. 2.
The diameter of microbeads can be varied using different gauge needles and alginate composition. LVM alginate formed smaller microbeads (a) than those formed with LVG (b). LVM microbeads using same needle gauge and alginate concentration formed smaller beads than LVG microbeads (c). * indicates statistical significance (P < 0.05) between LVG and LVM microbeads at the same needle gauge
3.2 Shape
Bead shape contributes to the biological and transport properties of alginate beads. A non-spherical bead can result in non-uniform diffusion within the microbead. In addition, the shape of the microbeads influences the risk of an adverse foreign body reaction which could lead to the eventual failure of the microbead system [22]. For alginate beads fabricated using a 23g needle, 3% LVM beads had a significantly higher shape factor (0.96 ± 0.01) and hence more spherical beads than 1% LVM beads (0.94 ± 0.03). Similarly, beads made with 2% LVG alginate (0.95 ± 0.02) had significantly higher shape factor than beads made with 1% LVG (0.92 ± 0.06) (Table 2). The effect of alginate type (LVM or LVG) on microbead shape was also investigated with various needle gauges but constant alginate concentration (Table 3). LVM beads were found to differ in shape from LVG beads only at needle gauge size 27g. Overall, the procedure resulted in beads with shape factor greater than or equal to 0.91 regardless of synthesis conditions.
Table 2.
Shape factor at varying alginate concentrations for LVG and LVM alginate
statistically significant difference from 1% at P < 0.05
Table 3.
Comparison of shape factor at varying needle gauge size for LVM and LVG at same alginate concentration
| 2% Alginate | 20g | 23g | 25g | 27g |
|---|---|---|---|---|
| LVM | 0.93 ± 0.04 | 0.95 ± 0.03 | 0.96 ± 0.02 | 0.94 ± 0.02a |
| LVG | 0.91 ± 0.06 | 0.95 ± 0.02 | 0.96 ± 0.06 | 0.96 ± 0.006 |
indicates statistically significant difference from LVG at P < 0.05
3.3 Volume fraction
Diffusion of solutes in hydrogels is a function of the liquid available for molecular movement; therefore, the dependence of volume fraction by varying alginate composition (LVG or LVM) as well as total alginate concentration, was investigated. A higher volume fraction indicates a greater fraction of alginate in the microbeads and less free volume available for diffusion. The present studies showed that microbead volume fraction increased with increasing alginate concentration. In addition, volume fraction was significantly higher for LVM than LVG under all conditions (P < 0.05, Table 4).
Table 4.
Volume fraction of alginate with varying concentration for LVM and LVG alginate
| % | LVM | LVG | P value |
|---|---|---|---|
| 3 | 0.052 ± 0.004 | 0.033 ± 0.003 | 1.224E-06 |
| 2 | 0.037 ± 0.01 | 0.025 ± 0.004 | 0.016 |
| 1 | 0.026 ± .008 | 0.017 ± 0.004 | 0.028 |
3.4 Release
A transport model was developed in order to determine whether the alginate parameters calculated can be used to predict the diffusion of a solute within alginate microbeads. In this model, the effective diffusion coefficient of FGF-1 is predicted based on microbead parameters (volume fraction, diameter) determined in the previous sections. Experimentally, microbeads were incubated in saline supplemented with physiological levels of calcium. Release profiles of radiolabeled FGF-1 (Fig. 3) show that the model agreed well with experimental results of FGF-1 release. It was also found that release was slowest for LVG 2% (25G) which had the largest diameter (d = 1414 μm). These results indicate that simple physical parameters can be used to predict solute transport within alginate microbead systems.
Fig. 3.
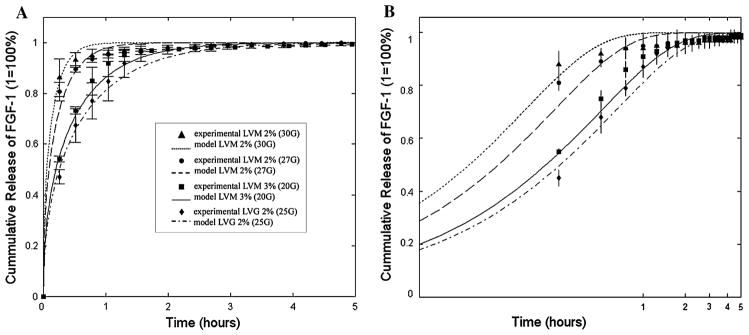
Change in alginate synthesis conditions altered release of radiolabeled FGF 1 from alginate microbeads and show good agreement with transport model of diffusion within the microbeads plotted in both (a) linear and (b) log scale
3.5 Microbead stability
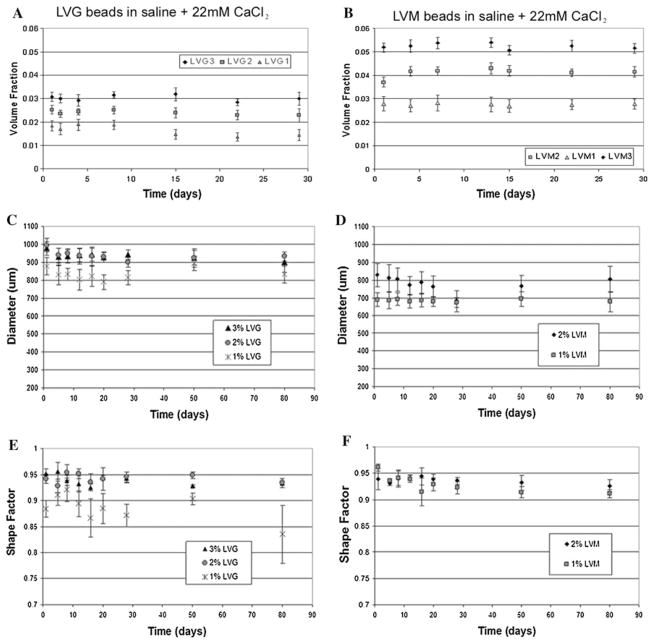
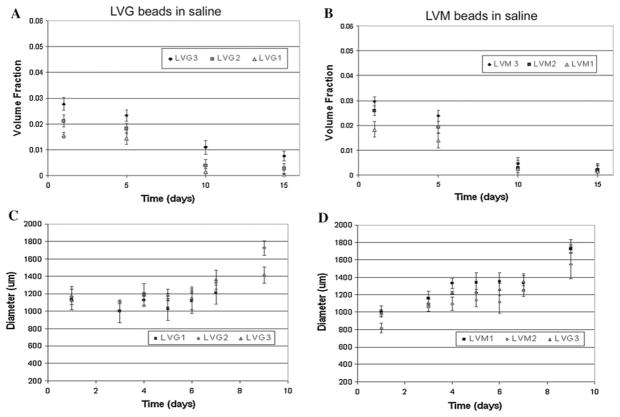
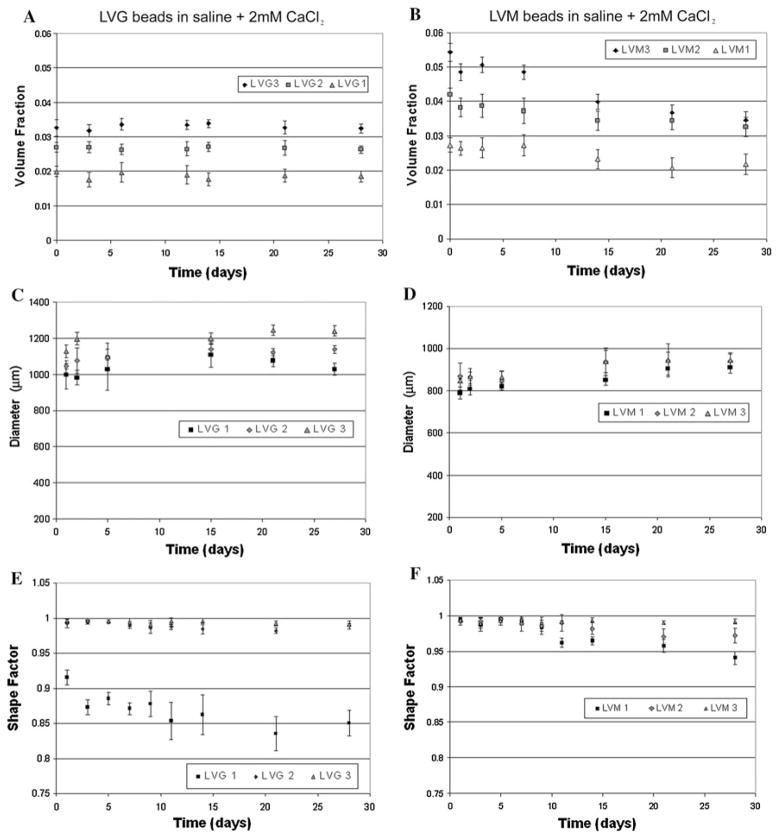
The previous studies investigated initial alginate properties, but these systems are often intended for long-term applications for cell encapsulation or delivery systems [1]. Variations in microbead properties with time can influence microbead function. Breakdown of microbeads can alter release kinetics and biological properties of the materials. Previous studies have provided conflicting results on the stability of microbeads in culture [23–26]. Our data suggest that the long-term stability of the alginate microbeads depends significantly on calcium levels in the microenvironment. Both LVG and LVM alginate beads remained stable with constant diameter (Fig. 4a, b) and volume fraction (Fig. 4c, d) during 80 days of incubation in saline supplemented with 22 mM (w/v) calcium. Shape factor values calculated from measured perimeters, demonstrated that at higher concentrations of LVG and at all concentrations of LVM, shape factors remained greater than 0.9 through 80 days (Fig. 4e, f). At low LVG concentration (1%) microbeads initially had the lowest shape factor, which decreased significantly over time. In saline, both LVG and LVM beads experienced an increase in diameter (Fig. 5a, b) with a corresponding decrease in volume fraction (Fig. 5c, d). By 2 weeks the beads incubated in saline alone had deteriorated regardless of synthesis conditions. When the beads were placed in saline supplemented with a physiological level of calcium (2 mM), a slight decrease in volume fraction was observed initially (Fig. 6a, b) as bead diameters gradually increased, reaching a plateau by the second week. Only microbeads made with LVM 1% continued increasing in diameter through 3 weeks (Fig. 6c, d).
Fig. 4.
Volume fraction (a, b) and bead diameters (c, d) remained constant for both LVM and LVG when incubated in saline with 22 mM CaCl2. Shape factor remained constant and greater than 0.9 for LVG concentrations greater than 2% and for all concentrations of LVM through 80 days (e, f)
Fig. 5.
Volume fraction of LVM and LVG beads in saline continually decreased (a, b) as microbead diameters increased for 2 weeks (c, d)
Fig. 6.
Beads stored in saline with 2 mM calcium (a, b) underwent a slight decrease in volume. Diameters (c, d) gradually increased in the first week but reached a plateau by the second week. Only LVM 1% continued increasing in diameter through 3 weeks. Shape factor remained constant and greater than 0.9 for all concentrations of LVM and concentrations of LVG greater than 2%
4 Discussion
The success of alginate microbead as delivery systems in therapeutic applications depends, in part, on the physical properties of the microbeads. In the present study, alginate microbead diameters varied with needle size and alginate composition. Bead diameter increased with decreasing needle gauge (i.e., increasing needle diameter). Additionally, beads fabricated using LVG alginate had larger diameters than corresponding LVM alginate beads. Other researchers have reported similar findings [27–29]. Different types of alginate vary in other properties (i.e. flexibility, strength, porosity), and therefore it is important to be able to synthesize microbeads of a particular diameter with either type of alginate.
A primary goal of this research was to investigate the stability of these properties over time. Calcium is one of the primary ions that influence microbead stability. Beads stored in saline undergo degradation in their structural integrity because Na+ ions in the surrounding solution undergo an ion exchange with Ca2+ ions in the cross-linked alginate. Under our experimental conditions, beads fabricated with LVM or LVG showed no signs of swelling or shrinking through 80 days of incubation in saline supplemented with 22 mM of CaCl2. These data are in agreement with results obtained by Amsden and Turner [25]. Other researchers that have examined alginate microbead stability have shown changes in volume and diameter of alginate beads [23, 24, 26]. These studies however, were performed in an incubation medium with low levels of calcium. Beads placed in pure distilled water showed no signs of swelling while those placed in saline showed a drastic increase in swelling after 3 h [24]. We found similar changes when beads were placed in saline, as evident in the increase in diameter, decrease in volume fraction, and complete breakdown of the beads by 2 weeks.
While our results are consistent with previous reports, in vitro conditions are limited in their reflection of the in vivo environment. Biomaterial degradation can be accelerated in vivo by oxidative, phagocytic, and enzymatic processes resulting from cell response to the implanted material. However, in the absence of chronic inflammation resulting from infection or other abnormal stimuli alginate polymers exhibit very slow degradation via hydrolytic, oxidative, or enzymatic mechanisms. A number of in vivo studies have shown that alginate microbeads are stable for greater than 6 weeks in a number of tissue locations [8, 9]. Alginate-based materials primarily resorb via ion exchange with the local microenvironment. Beads stored in saline supplemented with physiological levels of calcium underwent a slight change in volume fraction and diameter, but by 3 weeks the beads were still intact and these properties stabilized. These results suggest that an ion-exchange process may still occur in the presence of physiological levels of calcium, but that the effect may not be to the extent that has been reported previously when alginate microbeads are incubated in saline alone. Slight variations in volume fraction and diameter may alter solute transport within the beads which could alter behavior in vivo.
Synthesis conditions can be used to influence microbead volume fraction based on varying alginate concentration as well as alginate composition. Volume fraction is a ratio of the amount of alginate in the bead to the total volume of the bead. An increase in alginate content of the initial solution caused an increase in volume fraction in the resultant alginate microbeads. In addition, volume fractions were higher in alginate beads formed with LVM alginate when compared to corresponding LVG alginate. To our knowledge, there is no published data on the effects of alginate content in the pregelled solution on volume fraction of the resultant microbeads. Some investigators have found that solute diffusion is hindered more in alginates of high M/G ratio [23, 28, 30]. It has been suggested that these results may be explained by the fact that M chains do not actively participate in cross-linking, and may thus cause more significant obstructions to movement [23]. Additionally, high M alginate have less of the junction zones formed by G residues resulting in increased area for diffusion [28]. This suggests that an increase in M chains would cause decreased network size diffusion. Other investigators, however, have found that alginate preparations with high G result in slower diffusion [27, 31]. Our results show that an increase in M content leads to increased volume fractions, suggesting a decrease in both area for diffusion and network size.
A transport model of diffusion in alginate microbeads was developed and tested to evaluate whether these simple physical properties could be used to predict release kinetics. Experimental studies using radiolabeled FGF-1 were in agreement with the release trends predicted by the model. Diameters of the microbeads appear to have a greater influence on release duration than volume fraction, possibly due to the low volume fractions in all conditions. Despite the greater volume fraction of LVM 3% when compared to LVG 2%, because the diameter of LVG 2% fabricated with a 25G was greater than LVM 3% fabricated at 20G beads, the protein had a further distance to travel and hence a slower release profile was observed. The characteristic time for the protein to diffuse out of the bead is directly proportional to the path (i.e. the radius) squared and inversely proportional to its diffusion coefficient. In these studies, the change in the magnitude of the radius squared has a much larger effect than the change in diffusion coefficient due to change in volume fraction.
Although this model provides a relationship between alginate synthesis conditions and release profiles, it assumes infinite sink conditions. FGF-1 release occurred rapidly under conditions used for in vitro studies here approximating infinite sink conditions, but in vivo release are likely to occur over a longer time scale. When FGF-1-loaded beads were implanted in an animal model the protein was present in the microbeads at 3 weeks but not at 6 weeks [9]. In vivo, surrounding tissue may provide a diffusive resistance and slowing the released of the protein from the microbeads. While the conditions used for evaluating this model may not predict exact release protein concentrations in vivo, it helps in relating physical parameters to release profiles and serves as a useful tool in the design of a drug delivery system and, in turn, guiding release studies. In addition, the results suggests that the temporal changes in diameter and volume fraction observed may have a significant influence on solute transport and may need to be accounted for if long-term application is intended.
Whether alginate is used as a biomaterial to encapsulate cells or as a protein delivery conduit, variations in its physical properties are crucial to the viability of the product. In our studies, the focus was to investigate how variations in conditions used to synthesize alginate microbeads affected physical characteristics of the alginate microbeads that influence the transport of solutes within the beads and biological response to the material. In addition, we examined whether these properties would vary with time. These data can be used as a guide in the selection of microbead fabrication parameters for the design of alginate microbeads for cell encapsulation, drug delivery, or tissue engineering application. In summary, our data showed that alginate chemistry and alginate microbead synthesis conditions can be exploited to control physical properties of the resultant microbeads.
5 Conclusions
Alginate microbeads have been investigated clinically for a number of therapeutic interventions, including drug delivery, cell delivery, and cell encapsulation. They have shown promise in these applications but clinical success requires the ability to control and predict solute transport in the alginate matrix. The results presented here provide insight into how the properties of alginate-based microbeads can be controlled through synthesis conditions and how the properties vary with time. Future development of clinically applied alginate microbeads can use these findings as a guide for optimizing material design.
Acknowledgments
This research was supported, in part, by funding from the Gates Foundation (MLM), Mr. Edward Ross (MM, OK), Department of Veterans Affairs (EMB), National Science Foundation (Grant Nos: 0852048 and 0731201) and National Institutes of Health (Grant No: RO1 DK080897).
Contributor Information
Monica L. Moya, Pritzker Institute of Biomedical Science & Engineering, Illinois Institute of Technology, 3255 South Dearborn St, Chicago, IL 60616, USA. Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, USA
Michael Morley, Pritzker Institute of Biomedical Science & Engineering, Illinois Institute of Technology, 3255 South Dearborn St, Chicago, IL 60616, USA. Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, USA.
Omaditya Khanna, Department of Chemical and Biological Engineering, Illinois Institute of Technology, Chicago, IL, USA.
Emmanuel C. Opara, Wake Forest Institute for Regenerative Medicine, Wake Forest University Health Sciences, Winton-Salem, NC, USA
Eric M. Brey, Email: brey@iit.edu, Pritzker Institute of Biomedical Science & Engineering, Illinois Institute of Technology, 3255 South Dearborn St, Chicago, IL 60616, USA. Department of Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, USA. Research Service, Hines Veterans Administration Hospital, Hines, IL, USA
References
- 1.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. Design of a bioartificial pancreas(+) J Investig Med. 2010;58(7):831–7. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garfinkel, Harland RC, Opara EC. Optimization of the micro-encapsulated islet for transplantation. J Surg Res. 1998;76(1):7–10. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 3.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 4.Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95(2):632–40. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna O, Moya ML, Greisler HP, Opara EC, Brey EM. Multilayered microcapsules for the sustained-release of angiogenic proteins from encapsulated cells. Am J Surg. 2010;200(5):655–8. doi: 10.1016/j.amjsurg.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya ML, Garfinkel, Liu X, Lucas S, Opara EC, Greisler HP, Brey EM. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. 2010;160(2):208–12. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire TJ, Novik EI, Schloss R, Yarmush ML. Alginate encapsulation and hepatic differentiation of embryonic stem cells. Bioengineering conference, 2005. Proceedings of the IEEE 31st Annual Northeast; 2–3 April 2005; pp. 213–214. [Google Scholar]
- 8.Moya ML, Cheng MH, Huang JJ, Francis-Sedlak ME, Kao SW, Opara EC, Brey EM. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31(10):2816–26. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel, Huang JJ, Cheng MH, Opara EC, Brey EM. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78(2):142–7. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 11.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. doi: 10.1055/s-2007-973087. [DOI] [PubMed] [Google Scholar]
- 12.Amsden B. Solute diffusion in hydrogels.: an examination of the retardation effect. Polym Gels Netw. 1998;6(1):13–43. doi: 10.1016/S0966-7822(97)00012-9.. [DOI] [Google Scholar]
- 13.Goosen MFA. Physico-chemical and mass transfer considerations in microencapsulation. Ann NY Acad Sci. 1999;875(1):84–104. doi: 10.1111/j.1749-6632.1999.tb08496.x. [DOI] [PubMed] [Google Scholar]
- 14.Uludag H, De Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42(1–2):29–64. doi: 10.1016/S0169-409X(00)00053-3.. [DOI] [PubMed] [Google Scholar]
- 15.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–85. doi: 10.1016/S0169-409X(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 16.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, Simons M, Sellke FW. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J Thorac Cardiovasc Surg. 2002;124(1):28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 17.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65(6):1540–4. doi: 10.1016/S0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 18.Khurana R, Simons M. Insights from angiogenesis trials using fibroblast growth factor for advanced arteriosclerotic disease. Trends Cardiovasc Med. 2003;13(3):116–22. doi: 10.1016/S1050-1738(02)00259-1. [DOI] [PubMed] [Google Scholar]
- 19.Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344(20):1511–4. doi: 10.1056/NEJM200105173442004.. [DOI] [PubMed] [Google Scholar]
- 20.Cook WH, Smith DB. Molecular weight and hydrodynamic properties of sodium alginate. Can J Biochem Physiol. 1954;32(3):227–39. [PubMed] [Google Scholar]
- 21.Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31(23):8382–95. doi: 10.1021/ma980765f. [DOI] [Google Scholar]
- 22.Hobbs HA, Kendall WFJ, Darrabie M, Opara EC. Prevention of morphological changes in alginate microcapsules for islet xeno-transplantation. J Investig Med. 2001;49(6):572–5. doi: 10.2310/6650.2001.33722. [DOI] [PubMed] [Google Scholar]
- 23.Martinsen A, Skjåk-Bræk G, Smidsrød O. Alginate as immobilization material: i. correlation between chemical and physical properties of alginate gel beads. Biotechnol Bioeng. 1989;33(1):79–89. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 24.Bajpai SK, Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ions. React Funct Polym. 2004;59(2):129–40. doi: 10.1016/j.reactfunctpolym.2004.01.002. [DOI] [Google Scholar]
- 25.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65(5):605–10. doi: 10.1002/(SICI)1097-0290(19991205)65:5<605:AID-BIT14>3.0.CO;2-C[pii]. [DOI] [PubMed] [Google Scholar]
- 26.Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on microbead swelling. J Microencapsul. 2006;23(6):613–21. doi: 10.1080/02652040600687621. [DOI] [PubMed] [Google Scholar]
- 27.Klokk TI, Melvik JE. Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul. 2002;19(4):415–24. doi: 10.1080/02652040210144234. [DOI] [PubMed] [Google Scholar]
- 28.Yamagiwa K, Kozawa T, Ohkawa A. Effects of alginate composition and gelling conditions on diffusional and mechanical properties of calcium-alginate gel beads. J Chem Eng. 1995;28:462–7. doi: 10.1252/jcej.28.462. [DOI] [Google Scholar]
- 29.Kendall WF, Jr, Darrabie MD, El-Shewy HM, Opara EC. Effect of alginate composition and purity on alginate microspheres. J Microencapsul. 2004;21(8):821–8. doi: 10.1080/02652040400015452. [DOI] [PubMed] [Google Scholar]
- 30.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Control Release. 2004;96(3):463–72. doi: 10.1016/j.jconrel.2004.02.021S0168365904000999[pii].. [DOI] [PubMed] [Google Scholar]
- 31.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12(7):619–26. doi: 10.1016/0142-9612(91)90107-L. [DOI] [PubMed] [Google Scholar]
- 32.Dowd CJ, Cooney CL, Nugent MA. Heparan Sulfate Mediates bFGF Transport through Basement Membrane by Diffusion with Rapid Reversible Binding. J Biol Chem. 1999;274:5236–5244. doi: 10.1074/jbc.274.8.5236. [DOI] [PubMed] [Google Scholar]