Abstract
Background
Growth of new blood vessels (neovascularization) occurs naturally in the body, but the slow rate of the process may not be sufficient for survival of engineered tissues and transplanted cells, such as pancreatic islets. For transplanted islets, it is crucial that the transplantation site has sufficient vasculature to support the needs of the islets. Therefore, the specific aim of this research was quantify the effect of FGF-1 incorporation into alginate microbeads on neovascularization of such capsules in an in vivo rat transplant model.
Materials and Methods
Microbeads loaded with FGF-1 or control beads (beads without FGF-1) were implanted in the rat omental pouch model. Animals were sacrificed 7 d post-implantation.
Results
Microbeads loaded with FGF-1 stimulated a significant increase in vascular density compared with control rats implanted with control beads.
Conclusions
These results indicate that alginate microbeads loaded with FGF-1 enhance local neovascularization around implanted microbeads. These data provide a compelling impetus for experimental pursuit of FGF-loaded alginate microcapsules for vascularization of transplanted islets.
Keywords: alginate, FGF-1, protein delivery, neovascularization, microcapsules, cellular transplant
INTRODUCTION
Although immunoisolation of healthy transplanted islets by microencapsulation offers a promising treatment option for type 1 diabetes, a challenge to its clinical success is the inadequate nutrient supply to the encapsulated cells, which are routinely transplanted in the unmodified peritoneal cavity [1]. Enhancing the blood supply through neovascularization may improve the survival and function of microencapsulated islets at the transplantation site by allowing for adequate oxygen and nutrient exchange as well as removal of waste products between encapsulated islets and the systemic circulation. The use of growth factors for therapeutic stimulation of neovascularization has been investigated extensively, and current research suggests that sustained delivery of low levels of growth factor proteins is required for a persistent angiogenic response [2, 3]. However, sustained delivery in appropriate doses for optimal effect remains challenging.
Islets have a rich vascular network receiving up to 10%–15% of the pancreatic blood supply, despite making up only 1%–2% of the pancreatic mass [4]. Research has shown that a decreased blood supply owing to inadequate vascularization following transplantation can lead to death of β-cells or islet dysfunction [5, 6]. Revascularization at the transplantation site may not occur before ischemic damage impairs islet survival during the first crucial days post-transplantation. Extensive damage in islet grafts has been observed in the first 3 d after transplantation [5]. Bolus administrations of growth factors, such as nerve growth factor (NGF), have shown promising results for improving the viability of transplanted islets [7]. However, bolus administrations are not optimally effective, as they often require multiple administrations at abnormally high concentrations, resulting in abnormal vascular function and possible severe side effects [3]. A delivery strategy that focuses on controlled local delivery of therapeutic concentrations of growth factor may not only aid in controlling protein concentration at an appropriate level, but also could be used to target tissue of interest and, thus, minimize exposure to other sites [8].
In the present study, a new experimental model suitable for in vivo vascularization of alginate encapsulated islet transplants was examined. The use of the omentum pouch for islet transplantation has been previously investigated, but enhancing the vascularization of the omentum by delivery of growth factors from the encapsulation system has not been explored. The goal of the studies was to investigate if loading alginate microbeads with acidic fibroblast growth factor (aFGF, also known as FGF-1), an angiogenic protein, would induce rapid neovascularization in an in vivo rat omentum transplant model.
METHODS
Alginate Microbead Synthesis
Alginate microbeads without islets were synthesized using a modification of a technique developed for islet encapsulation [9]. Low viscosity alginate (20–200 mPa·s) of high mannuronic content (LVM) (NovaMatrix, Norway) was extruded from a custom-made 2-channel air droplet micro-encapsulator (at air jacket pressure of 10 psi and alginate jacket pressure of 15 psi) through 27 G needle into a 1.1% CaCl2 solution where alginate drops were crosslinked, thus forming solid spherical microbeads. Resultant beads were washed three times with a wash solution comprised of a mixture of 0.9% saline and 0.25% CaCl2 (wt/vol). Beads loaded with FGF-1 (total amount 150 ng) or without FGF-1 (control beads) were then used for animal studies.
Animal Studies
Lewis rats (n = 5 per group) were anesthetized using isoflurane, and 100 beads were implanted in an omental pouch via laparotomy. For this model, alginate beads were placed on the surface of the surgically exposed omentum (Fig. 1). The omental pouch was created by placing a purse-string suture along the edges of the omentum. Entire intact omenta were removed from rats at 7 d post-implantation, fixed in formalin, and paraffin embedded. Specimens were serially sectioned (5 μm thickness) for immunohistochemical analysis.
FIG. 1.
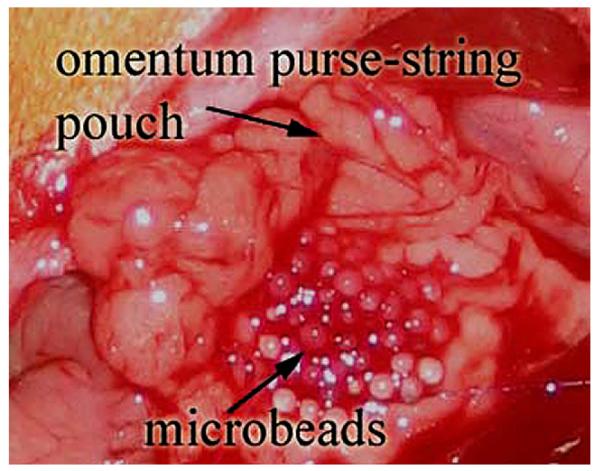
Omental pouch with microbeads created by placing a 6-0 nylon purse-string suture along edge of exposed omentum. Omental pouch was formed by pulling on the suture and tying. (Color version of figure is available online.)
Immunohistochemistry and Image Analyses
Serial sections were stained for CD31, a sensitive marker of ECs, and smooth muscle a actin (SMA), α mural cell marker [10]. Deparaffinized and rehydrated sections underwent steam antigen retrieval using DAKO target retrieval solution (DAKO, Carpinteria, CA) prior to immunohistological staining. Specimens were stained following an indirect procedure using rabbit anti-human CD31 (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-α smooth muscle actin (Abcam) and a biotinylated anti-rabbit secondary antibody using the Vectastain Elite ABC kit (Vector Labs, Burlingame, CA). Sections were digitally imaged (×20 objective, 0.017 μm/pixel) using an Axiovert 200 inverted microscope. Areas stained positive for CD31 were manually selected using Axiovision AC (Carl Zeiss, Germany). The percent vessel area was calculated using the following formula:
| (6) |
Four images (328 μm × 485 μm) per section were quantified for both CD31 and SMA analysis. Blood vessels per tissue view area were manually counted for CD31 as well as SMA stained sections. SMA coverage was calculated using the following formula:
| (7) |
Statistical Analysis
Data are expressed as means ± standard error. To determine significant differences between the two groups of data, Student’s t-test was performed. Differences were considered significant for P < 0.05.
RESULTS
Animal Studies
Microbeads were synthesized and either FGF-1-containing or empty microbeads were implanted in omentum pouches. The tissues were harvested at 1 wk. At the time of harvest, explanted omentum pouches were still intact, and alginate microbeads were visible through the thin omentum. Qualitatively, numerous vessels were present around microbeads in the omentum pouches, but no macroscopic differences were observed between groups.
Vascular Density
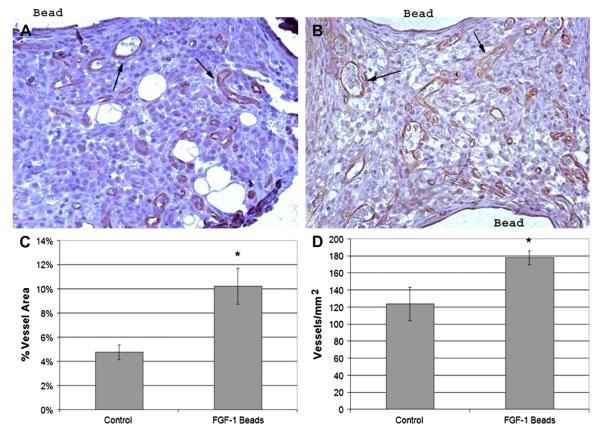
Specimens stained for CD31, a marker for endothelial cells, revealed vascularized omenta for both groups (Fig. 2 A and B). Rats implanted with FGF-1 loaded beads showed significantly higher tissue areas positive for CD31 vessels in the intercapsule regions of omental tissue than in those implanted with control beads (10.2% ± 1.5% versus 4.7% ± 0.6%, respectively P = 0.0005, Fig. 2 C). Similar results were observed when vascular density was quantified as number of vessels per tissue area (FGF-1 beads: 177.6 ± 8.1 vessels/mm2; control: 123.5 ± 19.6, P = 0.04, Fig. 2 D).
FIG. 2.
Positive CD31 stain in omentum sections from untreated rats (A) and treated rats (B).Treated animals showed significantly higher (*) vessel density than controls whether measured as percent vessel area (C) or number density (D). Statistical significance was defined as P < 0.05. Brown areas are positive for CD31. Arrows indicate select CD31 stained vessels. (Color version of figure is available online.)
Mural Cells
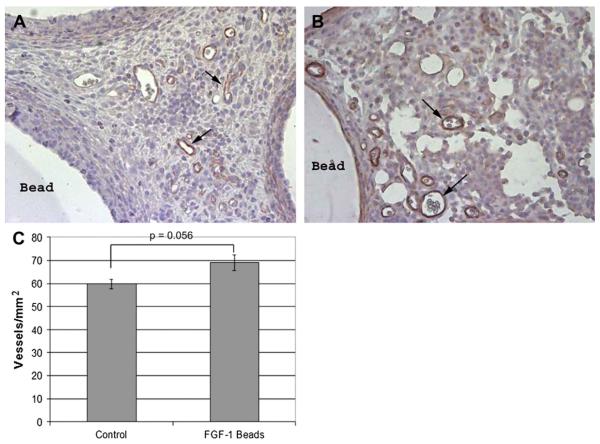
To assess vessel maturity, the presence of mural cells was determined by staining serial sections for SMA (Fig. 3 A and B). Both groups had greater than 40% of the vessels associated with SMA positive cells (FGF-1: 45.6% ± 2%; control: 69.1% ± 11.0%, P = 0.082). At 1 wk, there was a modest increase in the number of SMA stained vessels for animals implanted with FGF-1 loaded beads (62.3 ± 2.9) versus empty control beads (59.8 ± 2.0, Fig. 3 C), but these results were not found to be statistically significant (P = 0.056).
FIG. 3.
Positive SMA stain in omentum sections from untreated rats (A) and treated rats (B) showed no difference (C) at 1 wk in SMA coverage. Arrows indicate select SMA stained vessels. (Color version of figure is available online.)
DISCUSSION
A challenge to the survival of encapsulated islets or therapies based on transplanted cells is the need for an adequate blood supply for oxygen and nutrient exchange to the cells. Neovascularization using growth factors holds great promise; however, sustained delivery in doses required for optimal effect remains challenging. The ideal system would involve a material that could simultaneously provide a method for cell encapsulation and local delivery of angiogenic factors. In this study, we generated alginate microbeads using conditions that we have previously evaluated for islet encapsulation [9, 11, 12]. The microbeads without islets were evaluated for their ability to deliver FGF-1 for rapid neovascularization.
The alginate microbeads were implanted in a rat omentum flap model to investigate the effect of local delivery of FGF-1 on local neovascularization. At 1 wk post-implantation, FGF-1 loaded beads caused greater vessel density compared with the empty control alginate beads. Both vessel area and vessel number density were significantly greater in omentum pouches implanted with FGF-1 beads. Studies have demonstrated that transplanted naked islets revascularize within 10 to 14 d [13]. However, the delayed vascularization can result in significant damage to the islet grafts [5]. For this reason, it is vital that there is a rapid vascular response immediately after transplantation. In the present study, we have shown that vessel formation can be increased 2-fold within 7 d post-implantation by delivery of FGF-1. In a previous study by Sigrist et al., successful stimulation of angiogenesis in the rat’s omentum was achieved by transplanting encapsulated islets in collagen supplemented with one bolus of vascular endothelial growth factor (VEGF), but the enhanced angiogenesis decreased after 7 d perhaps because there was not a sustained delivery of VEGF [14].
Administration of high doses of growth factors has been shown to lead to unstable vessels, characterized by the absence of mural cells on the surface of capillaries. In capillaries, tubes of endothelial cells are surrounded or supported by pericytes (mural cells). Mural cell interactions with the newly formed microvascular network were determined by staining serial sections for SMA. SMA is often used as a marker for mural cells as an indication of vessel maturity. Mature vessels are less dependent on angiogenic factors for survival, and are important for proper vascular network function [15]. The results in our studies show that for both groups, greater than 40% of the vessels stained positive for SMA. For the FGF-1 bead group, there was only a modest increase in SMA stained vessels (P = 0.056) at 1 wk, which may be due to the small number of animals used in the experiments. Despite the increase in vessel formation for the FGF-1 group compared with the control group, the recruitment of mural cells was not found to differ significantly between the groups. In fact, mural cell levels of all three groups are much lower than levels in stable vessels of the omentum (89% ± 12%). At 1 wk, it is likely that this is a time of active angiogenesis, prior to vessel remodeling and maturation. While FGF-1 loaded microbeads were able to increase the vascular response, the trend of increased presence of vessels with low mural cell interactions suggests that additional recruitment of mural cells to vessels may require delivery of an additional growth factor. Richardson et al. showed that co-delivery of PDGF and VEGF led to rapid formation of a mature vascular network [16]. Future studies investigating not only the maturity and persistence of vessels at later time points but the functionality of these vessels formed would be of interest.
In these studies, we have shown that FGF-1 loaded beads stimulated an increase in vessel density compared with control empty beads after 1 wk of implantation. In the 1 wk time point, there did not appear to be a clear trend of increased vessel maturity. This 2D morphologic characterization of neovascularization provides information only on cells present in the vessels. Further studies are needed to asses the functionality of these vessels and to characterize their three-dimensional structure. These results nonetheless suggest that growth factors loaded in alginate microbeads may be used to vascularize the transplantation site to improve the survival of intraperitoneally transplanted islets, but more work remains to be done before successful clinical application. While not currently used clinically, intraperitoneal transplantation has been extensively studied as a prospective site for islet transplantation because of its potential to hold a large graft volume, technical ease of transplantation, and its proximity to portal circulation. Some limitations of using an unmodified peritoneal graft site for islet transplantation that have been reported include lack of paracrine factors, delayed release of insulin into the bloodstream after intraperitoneal infusion [17], and exposure to hypoxia upon implantation [18, 19]. We therefore sought to improve omental site for islet transplantation by inducing rapid vascularization in the graft using FGF-1 loaded microbeads. In this study, however, no islets were used; hence future studies using islets are necessary to fully assess if newly-formed vessels offer a benefit to the survival of transplanted islets.
In this study, we have demonstrated that FGF-1 loaded beads rapidly increased microvascular density, but at 1 wk time point, vessel maturity was not found to differ from control. The results presented in this paper provide a rationale for further experimental pursuit of protein-loaded alginate microcapsules to increase neovascularization, and potentially, viability of islet transplantation.
ACKNOWLEDGMENTS
The authors thank Monalee Shah and Jean Nothias for assistance with the animal studies. Special thanks to Nidal Younnes for his help with the animal model and Drs. Jung Ju Huang and Ming-Huei Cheng for their contribution to the image in Fig. 1. This research was supported by funding from the National Science Foundation (grants 0552896 and 0731201), National Institute of Health (Grant No: 1RO1 DK080897) the American Diabetes Association (1-04-ISLET-10) the U.S. Department of Veterans Affairs, and the Gates Foundation.
REFERENCES
- 1.Opara EC, Kendall WF., Jr. Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther. 2002;2:503. doi: 10.1517/14712598.2.5.503. [DOI] [PubMed] [Google Scholar]
- 2.Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am J Surg. 2006;192:604. doi: 10.1016/j.amjsurg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina PE. Endocrine physiology. McGraw-Hill Professional; New York: 2006. [Google Scholar]
- 5.Davalli AM, Scaglia L, Zangen DH, et al. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 6.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 7.Miao G, Mace J, Kirby M, et al. Beneficial effects of nerve growth factor on islet transplantation. Transplant Proc. 2005;37:3490. doi: 10.1016/j.transproceed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 8.August AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromolecular Biosci. 2006;6:623. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 9.Garfinkel MR, Harland RC, Opara EC. Optimization of the microencapsulated islet for transplantation. J Surg Res. 1998;76:7. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 10.Brey EM, McIntire LV, Johnston CM, et al. Three-dimensional, quantitative analysis of desmin and smooth muscle alpha actin expression during angiogenesis. Ann Biomed Eng. 2004;32:1100. doi: 10.1114/b:abme.0000036646.17362.c4. [DOI] [PubMed] [Google Scholar]
- 11.Charles K, Harland RC, Ching D, et al. Storage and microencapsulation of islets for transplantation. Cell Transplant. 2000;9:33. doi: 10.1177/096368970000900105. [DOI] [PubMed] [Google Scholar]
- 12.Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on micro-bead swelling. J Microencapsul. 2003;23:29. doi: 10.1080/02652040500286144. [DOI] [PubMed] [Google Scholar]
- 13.Menger MD, Vajkoczy P, Leiderer R, et al. Influence of experimental hyperglycemia on microvascular blood perfusion of pancreatic islet isografts. J Clin Invest. 1992;90:1361. doi: 10.1172/JCI116002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigrist S, Mechine-Neuville A, Mandes K, et al. Induction of angiogenesis in omentum with vascular endothelial growth factor: Influence on the viability of encapsulated rat pancreatic islets during transplantation. J Vasc Res. 2003;40:359. doi: 10.1159/000072700. [DOI] [PubMed] [Google Scholar]
- 15.Abramsson A, Berlin O, Papayan H, et al. Analysis of mural cell recruitment to tumor vessels. Circulation. 2003;105:112. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 16.Richardson TP, Peters MC, Ennett AB, et al. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 17.De Vos P, Vegter D, De Haan BJ, et al. Kinetics of intraperitone-ally infused insulin in rats. Functional implications for the bioartificial pancreas. Diabetes. 1996;45:1102. doi: 10.2337/diab.45.8.1102. [DOI] [PubMed] [Google Scholar]
- 18.Berman DM, O’Neil JJ, Coffey LC, et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9:91. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3:281. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]