Summary
Carbon dioxide (CO2) elicits an attractive host-seeking response from mosquitos [1–3] yet is innately aversive to Drosophila melanogaster [4, 5] despite being a plentiful byproduct of attractive fermenting food sources. Prior studies used walking flies exclusively, yet adults track distant food sources on the wing [6]. Here we show that a fly tethered within a magnetic field allowing free rotation about the yaw axis [7] actively seeks a narrow CO2 plume during flight. Genetic disruption of the canonical CO2-sensing olfactory neurons does not alter in-flight attraction to CO2; however, antennal ablation and genetic disruption of the Ir64a acid sensor do. Surprisingly, mutation of the obligate olfactory coreceptor (Orco [8]) does not abolish CO2 aversion during walking [4] yet eliminates CO2 tracking in flight. The biogenic amine octopamine regulates critical physiological processes during flight [9–11], and blocking synaptic output from octopamine neurons inverts the valence assigned to CO2 and elicits an aversive response in flight. Combined, our results suggest that a novel Orco-mediated olfactory pathway that gains sensitivity to CO2 in flight via changes in octopamine levels, along with Ir64a, quickly switches the valence of a key environmental stimulus in a behavioral-state-dependent manner.
Results and Discussion
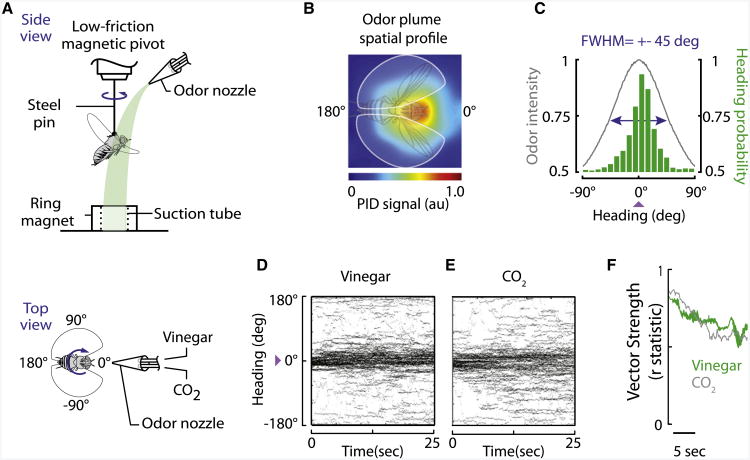
Here we explored the behavioral response of Drosophila melanogaster to CO2 using a flight simulator that tethers a fly within a magnetic field allowing for free rotational movement about the vertical body axis (yaw) in response to visual patterns projected from a panoramic array of LEDs and the presentation of localized odor stimuli [12]. On one side of the arena, an odor plume is delivered from a nozzle located above the fly's head, which is drawn beneath the fly with suction generating a spatially discrete gradient across the yaw plane (Figure 1A). We show via two methods that the plume is restricted to a narrow region of the arena, which enables us to clearly assess plume tracking behavior. First, we sampled the spatial distribution of plume intensity with a miniature photoionization detector (PID; Figure 1B), the results of which indicate that the full width at half maximum (FWHM) intensity occurs ±45 degrees from the plume source as measured along the arc defined by the yaw angle of the antennae (Figure 1C, gray line). Second, flies exposed to a vinegar-scented plume find it highly attractive, and the mean distribution of flight heading, binned across the azimuth, shows that the FWHM for behavioral data is considerably narrower than the PID measurement (Figure 1C, green bins). This indicates that over a 25 s trial, flies use the spatial gradient to actively and continuously track the plume of apple cider vinegar ([12] and Figure 1D), as well as other appetitive odors, such as banana, ethyl butyrate [13], mango volatiles, methyl salicylate, and ethyl acetate [14]. By contrast, flies in this apparatus continuously avoid or “antitrack” a plume of innately aversive benzaldehyde [15].
Figure 1. CO2 Is an Attractive Stimulus to Flies in Flight.
(A) A fly is tethered to a pin and suspended in a magnetic tether arena, where it can freely rotate in the yaw plane. Panels of LEDs surround the arena and display high-contrast stationary and moving visual patterns (not shown) used to orient each fly in a standard starting orientation and provide visual feedback during self-motion. The odor port at 0 degrees delivers a narrow stream of odor (indicated in green) that flows over the fly's head and is drawn downward and away by a vacuum through the aperture of the ring magnet. The angular heading of the fly is tracked by infrared video.
(B) The spatial cross-section of the plume, at the horizontal plane of the antennae, was measured for ethanol tracer with aminiature photoionization detector (PID), averaged over 11 repeated measurements across a 9 × 9 grid, smoothed with piecewise linear interpolation, normalized to the maximum ionization amplitude, and mapped onto a standard color scale (blue = 0, red = 1).
(C) The intensity profile from (B) was used to estimate the relative odor intensity across the arc formed at the radius of the antennae. The full width at half maximum (FWFM) of the odor intensity profile is indicated with blue arrows (±45 degrees). We tested a group of flies with a plume of vinegar and binned the resultant heading values at 9.5-degree increments, plotting the bin means in green for n = 85 trials, with one trial per individual fly. The purple arrowhead indicates the plume location, which also corresponds to the starting flight orientation of the flies (see Experimental Procedures). Note that the distribution of fly heading is considerably narrower than the relative photoionization measurements, suggesting that the flies are highly sensitive to the spatial odor gradient.
(D and E) Tethered wild-type (WT) flies track apple cider vinegar (vinegar, D) and carbon dioxide (CO2, E). Within-subjects design was used; heading trajectories of all individuals are plotted for a continuous odor plume of vinegar; n = 87 trials for (D) and n = 78 trials for (E). Heading distributions are not significantly different from each other (Kolmogorov-Smirnov, p = 0.9448).
(F) Flies track a vinegar and CO2 plume with the same robustness. Shown are vector strength measurements of the mean heading position over time for the time series data plotted in (D) and (E). Values closer to zero represent high variance in the mean tracking vector, whereas values closer to one indicate mean heading vector with near zero variance. See Experimental Procedures for the equation used to calculate the vector strength.
See also Movie S1.
Whereas walking flies find CO2 aversive (negative valence), we tested the hypothesis that flying animals either do not actively avoid this stimulus (as they do for benzaldehyde) or find it attractive (positive valence). In our first experiment, we used an innately attractive visual feature to position the fly directly within the plume at the start of each test trial (see Experimental Procedures) and presented sequences of vinegar and CO2 to each fly from the same nozzle in random order. We expected to confirm that flies robustly track vinegar. We also supposed that if the hedonic valence of CO2 is operating independent of behavioral state, then, like flies walking in a T-maze, flying flies would demonstrate antitracking behavior similar to their responses to benzaldehyde and quickly steer away from the plume, down the spatial gradient, and orient continuously in the direction directly opposite the nozzle [15]. Instead, we were surprised to find that flies remained centered in the CO2 plume in flight, and the resultant heading trajectories to CO2 closely emulated those observed in response to vinegar (Figures 1D and 1E). To quantify and compare the accuracy of tracking responses to vinegar and CO2, we computed vector strength, a circular statistical measure of angular heading variation over the duration of the trial. The high vector strength values and similarity for both odors indicate that as a population, flies are equally adept at maintaining their heading toward either the vinegar or CO2 plumes (Figure 1F).
For the experimental analysis showing CO2 tracking in Figure 1, each fly was presented with interspersed test plumes of 100% CO2 and the headspace of 100% apple cider vinegar. The two odor streams were controlled by a solenoid valve, pumped through separate parallel Teflon input tubes that converge within the tip of a pipette to form a single output stream (Figure 1A, top and bottom). The internal surface area of the nozzle was small, measuring 35 mm2, but nevertheless could have adsorbed vinegar molecules between trials with CO2, thereby contaminating these trials with vinegar scent. Whereas combining appetitive odor has been shown to weaken the CO2 aversion response in walking flies, it has never elicited a full switch in the valence of CO2 from aversive to attractive [5]. However, to further control for potential cross-contamination effects on CO2 tracking, we tested flies with a new nozzle and tubing system that were never exposed to vinegar, in which CO2 was interleaved with clean air. We confirmed that the time flies spent within the CO2 plume interspersed with air (1.56 ± 0.49 s) was statistically indistinguishable (p > 0.05 via two-way t test) from the vinegar-interleaved trials (2.96 ± 0.49 s).
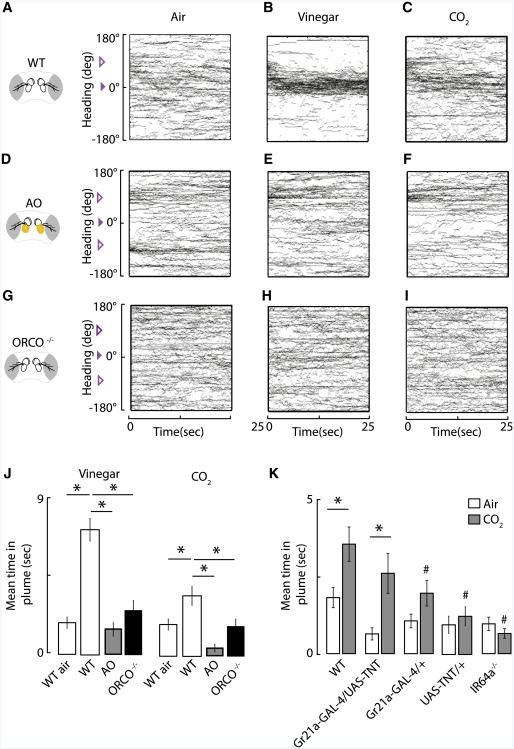
The flight trajectories that remain centered at the CO2 nozzle continuously for 25 s demonstrate that flies in flight do not find a high-intensity CO2 plume aversive (Figures 1D and 1E). But are they actively tracking the plume rather than merely passively failing to steer away from their start heading? To explicitly test for active plume acquisition, we started test trials with flies oriented at either ±90 degrees from the odor port, thereby requiring them to actively modify their flight heading to locate and track the plume. Under these conditions, wild-type (WT) flies evoke yaw turns oriented toward the vinegar plume ([12] and Figure 2B). Although the responses are not as robust as those to vinegar, WT flies actively steer toward the CO2 plume (Figure 2C) and wind up spending a significant amount of each trial within an envelope that we define as 610 degrees from the nozzle (Figure 2J; see Experimental Procedures). To confirm that flies were not tracking residual vinegar odor in the delivery nozzle, we exposed flies to an air plume delivered from the same nozzle using a within-subjects design. If residual vinegar were responsible for flight orientation toward the nozzle, then we would expect to see trajectories accumulate at the air plume in a manner similar to that seen in response to either vinegar or CO2 (Figures 2B and 2C). Instead, flies spend significantly less time in the air plume by comparison to either vinegar or CO2 (Figures 2A – 2J). They are actively and unequivocally tracking the CO2 plume.
Figure 2. Flies Require Functional Antennae and Orco to Track a Vinegar or CO2 Plume.
(A–I) Tethered wild-type (WT) flies seeka CO2 and vinegar plume. Shown are heading trajectories of all individuals presented with a continuous odor plume of air (A), vinegar (B), or CO2 (C). Tethered WT flies with both antennae occluded (AO) (D–F) or Orco mutants (G–I) fail to track either an apple cider vinegar plume (E and H) or a CO2 plume (F and I). The open purple arrowhead indicates the starting flight orientation of the flies, and the plume was defined as ±10 degrees around 0 degrees, the location of the odor nozzle indicated by the filled arrowhead (see Experimental Procedures). Antennae were occluded using UV-activated glue, highlighted in yellow (see Experimental Procedures). A within-subjects design for the air and two odorants was used for each anatomical or genetic manipulation. n > 52 trials.
(J) Wild-type flies require functional antennae and expression of the olfactory coreceptor Orco to successfully track a vinegar or CO2 plume in tethered flight. Shown is the mean time spent in the odor plume (0 degrees 6 10 degrees) over the length of the trial (25 s) for the flies shown in (A)–(I). Error bars indicate one SEM. *p < 0.05 via one-way ANOVA followed by Tukey's post hoc test.
(K) Wild-type flies require functional Ir64a receptors, but not synaptic output from Gr21a-expressing neurons, to track a CO2 plume in tethered flight. Shown is the mean time spent in the odor plume over the length of the trial (25 s). n > 48 trials. Error bars indicate one SEM. *p < 0.05 by one-way ANOVA, followed by Tukey's post hoc test for the experimental comparison connected by a horizontal bar; #p < 0.05 by one-way ANOVA, followed by Tukey's post hoc test for experimental comparison to WT flies in the CO2 condition. The Gr21a GAL4/+ flies result in p = 0.06 when comparing time spent in an air versus CO2 plume via a two sample t test.
See also Movie S1.
What is the underlying mechanism? There are two sensory pathways that transduce CO2 signals in flies. The third antennal segment (a3) contains sensory neurons that express olfactory receptors required for vinegar tracking in flight [16] and gustatory receptors that mediate CO2 aversion by walking flies [17]. We therefore examined whether the olfactory antennae are required for flies to track CO2 in flight by applying a small amount of UV-curing glue to both a3 antennal segments, thus occluding the olfactory sensillae. Previous work showed that this occlusion induces the loss of smell independently from the mechanical sensation of the glue [16]. Occluding the a3 segments prevents flies from actively sensing and tracking both vinegar and CO2 ([16] and Figures 2D – 2F). This result presumably excludes E409 gustatory cells previously shown to mediate an attractive behavioral response to CO2 from playing a central role in flight, because these taste cells are located on the proboscis, not on the occluded antennae [17].
Blocking the tracking behavior by removing antennal input suggests that the in-flight attraction to CO2 might somehow be mediated by input from the gustatory receptors Gr21a and Gr63a, which are expressed in antennal sensory neurons that respond to CO2 and are required for behavioral aversion in walking assays [18, 19]. We attempted to test mutants lacking the Gr63a CO2 gustatory receptor, but these transgenic flies were lethargic and would not take flight (no mutant for Gr21a currently exists). We instead used a Gr21a enhancer GAL4 to drive tetanus toxin (UAS-TNT) [20] to block synaptic output from neurons that coexpress Gr21a and Gr63a [18]. Surprisingly, these genetic reagents had no significant effect on CO2 tracking in flight (Figure 2J), suggesting that occluding the a3 segments (Figure 2) silences a noncanonical pathway for CO2 detection. Whereas the Gr21a-GAL4/+ and UAS-TNT/+ parent lines do not track CO2 as robustly as WT flies, neither do they spend less time in the CO2 plume by comparison to the Gr21a-GAL4/UAS-TNT flies, suggesting that the weakened CO2 tracking observed in the parent line does not contribute to the tracking behavior observed in their progeny. We next turned our attention to a previously identified acid sensor, the ionotropic receptor Ir64a that is expressed in coeloconic sensilla neurons and is activated by carbonic acid, a CO2 metabolite [21]. We examined Ir64a loss-of-function mutants [21] and observed a significant decrease in the amount of time these flies spent in a CO2 plume (Figure 2K).
The results thus far suggest that the ionotropic acid sensor Ir64a is required for CO2 tracking in flight but that the Gr21a/63a-expressing CO2 sensory neurons are not. Does occluding the antennae (Figure 2) simply knock out signaling to the Ir64a receptors, which mediates CO2 tracking autonomously? If so, then the obligate odorant coreceptor Orco [8], needed for proper functioning of olfactory receptor neurons of basiconic [22] and coeloconic sensilla [23] of the third antennal segment (a3), ought to be dispensable for CO2 tracking in flight. The loss of Orco should have no influence over CO2 attraction because neither Ir64a [21] nor the Gr21a/63a gustatory receptors require Orco [4, 18, 19, 24–26], and Orco has been shown to be dispensable for mediating CO2 aversion in walking assays [4]. In the positive control, as expected, loss of Orco abolished vinegar plume tracking (Figures 2H and 2J). Surprisingly, however, loss of Orco also abolished the CO2 plume tracking (Figures 2I and 2J). This is a peculiar result because it suggests that in addition to Ir64a, some component of sensory neurons expressing Orco, none of which have ever been shown physiologically to be sensitive to CO2, somehow gain sensitivity to this odorant in flight and cooperate with Ir64a receptors for attraction. It might seem reasonable to posit interactions between Orco-dependent olfactory receptor and Orco-independent ionotropic receptor-mediated chemosensory pathways, because recent work has shown that avoidance responses to carboxylic acids in Orco mutants are weakened by comparison to wild-type flies in walking assays [27]. Thus, it has been suggested that ionotropic receptor (IR)- and olfactory receptor (OR)-mediated olfactory pathways interact via basal activity of OR-expressing olfactory sensory neurons (OSNs) altering the downstream signals released from IR-expressing neurons [27]. Altering the gain of OR-expressing OSNs could potentially exert substantial downstream effects, resulting in varied synaptic output from both OR and IR subpopulations of olfactory neurons.
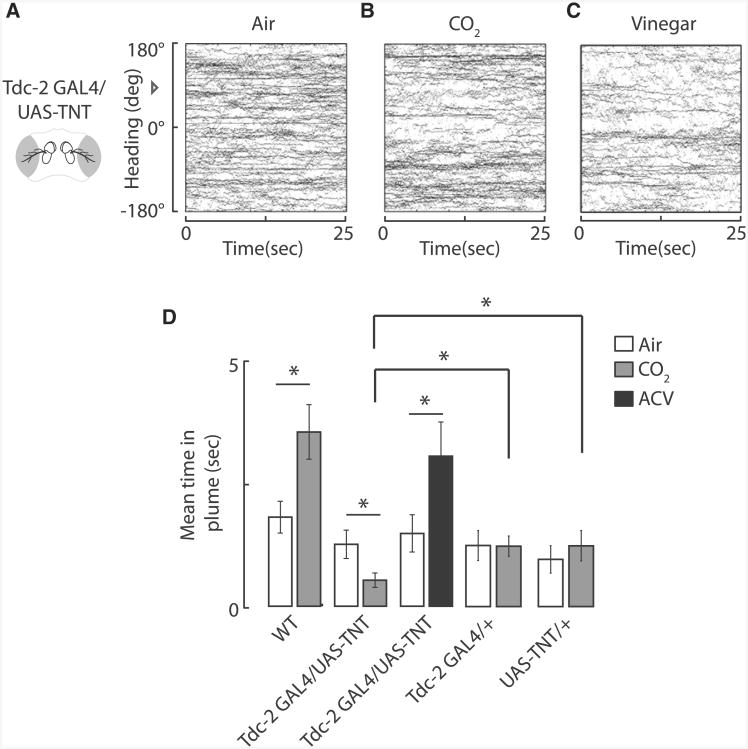
In addition to inter-receptor-class interactions, neuromodulatory pathways likely alter the effective input-output transformations of olfactory circuits. Octopamine, a potent biogenic amine, has recently been shown to modulate the response gain of identified visual interneurons in Drosophila via flight-induced activity of octopamine-releasing cells [11]. We reasoned that if CO2 sensing is modulated by flight, then blocking synaptic output from octopaminergic neurons using the Tdc2-GAL4 enhancer line driving tetanus toxin would simulate the lower levels of octopamine found in a quiescent or walking fly brain. Therefore, blocking octopamine signaling during flight should not disrupt vinegar tracking but should result in a walking-like aversive response to CO2. Indeed, Tdc2-GAL4/UAS-TNT flies actively avoid the CO2 plume (Figures 3B and 3D) while retaining attractive responses to vinegar (Figures 3C and 3D). We note that the Tdc2-GAL4/+ driver line does not spend a significantly greater amount of time in the CO2 plume by comparison to air, but this line, as well as the UAS-TNT/+ parent line, spends significantly more time in the CO2 plume in comparison to their progeny. Therefore, this experimental result cannot be fully attributable to the genetic background.
Figure 3. Loss of Synaptic Output from Octopamine Neurons Switches the Valence of CO2 from Attractive to Aversive.
(A–C) Blocking synaptic output of octopamine neurons with Tdc2-GAL4/UAS-TNT results in flies antitracking a CO2 plume, i.e., orienting away from the plume location (B) by comparison with an air plume (A) or vinegar plume (C) in tethered flight. The odor plume was positioned at 0 degrees ± 10 degrees. Flies began the trial at +90 degrees, indicated by the open arrowhead. Within-subjects design was used, heading trajectories of all individuals are plotted for a continuous odor plume of air, n = 86 trials (A), CO2, n = 84 trials (B), or vinegar, n = 50 trials (C).
(D) Tdc2-GAL4/UAS-TNT flies spend less time in a CO2 plume as compared to air. Shown is the mean time spent in the odor plume over the length of the trial (25 s) for the flies shown in (A)–(C). Error bars indicate one SEM. The same UAS-TNT/+ parent flies that were tested for CO2 plume tracking in Figure 2K were used for inactivating the octopamine neurons here. *p < 0.05 by two-way t test, n > 50 trials.
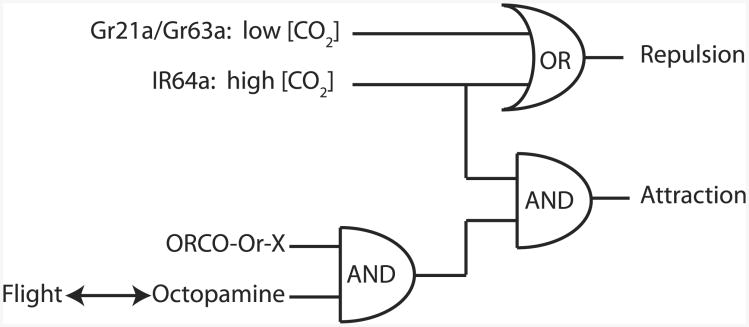
In summary, our findings demonstrate that whereas D. melanogaster may be repelled by CO2 in walking assays, they are attracted to it during flight (see Movie S1 available online). We have shown here that the in-flight tracking response requires several components, including octopamine signaling, the expression of the acid sensor Ir64a, and the olfactory coreceptor Orco (the logical organization of these interactions, as we currently understand them, is summarized in Figure 4). We envision two possible nonexclusive models of how IR and OR pathways might be working together to mediate CO2 attraction in flight. First, takeoff might engage sensitivity to CO2 within Orco-expressing olfactory receptor neurons by way of flight-initiated octopaminergic neuromodulation. This would be quite remarkable, because to date there are no candidate olfactory receptor neurons that both express Orco and respond to CO2 in quiescent recording preparations. Second, increased levels of octopamine associated with flying could change the operational gain of the OR-expressing neurons, increasing their basal activity and in turn acting to modulate IR-expressing OSNs sensitive to CO2, and vice versa. Subsequently, enhanced activity from these olfactory subpopulations could be integrated further downstream as previously hypothesized for the local interneuron network of the antennal lobe [27].
Figure 4. Summary Diagram for the Neural Mechanisms for the Switch from CO2 Aversion to Attraction.
Gr21a and Gr63a are required for mediating aversion to low concentrations of CO2, whereas Ir64 mediates aversion to high concentrations of CO2 in walking flies. A flight-induced increase in octopamine could confer CO2 sensitivity to a novel Orco-dependent olfactory receptor that along with activation of Ir64a mediates CO2 attraction during flight.
More generally, our results show that a single molecule can carry both negative and positive hedonic valence depending on the behavioral state of the animal. We posit that flight behavior is accompanied by neuromodulatory activation of the olfactory system by octopamine that rapidly shifts the function of olfactory sensory pathways in a manner similar to the operational gain and frequency response shifts triggered by locomotor activity in fly visual interneurons [9, 11, 28, 29] (Figure 4). Recent work in other organisms has identified similar roles for neuromodulators that serve to alter the state of neuronal circuits in a behaviorally contextual manner, thereby enabling computational flexibility and behavioral robustness to ever-changing internal and external environmental conditions [30]. Our findings unravel the paradox of why D. melanogaster would find an environmental signal indicating a potential food source repellent instead of attractive; for Drosophila gathered on the ground, under crowded social conditions, CO2 secreted as part of a stress pheromone releases an innate avoidance response [4, 31]. Taking flight appears to fully and rapidly switch the valence of this stimulus, triggering CO2 attraction consistent with the search for sugar-rich food resources undergoing fermentation that robustly attract D. melanogaster vinegar flies. These findings lay the groundwork for further exploring the neural substrate for a rapid and robust switch in hedonic valence.
Experimental Procedures
D. melanogaster (Meigen) reared in laboratory conditions for more than ten years were used as wild-type flies. Additional lines included Ir64a-/- and Orco-/- mutants as well as Gr21a-GAL4 and Tdc2-GAL4 driver flies and UAS-TNT (Bloomington Stock Center). Fly strains were maintained at 25°C under a 12hr:12hr light:dark cycle. Adult females 3–5 days posteclosion were selected for the experiments.
Magnetic Tether Flight Simulator and Odor Delivery
The olfactory magnetic tether arena has been previously described in detail [7,12,13, 32, 33]. Briefly, adult female flies were cold anesthetized (∼3°C), tethered to minutien pins (Fine Science Tools, item 26002-20) using UV glue (Plas-Pak Industries) cured with a UV light curing gun (ELC-410, Electro-Lite), and suspended between two rare-earth magnets, allowing for free rotation along the yaw axis. Odor was delivered using a mass-flow controlled gas multiplexer (Sable Systems) at a rate of 7 ml/min through a test tube containing filter paper saturated with 25 μl of odorant or 2 ml of aqueous solution. A vacuum set to 13 l/min (flow regulator, Cole Parmer Instruments) placed beneath the fly drew odor away. Odor intensity measurements across the horizontal plane at the level of the antennae, on a 9 × 9 mm grid at 500 mm increments, were made with a miniature photoionization detector (mini-PID) (Aurora Scientific). The tracer gas was ethanol, having an ionization potential of 10.62 eV. We sampled the grid 11 times and averaged the measurements at each point, before smoothing using piecewise linear interpolation in MATLAB (The MathWorks). A visual display of LEDs surrounded the fly in azimuth and reached 60 degrees above and below the visual horizon. Flies were illuminated for video tracking (Fire-I infrared firewire camera, Unibrain) via infrared LEDs (850 nm peak emission, DigiKey). Odor stimuli included apple cider vinegar (Ralphs grocery brand), room air, and medical-grade 100% CO2. All odorants were plumbed through nonadsorbing PTFE tubing (Small Parts), which was replaced between experiments.
Each experiment began by rotating a 30 degrees wide vertical stripe continuously around the arena for 60 s to verify that individual flies were able to freely yaw through any position within the arena. Data were collected only from flies that readily tracked the rotating bar throughout the full azimuthal extent of the arena. The vertical bar was then oscillated about a set point (either aligned with the odor nozzle at zero degrees azimuth or 90 degrees from the nozzle) for 8 s to engage a frontal fixation response to align the flies at a defined start position. The experimental odorant was activated during the initial visual positioning epoch, at which time the oscillating bar was switched off and a static visual grating (30 degrees spatial wavelength, 94% pattern contrast, 78 cd/m2) was presented for the 25 s duration of every experiment to provide visual feedback from self-motion. Each fly was run a maximum of three times through any given experiment, and individual trials were excluded if the fly stopped flying more than three times. Over 90% of the flies that were tested concluded the experiment. Antennal occlusions were performed by applying a thin layer of UV glue over the third antennal segment [16].
Analyses were performed with custom-written MATLAB software. Time in plume was calculated by summing the seconds (minimum of 1 s) spent within the defined 20-degree envelope around the nozzle.
Statistical Analyses
Comparisons of time spent in plume were made with a two-sample t test or a one-way ANOVA followed by Tukey's post hoc test. Comparisons of heading variance were compared by computing vector strength (v, [34]):
where n is the number frames in each trial and f is the mean heading at each frame. A two-sample Kolmogorov-Smirnov test was used to compare the heading distributions. All statistical analyses were performed using the MATLAB statistics toolbox.
Supplementary Material
Acknowledgments
M.A.F. is a Howard Hughes Medical Institute Early Career Scientist. We thank Daniel Malkin and Jade Nguyen for help with data collection and Brian Duistermars for contributing line art and data in Figures 1A and 1C.
Footnotes
Supplemental Information: Supplemental Information includes one movie and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2012.12.038.
References
- 1.Dekker T, Geier M, Cardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J Exp Biol. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- 2.Gillies MT. The role of carbon-dioxide in host-finding by mosquitos (Diptera, Culicidae):areview. Bull Entomol Res. 1980;70:525–532. [Google Scholar]
- 3.Takken W, Knols BGJ. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Suh GSB, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 5.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 6.Budick SA, Dickinson MH. Free-flight responses of Drosophila melanogaster to attractive odors. J Exp Biol. 2006;209:3001–3017. doi: 10.1242/jeb.02305. [DOI] [PubMed] [Google Scholar]
- 7.Duistermars BJ, Frye M. A magnetic tether system to investigate visual and olfactory mediated flight control in Drosophila. J Vis Exp. 2008:1063. doi: 10.3791/1063. http://dx.doi.org/10.3791/1063. [DOI] [PMC free article] [PubMed]
- 8.Vosshall LB, Hansson BS. Aunified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011;36:497–498. doi: 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- 9.de Haan R, Lee YJ, Nordström K. Octopaminergic modulation of contrast sensitivity. Front Integr Neurosci. 2012;6:55. doi: 10.3389/fnint.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sombati S, Hoyle G. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol. 1984;15:481–506. doi: 10.1002/neu.480150607. [DOI] [PubMed] [Google Scholar]
- 11.Suver MP, Mamiya A, Dickinson MH. Octopamine neurons mediate flight-induced modulation of visual processing in Drosophila. Curr Biol. 2012;22:2294–2302. doi: 10.1016/j.cub.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Duistermars BJ, Frye MA. Crossmodal visual input for odor tracking during fly flight. Curr Biol. 2008;18:270–275. doi: 10.1016/j.cub.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan P, Duistermars BJ, Frye MA. Odor identity influences tracking of temporally patterned plumes in Drosophila. BMC Neurosci. 2011;12:62. doi: 10.1186/1471-2202-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandawat V, Maimon G, Dickinson MH, Wilson RI. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J Exp Biol. 2010;213:3625–3635. doi: 10.1242/jeb.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasserman S, Lu P, Aptekar JW, Frye MA. Flies dynamically anti-track, rather than ballistically escape, aversive odor during flight. J Exp Biol. 2012;215:2833–2840. doi: 10.1242/jeb.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duistermars BJ, Chow DM, Frye MA. Flies requirebilateral sensory input to track odor gradients in flight. Curr Biol. 2009;19:1301–1307. doi: 10.1016/j.cub.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- 18.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 21.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 25.Stange G, Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc Res Tech. 1999;47:416–427. doi: 10.1002/(SICI)1097-0029(19991215)47:6<416::AID-JEMT5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Stensmyr MC, Giordano E, Balloi A, Angioy AM, Hansson BS. Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol. 2003;206:715–724. doi: 10.1242/jeb.00143. [DOI] [PubMed] [Google Scholar]
- 27.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung SN, Borst A, Haag J. Flight activity alters velocity tuning of fly motion-sensitive neurons. J Neurosci. 2011;31:9231–9237. doi: 10.1523/JNEUROSCI.1138-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 30.Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responsesof Drosophila to biogenic levelsof carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 32.Frye MA, Duistermars BJ. Visually mediated odor tracking during flight in Drosophila. J Vis Exp. 2009:1110. doi: 10.3791/1110. http://dx.doi.org/10.3791/1110. [DOI] [PMC free article] [PubMed]
- 33.Maimon G, Straw AD, Dickinson MH. A simple vision-based algorithm for decision making in flying Drosophila. Curr Biol. 2008;18:464–470. doi: 10.1016/j.cub.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 34.Batschelet E. Circular Statisticsin Biology. New York: Academic Press; 1981. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.