Abstract
CD13 is a large cell surface peptidase expressed on the monocytes and activated endothelial cells important for homing to and resolving the damaged tissue at sites of injury. We have previously shown that crosslinking of human monocytic CD13 with activating antibodies induces strong adhesion to endothelial cells in a tyrosine kinase- and microtubule-dependent manner. In the current study we examined the molecular mechanisms underlying these observations in vitro and in vivo. We found that crosslinking of CD13 on U937 monocytic cells induced phosphorylation of a number of proteins, including Src, FAK and ERK and inhibition of these abrogated CD13-dependent adhesion. We found that CD13 itself was phosphorylated in a Src dependent manner, an unexpected finding as its 7 amino acid cytoplasmic tail was assumed to be inert. Furthermore, CD13 was constitutively associated with the scaffolding protein IQGAP1 and CD13 crosslinkinginduced complex formation with the actin-binding protein α-actinin, linking membrane-bound CD13 to the cytoskeleton, further supporting CD13 as an inflammatory adhesion molecule. Mechanistically, mutation of the conserved CD13 cytoplasmic tyrosine to phenylalanine abrogated adhesion, Src, FAK and ERK phosphorylation and cytoskeletal alterations upon antibody crosslinking. Finally, CD13 was phosphorylated in isolated murine inflammatory peritoneal exudate cells and adoptive transfer of monocytic cell lines engineered to express the mutant CD13 were severely impaired in their ability to migrate into the inflamed peritoneum, confirming that CD13 phosphorylation is relevant to inflammatory cell trafficking in vivo. Therefore, this study identifies CD13 as a novel, direct activator of intracellular signaling pathways in pathophysiological conditions.
Introduction
During the inflammatory response following tissue injury or infectiona subset of blood leukocytes, the monocytes, migrate from the blood into tissues where they differentiate into macrophages or dendritic cells (1). These cells critically contribute to wound repair by removing matrix debris and dead cells and secreting cytokines and growth factors to enable activation of reparative cells and regeneration of damaged tissue (2-4). However, excessive or prolonged inflammation can also contribute to tissue damage, highlighting the significance of proper control of the inflammatory process. Adhesion molecules expressed on the surface of monocytes and endothelial cells play a vital role in regulating trafficking and many of the key players and regulatory processes in inflammatory trafficking have been identified (5). However, despite the extensive studies of monocyte trafficking and homing, current treatment is often inadequate suggesting that novel, alternate molecular mechanisms must exist to direct monocyte trafficking in vivo during infection and inflammation.
CD13 is a multifunctional cell surface ectopeptidase that is expressed on monocytes and macrophages, among others, and is significantly upregulated on endothelium at sites of inflammation (6). We have recently shown that independent of its enzymatic activity, CD13 can function as a homotypic adhesion molecule where it mediates monocyte/endothelial interactions in inflammatory trafficking in response to monoclonal antibody crosslinking [mAb, (7, 8)]. We and others have shown in vitro that crosslinking of monocytic CD13 alsoinduces calcium fluxes and activation of the Ras/MAPK pathway and PI-3K (Phosphatidylinositol 3-Kinase)(9, 10). Here we have examined the molecular consequences of CD13 crosslinking and signal transduction in detail and discovered that CD13 crosslinking leads to activation of the FAK, Src and ERK kinases and the tyrosine phosphorylation of CD13 itself. This phosphorylation enables CD13 to associate with cytoskeletal adapter proteins such as α-actinin and IQGAP and induces cytoskeletal changes, enabling tyrosine kinase-dependent adhesion. Importantly, mutation of the single tyrosine (Tyr6) to phenylalanine in the CD13 cytoplasmic tail completely abrogated crosslinking-induced monocytic adhesion when expressed in monocytic cells and significantly impaired trafficking to the inflamed peritoneum, further validating CD13 as a signal transducing monocytic adhesion molecule.
Materials and Methods
Reagents
Reagents were obtained from the following sources: U937 cells- ATCC (Manassas, VA); WEHI 78/24 monocytic cell line- Dr. Catherine Hedrick (La Jolla Institute for Immunology); mouse anti-human mAb CD13 (clone 452)- Dr. Meenhard Herlyn (The Wistar Institute of Anatomy and Biology, Philadelphia, PA); Anti-phosphotyrosine, anti-α actinin and anti-IQGAP1 antibodies-BD Biosciences (San Jose, CA); Control mouse IgG- Biolegend (San Diego, CA); TRITC-phalloidin, anti-GAPDH and α-tubulin antibody, fluorescent PKH26 and PKH67- Sigma (St. Louis, MO). Anti-β actin antibody- Abcam (Cambridge, MA), anti-phospho-Src and phospho-FAK (Cell Signaling, Danvers, MA). HRP-conjugated secondary antibodies- KPL (Gaithersburg, MD); Src kinase inhibitors (PP2 and Herbimycin), PD 98059 and Syk inhibitor- EMD Millipore (San Diego, CA) FAK inhibitor and anti-phospho-ERK (Santa Cruz Biotech).
Mice
CD13 global KO mice were generated at the Gene targeting and Transgenic Facility at University of Connecticut (8). For all experiments 6-8 week old FVB mice were used in accordance with Institutional and Office of Laboratory Animal Welfare guidelines.
Retroviral Vector Construction and Infection
Both full-length human (11) and mouse (12) CD13 cDNA were individually cloned into pcDNA/V5/GW/D-TOPO (Invitrogen, San Diego, CA). The V5 tagged CD13 was then excised and cloned into the retroviral expression vector pBM-IRES-Puro (13). Mutation of human and mouse CD13 tyrosine6 to phenylalanine (Y6F) was done using QuikChange II Site-Directed Mutagenesis Kits (Santa Clara, CA). High titer virus preparations were obtained using the Phoenix amphotropic packaging cell line (Orbigen, San Diego, CA) as previously described (14). For infection of WEHI-78/24 cells, 1 × 105 cells were resuspended in 5 mL virus stock in a 15 mL conical tube and centrifuged at 800 ×g for 30 min at 32 °C in the presence of 5 μg/ml polybrene. After infection, cells were cultured for 72 h in DMEM supplemented with 10% fetal bovine serum, antibiotics, L-glutamine. CD13-V5 overexpressing cells were enriched by puromycin selection (1 μg/ml for 36 h).
Quantitative cell adhesion assay and CD13 cross-linking
Monocyte adhesion assays were performed as described previously (7). In brief calcein labeled U937 monocytic cells were treated with activating anti-CD13 452 mAb for 30 min with or without kinase inhibitor pretreatment, washed and allowed to adhere to human CD13 expressing C33A monolayer cells for indicated time intervals, lysed and fluorescence read at 485/530 nm and expressed as relative fluorescence unit (RFU).
For cross-linking of CD13 on U937 or WEHI 78/24 monocytes, cells were incubated with control IgG or anti-CD13 452 mAb in buffer A (HBSS, 20.0 mM HEPES and 0.1 % BSA) or culture medium with 10.0% FBS for indicated time at 37°C in a humidified 5% CO2 incubator.
Immunoblotting
Immediately after cross-linking, the reaction was stopped by adding 5 mL of cold PBS and washed once. Cells were lysed in 1.0% NP-40 lysis buffer (20.0 mM HEPES pH 7.4, 150 mM NaCl and 1.0% NP-40) with protease inhibitor cocktail (Roche) and phosphatase inhibitors. Lysates were cleared by centrifugation at 7,000 rpm for 15 min. Proteins or immunoprecipitates were diluted with 4× sample buffer and resolved by 10% SDS-PAGE and electrotransfered onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA) followed by probing with the relevant primary Ab (1:1000), followed by HRP-conjugated secondary Ab (1:5000) and detected using the ECL- kit (Thermoscientific, USA). Bands were quantitated using NIH Image J software.
Immunoprecipitation
To study the CD13 tyrosine phosphorylation and interactions with α-actinin and IQGAP1, lysates were incubated overnight at 4°C with biotinylated anti-CD13 or biotinylated anti-phosphotyrosine antibody. Streptavidin-agarose beads were added to the lysates and incubated for 1 h at 4°C. Beads were washed three times with wash buffer (25.0 mM TrisCl pH 8.0, 140 mM NaCl and 0.1% NP-40) and resuspended in Laemmle sample buffer.
Immunofluorescence
WEHI monocytic cells were fixed in 4% PFA for 10 min and permeabilized with Triton X-100 (0.1% in PBS for 4 min), blocked with 2% BSA in PBS (37°C for 1 h). Cells were incubated with TRITC labeled phalloidin (for 1 h at 37°C), washed and cytospin. Images were acquired with Zeiss Axiocam camera (0.63× magnification) and processed by Zeiss Axiovision software.
Peritonitis
Thioglycollate (1 mL; 4%) was injected into the peritoneal cavity. Cells were collected at different times and used for western blot or flow cytometry analysis after adoptive transfer.
Flow Cytometry
Transfected WEHI monocytic cells were labeled with 452 anti-CD13 mAb to detect expression of human CD13 followed by FITC-conjugated goat anti-mouse secondary antibodies. Differentially labeled WEHI monocytes stably expressing murine CD13 (mCD13) or mCD13-Y6F accumulated in the peritoneal lavage or peripheral blood were analyzed by flow cytometry. Untransfected WEHI cells labeled with PKH26 (red) and PKH67 (green) were used as compensation controls. Flow cytometry was performed on either Calibur or LSRII (Becton Dickinson). Data were analyzed using Flow-Jo software (Tristar).
Statistical analysis
Statistical differences between groups were analyzed by using unpaired, two-tailed t test or one way ANOVA. Differences were considered significant at p<0.05.
Results
Key signaling molecules are phosphorylated upon in vitro cross-linking of CD13 in monocytic cell lines
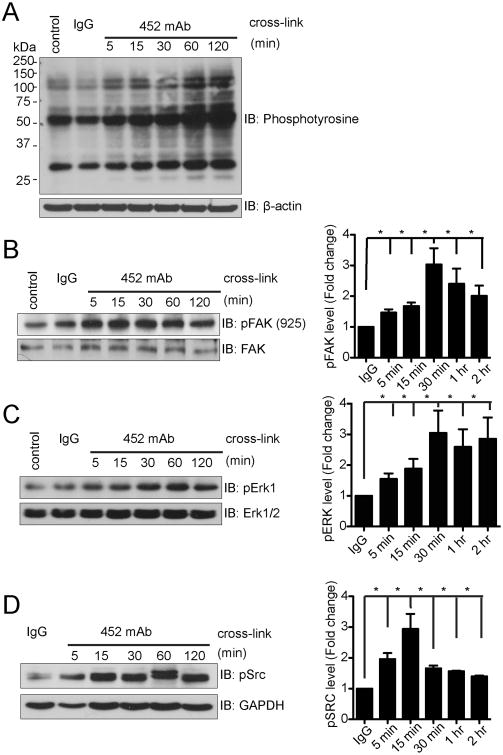
We have previously shown that monoclonal antibody crosslinking of CD13 on primary human monocytes or monocytic cell lines induces their homotypic adhesion to endothelial cells or epithelial cell lines engineered to express human CD13 in a tyrosine kinase-dependent manner (7, 10). To begin to characterize the signal transduction pathways induced by CD13 crosslinking, we probed lysates of U937 human monocytic cells for tyrosine-phosphorylated proteins following treatment with the activating mAb 452. Results indicated thata number of distinct proteins were tyrosine phosphorylated in a time dependent manner (Fig. 1A). The molecular weights of several of the phosphorylated bands correlated with known phosphoproteins that are associated with adhesion, such as focal adhesion kinase (FAK, 125 kD), Extracellular signal-regulated kinase (ERK1/2, 42-44 kD) and Src kinase (60 kD). Further analysis indicated that indeed, treatment of U937 cells with the activating CD13 mAb 452 resulted in a temporal increase in phosphorylation of FAK, ERKand Src kinases (Figs. 1B-D) suggesting that these kinases participate in CD13-induced signal cascades.
Figure 1. CD13 crosslinking induces tyrosine phosphorylation.
U937 monocytic cells were stimulated by crosslinking CD13 with anti CD13 452 mAb (1.0 μg/mL) or control IgG (1.0 μg/mL) for the indicated periods of time. Samples were resolved on SDS-PAGE gels and analyzed by Western blotting using: A) anti-phosphotyrosine, B) anti-phospho-FAK Tyr925 and C) anti-phospho ERK1, D) anti-phospho Src Tyr416. Graphs of quantitative data combine 3 separate experiments.
Monocytic CD13 is phosphorylated in response to crosslinking
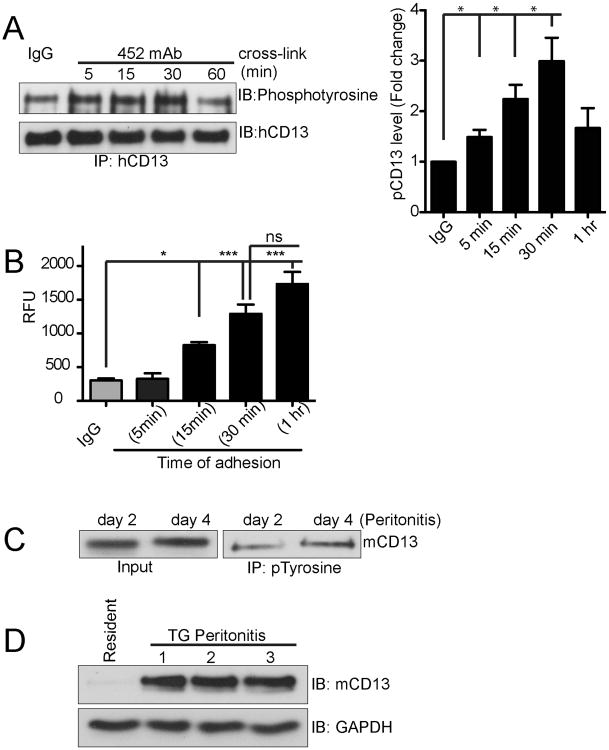
Antibody-mediated cross-linking of a number of classical cell surface adhesion molecules stimulates their phosphorylation (15, 16). We observed that the size of a fourth prominently phosphorylated band was consistent with CD13 itself (130-150kD). Interestingly, probing complexes immunoprecipitated from 452 mAb-treated U937 cells using an epitope-distinct anti-CD13 mAb for phosphotyrosine showed thatCD13is inducibly phosphorylated upon crosslinkingin a time dependent manner, peaking at 30 minutes and decreasing thereafter (Fig. 2A). This pattern mimics the kinetics of crosslinking-induced U937 adhesion to C33A epithelial monolayers (CD13 negative) engineered to express humanCD13 [Fig 2B and ref (7)]. To establish the potential in vivo relevance of CD13 phosphorylation in inflammatory conditions where monocyte adhesion to endothelial cells is critical, we immunoprecipitated cell lysates ofthe infiltrating peritoneal exudate cells of thioglycollate-treated animals with anti-phosphotyrosine and probed for CD13 (Fig. 2C), indicating that CD13 is phosphorylated in vivo under inflammatory conditions and thus may participate in adhesion of monocytes during inflammation. In addition, CD13 expression is markedly higher in activated peritoneal exudate cells when compared to resting cells (Fig 2D), consistent with the expression of CD13 on infiltrating inflammatory cells at inflammatory sites.
Figure 2. CD13 is tyrosine phosphorylated upon 452-mAb crosslinking.
A) U937 cells were treated for the indicated times with 452 mAb (1.0 μg/mL) or control IgG (1.0 μg/mL). Lysates were immunoprecipitated with biotinylated anti-CD13 mAbs. Samples were resolved on SDS-PAGE gels and analyzed by Western blotting using anti-phosphotyrosine and CD13 antibodies. Quantitative data combine 3 separate experiments. B) Time course of CD13 crosslinking-induced adhesion of U937 monocytes. Calcein labeled U937 monocytes were treated with activating 452 mAb for 30 min and after washing, allowed to adhere to human CD13 expressing C33A monolayer cells for indicated periods of time, lysed and calcein fluorescence read at 485/530 nm and expressed as relative fluorescence unit (RFU). *; p<0.05, ***; p<0.001, ns; not significant. C) Peritoneal lavage cells collected from mice with thioglycollate induced peritonitis at day 2 or 4, were lysed and immunoprecipitated with biotinylated anti-pTyr antibody and probed with the anti-mouse CD13 antibody, SL13. D) Peritoneal lavage cells were collected from thioglycollate treated (48h) or untreated controls (resident immune cells) and probed for mCD13 by western blot analysis. n=3 mice for TG treated, resident cells were pooled from 3 mice.
Src kinase mediates crosslinking stimulated CD13 phosphorylation and cell adhesion
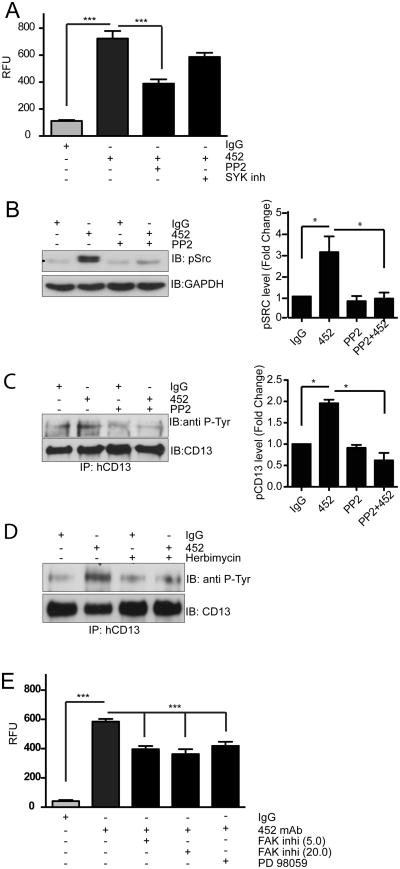
Src and spleen tyrosine kinase (SYK) are the primary tyrosine kinases that phosphorylate the cell surface molecules mediating leukocyte adhesion (17). To determine whether these kinases werealso responsible for CD13 phosphorylation and cell adhesion, we initially investigated the effect of specific inhibitors of these kinases on the 452-activated adhesion of U937 monocytes to CD13-expressing monolayers. The Src kinase inhibitor PP2 significantly blocked 452 mAb-induced adhesion while inhibition of SYK had no effect (Fig. 3A). Furthermore, pretreatment of 452 mAb-induced monocytic cells with the Src kinase inhibitors, PP2 or Herbimycin, blocked Src activation as well as CD13 phosphorylation, supporting an important role for Src in CD13 crosslinking-induced adhesion (Figs 3B-D). Finally, treatment of mAb-induced U937 cellswith specific inhibitors of FAK and MEK also decreased their adhesion to CD13+ monolayers, suggesting that activation of these kinases by CD13 crosslinking is required for CD13-dependent monocyte adhesion (Fig 3E).
Figure 3. Src Kinase mediates CD13 tyrosine phosphorylation and adhesion.
A) U937 monocytes were pretreated with vehicle or the Src kinase inhibitor PP2 (10.0 μM) or the SYK kinase inhibitor (10.0 μM) for 30 min, crosslinked with 452 mAb and allowed to adhere to human CD13 expressing C33A monolayer cells, lysed and fluorescence quantitated. Results were expressed as relative fluorescence units (RFU). ***; p<0.001.B-D) U937 monocytes were pretreated with vehicle or Src Kinase inhibitors PP2 (B, C 10.0 μM) or Herbimycin (D, 10.0 μM) for 30 min, crosslinked with 452 mAb for 30 min and lysates probed for B) phospho-Src or C, D) immunoprecipitated with biotinylated anti-CD13 Abs. Immunoprecipitated samples were probed with anti-pTyr or anti-CD13 antibodies. E) U937 cells were pretreated with vehicle, FAK inhibitor 14 (5.0/20.0 μM) or MEK1 inhibitor PD98059 (10.0 μM) for 30 min, crosslinked with 452 mAb and allowed to adhere to human CD13-expressing C33A monolayer cells, lysed and fluorescence read at 485/530 nm. Results were expressed as relative fluorescence unit (RFU). ***; p<0.001. Graphs of quantitative Western blot data combine 3 separate experiments.
CD13 associates with cytoskeletal adaptor proteins
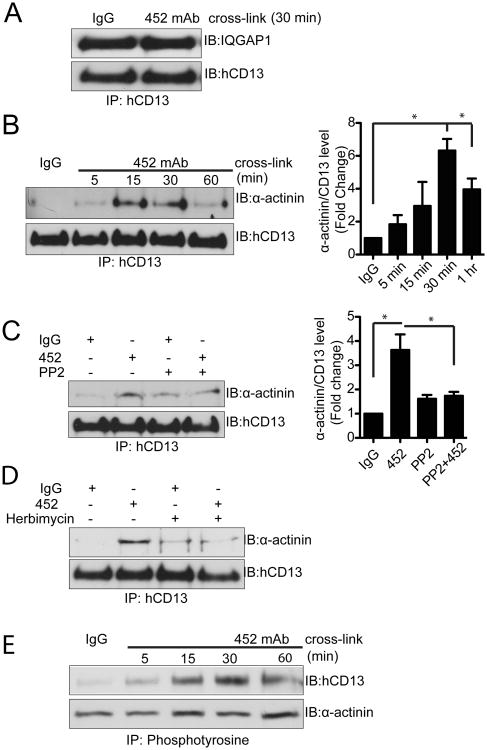
Actin polymerization is strictly required for monocyte adhesion to target cells and binding affinity is often mediated by the nature of the cytoskeletal adaptor proteins that link adhesion molecules to the cytoskeleton (18, 19). For example, “inside-out” activation of the integrin adhesion molecules involves exchange of intracellular adaptor proteins which induces conformational changes in the integrin extracellular domains, enabling high affinity interactions (20). Similarly, antibody activation induces the formation of numerous actin rich protrusions and that inhibition of microfilament assembly in monocytic cells abrogates CD13-induced adhesion, demonstrating that CD13-induced signal transduction induces cytoskeletal alterations that contribute to adhesion (7). To determine if CD13 also interacts with cytoskeletal adaptors, we analyzed proteinsthat co-immunoprecipitated with CD13 in lysates from U937 monocytes treated with activating mAb by Mass Spectrometry (data not shown). One protein identified in our proteomic analysis of the CD13-containing complex was the scaffold protein IQGAP1 that coordinates protein-protein interactions to regulate microtubule networks, actin cytoskeletal organization and cell-cell adhesion (21-23). Co-immunoprecipitation and Western blot analysis indicated that CD13 and IQGAP1 are present in the same complex, and that this interaction appears to be constitutive (Fig 4A). Alternatively, tyrosine phosphorylation of a second protein identified in our analysis, α-actinin, regulates its binding to integrin and thus controls inside out signaling (24). We confirmed that CD13 and α-actinin do indeed co-immunoprecipitate by western blotting (Fig. 4B) and that this interaction isdependent upon Src kinase phosphorylation astreatment withPP2 or herbimycin blocks the interaction (Figs 4C, D). Finally, both CD13 and α-actinin are tyrosine phosphorylated in the complex (Fig 4E). This crosslinking-dependent complex formation may suggest that, similar to integrins, alterations in CD13 cytoskeletal tethers upon crosslinkingmay be responsible for the increase in adhesion. Taken together, these data would support a model where CD13 is linked to the cytoskeleton in resting cells via a complex including IQGAP1 and thatCD13 crosslinking induces adhesion by enabling its interaction with α-actinin and perhaps other cytoplasmic adaptor proteins to affect cytoskeleton and microtubule-polymerization, resulting in increased adhesion.
Figure 4. CD13 forms complexes with cytoskeletal adapter proteins.
U937 cells were crosslinked with control IgG or anti-CD13 452 mAb (1.0 μg/mL) for indicated period of time, lysates immunoprecipitated with biotinylated anti-CD13 mAbs and probed for A) IQGAP1 and CD13 or B)α-actinin and CD13. C, D) U937 monocytes were pretreated with vehicle or Src Kinase inhibitors PP2 or Herbimycin for 30 min, crosslinked with control IgG or 452 mAb for 30 min, lysates were immunoprecipitated with biotinylated anti-CD13 mAbs and probed for α-actinin and CD13. E) U937 cells were cross-linked with control IgG or anti CD13 452 mAb for the indicated periods of time, lysates immunoprecipitated with biotinylated anti-pTyr antibody and probed for α-actinin1 and CD13. Graphs of quantitative Western blot data combine 3 separate experiments.
Tyrosine 6 in the CD13 cytoplasmic tail is essential for crosslinking mediated adhesion and activation of SRC, FAK and ERK kinases
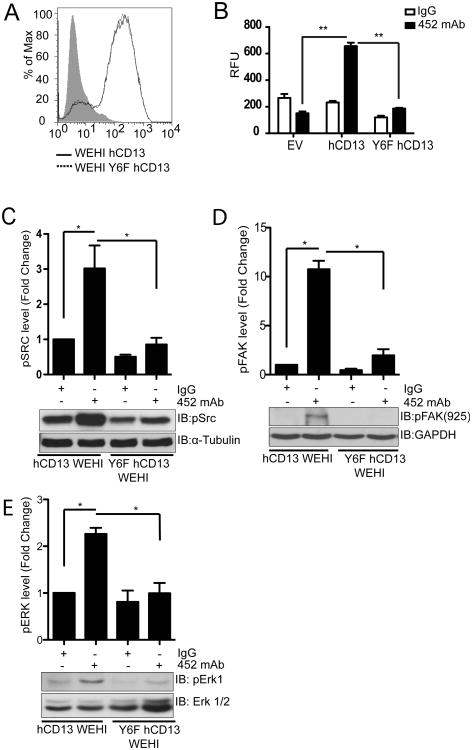
Our next task was to identify which tyrosine residue in CD13 is phosphorylated and the functional relevance of this modification. While CD13 contains a total of 45 tyrosine residues, only Tyr6 (in a region conserved in humans and mice) is intracellular and thus likely to be modified. We produced expression constructs encoding either wild type human CD13 cDNA or containing a point mutation converting Tyr6 to a nonphosphorylatable phenylalanine residue (Y6F) and created murine monocytic WEHI 78/24 cell lines (CD13lo) stably expressing either wild type or Y6F human CD13. FACS analysis indicated cell surface expression of both constructs at equivalent levels (Fig 5A). Analysis of these cell lines in our adhesion assay revealed that mutation of Tyr6 in the cytoplasmic tail completely abrogated crosslinking induced U937 monocyte adhesion to hCD13 expressing C33A monolayer cells (Fig. 5B).
Figure 5. Mutation of the CD13 cytoplasmic tail attenuates crosslinking-induced monocyte adhesion and signaling.
A) WEHI 78/24 mouse monocytic cells stably expressing human CD13 (hCD13) or human CD13 with tyrosine6 mutated to phenylalanine (Y6F) were analyzed for hCD13 expression by flow cytometry. B) Calcein labeled empty vector (EV) or hCD13 or Y6F hCD13 expressing cells were treated with control IgG or activating 452 mAb (5.0 μg/mL) for 30 min, washed, allowed to adhere to human CD13 expressing monolayer cells for 30 min, lysed and fluorescence read at 485/530 nm. **; p<0.01. C-E) hCD13 or Y6F hCD13 expressing WEHI cells were treated with IgG or 452 mAb for 30 min. and analyzed by western blot using C) anti-phospho-Src, D) anti phospho-FAK Tyr925 and E) anti-phospho ERK1. Data combine 3 separate experiments.
Because CD13 crosslinking in monocytes induced activation of the Src, FAK and ERK kinases and their inhibition blocked subsequent adhesion, we asked if CD13 phosphorylation was also required for the activation of these kinases. Crosslinking of the WEHI cell lines expressing WT-hCD13 and the Y6F mutant with the 452 mAb demonstrated that levels of Src, FAK and ERK phosphorylation were clearly reduced in Y6F mutants after cross-linking (Figs.5C-E), indicating that CD13 phosphorylation at Tyr6 induces signaling cascades resulting in increased adhesion.
CD13 phosphorylation is required for crosslinking-induced cytoskeletal changes
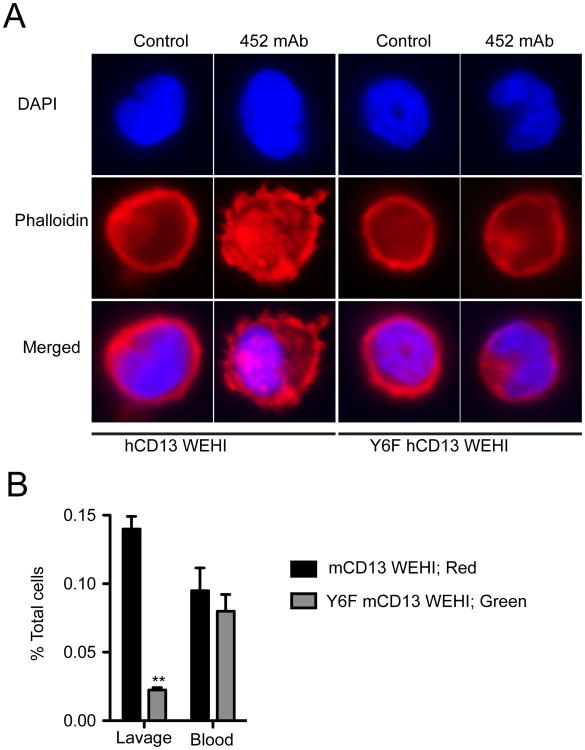
As mentioned previously, we have shown that CD13 crosslinking induces striking cytoskeletal changes as detected by phalloidin staining (7). To determine if CD13 phosphorylation was necessary for this aspect of monocyte activation, we treated the wild type or Y6F CD13 cell lines with mAb 452, stained with TRITC-phalloidin and DAPI and observed them by confocal microscopy (Fig 6A). Indeed, abrogation of CD13 phosphorylation disrupts the actin rearrangements induced in cells expressing wild type CD13, consistent with the notion that crosslinking-dependent phosphorylation of CD13 enables its linkage to the actin cytoskeleton and increases adhesion.
Figure 6. CD13 tyrosine mutants (Y6F) do not undergo cytoskeletal changes and show impaired trafficking in vivo.
A) hCD13 or Y6F hCD13 expressing WEHI cells were treated with antiCD13 452 mAb (5.0 μg/mL) for 15 min at 37°C, fixed, permeabilized, and stained with TRITC-conjugated phalloidin. Blue; DAPI (nucleus) and Red; Phalloidin. Data are representative of 2 separate experiments. B) Equal numbers of WEHI CD13 negative mouse monocytes stably expressing murine CD13 (mCD13) or mCD13-Y6F were differentially labeled with the fluorescent dyes PKH26 (red) and PKH67 (green) and adoptively transferred into mice via tail vein, followed by thioglycollate injection. After 48h, peritoneal lavage and peripheral blood cells were subjected to flow cytometric analysis (n=4 mice). **; p<0.01.
CD13 phosphorylation is required for monocytic cell trafficking to inflammatory sites in vivo
Ligand binding by many receptors induces their dimerization or clustering to initiate downstream signaling and these signals can often be recapitulated by antibody treatment (16, 25). While we believe that monoclonal antibody crosslinking of CD13 similarly mimics the response to ligand, to date it remains an orphan receptor. To determine the in vivo relevance of CD13 activation, we produced additional WEHI cell lines expressing wild type or Y6F CD13murine cDNA. Adoptive transfer of mixtures of equal numbers of differentially labeled populations into wild type animals subjected to thioglycollate-induced peritonitis showed a marked inability of the mutant cells to infiltrate the peritoneal cavity when compared to the wild type cells (Fig 6B). Importantly, experiments in which the dyes were switched confirmed this data (not shown). Finally, equivalent numbers of both groups of labeled cells remaining in the peripheral blood argues against an increase in antibody dependent cell death upon mAb treatment of the cells prior to injection. Taken together, these results indicate that crosslinking of CD13 leads to its phosphorylation, activation of downstream kinases, interactions with cytoskeletal adaptor proteins which are required for monocyte adhesion and trafficking to inflammatory sites (Fig 7).
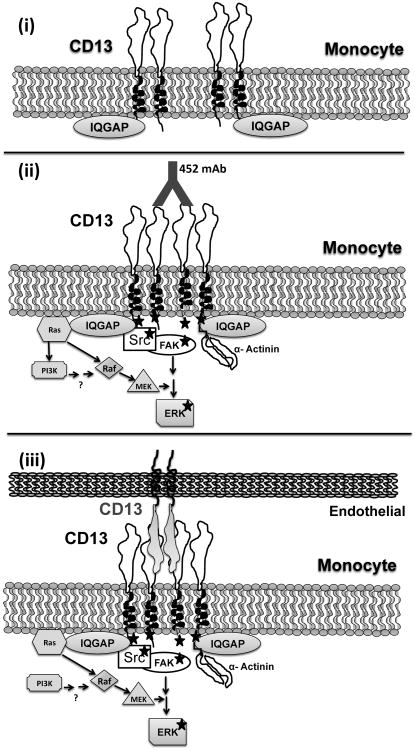
Figure 7. Schematic representation of CD13 crosslinking-induced signaling and adhesion.
i) In resting monocytic cells CD13 is present in association with IQGAP1. ii) mAb crosslinking of CD13 induces clustering of CD13, activation of Src kinase, tyrosine phosphorylation of CD13, activation of FAK and ERK kinases and complex formation between α-actinin and CD13, and potentially other components of the Ras/MAPK and PI-3K pathways as has been reported (9, 10), resulting in iii), increased adhesion of monocytic CD13 to endothelial or monolayer CD13.
Discussion
Adhesion of monocytes to endothelial cells during homeostasis and inflammation is a carefully regulated process that involves the participation of cell surface adhesion molecules, which are clustered and tyrosine phosphorylated upon ligand binding to increase their adhesive capacity (15, 16). Independent of its enzymatic activity, we have found that CD13 is also a homotypic adhesion molecule where crosslinking of monocytic CD13 with an activating monoclonal antibody increases its adhesion to endothelial cells in a tyrosine kinase and microtubule-dependent manner (7, 10). The goal of the present study was to dissect the molecular consequences of CD13 crosslinking and the mechanisms regulating this increased adhesiveness. Using the U937 monocytic cell line and the activating mAb 452 we found that mAb treatment induces activation of the FAK, ERK and Src tyrosine kinases and Src-dependent tyrosine phosphorylation ofTyrosine6 in the CD13 cytoplasmic domain. We demonstrate that CD13 constitutively complexes with the multifunctional cytoskeletal adapter protein IQGAP and inducibly with the actin-binding protein α-actinin in a Src kinase-dependent manner. Mutation of Tyr6to phenylalanine in the CD13 cytoplasmic tail abrogates 452 mAb-stimulated FAK, ERK and Src activation and abrogates monocytic cell adhesion in vitro and in vivo, providing further evidence for CD13as a bona fide inflammatory adhesion molecule.
Although it has been previously shown that crosslinking CD13 in monocytes activates calcium fluxes, the Ras-MAP kinase pathway and PI-3 kinase (9, 10) the molecular mechanisms and functional consequences of these observations have not been addressed. The participation of both of these pathways likely reflects the extensive crosstalk and feedback regulation between ERK and PI3K signaling downstream of RAS [reviewed in ref (26)]. In the current study, we added an integral component of the Ras-MAPK pathway, ERK, to the list of downstream molecules activated by CD13 and illustrated that it is required forCD13-mediated adhesion. Interestingly, the other kinases implicated in CD13 adhesion are also closely interconnected. Src has been found to bind to FAK via the Src-SH2 and stimulate subsequent FAK phosphorylation, generating binding sites on FAK for SH2 domains of other downstream molecules, including PI3K (Phosphatidylinositol 3-Kinase) and Rac. Rac and Raf promote activation of the ERKs through phosphorylation of MEK1 and Raf1 to activate ERK1/2 via MEK1/2 (27). Furthermore, FAK has been implicated in a number of PI3K-dependent functions (26). Finally, Ras and Src have been implicated in the induction of calcium fluxes in various cell types (28-30), further linking our findings with the published studies.
Tyrosine phosphorylation of surface proteins upon crosslinking of cell surface molecules by mAbs or ligand is one of the earliest events in the activation of leukocytes during inflammation (31-33). However, CD13's extremely short cytoplasmic tail (seven amino acids) has long been assumed to be too small to be modified or to interact with the cytoskeleton in any meaningful way (6). Surprisingly, we found that the single juxtamembrane tyrosine residue of the CD13 cytoplasmic tail was indeed phosphorylated and that this modification was necessary for both the increased adhesion in response to mAb crosslinking and the trafficking of monocytes to the inflamed peritoneum. Furthermore, we demonstrated that CD13 is likely phosphorylated by the Src nonreceptor protein tyrosine kinase that is commonly activated in response to stimulation of a variety of cell surface receptors such as tyrosine kinase receptors, integrin receptors, and G protein-coupled receptors, and by cellular stress (34). Src also phosphorylates well-known adhesion molecules like ICAM1 and CD31 upon antibody cross-linking (16, 25). Finally, the activation of adhesion molecules stimulates downstream signaling pathways that are required for adhesion. Similarly, we found that mAb crosslinking of monocytic CD13 induced the activation of the Src, FAK and ERK intracellular signaling proteins that have well-established roles in inflammatory adhesion (35). Therefore, mechanisms induced by crosslinking of CD13 appear to replicate the mechanisms and interactions of classical adhesion processes.
We found that CD13 is present in a complex with the cytoskeletal adaptor proteins IQGAP1 and α-actinin. IQGAP1 is a multifunctional scaffolding protein that has been implicated in numerous cellular processes underlying receptor activation, signal transduction and cell adhesion [reviewed in ref (36)]. Interaction of IQGAP with its various transmembrane receptor partners can be constitutive or regulated by ligand binding, and is often required for receptor activation. Its constitutive association with CD13 in U937 cells suggests that CD13 activation is not necessary for complex formation, but whether this interaction is necessary for CD13 phosphorylation remains to be determined. Interestingly, c-Src has been shown to bridge interactions between IQGAP and VEGFR2 (37), which may be relevant to Src-dependent CD13 phosphorylation. IQGAP1 is a prominent regulatory scaffold for the MAPK pathway where it directly binds MEK-1 and ERK1/2 which again may be pertinent to signaling downstream of CD13. Finally, IQGAP coordination of the Rho family of small GTPases as well as direct interactions with actin, microtubules and cell adhesion molecules allow it to regulate many processes underlying cell spreading, adhesion, and migration (36, 38, 39).
Similarly, α-actinin is a prominent actin binding protein that interacts with many additional partners to regulate cell-cell adhesion among many other cellular processes. α-actinin is localized to the cytoplasmic face of many sites of cell-cell and cell extracellular matrix adhesion, including leukocyte-endothelial contact sites where it binds to the cytoplasmic tails of the classical as well as non-classical adhesion molecules to link them to actin and microtubules (40-45). At these sites α-actinin serves to strengthen the site, maintain cell shape, bind and organize signaling molecules at adhesion sites, increase avidity by clustering adhesion molecules as well as control the activity of independent receptors. The notion that similar interactions may regulate CD13-dependent adhesion is supported by previous studies from our laboratory demonstrating that CD13 crosslinking in U937 cells induced the formation of numerous actin rich protrusions visualized by staining with fluorescently-labeled phalloidin (7). In addition, disruption of microtubule organization by cytochalasin D completely blocked crosslinking-induced adhesion of U937 monocytic cells (7, 10), suggesting these interactions are important for adhesion. Tyrosine phosphorylation also regulates α-actinin's function. Phosphorylation of α-actinin at Y12 enables the recruitment of Src to β1-integrin, and the subsequent interaction of FAK with Src to augment FAK phosphorylation, thus contributing to cell adhesion (46, 47). In other studies, α-actinin is phosphorylated by integrin-activated FAK, reducing its binding to actin (48). We demonstrate that a tyrosine-kinase dependent interaction complex forms upon CD13 activation that contains both phosphorylated CD13 and α-actinin, but does not distinguish if phosphorylation of both is required for these interactions. Taken together, the association of IQGAP and α-actinin with CD13 may underlie many of the adhesive and cytoskeletal changes seen upon CD13 crosslinking.
The activation of a number of cell surface adhesion molecules induces a rearrangement of the adaptor proteins linking their cytoplasmic domains to the actin cytoskeleton (49-52). First described for integrin heterodimers which are in a closed, low affinity conformation in the resting state; activation induces unclasping of their cytoskeletal tethers, forcing a spatial separation of the alpha and beta chain cytoplasmic domains leading to extracellular conformational changes and increased binding affinity (53-55). Similarly, it is possible that clustering-induced CD13 activation may provoke an analogous conformational change to facilitate adhesion. Supporting evidence comes from the recent solution of the X-ray structure of the human CD13 homodimer, revealing that it forms an arch-like structure on the cell surface that can also assume both an open and closed conformation (56). Conversion from one conformation to the other leads to a results in a large, 50Å spatial change in the interval between the membrane anchoring domains, a conformational change that the authors predicted to be sufficient to trigger signal transduction. While no predictions were made regarding the adhesion capabilities of the open and closed conformations, the potential for conformational change upon activation is consistent with known properties of inflammatory adhesion molecules.
Finally, while antibody crosslinking of receptors and adhesion molecules often mimics the ligand binding-dependent activation of signal transduction cascades, no ligand has yet been identified for CD13. Our demonstration that CD13 is phosphorylated in the infiltrating exudate cells of the inflammatory peritoneum and that mutation of the Tyr6 phosphorylation site abrogates the homing of adoptively transferred, untreated monocytic cells supports the in vivo relevance of the current study and confirms that monocytic CD13 is activated under inflammatory conditions in vivo. In support of this notion, CD13 is the receptor for the human coronavirus HCov229E and we have shown that virus binding induces monocyte adhesion (7, 57). The fact that a number of viruses exploit adhesion molecules as receptors to gain access to tissues (58-64) reinforces CD13-activated adhesion as a physiological process. Therefore, our demonstration that CD13 phosphorylation underlies its signal transduction and inflammatory adhesion functions licenses CD13 as a novel, direct activator of intracellular signaling pathways in pathophysiological conditions. Further studies will determine how this unique inflammatory adhesion and signaling molecule integrates with classical mechanisms of inflammatory cell trafficking.
Acknowledgments
The authors express our appreciation to Dr. Kevin Claffey's laboratory for use of their microscope. In addition we thank the staff of the Center for Comparative Medicine (CCM) and Gene Targeting and Transgenic Facility.
Sources of funding: This work was supported by Public Health Service grants CA-106345 from the National Cancer Institute and HL-70694 (LHS) from the National Heart, Lung and Blood Institute.
References
- 1.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 4.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 5.Muller WA. Mechanisms of leukocyte transendothelial migration. Annual review of pathology. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 7.Mina-Osorio P, Winnicka B, O'Conor C, Grant CL, Vogel LK, Rodriguez-Pinto D, Holmes KV, Ortega E, Shapiro LH. CD13 is a novel mediator of monocytic/endothelial cell adhesion. J Leukoc Biol. 2008;84:448–459. doi: 10.1189/jlb.1107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winnicka B, O'Conor C, Schacke W, Vernier K, Grant CL, Fenteany FH, Pereira FE, Liang B, Kaur A, Zhao R, Montrose DC, Rosenberg DW, Aguila HL, Shapiro LH. CD13 is dispensable for normal hematopoiesis and myeloid cell functions in the mouse. J Leukoc Biol. 2010;88:347–359. doi: 10.1189/jlb.0210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos AN, Langner J, Herrmann M, Riemann D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell Immunol. 2000;201:22–32. doi: 10.1006/cimm.2000.1629. [DOI] [PubMed] [Google Scholar]
- 10.Mina-Osorio P, Shapiro LH, Ortega E. CD13 in cell adhesion: aminopeptidase N (CD13) mediates homotypic aggregation of monocytic cells. J Leukoc Biol. 2006;79:719–730. doi: 10.1189/jlb.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Look AT, Ashmun RA, Shapiro LH, Peiper SC. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. The Journal of clinical investigation. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Kinzer CA, Paul WE. p161, a murine membrane protein expressed on mast cells and some macrophages, is mouse CD13/aminopeptidase N. J Immunol. 1996;157:2593–2600. [PubMed] [Google Scholar]
- 13.Caromile LA, Oganesian A, Coats SA, Seifert RA, Bowen-Pope DF. The neurosecretory vesicle protein phogrin functions as a phosphatidylinositol phosphatase to regulate insulin secretion. J Biol Chem. 2010;285:10487–10496. doi: 10.1074/jbc.M109.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Florey O, Durgan J, Muller W. Phosphorylation of leukocyte PECAM and its association with detergent-resistant membranes regulate transendothelial migration. J Immunol. 2010;185:1878–1886. doi: 10.4049/jimmunol.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood. 2012;120:1942–1952. doi: 10.1182/blood-2011-12-397430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Munoz ME, Salas-Vidal E, Salaiza-Suazo N, Becker I, Pedraza-Alva G, Rosenstein Y. The CD43 coreceptor molecule recruits the zeta-chain as part of its signaling pathway. J Immunol. 2003;171:1901–1908. doi: 10.4049/jimmunol.171.4.1901. [DOI] [PubMed] [Google Scholar]
- 18.Watson JM, Harding TW, Golubovskaya V, Morris JS, Hunter D, Li X, Haskill JS, Earp HS. Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J Biol Chem. 2001;276:3536–3542. doi: 10.1074/jbc.M006916200. [DOI] [PubMed] [Google Scholar]
- 19.Vicente-Manzanares M, Sanchez-Madrid F. Role of the cytoskeleton during leukocyte responses. Nat Rev Immunol. 2004;4:110–122. doi: 10.1038/nri1268. [DOI] [PubMed] [Google Scholar]
- 20.Hu P, Luo BH. Integrin bidirectional signaling across the plasma membrane. J Cell Physiol. 2012 doi: 10.1002/jcp.24154. [DOI] [PubMed] [Google Scholar]
- 21.Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO reports. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 23.Mateer SC, Wang N, Bloom GS. IQGAPs: integrators of the cytoskeleton, cell adhesion machinery, and signaling networks. Cell motility and the cytoskeleton. 2003;55:147–155. doi: 10.1002/cm.10118. [DOI] [PubMed] [Google Scholar]
- 24.Tadokoro S, Nakazawa T, Kamae T, Kiyomizu K, Kashiwagi H, Honda S, Kanakura Y, Tomiyama Y. A potential role for alpha-actinin in inside-out alphaIIbbeta3 signaling. Blood. 2011;117:250–258. doi: 10.1182/blood-2009-10-246751. [DOI] [PubMed] [Google Scholar]
- 25.Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- 26.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes & cancer. 2011;2:261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuderland D, Seger R. Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Molecular biotechnology. 2005;29:57–74. doi: 10.1385/MB:29:1:57. [DOI] [PubMed] [Google Scholar]
- 28.Rausch DM, Lewis DL, Barker JL, Eiden LE. Functional expression of dihydropyridine-insensitive calcium channels during PC12 cell differentiation by nerve growth factor (NGF), oncogenic ras, or src tyrosine kinase. Cell Mol Neurobiol. 1990;10:237–255. doi: 10.1007/BF00734577. [DOI] [PubMed] [Google Scholar]
- 29.Rosado JA, Graves D, Sage SO. Tyrosine kinases activate store-mediated Ca2+ entry in human platelets through the reorganization of the actin cytoskeleton. Biochem J. 2000;351(Pt 2):429–437. [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J, Park JH, Kwon OY, Kim S, Chung JH, Lim DS, Kim KS, Rhim H, Han YS. T-type calcium channel trigger p21ras signaling pathway to ERK in Cav3.1-expressed HEK293 cells. Brain Res. 2005;1054:22–29. doi: 10.1016/j.brainres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Hegen M, Kameoka J, Dong RP, Schlossman SF, Morimoto C. Cross-linking of CD26 by antibody induces tyrosine phosphorylation and activation of mitogen-activated protein kinase. Immunology. 1997;90:257–264. doi: 10.1046/j.1365-2567.1997.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Buono N, Parrotta R, Morone S, Bovino P, Nacci G, Ortolan E, Horenstein AL, Inzhutova A, Ferrero E, Funaro A. The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J Biol Chem. 2011;286:18681–18691. doi: 10.1074/jbc.M111.227876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jewell K, Kapron-Bras C, Jeevaratnam P, Dedhar S. Stimulation of tyrosine phosphorylation of distinct proteins in response to antibody-mediated ligation and clustering of alpha 3 and alpha 6 integrins. J Cell Sci. 1995;108(Pt 3):1165–1174. doi: 10.1242/jcs.108.3.1165. [DOI] [PubMed] [Google Scholar]
- 34.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 35.Abram CL, Lowell CA. Leukocyte adhesion deficiency syndrome: a controversy solved. Immunology and cell biology. 2009;87:440–442. doi: 10.1038/icb.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White CD, Erdemir HH, Sacks DB. IQGAP1 and its binding proteins control diverse biological functions. Cellular signalling. 2012;24:826–834. doi: 10.1016/j.cellsig.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNulty DE, Li Z, White CD, Sacks DB, Annan RS. MAPK scaffold IQGAP1 binds the EGF receptor and modulates its activation. J Biol Chem. 2011;286:15010–15021. doi: 10.1074/jbc.M111.227694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neel NF, Sai J, Ham AJ, Sobolik-Delmaire T, Mernaugh RL, Richmond A. IQGAP1 is a novel CXCR2-interacting protein and essential component of the “chemosynapse”. PLoS One. 2011;6:e23813. doi: 10.1371/journal.pone.0023813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell motility and the cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 43.Jevnikar Z, Obermajer N, Kos J. LFA-1 fine-tuning by cathepsin X. IUBMB life. 2011;63:686–693. doi: 10.1002/iub.505. [DOI] [PubMed] [Google Scholar]
- 44.Stanley P, Smith A, McDowall A, Nicol A, Zicha D, Hogg N. Intermediate-affinity LFA-1 binds alpha-actinin-1 to control migration at the leading edge of the T cell. The EMBO journal. 2008;27:62–75. doi: 10.1038/sj.emboj.7601959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jevnikar Z, Obermajer N, Pecar-Fonovic U, Karaoglanovic-Carmona A, Kos J. Cathepsin X cleaves the beta2 cytoplasmic tail of LFA-1 inducing the intermediate affinity form of LFA-1 and alpha-actinin-1 binding. European journal of immunology. 2009;39:3217–3227. doi: 10.1002/eji.200939562. [DOI] [PubMed] [Google Scholar]
- 46.Craig DH, Haimovich B, Basson MD. α-Actinin-1 phosphorylation modulates pressure-induced colon cancer cell adhesion through regulation of focal adhesion kinase-Src interaction. American Journal of Physiology - Cell Physiology. 2007;293:C1862–C1874. doi: 10.1152/ajpcell.00118.2007. [DOI] [PubMed] [Google Scholar]
- 47.Chacón MR, Fernández G, Rico B. Focal adhesion kinase functions downstream of Sema3A signaling during axonal remodeling. Molecular and Cellular Neuroscience. 2010;44:30–42. doi: 10.1016/j.mcn.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Izaguirre G, Aguirre L, Hu YP, Lee HY, Schlaepfer DD, Aneskievich BJ, Haimovich B. The cytoskeletal/non-muscle isoform of alpha-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J Biol Chem. 2001;276:28676–28685. doi: 10.1074/jbc.M101678200. [DOI] [PubMed] [Google Scholar]
- 49.Tsuiji H, Xu L, Schwartz K, Gumbiner BM. Cadherin conformations associated with dimerization and adhesion. J Biol Chem. 2007;282:12871–12882. doi: 10.1074/jbc.M611725200. [DOI] [PubMed] [Google Scholar]
- 50.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annual review of cell and developmental biology. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 51.Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. The Journal of experimental medicine. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Current opinion in cell biology. 2000;12:542–547. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 53.Oxvig C, Lu C, Springer TA. Conformational changes in tertiary structure near the ligand binding site of an integrin I domain. Proceedings of the National Academy of Sciences. 1999;96:2215–2220. doi: 10.1073/pnas.96.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JO, Bankston LA, Arnaout MA, Liddington RC. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure. 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 55.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 56.Wong AH, Zhou D, Rini JM. The X-ray crystal structure of human aminopeptidase N reveals a novel dimer and the basis for peptide processing. J Biol Chem. 2012 doi: 10.1074/jbc.M112.398842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, Holmes KV. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasseville VG, Newman W, Brodie SJ, Hesterberg P, Pauley D, Ringler DJ. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994;144:27–40. [PMC free article] [PubMed] [Google Scholar]
- 59.Hartshorn KL, White MR. Influenza A virus up-regulates neutrophil adhesion molecules and adhesion to biological surfaces. J Leukoc Biol. 1999;65:614–622. doi: 10.1002/jlb.65.5.614. [DOI] [PubMed] [Google Scholar]
- 60.Kim SJ, Nair AM, Fernandez S, Mathes L, Lairmore MD. Enhancement of LFA-1-mediated T cell adhesion by human T lymphotropic virus type 1 p12I1. J Immunol. 2006;176:5463–5470. doi: 10.4049/jimmunol.176.9.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehl AM, Floettmann JE, Jones M, Brennan P, Rowe M. Characterization of intercellular adhesion molecule-1 regulation by Epstein-Barr virus-encoded latent membrane protein-1 identifies pathways that cooperate with nuclear factor kappa B to activate transcription. J Biol Chem. 2001;276:984–992. doi: 10.1074/jbc.M003758200. [DOI] [PubMed] [Google Scholar]
- 62.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. Roles of CD18 and intercellular adhesion molecule-1. J Immunol. 1996;156:4774–4782. [PubMed] [Google Scholar]
- 63.Marker O, Scheynius A, Christensen JP, Thomsen AR. Virus-activated T cells regulate expression of adhesion molecules on endothelial cells in sites of infection. J Neuroimmunol. 1995;62:35–42. doi: 10.1016/0165-5728(95)00099-n. [DOI] [PubMed] [Google Scholar]
- 64.Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]