Abstract
Lemur tyrosine kinase-3 (LMTK3) was recently identified as estrogen receptor (ER) -α modulator related to endocrine therapy resistance, and its polymorphisms rs9989661 (T>C) T/T genotype and rs8108419 (G>A) G/G or A/G genotype predicted improved outcomes in breast cancer. Since different predominant ERs distributions link to breast and gastric cancer and little is known of the prognostic role of LMTK3 in gastric cancer, this study was conducted to clarify the prognostic role of these polymorphisms in gastric cancer. One-hundred and sixty-nine Japanese and one-hundred and thirty-seven United States (US) patients with localized gastric adenocarcinoma were enrolled. Genomic DNA was extracted from blood or tissue, and all samples were analyzed by PCR-based direct DNA-sequencing. Overall, these polymorphisms were not associated with survival in both cohorts. When gender was considered, in multivariate analysis, harboring rs9989661 T/T genotype was associated with disease-free survival (HR 4.37; 95% CI, 2.08–9.18; p<0.0001) and overall survival (OS) (HR 3.69; 95% CI, 1.65–8.24; p=0.0014) in the Japanese males and time to recurrence (HR 7.29; 95% CI, 1.07–49.80; p=0.043) in the US females. Meanwhile, harboring rs8108419 G/G genotype was associated with OS in the Japanese females (HR 3.04; 95% CI, 1.08–8.56; p=0.035) and the US males (HR 3.39; 95% CI, 1.31–8.80; p=0.012). The prognostic role of these polymorphisms may be negative in gastric cancer. These findings suggest that the estrogen pathway may play a prognostic role in patient with gastric cancer but this may be dependent on the regional differences both in physiology and genetic alterations of gastric cancer.
Keywords: LMTK3, estrogen, estrogen receptor, gastric cancer, prognostic factor
Introduction
Gastric cancer is currently the fourth most common malignancy and second leading cause of cancer-related deaths worldwide, with 939,600 new cases diagnosed annually and 738,000 patients succumbing to this disease (1). The National Cancer Center in Japan reported that 117,320 cases were diagnosed and 50,597 people died from this disease in Japan in 2007. It is estimated that 133,900 new cases will be diagnosed and 49,400 people will die from this disease each year from 2010 to 2014. On the other hand, the American Cancer Society estimated that 21,320 new cases will be diagnosed and 10,540 people will die from this disease in the United States (US) in 2012 (2, 3).
Gastric cancer is a heterogeneous disease, and there are regional differences in epidemiology and clinicopathologic features. Despite the overall decline in the incidence of gastric cancer over the past several decades, rates of gastro-esophageal junction (GEJ) cancer has increased since the 1970s in Western countries, especially in Caucasian males in the US (4, 5). Meanwhile, both distal gastric cancer and intestinal-type, characterized by atrophic gastritis with intestinal metaplasia due to Helicobactor pylori infection, are more common in East Asia, Eastern Europe, and Central and South America (6). In addition, specific genetic alternations are associated with clinicopathologic features and prognosis in gastric cancer (7). HER2 overexpression is associated with the intestinal-type and GEJ cancers and EGFR overexpression is more likely to be found in the intestinal-type (8–10), while loss-of-function of E-cadherin and c-MET overexpression are more likely to be found in the diffuse-type (11, 12). These molecular diversities of genetic alternations, which reflect clinicopathologic features based on regional differences, have led to different prognosis with a lack of standard chemotherapeutic strategies across the world for gastric cancer patients (13–15).
In contrast to breast cancer, accumulated epidemiologic studies have suggested that estrogen may have protective effects against gastrointestinal (GI) cancer. Male predominant prevalence in GI cancer and better survival of young women in colorectal cancer (CRC) and esophageal cancer have been shown (1, 16, 17). Postmenopausal hormone replacement therapy (HRT) reduced the incidence of CRC and gastric cancer (18, 19), and conversely, adjuvant anti-estrogen, tamoxifen, therapy for breast cancer increased second primary CRC and gastric cancer (19–21). Since an incidence of gastric cancer in males is more than 2 folds higher than females and the onset of gastric cancer in males is 10 to 17 years earlier compared with females (22, 23), estrogen may critically influence gastric cancer incidence, development and progression. This epidemiologic evidence suggests a protective estrogen effect against development of gastric cancer; however, its mechanism remains to be elucidated. The effects of estrogen are typically mediated by estrogen receptors (ERs): ER alpha (ERα) and ER beta (ERβ). In contrast to the reproductive system where ERα is predominantly expressed, ERβ is the predominant ER expressed in the GI tract (24, 25). Expression rates of ERα and ERβ in gastric cancer by immunohistochemical staining (IHC) are 0–36% and 11–100%, respectively, whereas expression rates of ERα and ERβ in corresponding normal stomach tissue are 0–25.6% and 34–100%, respectively (26–30). Although ERα is well known to promote growth and is associated with aggressive forms of breast cancer, recent biological evidence suggests that ERβ has a suppressive effect against ERα. It was shown that ERβ signaling up-regulates integrin gene-expression and down-regulates several genes in vitro, including IL-6, cyclin D1, VEGF, and Bcl-2, suggesting that ERβ signaling may play a critical role in regulating the inflammatory reaction, proliferation, migration, angiogenesis, and apoptosis (31–34). In addition, loss of ERβ expression in gastric cancer was associated with diffuse-type histology, advanced stage, peritoneal invasion, and worse prognosis (28–30). These molecular biological data further support the protective role of estrogen via ERβ pathway in gastric cancer.
Lemur tyrosine kinase-3 (LMTK3) belongs to the family of serine-threonine-tyrosine kinases and was recently identified as a regulatory target associated with endocrine therapy resistance in adjuvant breast cancer (35, 36). LMTK3 phosphorylates and protects ERα from proteosomal degradation; consequently, leading to ERα stabilization and activation. Lower LMTK3 protein expression and its germline polymorphisms rs9989661 (T>C) T/T genotype and rs8108419 (G>A) G/G or A/G genotypes were associated with favorable clinicopathological profiles and better prognosis in ERα+ breast cancer (35, 37). Meanwhile, we recently reported inverse results that rs9989661 T/T genotype was associated with worse prognosis in CRC (38, 39). Given these opposing results which reflect the different biology based on predominant ERs distributions among breast and CRC and growing data suggesting an important role of estrogen in gastric cancer, we hypothesized that LMTK3 polymorphisms may have different prognostic roles in breast and GI cancers. Since the prognostic role of LMTK3 polymorphisms in gastric cancer is unknown and the regional differences in epidemiology and clinicopathologic features are recognized, we tested whether LMTK3 polymorphisms in gastric cancer will be associated with outcome in two ethnically and epidemiologically different cohorts from Japan and the US.
Materials and Methods
Patients
One-hundred and sixty-nine Japanese (n=169) and one-hundred and thirty-seven (n=137) US patients with histopathologically-confirmed localized (stage Ib to IV) gastric adenocarcinoma were enrolled from Japan (Fukushima Red Cross Hospital and Kitasato University East Hospital), and from the US (University of Southern California/Norris Comprehensive Cancer Center, Los Angeles County Hospital, and Memorial Sloan-Kettering Cancer Center), respectively, between 1991 and 2010. Japanese gastric cancer patients were treated with D2 lymphadenectomy based surgery alone or surgery plus S-1 or fluoropyrimidine based adjuvant chemotherapy without radiation therapy, while US patients were treated with D-1 based surgery alone or surgery plus fluoropyrimidine based adjuvant (radio)-chemotherapy. Patients were followed clinically every 3 months for the first two years and then every 6 months. Pathological stage was decided according to TNM classification 6th edition in both cohorts. This study was approved by the Institutional Review Boards of each institute, and all patients signed an informed consent for the analysis of molecular correlates. This study was conducted adhering to the REporting recommendations for tumor MARKer prognostic studies (REMARK) (40).
LMTK3 rs9989661 and rs8108419 genotyping
Genomic DNA was extracted from peripheral blood or formalin fixed paraffin embedded (FFPE) tissue derived from tumor samples using the QIAmp-kit (Qiagen, Valencia, CA) according to manufacturer’s protocol. Extracted DNA was amplified using following primer set: forward; 5’-GGG CCT TCC CAA GTG GTT-3’, and reverse; 5’-ATC CAA GCC TGG GGT GAG-3’ for rs9989661, forward; 5’-GAG GAC GAG GCT AGA ATC CA-3’, and reverse; 5’-GTT GGT GTG AAC CAG AGC AG-3’ for rs8108419. All samples were analyzed by means of PCR-based direct DNA sequencing. For quality control purposes, a random selection of 10% of the samples was examined for each polymorphism and genotype concordance rate was 100%.
Immunohistochemistry
Seventeen (n=17) of FFPE adjuvant gastric cancer samples from the US cohort were subjected to IHC to detect LMTK3 protein expression. IHC was conducted at the University of Southern California Immunohistochemistry Clinical Laboratory using the LMTK3 monoclonal antibody (I-17; Santa Cruz Biotechnology, Inc) at a concentration of 2µg on full-face excisional tissue sections as previously described (35, 37). Slides were cut at 4µm thick FFPE adjuvant gastric cancer samples. Negative controls were performed by omission of the primary antibody. Positive controls were conducted with breast cancer samples (n=5). LMTK3 immuno-reactivity was detected in the nucleus and in the cytoplasm to a variable degree in gastric cancer samples by digital imaging (Leica ICC50) at magnification (x200) with a computer-based interface (Leica Acquire 1.0). Protein expression levels of LMTK3 were determined according to previous reports (35, 37).
Statistical Analysis
The primary endpoints were disease-free survival (DFS) in the Japanese cohort and time to recurrence (TTR) in the US cohort, and the secondary endpoint was overall survival (OS) in both cohorts. DFS was defined between the date of surgery and first documented recurrence or death from any cause, while TTR was defined between the date of diagnosis and first documented recurrence. OS in the Japanese cohort was defined between the date of surgery and death from any cause, while OS in the US cohort was defined between the date of diagnosis and death from any cause. If patients did not meet any endpoints until April 20, 2011, they were censored at the time of last contact. Allelic distribution of the polymorphisms by ethnicity was tested for deviation from Hardy-Weinberg equilibrium using χ2-test with 1 degree of freedom. Linkage disequilibrium among two polymorphisms was assessed using D’ and r2 values. To evaluate the prognostic value of these polymorphisms, endpoints were estimated using by Kaplan-Meier methods and compared by the log-rank test. The Cox proportional hazards regression model with stratification factors were fitted to re-evaluate the association between LMTK3 polymorphisms and outcomes considering the imbalanced in the distributions of baseline characters in both cohorts. The baseline demographic and clinical markers that remained significantly associated with endpoints in the multivariate analyses (p<0.1) were included in the final model. With 169 patients in the Japanese cohort and 137 patients in the US cohort, we would have 80% power to detect a minimum hazard ratio 1.8–2.0 in DFS and TTR across a range of the variant allele frequencies (0.2–0.5) in a dominant model using a 0.05 level two-sided log-rank test. For a recessive model, a minimum hazard ratio is <3.8 when the variant allele frequency is 0.2 and approaches 1.8–2.0 when the allele frequency is 0.5. The level of significance was set to p<0.05, and all statistical tests were two-sided and performed using the SAS statistical package version 9.2 (SAS Institute, Cary, NC).
Results
A total of 306 patients, 169 Japanese and 137 US patients, with localized gastric cancer were enrolled in this study. The clinicopathologic characteristics and outcomes in both cohorts were summarized in Table 1 and 2. The clinicopathologic baselines of both cohorts varied considerably. Briefly, the US cohort was more likely to have young, advanced stage, poorly differentiated pathology and worse general condition compared with the Japanese cohort. With respect to primary tumor site, significantly higher incidence of GEJ cancer in males was found in the US cohort (Suppl. table 1). Furthermore, adjuvant treatments of both cohorts were lacking of unity. The median follow-up periods were 4.0 years in the Japanese cohort and 3.3 years in the US cohort, respectively. The median DFS and OS in the Japanese cohort were 4.8 and 5.8 years, whereas the median TTR and OS in the US cohort were 2.8 and 4.7 years, respectively. All patients in the Japanese cohort and 131 patients (96%) in the US cohort were followed until death or the end of the study period. One-hundred and fifty-five patients (90%) in the Japanese cohort and 127 patients (93%) in the US cohort complied with the follow-up schedule. Age, stage, T-category, N-category, performance status, and adjuvant chemotherapy were significantly associated with DFS in the Japanese cohort; on the other hand, stage, T-category, N-category, tumor site, and adjuvant chemotherapy were also significantly associated with TTR in the US cohort.
Table 1.
Japanese cohort characteristics and clinical outcome: disease-free survival and overall survival
| Disease-free survival |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | N (%) | Median DFS, years (95% CI) |
Hazard Ratio (95% CI) |
P value * | Median OS, years (95% CI) |
Hazard Ratio (95% CI) |
P value * |
| Age | 0.001 | <.001 | |||||
| <65 | 65 (38%) | 20.1+ (4.8, 20.1+) | 1 (Reference) | 20.1+ (5.7, 20.1+) | 1 (Reference) | ||
| 65–74 | 60 (36%) | 9.0+ (1.7, 9.0+) | 1.59 (0.92, 2.75) | 4.3 (2.8, 9.0+) | 1.97 (1.08, 3.60) | ||
| ≥75 | 44 (26%) | 1.7 (1.2, 2.5) | 2.67 (1.53, 4.65) | 2.1 (1.5, 2.8) | 4.04 (2.18, 7.49) | ||
| Sex | 0.98 | 0.50 | |||||
| Male | 109 (64%) | 4.8 (2.0, 20.1+) | 1 (Reference) | 5.7 (3.4, 20.1+) | 1 (Reference) | ||
| Female | 60 (36%) | 16.1+ (1.9, 16.1+) | 1.01 (0.64, 1.59) | 3.9 (2.7, 16.1+) | 1.18 (0.73, 1.91) | ||
| Stage | <.001 | <.001 | |||||
| IB | 28 (16%) | 20.1+ | 1 (Reference) | 20.1+ | 1 (Reference) | ||
| II | 53 (31%) | 16.1+ (2.4, 16.1+) | 4.56 (1.38, 15.11) | 16.1+ (3.4, 16.1+) | 3.96 (1.20, 13.12) | ||
| III | 60 (36%) | 2.5 (2.0, 6.3+) | 6.45 (2.00, 20.80) | 4.3 (2.7, 6.3+) | 5.27 (1.63, 17.03) | ||
| IV | 28 (16%) | 0.7 (0.4, 1.6) | 17.30 (5.22, 57.29) | 1.2 (0.8, 2.7) | 15.71 (4.72, 52.27) | ||
| Tumor stage | 0.001 | ||||||
| T1 † | 12 (7%) | 0.003 | |||||
| T2 † | 66 (39%) | 20.1+ | 1 (Reference) | 20.1+ (4.3, 20.1+) | 1 (Reference) | ||
| T3 ‡ | 88 (52%) | 2.0 (1.6, 4.8) | 2.09 (1.31, 3.32) | 3.4 (2.2, 16.1+) | 2.06 (1.26, 3.37) | ||
| T4 ‡ | 3 (2%) | ||||||
| N | <.001 | 0.001 | |||||
| N0 | 36 (21%) | 20.1+ | 1 (Reference) | 20.1+ | 1 (Reference) | ||
| N1 | 85 (50%) | 15.4 (2.2, 15.4+) | 2.00 (0.97, 4.13) | 15.4 (3.4, 15.4+) | 1.75 (0.84, 3.65) | ||
| N2 | 33 (20%) | 2.2 (1.6, 4.8) | 3.16 (1.45, 6.90) | 3.4 (2.3, 5.7) | 2.70 (1.21, 6.01) | ||
| N3 | 15 (9%) | 1.3 (0.6, 2.2) | 5.95 (2.53, 14.00) | 2.3 (0.4, 3.1) | 4.74 (1.94, 11.57) | ||
| Tumor Site | 0.23 | 0.19 | |||||
| Lower | 55 (32%) | 2.3 (1.6, 16.1+) | 1 (Reference) | 16.1+ (2.1, 16.1+) | 1 (Reference) | ||
| Middle | 59 (35%) | 20.1+ (2.0, 20.1+) | 0.87 (0.51, 1.46) | 4.1 (3.0, 20.1+) | 0.89 (0.51, 1.54) | ||
| Upper | 49 (29%) | 15.2+ (3.0, 15.2+) | 0.69 (0.38, 1.23) | 5.7 (3.9, 15.2+) | 0.66 (0.35, 1.22) | ||
| GEJ | 3 (2%) | 2.2 (1.1, 2.3) | 1.99 (0.60, 6.58) | 2.8 (1.4, 2.9+) | 2.28 (0.68, 7.61) | ||
| UML | 3 (2%) | 0.9 (0.9–2.0) | 2.22 (0.52, 9.44) | 4.0+ | − | ||
| Tumor Differentiation | 0.67 | 0.63 | |||||
| Well – Moderate | 68 (40%) | 20.1+ (1.9, 20.1+) | 1 (Reference) | 20.1+ (3.4, 20.1+) | 1 (Reference) | ||
| Poor | 101 (60%) | 3.0 (2.2, 7.2+) | 1.10 (0.70, 1.74) | 4.3 (3.0, 7.2+) | 1.12 (0.69, 1.82) | ||
| PS | <.001 | <.001 | |||||
| ECOG 0 | 157 (93%) | 20.1+ (2.9, 20.1+) | 1 (Reference) | 20.1+ (3.9, 20.1+) | 1 (Reference) | ||
| ECOG 1 | 12 (7%) | 1.2 (0.3, 1.9) | 3.41 (1.79, 6.47) | 1.2 (0.2, 2.3) | 4.75 (2.45, 9.22) | ||
| Chemotherapy | 0.008 | 0.067 | |||||
| No | 60 (36%) | 20.1+ | 1 (Reference) | 20. 1+ | 1 (Reference) | ||
| Yes | 109 (64%) | 2.3 (1.9, 4.8) | 1.97 (1.18, 3.30) | 3.4 (2.8, 16.1+) | 1.62 (0.96, 2.75) | ||
Based on log-rank test
Estimates were not reached.
No events occurred and estimates were not obtained.
Grouped together for the estimates of hazard ratio
Abbreviations: DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; GEJ, Gastro-esophageal junction; OS, overall survival; PS, performance status; UML, Upper-Middle-Lower.
Table 2.
The US cohort characteristics and clinical outcome: time to recurrence and overall survival
| Time to recurrence |
Overall Survival |
||||||
|---|---|---|---|---|---|---|---|
| Characteristics | N (%) | Median TTR, years (95% CI) |
Hazard Ratio (95% CI) |
P value * | Median OS, years (95% CI) |
Hazard Ratio (95% CI) |
P value * |
| Age, years | 0.42 | 0.65 | |||||
| < 60 | 80 (58%) | 2.2 (1.5, 14.5+) | 1 | 4.7 (3.8, 14.6+) | 1 | ||
| ≥ 60 | 57 (42%) | 3.7 (2.1, 12.3+) | 0.81 (0.48, 1.36) | 4.5 (3.3, 7.3) | 1.14 (0.64, 2.05) | ||
| Sex | 0.85 | 0.32 | |||||
| Male | 83 (61%) | 2.3 (1.8, 7.0) | 1 | 4.1 (3.3, 7.3) | 1 | ||
| Female | 54 (39%) | 7.0 (1.5, 8.3+) | 0.95 (0.56, 1.63) | 7.3 (3.8, 8.3+) | 0.72 (0.37, 1.39) | ||
| Race | 0.085 | 0.040 | |||||
| White | 63 (46%) | 1.7 (1.2, 4.4) | 1 | 3.8 (2.7, 5.5) | 1 | ||
| African American | 1 (1%) | 0.5+ | — | 0.5+ | — | ||
| Asian | 28 (20%) | 7.0 (2.3, 14.5+) | 0.45 (0.23, 0.91) | 7.3 (3.3, 14.6+) | 0.45 (0.20, 1.03) | ||
| Hispanic | 45 (33%) | 3.7 (2.1, 10.7+) | 0.63 (0.34, 1.17) | 10.7+ (3.6, 10.7+) | 0.36 (0.15, 0.85) | ||
| Stage | 0.030 | 0.32 | |||||
| IB | 12 (9%) | 4.3+ (2.2, 4.3+) | 1 | 4.4+ | — | ||
| II | 36 (26%) | 7.0 (2.9, 10.7+) | 1.56 (0.35, 6.98) | 5.4 (4.1, 10.7+) | 1 | ||
| III | 71 (52%) | 1.8 (1.4, 2.8) | 3.24 (0.78, 13.5) | 3.8 (2.8, 7.3) | 1.31 (0.69, 2.50) | ||
| IV | 18 (13%) | 1.6 (1.2, 3.8+) | 4.00 (0.86, 18.5) | 7.3+ (1.4, 7.3+) | 1.33 (0.43, 4.09) | ||
| Tumor stage | 0.013 | 0.30 | |||||
| T1 † | 4 (3%) | ||||||
| T2 † | 44 (32%) | 8.3+ (2.9, 8.3+) | 1 | 5.4 (4.1, 8.3+) | 1 | ||
| T3 ‡ | 79 (58%) | 1.7 (1.4, 4.4) | 2.04 (1.14, 3.67) | 4.5 (3.3, 7.3) | 1.40 (0.73, 2.68) | ||
| T4 ‡ | 10 (7%) | ||||||
| N | 0.004 | 0.088 | |||||
| N0 | 27 (20%) | 7.0 (1.8, 10.7+) | 1 | 7.3 (3.4, 10.7+) | 1 | ||
| N1 | 64 (47%) | 4.4 (2.2, 14.5+) | 0.99 (0.47, 2.11) | 5.5 (4.1, 14.6+) | 1.07 (0.46, 2.47) | ||
| N2 | 31 (23%) | 1.3 (1.1, 2.3) | 2.62 (1.15, 5.94) | 3.3 (2.0, 5.7+) | 2.27 (0.85, 6.07) | ||
| N3 | 15 (11%) | 1.6 (1.0, 3.8+) | 1.96 (0.73, 5.32) | 2.4 (1.1, 3.8+) | 2.35 (0.66, 8.41) | ||
| PS | 0.77 | 0.25 | |||||
| ECOG 0 | 62 (45%) | 2.5 (1.7, 10.7+) | 1 | 4.5 (2.8, 10.7+) | 1 | ||
| ECOG 1 | 65 (47%) | 2.3 (1.6, 14.5+) | 1.00 (0.60, 1.68) | 5.7 (3.8, 14.6+) | 0.68 (0.37, 1.25) | ||
| ECOG 2 | 10 (7%) | 2.2+ (1.7, 2.2+) | 0.60 (0.14, 2.53) | 2.2+ (1.2, 2.2+) | 1.67 (0.36, 7.75) | ||
| Differentiation | 0.15 | 0.098 | |||||
| Moderate | 27 (20%) | 2.1 (1.1, 3.8+) | 1 | 4.1 (2.7, 4.7) | 1 | ||
| Poor/Moderate ‡ | 10 (7%) | 3.7 (2.1, 14.5+) | 0.65 (0.36, 1.19) | 7.3 (3.8, 14.6+) | 0.59 (0.31, 1.13) | ||
| Poor ‡ | 97 (71%) | ||||||
| Lauren | 0.87 | 0.74 | |||||
| Diffuse | 40 (29%) | 3.7 (1.8, 8.9+) | 1 | 7.3 (5.4, 8.9+) | 1 | ||
| Intestinal | 50 (36%) | 7.0 (2.1, 14.5+) | 0.87 (0.45, 1.67) | 5.7 (3.8, 14.6+) | 1.10 (0.48, 2.51) | ||
| Mixed | 21 (15%) | 12.3+ (1.7, 12.3+) | 1.04 (0.45, 2.41) | 3.6 (1.9, 12.3+) | 1.52 (0.51, 4.58) | ||
| Tumor Site | 0.020 | 0.003 | |||||
| Stomach | 88 (64%) | 4.4 (2.1, 14.5+) | 1 (Reference) | 7.3 (3.8, 14.6+) | 1 (Reference) | ||
| GEJ | 38 (28%) | 1.6 (1.1, 2.9) | 1.97 (1.15, 3.37) | 3.4 (2.4, 4.5) | 2.50 (1.35, 4.60) | ||
| Unknown | 11 (8%) | 4.4+ (3.2, 4.4+) | 0.77 (0.24, 2.54) | 4.4+ (3.3, 4.4+) | 0.51 (0.07, 3.89) | ||
| Type of chemotherapy | <0.001 | 0.009 | |||||
| 5-FU/LV | 70 (51%) | 7.0 (2.8, 10.6+) | 1 | 7.3 (3.8, 10.6+) | 1 | ||
| 5-FU/LV/oxaliplatin | 19 (14%) | 1.6 (1.1, 2.9) | 2.65 (1.22, 5.75) | 2.4 (1.2, 4.5+) | 4.07 (1.62, 10.19) | ||
| 5-FU, CDDP, CPT-11 | 23 (17%) | 14.5 (1.2, 14.5) | 1.25 (0.58, 2.69) | 4.1 (2.2, 14.6+) | 1.32 (0.57, 3.05) | ||
| None | 25 (18%) | 2.1 (0.8, 2.5) | 2.99 (1.62, 5.51) | 4.7 (2.8, 5.7) | 1.95 (0.93, 4.07) | ||
| Radiation | 0.92 | 0.68 | |||||
| Yes | 88 (64%) | 2.5 (1.8, 14.5+) | 1 | 4.5 (3.3, 14.6+) | 1 | ||
| No | 48 (35%) | 3.7 (1.7, 12.3+) | 1.03 (0.60, 1.76) | 5.4 (3.8, 12.3+) | 0.89 (0.48, 1.63) | ||
Based on log-rank test
Estimates were not reached.
No events occurred and estimates were not obtained.
Grouped together for the estimates of hazard ratio
Abbreviations: CDDP, cisplatin; DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; 5-FU, fluorouracil; GEJ, Gastro-esophageal junction; LV, Levofolinate; OS, overall survival; PS, performance status; TTR, time to recurrence.
LMTK3 rs9989661 and rs8108419 genotyping
Final success rates of LMTK3 genotyping in the Japanese and the US cohorts were 167 (99%) and 125 (91%) for rs9989661 and 165 (98%) and 127 (93%) for rs8108419, respectively. The allelic frequencies of rs9989661 were not within the probability limits of Hardy-Weinberg equilibrium in both cohorts (Chi-Square p value <0.05). In addition, significantly different allelic distributions in LMTK3 rs9989661 were found between both cohorts (Suppl. table 2). Moreover, strong linkage disequilibrium between rs9989661 C allele and rs8108419 G allele was found in the Japanese cohort (D’ = 0.967, r2 = 0.338), while weak linkage disequilibrium between rs9989661 T allele and rs8108419 G allele was found in the US cohort (D’ = 0.512, r2 = 0.042). There were no significant differences between genotypes of these polymorphisms and clinical characteristics including differentiation, Lauren classification, and tumor site in both cohorts (All Chi-Square p values>0.05; data were not shown).
Univariate analysis for LMTK3 polymorphisms
In both polymorphisms, no significant differences of endpoints in overall patients were found in both cohorts (Suppl. table 3). After analyzing according to gender, in rs9989661, the Japanese males harboring T/T genotype had shorter median DFS of 0.9 years (95% CI, 0.3–6.1+ years) and median OS of 1.7 years (95% CI, 0.6–6.1+ years) compared with median DFS of 20.1+ years (95% CI, 2.4–20.1+ years) (HR 2.05; 95% CI, 1.05–4.00; p=0.030.) and OS of 20.1+ years (95% CI, 3.9–20.1+ years) (HR 2.07; 95% CI, 1.02–4.20; p=0.039) for CT or CC genotype (Table 3). The US females harboring T/T genotype had a shorter median TTR of 1.7 years (95% CI, 0.7–7.0+ years) compared with 7.0 years (95% CI, 3.7 −8.3+ years) for CT or CC genotype (HR 2.70; 95% CI, 1.01–7.19; p=0.025), however, no significant difference was found in OS (Table 4). With respect to the US males and the Japanese females in univariate analysis, no significant differences were found in DFS, TTR and OS in terms of both polymorphisms (Table 3, 4). On the other hand, even upon considering gender, rs8108419 showed no significant differences in the endpoints in both cohorts.
Table 3.
LMTK3 polymorphisms and survival in Japanese cohort by gender
| LMTK3 polymorphisms and disease-free survival and overall survival in Japanese male patients with gastric cancer | |||||||
|---|---|---|---|---|---|---|---|
| Disease-Free Survival |
Overall survival |
||||||
| N | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI)‡ | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | |
| LMTK3 rs8108419 |
|||||||
| A/A* | 4 | ||||||
| A/G* | 26 | 2.3 (1.2, 20.1+) | 1 (Reference) | 1 (Reference) | 20.1+ (2.3, 20.1+) | 1 (Reference) | 1 (Reference) |
| G/G | 78 | 4.8 (2.0, 15.2+) | 0.86 (0.47, 1.57) | 0.79 (0.42, 1.47) | 5.7 (3.4, 15.2+) | 0.98 (0.51, 1.91) | 0.86 (0.43, 1.70) |
| P value† | 0.62 | 0.46 | 0.96 | 0.66 | |||
| LMTK3 rs9989661 |
|||||||
| C/C* | 53 | ||||||
| C/T* | 37 | 20.1+ (2.4, 20.1+) | 1 (Reference) | 1 (Reference) | 20.1+ (3.9, 20.1+) | 1 (Reference) | 1 (Reference) |
| T/T | 18 | 0.9 (0.3, 6.1+) | 2.05 (1.05 4.00) | 4.37 (2.08, 9.18) | 1.7 (0.6, 6.1+) | 2.07 (1.02, 4.20) | 3.69 (1.65, 8.24) |
| P value† | 0.030 | <.0001 | 0.039 | 0.0014 | |||
| LMTK3 polymorphisms and disease-free survival and overall survival in Japanese female patients with gastric cancer | |||||||
|---|---|---|---|---|---|---|---|
| Disease-Free Survival |
Overall survival |
||||||
| N | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | |
| LMTK3 rs8108419 |
|||||||
| A/A* | 4 | ||||||
| A/G* | 17 | 2.5 (1.6, 16.1+) | 1 (Reference) | 1 (Reference) | 3.4 (1.8, 16.1+) | 1 (Reference) | 1 (Reference) |
| G/G | 36 | 15.4+ (1.9, 15.4+) | 0.88 (0.40, 1.95) | 2.12 (0.80, 5.61) | 15.4+ (2.3, 15.4+) | 0.95 (0.42, 2.15) | 3.04 (1.08, 8.56) |
| P value† | 0.76 | 0.13 | 0.90 | 0.035 | |||
| LMTK3 rs9989661 | |||||||
| C/C* | 19 | ||||||
| C/T* | 27 | 15.4+ (1.9, 15.4+) | 1 (Reference) | 1 (Reference) | 3.4 (2.3, 15.4+) | 1 (Reference) | 1 (Reference) |
| T/T | 13 | 16.1+ (0.7, 16.1+) | 1.13 (0.46, 2.79) | 0.92 (0.36, 2.34) | 16.1+ (0.9, 16.1+) | 0.92 (0.35, 2.44) | 0.71 (0.26, 1.92) |
| P value† | 0.79 | 0.86 | 0.87 | 0.50 | |||
Combined in the analysis in the dominant or recessive model.
Based on the log-rank test in the univariate analysis and Wald test in the multivariate analysis within Cox regression model.
Estimates were not reached.
Adjusted for stage (I, II, III, and IV as categorical), age (<65, 65–74, ≥75 as ordinal), and type of adjuvant therapy (no vs yes.).
Table 4.
LMTK3 polymorphisms and survival in the US cohort by gender
| LMTK3 polymorphisms and time to recurrence and overall survival in the US male patients with gastric cancer | |||||||
|---|---|---|---|---|---|---|---|
| Time to recurrence |
Overall survival |
||||||
| N | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI)‡ | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | |
| LMTK3 rs8108419 |
|||||||
| A/A* | 7 | ||||||
| A/G* | 23 | 2.5 (1.8, 14.5+) | 1 (Reference) | 1 (Reference) | 5.7 (2.8, 14.6+) | 1 (Reference) | 1 (Reference) |
| G/G | 48 | 2.3 (1.5, 7.0) | 1.48 (0.74, 2.95) | 1.70 (0.76, 3.78) | 3.4 (2.8, 5.5) | 1.68 (0.79, 3.57) | 3.39 (1.31, 8.80) |
| P value† | 0.24 | 0.20 | 0.17 | 0.012 | |||
| LMTK3 rs9989661 |
|||||||
| C/C* | 12 | ||||||
| C/T* | 15 | 2.5 (2.1, 7.0) | 1 (Reference) | 1 (Reference) | 4.5 (2.8, 14.6+) | 1 (Reference) | 1 (Reference) |
| T/T | 45 | 2.1 (1.5, 10.7+) | 1.04 (0.52, 2.09) | 1.09 (0.35, 3.38) | 4.1 (3.3, 10.7+) | 1.12 (0.52, 2.41) | 1.03 (0.31, 3.40) |
| P value† | 0.90 | 0.88 | 0.77 | 0.96 | |||
| LMTK3 polymorphisms and time to recurrence and overall survival in the US female patients with gastric cancer | |||||||
|---|---|---|---|---|---|---|---|
| Time to recurrence |
Overall survival |
||||||
| N | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | Median, yrs (95% CI) | HR (95% CI) | HR (95% CI) ‡ | |
| LMTK3 rs8108419 |
|||||||
| A/A* | 4 | ||||||
| A/G* | 15 | 7.0 (1.5, 7.0+) | 1 (Reference) | 1 (Reference) | 7.3+ (1.9, 7.3+) | 1 (Reference) | 1 (Reference) |
| G/G | 30 | 3.7 (1.7, 8.3+) | 1.17 (0.42, 3.26) | 0.75 (0.15, 3.76) | 5.4 (3.8, 8.3+) | 1.41 (0.36, 5.49) | 5.81 (0.46, 74.00) |
| P value† | 0.76 | 0.72 | 0.62 | 0.18 | |||
| LMTK3 rs9989661 |
|||||||
| C/C* | 9 | ||||||
| C/T* | 16 | 7.0 (3.7, 8.3+) | 1 (Reference) | 1 (Reference) | 7.3 (3.8, 8.3+) | 1 (Reference) | 1 (Reference) |
| T/T | 28 | 1.7 (0.7, 7.0+) | 2.70 (1.01, 7.19) | 7.29 (1.07, 49.80) | 4.5 (2.2, 7.0+) | 1.47 (0.42, 5.13) | 1.27 (0.08, 20.38) |
| P value† | 0.025 | 0.043 | 0.51 | 0.87 | |||
Combined in the analysis in the dominant or recessive model.
Based on the log-rank test in the univariate analysis and Wald test in the multivariate analysis within Cox regression model.
Estimates were not reached.
Adjusted for N stage (2 groups: N0, N1 vs N2, N3) and stratified by race (4 groups: White; African American; Asian; Hispanic) and adjuvant therapy (4 groups: 5-FU/LV; 5-FU/LV/oxaliplatin; 5-FU, CDDP, CPT-11; None).
Multivariate analysis for LMTK3 polymorphisms
Multivariate analysis for LMTK3 rs9989661 and rs8108419 was stratified by age, stage and adjuvant chemotherapy in the Japanese cohort and by N stage, race, and adjuvant chemotherapy in the US cohort, LMTK3 rs9989661 remained significantly associated with DFS (HR 4.37; 95% CI, 2.08–9.18; p<0.0001) and OS (HR 3.69; 95% CI, 1.65–8.24; p=0.0014) in the Japanese males and TTR (HR 7.29; 95% CI, 1.07–49.80; p=0.043) in the US females (Table 3, 4). LMTK3 rs8108419 was also associated with only OS in the Japanese females (HR 3.04; 95% CI, 1.08–8.56; p=0.035) and in the US males (HR 3.39; 95% CI, 1.31–8.80; p=0.012) (Table 3, 4).
Immunohistochemistry
In addition to detection of LMTK3 polymorphisms, we conducted IHC to detect LMTK3 protein expression in gastric cancer tissue. All 17 samples were stained positive for LMTK3 protein expression, with 64.7% (n=11) staining cytoplasmic only, 11.7% (n=2) nuclear only and 23.5% (n=4) staining positive for both nuclear and cytoplasmic (Fig. 1).
Figure 1.
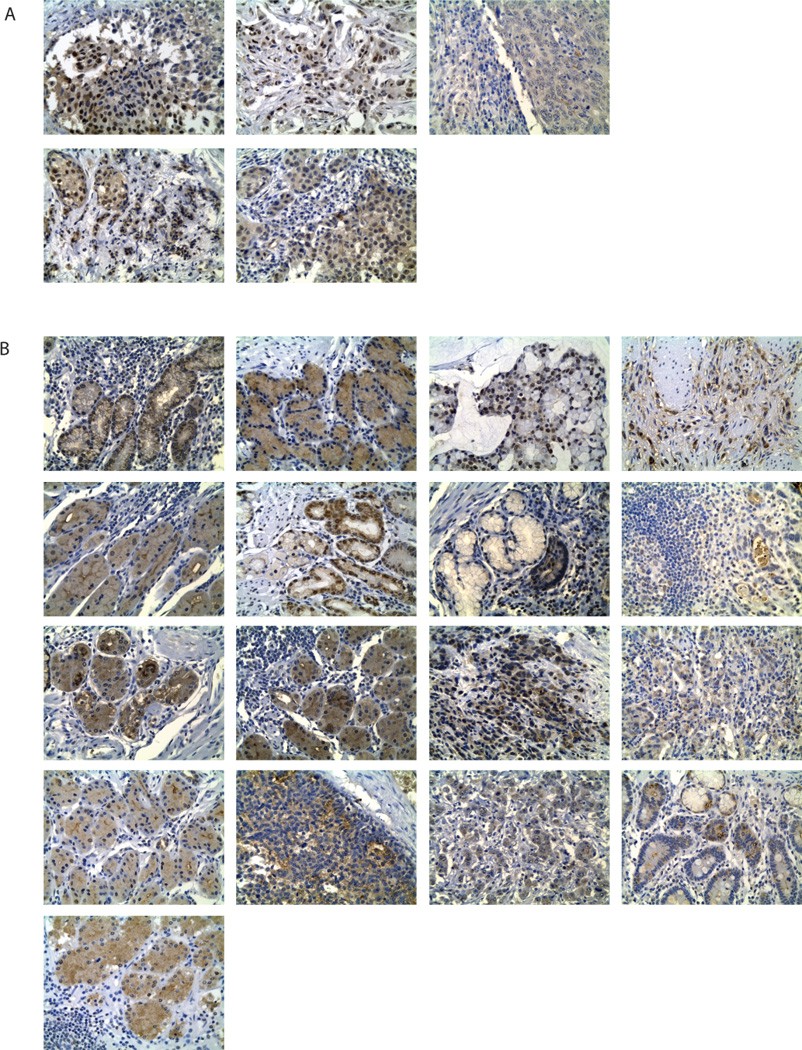
Immunohistochemical staining of LMTK3 with the anti-LMTK3 mouse monoclonal antibody (Santa Cruz Biotechnology). Specimens were processed as described in Materials and Methods. A, LMTK3 protein expression in breast cancer samples were utilized as positive controls (x200; Leica ICC50). Negative controls were performed by omission of the primary antibody (not shown). B, LMTK3 protein expression in adjuvant gastric cancer samples from USC cohort (x200; Leica ICC50).
Discussion
Accumulated epidemiological data suggest female have an advantage in GI cancer with a lower incidence of GI cancer reported in females and longer survival in young women particular in CRC and esophageal cancer have been shown (1, 16, 17). Moreover, postmenopausal HRT reduced the incidence of CRC and gastric cancer (18, 19). Because of the significant lower incidence and delayed onset in females compared with males, gastric cancer may be strongly affected by estrogen (22, 23). This epidemiological evidence strongly suggests the protective role of estrogen in gastric cancer. This protective estrogen effect in gastric cancer should be affected by plasma estradiol (E2) levels and ERs expression in stomach. Plasma E2 levels in postmenopausal women can be affected by adiposity, ethnicity, genetic variation, and lifestyle factors, such as age at menarche, type of menopause, parity, using hormone therapy, diet, consumption of alcohols, and smoking, but not all consistent (41–43). Among them, the most consistent evidence is a positive association between body mass index (BMI) and plasma E2 levels with rationale as follows; in men and postmenopausal women, androgenic steroids are converted to estrogen by adipose tissue aromatase and an amount of adipose tissue aromatase depends on an amount of adipose tissue (41). When compared with females, males have higher E2 levels than females in their sixties, and higher E2 levels in US people are reported in each gender as for reflecting higher BMI compared with those in Japanese (Suppl. table 4) (44, 45). Meanwhile, predominant expression of ERβ, which demonstrates a protective effect when compared to ERα, has been previously shown in normal stomach tissue. There was a trend that gain of ERα and loss of ERβ expression in gastric cancer tissue was observed during gastric carcinogenesis (28, 30). Expression rates of ERα and ERβ in gastric cancer by IHC in the Asian population are 0–22.7% and 43.6–100%, respectively (26, 28–30), whereas those in Western population are 36% and 11%, respectively (27). Moreover, ERβ expression in gastric cancer is associated with higher age, early stage, intestinal-type, and better survival and is conversely correlated with peritoneal invasion (28–30). Since the intestinal-type is more common in Asia (6), Asian patients may have more prevalent ERβ expression than Western patients in gastric cancer. These physiological and biological differences among gender and region may impact the protective estrogen effect in gastric cancer.
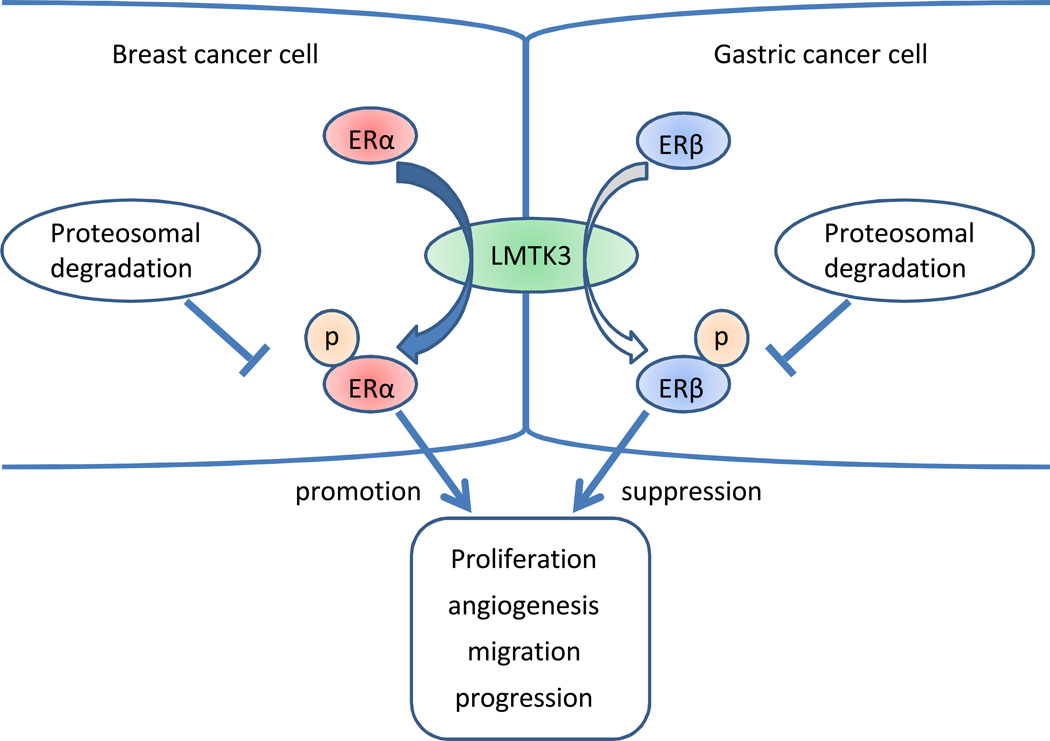
LMTK3 polymorphisms were associated with the better outcome in ERα+ breast cancer but worse outcome in CRC (35–39). As for gastric cancer, our results are consistent with previous CRC results. These opposite results between breast and GI cancers in LMTK3 polymorphisms are consistent with the different ERs distributions in the breast and in the GI tract (24, 25), suggesting that the prognostic role of LMTK3 polymorphisms reflects different predominant ERs distributions in different organs. LMTK3 enhances the estrogen pathway at least partly via ERα phosphorylation (35–37); however, few data are available in terms of a regulation of ERβ phosphorylation. Extracellular signal-regulated kinase 1/2 phosphorylated both ERα and ERβ, indicating that one kinase is possibly responsible for phosphorylation of both ERs (46, 47). Moreover, it was reported that phosphorylation of ERβ was associated with better survival in ERα+ breast cancer via posttranslational modifications (48). These data may suggest the hypothesis that in GI, LMTK3 could phosphorylate ERβ, resulting in its stabilization and activation in the same manner as ERα (35–37); consequently, leading to better survival by enhancing ERβ pathway (Fig. 2). The functions of these polymorphisms remain unclear; hence, the F-SNP database was used for the LMTK3 rs9989661 and rs8108419. F-SNP predicted changes in transcriptional regulation for both polymorphisms (49). Since these polymorphisms are an intronic germline polymorphism, it is possible for these variations to impact transcription factor binding sites. These findings suggest that these polymorphisms may affect LMTK3 gene expression levels (50). Besides polymorphic results, we confirmed that LMTK3 protein was highly expressed in gastric cancer tissue with variable staining patterns. To our knowledge, this is the first evidence that LMTK3 protein exists in GI tract. These results indicate not only the rationale of this study but also the possibility that LMTK3 protein expression levels might be useful to predict outcome in gastric cancer. Further molecular and biological analysis in vitro and vivo are ongoing including the study of the functional role of these SNPs.
Figure 2.
Schema of the relationship between LMTK3 and ERs in breast cancer and gastric cancer. ERα and ERβ have opposing effects in each other. ERα is predominantly expressed in breast cancer, while ERβ is predominant expression in gastric cancer. LMTK3 phosphorylates ERα and protects it from proteosomal degradation in breast cancer. It is proposed that LMTK3 may phosphorylate ERβ, in a similar mechanism shown for ERα in breast cancer, leading to its activation. Abbreviations; ERα, estrogen receptor α; ERβ, estrogen receptor β; LMTK3, lemur tyrosine kinase-3; P, phosphate.
In this study, rs9989661 T/T genotype and rs8108419 G/G genotype were associated with worse outcome, especially rs9989661 T/T genotype predicted recurrence of gastric cancer; hence, rs9989661 may reflect more cancer-specific prognosis in gastric cancer. On the other hand, rs8108419 G/G genotype was not significantly associated with OS in the univariate analysis; however, it became significant in the multivariate analysis in Japanese females and US males. Because Japanese females carrying the G allele are more likely to be younger than those carrying the A allele and younger Japanese patients showed a longer OS, this genotype would not be significantly associated with OS in the univariate analysis. On the other hand, in the US cohort, Asian and Hispanic patients were more likely to have a G allele and these patients showed a longer OS than the others. Consequently, G/G genotype would also not be significantly associated with OS in the univariate analysis. After adjusting for these categories in each cohort, they then became significant. The multivariate analysis of rs8108419 revealed the true association with OS when adjusting the imbalance in the distribution of the baseline prognostic factors; hence, rs8108419 G/G genotype might serve as negative prognostic factors in OS.
However, this association in rs9989661 was found only in the Japanese males and the US females. Postmenopausal Japanese women have the lowest estradiol levels among subgroups in this study, according to previous reports (Suppl. table 4) (44, 45). Lower incidence of postmenopausal breast cancer was found in Japanese females compared to US females (51), and there is a relatively small frequency of HRT in Japan compared with Western countries (less than 10% and more than 40%, respectively), according to case control studies (51, 52). Furthermore, HRT increased postmenopausal breast cancer in Western countries, while this evidence was not confirmed in Japan (18, 51, 52). Moreover, adjuvant anti-estrogen treatment for breast cancer increased second primary GI cancer in Western countries (19–21), while this evidence was not shown to be significant in Japan (53). This evidence is consistent with lower estrogen levels of postmenopausal women in Japan compared with those of the US and supports that postmenopausal Japanese females have less estrogen effect; therefore, Japanese females with this genetic variation may not show a positive impact. Meanwhile, despite the highest estradiol level, the US males were not impacted either. This may in part be explained by the fact that the US males had the highest incidence of GEJ cancer in this study (Suppl. table 1) and higher rate of HER2 overexpression is expected in GEJ cancers (24–32%) (8, 9). In ER-positive operable breast cancer treated with adjuvant tamoxifen, HER2 overexpression was associated with poor prognosis (54). Also, HER2-positive metastatic breast cancer is less responsive to any type of endocrine treatment (55). Moreover, increasing data from preclinical studies have shown that cross-talk between HER2 and ER leads to a hormone-independent state in HER2 co-overexpression in breast cancer cells. This occurs via the redistribution of ER nuclear to cytoplasmic or up-regulation of extracellular signal-regulated kinase 1/2, probably resulting in the development for the endocrine resistance in ER-positive breast cancer (56). These data suggest that the GEJ cancer may not be or less influenced by estrogen efficacy. An alternative possibility, although there are no specific data, is that the expression rate of ERβ in GEJ cancer may be lower than those of stomach cancer. These diversities based on physiologic and clinicopathologic backgrounds among gender and region may account for gender and regional specific outcomes in this study. On the other hand, rs8108419 G/G genotype was associated with OS without tumor recurrence in the US males and the Japanese females. These results were also opposite to the data in breast cancer results, suggesting different predominant ERs distributions between the breast and the GI tract. However, it is not clear why these two polymorphisms were associated with different endpoints, DFS/TTR or OS, in US and Japanese populations. Significant different allele frequencies in rs9989661 in the two cohorts and different linkage disequilibrium, rs9989661 C allele and rs8108419 G allele in the Japanese cohort and rs9989661 T allele and rs8108419 G allele in the US cohort, may explain these data. In addition, the small number of patient population along with different definitions of endpoints, clinicopathologic baselines, surgical technique of lymphadenectomy, and different adjuvant treatment in the two cohorts might impact outcomes and associations. Further experiments are warranted to elucidate the molecular mechanisms how these polymorphisms exert their biological effect.
The prognostic role of LMTK3 polymorphisms reflects predominant ER distribution in each organ, and its prognostic impact should be taken into account given the complexities consisting of regional differences both in physiology and genetic alternations of gastric cancer.
There are several limitations in this study. We must recognize that there are differences in standard clinical practices between Japan and the US. Some clinical information is missing due to the retrospective nature of data collection, leading to different endpoints and different definitions of endpoints. In addition, not all patients completed to follow-up schedules; therefore, potential selection bias should be considered. These issues may keep firm conclusions at a distance; nevertheless, our consistent results among breast and GI cancers reflecting predominant ERs may set a precedent for future researches in the new field of GI cancer.
Our results in the LMTK3 polymorphisms analysis in gastric cancer are the first in GI cancer and shed new light on the differences between the responses on ERα versus ERβ expressing cancers; however, several biological issues remain to be elucidated with the goal of shedding light on the new possibility of prevention and possible treatment in GI cancer. Further biological molecular studies will elucidate these complexities. In conclusion, LMTK3 polymorphisms may serve as a prognostic factor candidate in gastric cancer and may help to select patients who benefit from more careful observation or aggressive treatment. These data suggest that the estrogen pathway may be a novel target for treatment strategy in GI cancer. Further functional correlative preclinical analyses and external clinical validation studies are needed to validate these results.
Supplementary Material
Acknowledgments
Financial support: H.J. Lenz received P30 CA014089-27S1, from the NIH/NCI. H.J. Lenz received a donation by the Dhont Foundation, San Pedro Guild, and Yvonne Bogdanovich.
Abbreviations list
- BMI
body mass index
- CRC
colorectal cancer
- DFS
disease-free survival
- E2
estradiol
- ER
estrogen receptor
- FFPE
formalin fixed paraffin embedded
- GEJ
gastro-esophageal junction
- GI
gastrointestinal
- HRT
hormone replacement therapy
- IHC
immunohistochemical staining
- LMTK3
Lemur tyrosine kinase-3
- OS
overall survival
- REMARK
reporting recommendations for tumor marker prognostic studies
- TTR
time to recurrence
- US
United States
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
Reference
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Center for Cancer Control and Information Services, National Cancer Center Japan. [cited 2012 Accessed on May 23rd ]; Available from: http://ganjoho.jp/professional/statistics/statistics.html#08.
- 3.The American Cancer Society. [cited 2012 Accessed on May 23rd]; Available from: http://www.cancer.org/cancer/stomachcancer/detailedguide/stomach-cancer-key-statistics.
- 4.Yao JC, Schnirer II, Reddy S, Chiang S, Najam A, Yu C, et al. Effects of sex and racial/ethnic group on the pattern of gastric cancer localization. Gastric Cancer. 2002;5:208–212. doi: 10.1007/s101200200036. [DOI] [PubMed] [Google Scholar]
- 5.Yao JC, Tseng JF, Worah S, Hess KR, Mansfield PF, Crane CH, et al. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution's experience over 15 years. J Clin Oncol. 2005;23:3094–3103. doi: 10.1200/JCO.2005.08.987. [DOI] [PubMed] [Google Scholar]
- 6.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 9.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 10.Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of Expression of Human Epidermal Growth Factor Receptors EGFR and ERBB2 on Survival in Stage II/III Gastric Cancer. Clin Cancer Res. 2012;18:5992–6000. doi: 10.1158/1078-0432.CCR-12-1318. [DOI] [PubMed] [Google Scholar]
- 11.Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol. 2003;14:1705–1713. doi: 10.1093/annonc/mdg486. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 15.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 16.Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–6397. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohanes P, Yang D, Chhibar RS, Labonte MJ, Winder T, Ning Y, et al. Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol. 2012;30:2265–2272. doi: 10.1200/JCO.2011.38.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20–38. doi: 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 21.Chandanos E, Lindblad M, Jia C, Rubio CA, Ye W, Lagergren J. Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: a population-based cohort study of breast cancer patients in Sweden. Br J Cancer. 2006;95:118–122. doi: 10.1038/sj.bjc.6603214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 23.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 24.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson JA. Estrogen receptor beta--a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, et al. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319–324. doi: 10.1007/s00432-002-0336-3. [DOI] [PubMed] [Google Scholar]
- 27.Chandanos E, Rubio CA, Lindblad M, Jia C, Tsolakis AV, Warner M, et al. Endogenous estrogen exposure in relation to distribution of histological type and estrogen receptors in gastric adenocarcinoma. Gastric Cancer. 2008;11:168–174. doi: 10.1007/s10120-008-0475-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33:195–201. doi: 10.1016/j.ejso.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY, et al. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010;17:2503–2509. doi: 10.1245/s10434-010-1031-2. [DOI] [PubMed] [Google Scholar]
- 30.Ryu WS, Kim JH, Jang YJ, Park SS, Um JW, Park SH, et al. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol. 2012;106:456–461. doi: 10.1002/jso.23097. [DOI] [PubMed] [Google Scholar]
- 31.Edvardsson K, Strom A, Jonsson P, Gustafsson JA, Williams C. Estrogen receptor beta induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol Endocrinol. 2011;25:969–979. doi: 10.1210/me.2010-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg K, Strom A, Lock JG, Gustafsson JA, Haldosen LA, Helguero LA. Expression of estrogen receptor beta increases integrin alpha1 and integrin beta1 levels and enhances adhesion of breast cancer cells. J Cell Physiol. 2010;222:156–167. doi: 10.1002/jcp.21932. [DOI] [PubMed] [Google Scholar]
- 33.Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, et al. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 34.Helguero LA, Faulds MH, Gustafsson JA, Haldosen LA. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene. 2005;24:6605–6616. doi: 10.1038/sj.onc.1208807. [DOI] [PubMed] [Google Scholar]
- 35.Giamas G, Filipovic A, Jacob J, Messier W, Zhang H, Yang D, et al. Kinome screening for regulators of the estrogen receptor identifies LMTK3 as a new therapeutic target in breast cancer. Nat Med. 2011;17:715–719. doi: 10.1038/nm.2351. [DOI] [PubMed] [Google Scholar]
- 36.Stebbing J, Filipovic A, Lit LC, Blighe K, Grothey A, Xu Y, et al. LMTK3 is implicated in endocrine resistance via multiple signaling pathways. Oncogene. 2012 doi: 10.1038/onc.2012.343. [DOI] [PubMed] [Google Scholar]
- 37.Stebbing J, Filipovic A, Ellis IO, Green AR, D'Silva TR, Lenz HJ, et al. LMTK3 expression in breast cancer: association with tumor phenotype and clinical outcome. Breast Cancer Res Treat. 2012;132:537–544. doi: 10.1007/s10549-011-1622-z. [DOI] [PubMed] [Google Scholar]
- 38.Loupakis F, Zhang W, Gerger A, Yang D, Bohanes P, Labonte MJ, et al. ASCO Gastrointestinal Meeting. J Clin Oncol. 4. Vol. 30. San Francisco: 2012. LMTK3 polymorphism in patients with metastatic colon cancer. abstr 471; 2012. [Google Scholar]
- 39.Zhang W, Gerger A, Labonte MJ, Yang D, Bohanes P, Ning Y, et al. ASCO Gastrointestinal Meeting. J Clin Oncol. 4. Vol. 30. San Francisco: 2012. Association of gender-related tumor recurrence with a polymorphic variant of LMTK3 in stage II and III colon cancer. abstr 454; 2012. [Google Scholar]
- 40.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66:33–38. doi: 10.1016/j.maturitas.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Wu AH, Stanczyk FZ, Seow A, Lee HP, Yu MC. Soy intake and other lifestyle determinants of serum estrogen levels among postmenopausal Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2002;11:844–851. [PubMed] [Google Scholar]
- 43.Kim C, Golden SH, Mather KJ, Laughlin GA, Kong S, Nan B, et al. Racial/Ethnic Differences in Sex Hormone Levels among Postmenopausal Women in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2012;97:4051–4060. doi: 10.1210/jc.2012-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, et al. Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clin Chim Acta. 2008;398:43–47. doi: 10.1016/j.cca.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Vaidya D, Dobs A, Gapstur SM, Golden SH, Cushman M, Liu K, et al. Association of baseline sex hormone levels with baseline and longitudinal changes in waist-to-hip ratio: Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weitsman GE, Weebadda W, Ung K, Murphy LC. Reactive oxygen species induce phosphorylation of serine 118 and 167 on estrogen receptor alpha. Breast Cancer Res Treat. 2009;118:269–279. doi: 10.1007/s10549-008-0221-0. [DOI] [PubMed] [Google Scholar]
- 47.Lam HM, Suresh Babu CV, Wang J, Yuan Y, Lam YW, Ho SM, et al. Phosphorylation of human estrogen receptor-beta at serine 105 inhibits breast cancer cell migration and invasion. Mol Cell Endocrinol. 2012;358:27–35. doi: 10.1016/j.mce.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamilton-Burke W, Coleman L, Cummings M, Green CA, Holliday DL, Horgan K, et al. Phosphorylation of estrogen receptor beta at serine 105 is associated with good prognosis in breast cancer. Am J Pathol. 2010;177:1079–1086. doi: 10.2353/ajpath.2010.090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36:D820–D824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagani F, Baralle FE. Genomic variants in exons and introns: identifying the splicing spoilers. Nat Rev Genet. 2004;5:389–396. doi: 10.1038/nrg1327. [DOI] [PubMed] [Google Scholar]
- 51.Saeki T, Sano M, Komoike Y, Sonoo H, Honjyo H, Ochiai K, et al. No increase of breast cancer incidence in Japanese women who received hormone replacement therapy: overview of a case-control study of breast cancer risk in Japan. Int J Clin Oncol. 2008;13:8–11. doi: 10.1007/s10147-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 52.Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 53.Matsuyama Y, Tominaga T, Nomura Y, Koyama H, Kimura M, Sano M, et al. Second cancers after adjuvant tamoxifen therapy for breast cancer in Japan. Ann Oncol. 2000;11:1537–1543. doi: 10.1093/oxfordjournals.annonc.a010406. [DOI] [PubMed] [Google Scholar]
- 54.Moon YW, Park S, Sohn JH, Kang DR, Koo JS, Park HS, et al. Clinical significance of progesterone receptor and HER2 status in estrogen receptor-positive, operable breast cancer with adjuvant tamoxifen. J Cancer Res Clin Oncol. 2011;137:1123–1130. doi: 10.1007/s00432-011-0976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 56.Yang Z, Barnes CJ, Kumar R. Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cells. Clin Cancer Res. 2004;10:3621–3628. doi: 10.1158/1078-0432.CCR-0740-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.