Abstract
Dickkopf-related protein 1 (DKK1) is essential to maintain skeletal homeostasis as an inhibitor of Wnt signaling and osteogenic differentiation. The purpose of this study was to investigate the molecular mechanisms underlying the developmental stage–specific regulation of the DKK1 protein level. We performed a series of studies including luciferase reporter assays, micro-RNA microarray, site-specific mutations, and gain- and loss-of-function analyses. We found that the DKK1 protein level was regulated via DKK1 3′ UTR by miRNA control, which was restricted to osteoblast-lineage cells. As a result of decreased DKK1 protein level by miR-335-5p, Wnt signaling was enhanced, as indicated by elevated GSK-3β phosphorylation and increased β-catenin transcriptional activity. The effects of miR-335-5p were reversed by anti-miR-335-5p treatment, which downregulated endogenous miR-335-5p. In vivo studies showed high expression levels of miR-335-5p in osteoblasts and hypertrophic chondrocytes of mouse embryos, indicating a pivotal role of miR-335-5p in regulating bone development. In conclusion, miR-335-5p activates Wnt signaling and promotes osteogenic differentiation by down-regulating DKK1. This cell- and development-specific regulation is essential and mandatory for the initiation and progression of osteogenic differentiation. miR-335-5p proves to be a potential and useful targeting molecule for promoting bone formation and regeneration.
Keywords: MIR-335-5P, OSTEOGENIC DIFFERENTIATION, DKK1, WNT, BONE FORMATION
Introduction
Wnt proteins are a family of secreted, cysteine-rich 39- to 46-kDa glycoproteins.(1-3) Activation of canonical Wnt signaling is associated with phosphorylation-mediated inhibition of a kinase-designated glycogen synthase kinase 3β (GSK-3β). This is the principal kinase that induces degradation of cytosolic β-catenin.(4) As a consequence of GSK-3β phosphorylation and inhibition by canonical Wnt signaling, β-catenin accumulates in the cytoplasm and is translocated to the nucleus, where it associates with the Tcf/Lef family of transcription factors to regulate a cohort of canonical Wnt target genes.(5-7) Canonical Wnt signaling is functionally linked to key parameters of osteoblast commitment, proliferation, and apoptosis.(8-10) Biologic relevance is reflected by the absence of skeletal structures derived from the cranial neural crest in β-catenin null mice and arrest of osteoblast differentiation in conditional β-catenin mutants animals.(11-13) Wnt signaling has been reported to directly enhance the expression of Runx2, the master regulatory gene for osteogenic differentiation, via a functional TCF regulatory element in the Runx2 gene promoter (−97 to −93).(14)
Among the extracellular antagonists of canonical Wnt signaling, DKK1 was reported to act through binding to and inactivating signaling from LRP5/6 receptors.(15-17) DKK1 plays a critical role in embryonic bone development and adult bone remodeling.(18) DKK1-regulated canonical Wnt signaling is essential to sustaining proper skeletal homeostasis, and incremental decreases in DKK1 expression result in concomitant incremental increases in bone mass.(19,20) In addition, dexamethasone-induced suppression of alkaline phosphatase activity, osteocalcin expression, and rate of bone formation is mediated by DKK1.(21) DKK1 is emerging as an important molecular component to the development of malignant bone lesions by inhibiting osteoblast differentiation(22) and has been found to be a major determinant of bone and joint pathology in rheumatoid arthritis.(23) The binding of DKK1 to LRP5 has been characterized as the most extensively used mechanism of modulation for control of Wnt signaling in human bone tissues.(24)
Micro-RNAs (miRNAs) are a family of small, noncoding RNAs that posttranscriptionally regulate gene expression for control of a broad spectrum of biologic processes, including stem cell self-renewal, development, differentiation, growth, and metabolism.(25,26) By imperfect or perfect base pairing with specific sequences in the 3′ UTR of target mRNAs, miRNAs induce either translational repression or cleavage of the target mRNAs, respectively.(26) In addition to studies reporting selective expression of miRNAs in mesenchymal stem cells, osteoblasts, or osteoclasts,(27,28) miRNAs also have been implicated in osteogenic differentiation. Lentivirus expression of shRNAs to downregulate miRNA processing enzymes (Dicer and Drosha), as well as in vivo excision of Dicer in osteoblast lineage cells, inhibits mesenchymal stem cell differentiation into osteoblasts.(29,30) Two miRNAs, respectively, targeting Runx2 and Smad5 were identified. These miRNAs, miR-133 and miR-135, are down-regulated following osteogenic differentiation by bone morphogenetic protein 2 (BMP-2) treatment.(31) Using human adipose tissue–derived stem cells (hADSCs), miR-26a was shown to target SMAD1 and inhibit hADSC differentiation toward the osteogenic lineage induced by treatment with dexamethasone, ascorbic acid, and β-glycerol phosphate.(32)
In contrast to lower eukaryotic model systems, our knowledge of miRNA function in mammals is limited, and there is minimal understanding of miRNA regulatory activity in bone research. In this study, we investigated the regulatory role of miRNAs targeting DKK1 in controlling Wnt signaling that is confined to osteogenic differentiation.
Materials and Methods
Cell culture
C3H10T-1/2 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. MC3T3-E1 cells were maintained in α modified essential medium (α-MEM) with 10% FBS and antibiotics. MLO-A5 cells were cultured in α-MEM with 5% FBS, 5% iron-supplemented calf serum (iCS; HyClone Laboratories, Logan, UT, USA) and antibiotics. MLO-Y4 cells were cultured in α-MEM with 2.5% FBS, 2.5% iCS, and antibiotics. Osteogenesis was induced with 50 μg/mL of ascorbic acid, as described previously.(33) MLO-A5 and MLO-Y4 cells were provided by Dr Lynda Bonewald (University of Missouri, Kansas City, MO, USA). Primary calvarial osteoblasts were prepared by enzymatic digestion, as described previously,(34) and routinely cultured in α-MEM (Life Technologies, Rockville, MD, USA) supplemented with 10% HIFBS (Life Technologies), 2 mM l-glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin.
miRNA microarray analysis
Total RNA was extracted from MC3T3-E1 and MLO-A5 cell cultures treated with or without 50 μg/mL of ascorbic acid for 10 days using the miRNeasy Mini Kit (Qiagen, Valencia, CA, USA). Five micrograms of total RNA from each cell line was sent to LC Sciences (Houston, TX, USA) for microarray analysis. Image processing and data extraction and analysis also were performed by LC Sciences using their established protocols.(35)
Luciferase reporter constructs
The luciferase–3′ UTR constructs were created by subcloning the 3′ UTR polymerase chain reaction (PCR) fragments into pMIR-REPORT miRNA luciferase reporter vector (Ambion, Austin, TX, USA). Site mutations in DKK1 3′ UTR were performed using the Quickchange XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). The mutated site was confirmed by digestion of the mutated construct with HindIII, and all the newly constructed plasmids were sequenced to confirm correct DNA sequence and orientation (Tufts University Core Facility, Boston, MA, USA). The primer sets are listed in Supplemental Table S1. The β-catenin reporter construct (p4xTRE1-Luc) was purchased from Addgene (Addgene ID: 16535, Cambridge, MA, USA).(36)
Cell transfection and luciferase assay
For transient transfection, cells were cotransfected with the luciferase–3′ UTR plasmids and pMIR-REPORT β-gal control plasmid (pMIR-β-gal) using lipofectamine (Invitrogen, Carlsbad, CA, USA). Forty-eight hours after transfection, luciferase and β-gal levels were determined using a luminometer (Lumat LB 9501; EG&G Berthold, Bad Wildbach, Germany) and β-Gal Detection Kit II (BD Clontech, Palo Alto, CA, USA), respectively. Luciferase activity was normalized to β-galactosidase activity. For stable transfection, cells were transfected with luciferase-DKK1-3′UTR construct and selected with 1 μg/mL of puromycin for 2 weeks. Luciferase level was determined and normalized for total protein. For miRNA transfection, cells were transfected with miR-335-5p precursor hairpin, negative control pre-miR, anti-miR-335-5p, or anti-negative control pre-miR (Ambion, Austin, TX, USA) using siPORT NeoFX transfection reagent (Ambion).
Real-time RT-PCR for mRNA and miRNA analysis
Quantitative real-time reverse-transcriptase PCR (qRT-PCR) assay for mRNA analysis was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on a Bio-Rad iQ5 thermal cycler (Bio-Rad Laboratories). The evaluation of relative differences in PCR product amounts was carried out by the comparative cycle threshold (CT) method using GAPDH as a control. For miRNA analysis, total RNA was extracted using the miRNeasy Mini Kit (Qiagen), and cDNA was synthesized using an NCode miRNA First-Strand cDNA Synthesis Kit (Invitrogen). qRT-PCR was performed on a Bio-Rad iQ5 thermal cycler using an NCode Express SYBR GreenER miRNA qRT-PCR Kit (Invitrogen). The relative differences in PCR product amounts were evaluated by the comparative cycle threshold (CT) method using U6 snRNA as a control. Primers used for amplification are listed in Supplemental Table S2.
Western blot
Whole-protein lysates were prepared essentially as described previously.(37) Antibodies for DKK1 or β-actin were obtained from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA, USA). Antibodies for GSK-3β or phospho-GSK-3β (serine 9) were obtained from Cell Signaling Technology (Beverly, MA, USA). The secondary antibodies were horseradish peroxidase (HRP)–linked goat-anti rabbit IgG (Santa Cruz Biotechnologies, Inc.). Blots were visualized using ECL chemiluminescence reagents from Pierce Biotechnology (Rockford, IL, USA).
In situ hybridization and ALP staining
Nonradioactive in situ hybridization on paraffin sections was performed using DIG-labeled miRCURY detection probes (Exiqon, Vedbaek, Denmark). Briefly, embryo tissue sections (kindly provided by Dr Yiping Chen, Tulane University, New Orleans, LA, USA) were deparaffinized, deproteinated, and hybridized with DIG-labeled probes specifically targeting miR-335-5p. Positive signals were visualized using immunologic detection with 1:2000 anti-DIG-AP Fab fragments and color reaction with light-sensitive NBT/BCIP. Alkaline phosphatase (ALP) histochemistry was performed as described previously with minor modifications.(38)
Statistical analysis
Generally, results are shown as the mean ± SEM of three or more experiments. One-way ANOVA was used to test significance using the software package Origin 6.1 (OriginLab, Northampton, MA, USA). Values of p lower than .01 or .05, as specifically indicated in the legends, were considered statistically significant.
Results
miRNAs exhibit expression profiles that provide signatures for differentiation
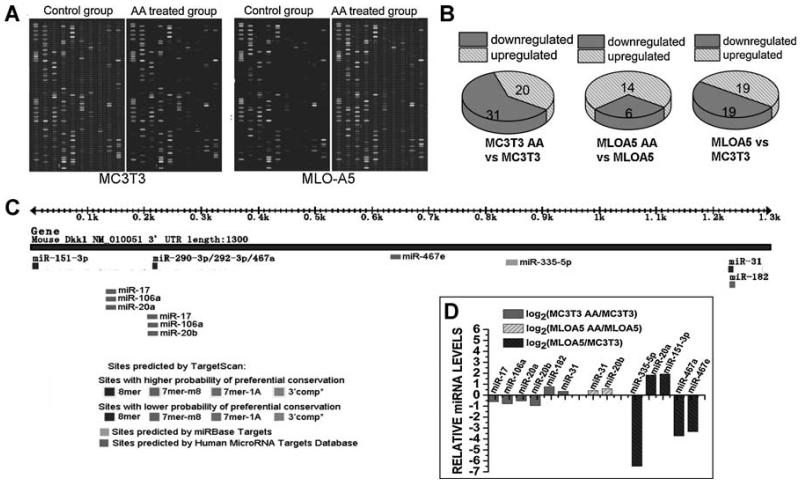
We selected two well-studied osteoblast cell lines, MC3T3-E1 murine osteoblast-like cells and MLO-A5 murine preosteocyte-like cells, representing two key developmental stages of the osteoblast for miR profiling. After 10 days of osteogenic induction by 50 μg/mL of ascorbic acid, both MLO-A5 and MC3T3-E1 cells exhibit unique miRNA expression profiles when compared with corresponding control cells (Fig. 1A). Among 600 miRNAs investigated, expression levels of 51 miRNAs were significantly altered by ascorbic acid treatment in MC3T3-E1 cells, with 31 miRNAs being decreased. In MLO-A5 cells, expression levels of 20 miRNAs were altered by ascorbic acid treatment, with 6 miRNAs being downregulated (Fig. 1B). Statistically significant differences in the expression of 38 miRNAs between MLO-A5 and MC3T3-E1 cells also were observed (Fig. 1B). With DKK1 being identified as a preferential target, miRNA target prediction was carried out using a combination of the following computational algorithms: www.targetscan.org,(39) Sloan-Kettering Cancer Center Human MicroRNA Targets Database (www.microRNA.org),(40) and miRBase Targets (http://microrna.sanger.ac.uk/targets/v5). Among miRNAs being predicted to target DKK1 3′ UTR (Fig. 1C), we selected the ones that are up- or downregulated during osteogenic differentiation for further investigation. Changes in expression levels of these miRNAs were provided by miRNA microarray analysis and are shown in Fig. 1D.
Fig. 1.
Changes in miRNA expression profiles as indicated by miRNA microarray analysis. (A) Original images of the chips. The chip detects miRNA transcripts listed in Sanger miRBase Release 11.0. (B) Data analysis revealed groups of miRNAs that were down- or upregulated after ascorbic acid treatment, indicating that these miRNAs may be actively involved in the regulation of osteogenic differentiation. (C) miRNA target prediction using a combination of the following computational algorithms: TargetScan, Sloan-Kettering Cancer Center Human MicroRNA Targets Database, and miRBase Targets. (D) Expression levels of some miRNAs predicted to target DKK1 by the aforementioned computer algorithms also were found to be regulated by ascorbic acid treatment, as indicated by miRNA microarray analysis.
miRNAs are functionally linked to the control of DKK1 expression in a cell- and developmental stage–specific manner
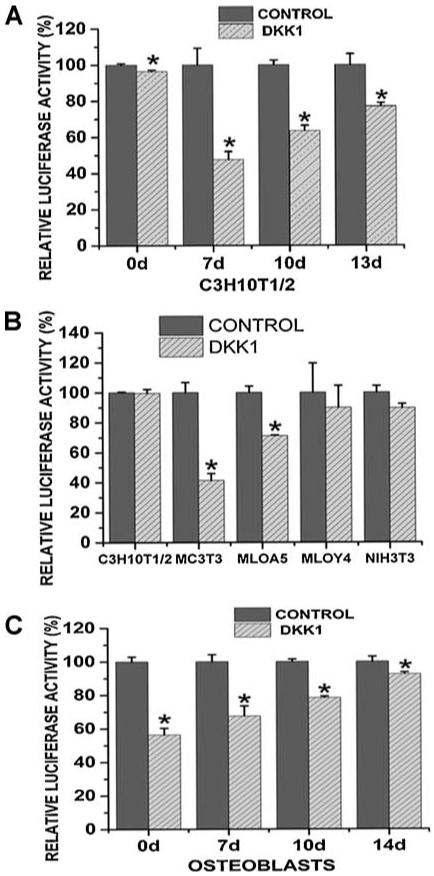
To address the functional regulation of DKK1 by miRNAs as related to the progression of osteoblastogenesis, we examined activity of a luciferase-DKK1-3′UTR construct (luc-DKK1-UTR) in different cell types. C3H10T1/2 murine mesenchymal stem cells were treated with 50 μg/mL of ascorbic acid for 13 days to increase osteoprogenitor differentiation and were transiently transfected with luc-DKK1-UTR at different time points. We found that in undifferentiated C3H10T-1/2 cells transfected with luc-DKK1-UTR (on day 0), the luciferase level was only slightly lower than that in control cells. However, a dramatic decrease in luciferase level was observed when the cells were treated with ascorbic acid for 7 days. At later time points following ascorbic acid treatment, luc-DKK1-UTR-transfected cells demonstrated a significant but less prominent decrease in luciferase levels compared with control cells (Fig. 2A). These results provide the first evidence that during osteogenic differentiation, the protein level of DKK1 is regulated in response to endogenous miRNAs through activity of DKK1 3′ UTR sequence in a differentiation-specific manner. Under the regulation of endogenous miRNAs, the protein level of DKK1 was decreased at initial stages of osteogenic differentiation and subsequently increased.
Fig. 2.
DKK1 expressions were regulated by the interactions between miRNAs and 3 ′UTR sequences in a stage- and cell-specific way. (A) C3H10T1/2 cells were treated with 50 μg/mL of ascorbic acid (AA) and were transfected with DKK1 3′ UTR construct (DKK1) or pMIR-REPORT (Control) at different time points. pMIR-β-gal was cotransfected to determine the transfection efficiency. Forty-eight hours after transfection, luciferase and β-gal levels were determined, and luciferase activity was normalized to β-galactosidase activity. Three independent experiments were performed in triplicates, and data are represented as mean ± SEM. *p < .05 versuscontrol group. (B) C3H10T1/2, MC3T3-E1, MLO-A5, MLO-Y4, and NIH3T3 cells were transfected with pMIR-REPORT (Control) or pMIR-REPORT-DKK1 3′ UTR (DKK1). Lucifearse and β-gal levels were determined as stated in panel A. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .01 versus control group. (C) Primary calvarial osteoblasts were treated with 50 μg/mL of ascorbic acid (AA) for 14 days and were transiently transfected with DKK1 3′ UTR construct (DKK1) or pMIR-REPORT (Control) at different time points. Lucifearse and β-gal levels were determined as stated in panel A. Three independent experiments were performed in triplicates, and data are represented as mean ± SEM. *p < .05 versuscontrol group.
To further investigate the role of endogenous miRNAs as a function of osteogenic differentiation, we transiently transfected luc-DKK1-UTR into distinct cell lines, including C3H10T-1/2 murine mesenchymal stem cells, MC3T3-E1 murine osteoblast-like cells, MLO-A5 murine preosteocyte-like cells, MLO-Y4 murine osteocyte-like cells, and NIH3T3 murine fibroblasts. In Fig. 2B we show the percentage changes in luciferase activity determined in cells transfected with luc-DKK1-UTR (DKK1) compared with cells transfected with the empty vector (CONTROL). For comparison, luciferase activity in cells transfected with the empty vector were arbitrarily assigned a value of 100%. We found that the decreased luciferase levels that resulted from the insertion of DKK1 3′ UTR were barely detectable in C3H10T-1/2 mesenchymal stem cells (99.56%) and NIH3T3 fibroblasts (89.62%). However, in MC3T3-E1 cells transfected with luc-DKK1-UTR, the luciferase level decreased to 41.33% compared with control cells. At the terminal stages of osteogenic differentiation, as observed in MLO-A5 and MLO-Y4 cells, luciferase levels were restricted to 71.11% and 89.84%, respectively. We also performed similar experiments using primary calvarial osteoblasts. For osteogenic induction, these primary osteoblasts were treated with 50 μg/mL of ascorbic acid for 14 days and were transiently transfected with luc-DKK1-UTR at different time points. Using these cells, we observed that the decrease in luciferase activity resulting from insertion of the DKK1 3′ UTR became less prominent as cells were induced to differentiate toward the terminal stage of osteogenic differentiation (Fig. 2C). These findings confirm that the expression of DKK1 is posttranscriptionally regulated through interactions between the DKK1 3′ UTR sequence and specific endogenous miRNAs. Furthermore, the inhibitory effects of these miRNAs on DKK1 protein levels are unique in certain cell types, suggesting that the regulatory role of these miRNAs is selective, functioning in a cell- and stage-specific manner, as demonstrated in the regulation of osteogenic differentiation.
DKK1 expression is subtly regulated during osteogenic differentiation
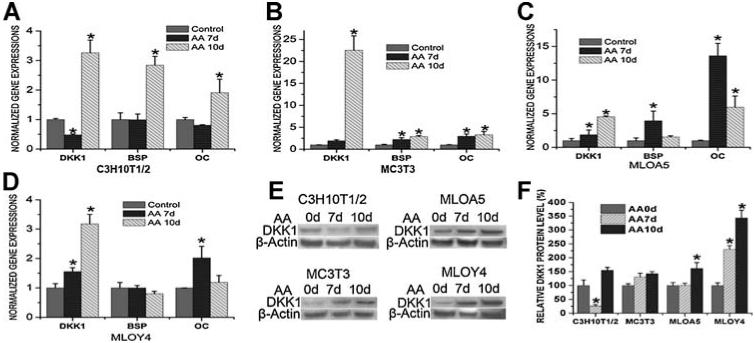
Expression levels of DKK1 were monitored in response to the induction of osteogenic differentiation by ascorbic acid in a series of cell lines. In MC3T3-E1, MLO-A5, and MLO-Y4 cells, ascorbic acid treatment resulted in a significant increase in DKK1 mRNA levels (Fig. 3B, D). However, in C3H10T-1/2 cells, the DKK1 mRNA level decreased 7 days after ascorbic acid treatment and was restored subsequently (Fig. 3A). Expression of bone marker genes, reflected by mRNA levels of bone sialoprotein (BSP) and osteocalcin (OC), were monitored to document stages of osteogenic differentiation (Fig. 3A, D). The changing levels in DKK1 expression suggest that as the osteoblast becomes fully differentiated, there is a requirement to attenuate Wnt signaling.
Fig. 3.
Real-time RT-PCR (A–D) and Western blot (E, F) analyses indicated mRNA and protein levels of DKK1 before and after ascorbic acid (AA) treatment. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05, AA-treated cells versus control cells.
As revealed by Western blot analysis, changes in DKK1 protein levels showed similar responses (Fig. 3E, F). While, in MC3T3-E1 cells, the DKK1 protein level increased after osteogenic induction, the change was not statistically significant. In MLO-A5 cells, 10 days of ascorbic acid treatment resulted in an approximately 1.5-fold increase in DKK1 protein levels (Fig. 3F). In contrast, the increased DKK1 mRNA levels in response to ascorbic acid treatment in C3H10T-1/2 cells, MC3T3-E1 cells, and MLO-A5 cells ranged from 3- to 22-fold (Fig. 3A, C). When osteogenic differentiation reached the terminal stage, as represented by the MLO-Y4 late osteocyte cells, both the DKK1 mRNA level and the DKK1 protein level increased dramatically to a similar extent (Fig. 3D, F). These results clearly indicate that additional posttranscriptional inhibitors are involved in the regulation of DKK1 protein level at certain stages of osteogenic differentiation. The regulatory effects of these posttranscriptional inhibitors, including specific endogenous miRNAs, may be more pronounced at early stages of osteogenic differentiation.
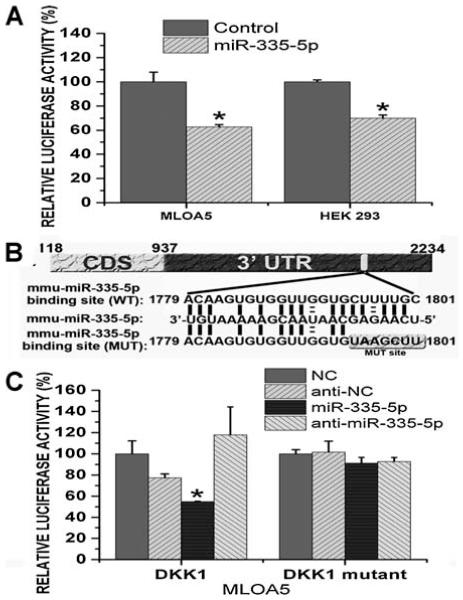
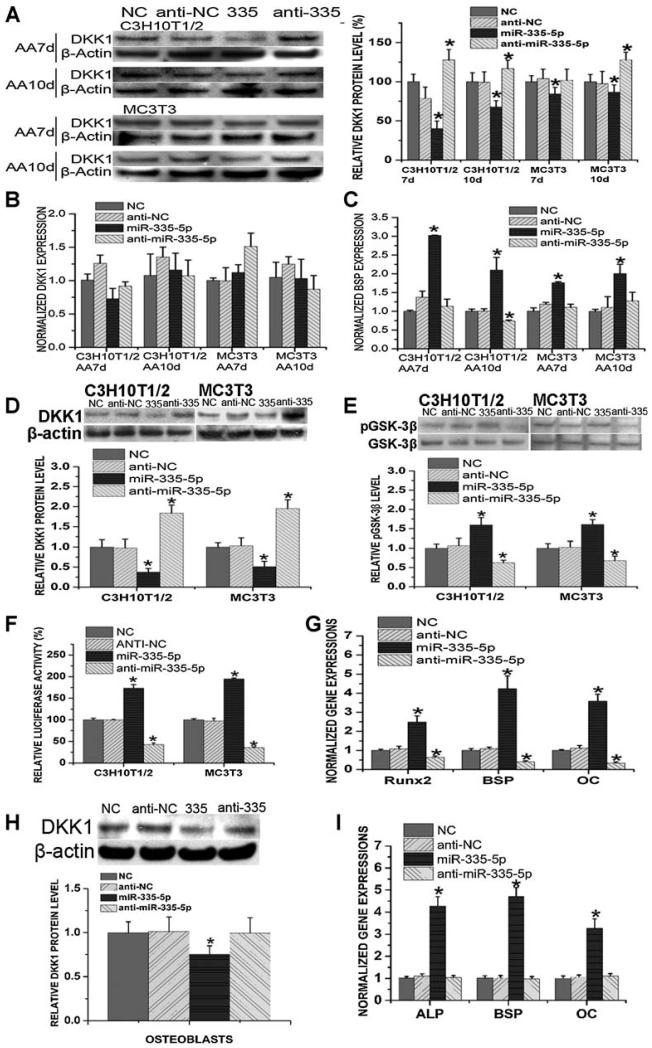
miR-335-5p specifically targets DKK1 3′ UTR and controls DKK1 expression, canonical Wnt signaling activity, and osteogenic differentiation
MLO-A5 cells stably expressing luc-DKK1-UTR (MLO-A5-DKK1UTR) were transfected with negative control pre-miR and some of the miRNA precursor hairpins listed in Fig. 1D (Ambion). Among the miRNAs investigated, including miR-335-5p, miR-106a, and miR-17, we found that miR-335-5p decreased luciferase activity by 40% in MLO-A5-DKK1UTR compared with the negative control (Fig. 4A). We also transiently cotransfected luc-DKK1-UTR with miR-335-5p precursor hairpin or with negative control pre-miR into HEK 293 cells using siPORT NeoFX as the transfection reagent. In these cells, miR-335-5p decreased luciferase activity by 31% compared with the negative control (Fig. 4A). However, we failed to observe any statistically significant changes in luciferase activity in MLO-A5-DKK1UTR transfected with miR-106a or miR-17 compared with control cells (data not shown).
Fig. 4.
miR-335-5p specifically targets DKK1 3′ UTR. (A) Luciferase activity in MLO-A5 cells stably expressing luciferase-DKK1 3′ UTR construct was decreased after transfected with miR-335-5p precursor hairpin (miR-335-5p) when compared with cells transfected with negative control pre-miR (Control). Luciferase level was normalized to total protein. Luciferase-DKK1 3′ UTR construct and pMIR-REPORT β-gal control also were transiently cotransfected with miR-335-5p precursor hairpin (miR-335-5p) or with negative control pre-miR (Control) into HEK 293 cells using siPORT NeoFX transfection reagent. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .01 versus control cells. (B) Schema and sequence of predicted miR-335-5p binding site, sequence of miR-335-5p, and sequence of mutated DKK1 3′ UTR. (C) The inhibitory effect of miR-335-5p on wild-type DKK1 3′ UTR disappeared in mutated DKK1 3′ UTR. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05 versus control cells. NC = negative control.
Site-specific mutations then were introduced into the predicted binding site of miR-335-5p in the DKK1 3′ UTR sequence (Fig. 4B). This luciferase construct with mutated DKK1 3′ UTR (DKK1 mutant) was stably transfected into MLO-A5 cells. The inhibitory effect of miR-335-5p observed on wild-type DKK1 3′ UTR disappeared in mutated DKK1 3′ UTR (Fig. 4C), which convincingly substantiated the idea that the regulatory function of miR-335-5p in control of DKK1 protein level is direct and site-specific. We also transfected anti-miR-335-5p into MLO-A5 cells stably expressing wild-type or mutated DKK1 3′ UTR constructs. However, we did not observe statistically significant changes in luciferase levels. The insertion of DKK1 UTR only resulted in a nearly 30% reduction in luciferase activity in MLO-A5 cells (Fig. 2B), suggesting that the level of endogenous miR-335-5p in MLO-A5 cells may be relatively lower than in cells at earlier stages of differentiation. This may account for the absence of observed changes in luciferase activity in MLO-A5 cells after anti-miR-335-5p treatment.
We then transfected miR-335-5p or anti-miR-335-5p into C3H10T-1/2 cells and MC3T3-E1 cells at different time points during osteogenic differentiation and determined the DKK1 mRNA and DKK1 protein levels. BSP mRNA levels also were determined to evaluate the osteogenic status. Consistent with previous findings showing that miRNAs principally induce translational repression through 3′ UTR inhibition in mammals,(31) we found that miR-335-5p did not have an inhibitory effect on DKK1 mRNA levels (Fig. 5B). However, miR-335-5p significantly decreased DKK1 protein levels in both cell lines, whereas anti-miR-335-5p increased DKK1 protein levels (Fig. 5A). Consequently, BSP expression levels were elevated by miR-335-5p treatment (Fig. 5C).
Fig. 5.
Functional analysis of miR-335-5p in C3H10T1/2 cells and MC3T3-E1 cells. (A–C) C3H10T-1/2 cells and MC3T3 cells were transfected with miR-335-5p or anti-miR-335-5p once over 7 or 10 days of ascorbic acid treatment (transfected on day 5 or day 8). DKK1 protein (A) and DKK1 mRNA (B) levels were determined. BSP mRNA levels (C) also were determined to evaluate the osteogenic status. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05 versus control cells. NC = negative control; AA = ascorbic acid. (D–G) C3H10T1/2 cells and MC3T3 cells were transfected with miR-335-5p or anti-miR-335-5p for three times over 8 days of ascorbic acid treatment (transfected on days 0, 3, and 6). (D) Western blot analysis indicated the changes in DKK1 protein levels. (E) Western blot analysis indicated the changes in phospho-GSK-3β levels. (F) On day 6, β-catenin reporter construct (p4xTRE1-Luc) was cotransfected with miR-335-5p or anti-miR-335-5p. Luciferase assays showed the changes in transcriptional activity of β-catenin. (G) RT-PCR showed mRNA expression changes of Wnt target genes, including Runx2, BSP, and OC (in C3H10T-1/2 cells). Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05 versus control cells. NC = negative control. (H, I) Calvarial osteoblasts were transfected with miR-335-5p or anti-miR-335-5p once over 14 days of ascorbic acid treatment (transfected on day 12). DKK1 protein level (H) and mRNA levels of ALP, BSP, and OC (I) were determined. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05 versus NC group. NC = negative control.
We also transfected C3H10T-1/2 and MC3T3-E1 cells with miR-335-5p or anti-miR-335-5p at three time points during 8 days of ascorbic acid modulation of osteogenic differentiation (on days 0, 3, and 6) and monitored canonical Wnt signaling activity. Using Western blot analysis, we directly determined the protein level of DKK1 and assayed the phosphorylation level of GSK-3β at serine 9 as an indication of GSK-3β inactivation. Multiple transfections with miR-335-5p or anti-miR-335-5p resulted in dramatic changes in DKK1 protein level (Fig. 5D). Additionally, although miR-335-5p or anti-miR-335-5p did not influence the level of total GSK-3β (data not shown), miR-335-5p significantly increased the phosphorylation level of GSK-3β, which was notably decreased by anti-miR-335-5p (Fig. 5E). To evaluate the transcriptional activity of β-catenin, we transfected C3H10T-1/2 and MC3T3-E1 cells with miR-335-5p or anti-miR-335-5p at three time points during 8 days of ascorbic acid–induced osteogenic differentiation (on days 0, 3, and 6), and cotransfected β-catenin reporter constructs (p4xTRE1-Luc) with miR-335-5p or anti-miR-335-5p on day 6. Luciferase assays indicated that the transcriptional activity of β-catenin was drastically enhanced by miR-335-5p treatment but decreased by anti-miR-335-5p (Fig. 5F). We then monitored the effect of miR-335-5p on expression levels of several Wnt target genes, including Runx2, BSP, and OC in C3H10T-1/2 cells transfected with miR-335-5p or anti-miR-335-5p at three time points during 8 days of ascorbic acid–induced osteogenic differentiation (on days 0, 3, and 6). Consistent with the aforementioned results indicating enhanced Wnt signaling after miR-335-5p treatment, expression levels of these Wnt target genes were increased dramatically in cells transfected with miR-335-5p (Fig. 5G).
We also confirmed our findings using primary osteoblasts in the form of calvarial cells. Briefly, calvarial osteoblasts were transfected with miR-335-5p or anti-miR-335-5p once over 14 days of ascorbic acid treatment (transfected on day 12). Consistent with previous findings, DKK1 protein level was downregulated by miR-335-5p treatment in these cells (Fig. 5H). As a result, mRNA levels of osteogenic markers, including ALP, BSP, and OC, were increased by miR-335-5p overexpression (Fig. 5I). However, no significant changes in DKK1 protein level or mRNA levels of ALP, BSP, and OC were detected after the transfection of anti-miR-335-5p (Fig. 5I, H), which may be the result of a lower endogenous miR-335-5p level at this time point of osteogenic differentiation.
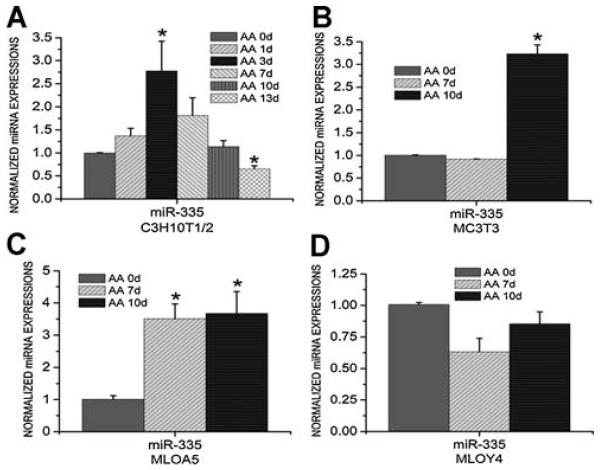
Expression levels of miR-335-5p in different cell lines then were determined. Consistent with the Western blot results showing that protein levels of DKK1 in C3H10T1/2 cells were decreased at an earlier time point during ascorbic acid–induced osteogenic differentiation but increased at a later time point (Fig. 3F), expression levels of miR-335-5p were increased in C3H10T1/2 cells on days 3 and 7 after ascorbic acid treatment but decreased thereafter (Fig. 6A). Similarly, miR-335-5p expression levels in MC3T3-E1 cells (Fig. 6B) and MLO-A5 cells (Fig. 6C) increased in response to ascorbic acid, which may explain why DKK1 protein levels are not increased in parallel with DKK1 mRNA levels in these cells (Fig. 3B, C, F). However, when osteogenic differentiation moves toward the terminal stage, as occurs in MLO-Y4 cells, miR-335-5p expression was not increased in response to ascorbic acid (Fig. 6D), which is consistent with the Western blot results indicating that ascorbic acid induces similar increases in DKK1 mRNA and protein levels in MLO-Y4 cells (Fig. 3D, F). These results corroborate evidence demonstrating that miR-335-5p plays a significant role during osteogenic differentiation through targeting the DKK1 3′ UTR sequence to dampen the increase in DKK1 protein levels. Such a mechanism may be physiologically relevant to maintaining osteogenic competency in immature bone-forming cells.
Fig. 6.
Real-time RT-PCR analysis showed the expression levels of miR-335-5p in C3H10T1/2 cells (A), MC3T3 cells (B), MLO-A5 cells (C), and MLO-Y4 cells (D) before and after ascorbic acid (AA) treatment. Three independent experiments were performed in triplicate, and data are represented as mean ± SEM. *p < .05, AA-treated cells versus control cells.
miR-335-5p is robustly expressed in osteoblasts during bone development
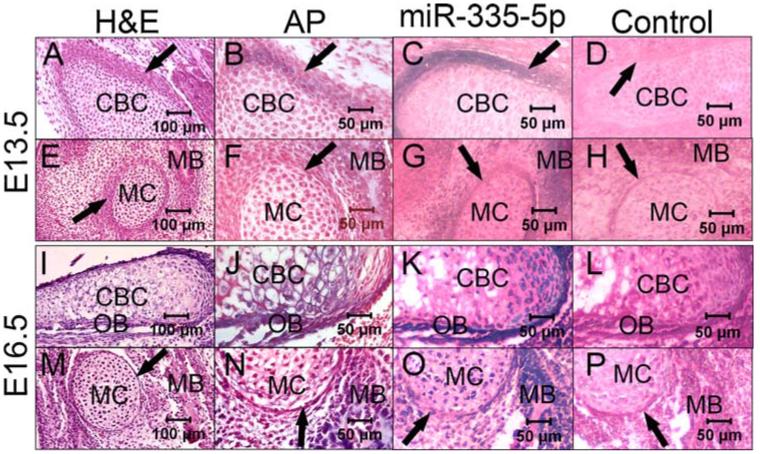
To initially explore the in vivo function of miR-335-5p, we performed in situ hybridization to monitor the in vivo distribution of miR-335-5p during bone development. ALP staining also was performed as an indicator of new bone formation. At E13.5, perichondrium was observed to surround the cranial base cartilage and Meckel’s cartilage, which consists of mature chondrocytes (Fig. 7A, E). As shown by ALP staining, a component of the cells residing in or adjacent to the perichondrium began to differentiate toward osteoblasts, and the formation of mandibular bone could be observed around Meckel’s cartilage (Fig. 7B, F). In situ hybridization for the detection of miR-335-5p indicats subtle positive staining in the developing mandible and in some of the cells residing in the perichondrium in E13.5 embryos. The expression of miR-335-5p is minimally detectable in mature chondrocytes (Fig. 7C, G).
Fig. 7.
In situ hybridization showing in vivo distribution of miR-335-5p in E13.5 and E16.5 mouse embryos. (A–D) E13.5 cranial base cartilage by hematoxylin and eosin (H&E) staining (A), ALP staining (B), in situ hybridization for miR-335-5p (C), and in situ hybridization for negative control (D). (E–H) E13.5 Meckel’s cartilage by H&E staining (E), ALP staining (F), in situ hybridization for miR-335-5p (G), and in situ hybridization for negative control (H). (I–L) E16.5 cranial base cartilage by H&E staining (I), ALP staining (J), in situ hybridization for miR-335-5p (K), and in situ hybridization for negative control (L). (M–P) E13.5 Meckel’s cartilage by H&E staining (M), ALP staining (N), in situ hybridization for miR-335-5p (O), and in situ hybridization for negative control (P). CBC = cranial base cartilage; MC = Meckel’s cartilage; MB = mandibular bone; OB = osteoblasts; arrow = perichondrium.
At E16.5, the cranial base cartilage and Meckel’s cartilage are well developed and consist of mature and hypertrophic chondrocytes. Osteoblasts align on the surface of the cranial base cartilage and the newly formed mandibular bone (Fig. 7I, M). ALP staining reveals stronger positive staining in osteoblasts and in components of the hypertropic chondrocytes (Fig. 7J, N). Consistent with the ALP staining results, intense staining for miR-335-5p was detected at this stage in some hypertropic chondrocytes and in osteoblasts aligned on the surface of the cranial base cartilage. The E16.5 mandible also was positive with intense staining for miR-335-5p (Fig. 7K, O).
Discussion
Numerous lines of evidence indicated stringent up- or down-regulation of Wnt signaling at specific stages of osteogenic differentiation, and an altered Wnt signal even could compromise bone regeneration.(41) For example, Wnt signaling is endogenously activated in undifferentiated mouse embryonic stem cells (ESCs) to maintain pluripotency and self-renewal in these cells,(42-45) and on differentiation of ESCs toward precursor cells, Wnt signaling is downregulated.(42,43) However, upregulation of Wnt signaling is necessary for osteoprecursor cells to differentiate into osteoblasts rather than chondrocytes.(12) When the osteoblasts differentiate further toward the terminal stage of bone-forming cells, Wnt signaling is inhibited again, as indicated by the upregulation of Wnt antagonists in the postproliferative preosteocytic stage or osteocytic stage to limit excessive mineralization.(46-48) In this context, a larger number of miRNAs increase during progression of osteblast differentiation that potentially target components of Wnt signaling.(49)
Consistent with these findings, we found that DKK1 expression is regulated in a stage-specific manner. DKK1 mRNA and DKK1 protein levels are decreased initially when osteogenic differentiation is initiated in mesenchymal stem cells (C3H10T-1/2). Consequently, Wnt signaling is more efficiently activated at the onset of osteogenic differentiation. Cartilage-forming chondrocytes and bone-forming osteoblasts are both derived from common osteochondroprogenitor cells.(12) While Runx2 is widely accepted as the master regulatory gene for osteogenic differentiation,(50) Sox9 is considered to be the most important regulator for chondrogenic differentiation.(51) Furthermore, the determination for osteochondroprogenitor cells to differentiate along the chondrogenic path rather than the osteogenic path is controlled by the relative levels of Sox9 and Runx2.(52) Several lines of evidence indicate that expression levels of both Sox9 and Runx2 are directly regulated by Wnt signaling.(12,14,53) Together with previous findings, our results suggest that Wnt signaling is activated when osteogenic differentiation is initiated from mesenchymal stem cells. Physiologic control may be mediated at least in part by downregulation of the Wnt signaling antagonist DKK1. As a result, cellular levels of Sox9 are decreased and remain low relative to Runx2 levels. This mechanism supports preferential commitment of the progenitor cells to osteogenic differentiation. When osteogenic differentiation reaches the terminal stage, cellular levels of DKK1 protein are again upregulated. Osteogenic differentiation is thereby slowed down by DKK1-mediated inhibition of Wnt signaling to limit excessive mineralization.
Interestingly, the changes in DKK1 protein and DKK1 mRNA levels were not completely consistent. Although after osteogenic induction, DKK1 mRNA levels in MC3T3-E1 and MLO-A5 cells were increased by 3- to 22-fold, and DKK1 protein levels did not increase significantly. Statistically significant changes were not observed in osteoblastic MC3T3-E1 cells, and less than a 2-fold increase was observed in osteocytic MLO-A5 cells. These results suggest that additional posttranscriptional control may be operative in the regulation of DKK1 protein levels.
Using a luciferase-DKK1 3′ UTR reporter construct, we showed that stage-specific changes in DKK1 protein levels are, at least in part, regulated posttranscriptionally by miRNAs that specifically target DKK1. On transfection with a luciferase-DKK1 3′ UTR construct, bone-forming cell lines at various stages of osteoblast differentiation exhibit different levels of luciferase activity that are consistent with the changes observed in DKK1 mRNA and DKK1 protein levels after osteogenic differentiation. To exclude confounding contributions that can be attributed to characteristics of each cell line, we transfected the DKK1 3′ UTR construct into C3H10T-1/2 cells at different time points following osteogenic induction and monitored luciferase levels. Luciferase levels in C3H10T-1/2 cells transfected with the DKK1 3′ UTR construct were decreased initially when C3H10T-1/2 cells were induced to differentiate toward bone-forming cells and subsequently increased again at later time points as osteoblast differentiation proceeded. These findings not only indicated that miRNAs are actively involved in the regulation of DKK1 expression but also suggested that the inhibitory roles of these miRNAs are compromised when osteogenic differentiation progresses to the terminal stages and prevent excessive mineralization. In agreement with this interpretation, a minimally detectable decrease in luciferase levels resulted from insertion of DKK1 3′ UTR in mesenchymal stem cells and fibroblasts, indicating that the regulatory role of these miRNAs occurs in a cell-specific manner and is particularly important in control of osteogenic differentiation.
Combining miRNA microarray analysis, computational target prediction, and functional analysis, we observed that miR-335-5p negatively regulates protein levels of DKK1, one of the Wnt antagonists, through targeting a specific binding site in the DKK1 3′ UTR sequence. As a result of downregulation of the DKK1 protein level, Wnt signaling is enhanced significantly, as indicated by elevated phosphorylation of GSK-3β and increased β-catenin transcriptional activity. Consequently, expression levels of Wnt target genes that include Runx2, BSP, and OC are significantly upregulated, promoting osteoblast differentiation.
Further analysis revealed that in C3H10T-1/2 cells, expression of miR-335-5p was increased at earlier time points following ascorbic acid induction of osteoblast differentiation and decreased subsequently. In agreement, Western blot analysis showed that DKK1 protein levels change in C3H10T1/2 cells following ascorbic acid treatment. Similarly, expression levels of miR-335-5p were increased in MC3T3-E1 cells and MLO-A5 cells, whereas changes in miR-335-5p expression were not detected in MLO-Y4 cells.
Taken together, these findings suggest that when mesenchymal stem cells initiate differentiation along the osteoblast lineage, the protein level of DKK1 is selectively decreased by inhibitory miRNAs. When differentiation progresses, as observed for late-stage osteoblasts and osteocytes, the protein levels of DKK1 are upregulated in response to the downregulation of inhibitory miRNAs. It appears that at early stages of osteogenic differentiation, miRNAs specifically target DKK1 to maintain the osteogenic state and competency for osteoprogeniter cells to form bone. In contrast, at later stages of differentiation, the silencing miRNAs are downregulated, resulting in elevated levels of DKK1 protein and a consequential suppression of osteogenic differentiation to avoid excessive mineralization. This regulation of DKK1 and Wnt signaling is mediated by miR-355-5p. Thus our discovery of a specific microRNA, miR-355-5p, developmentally upregulated at the onset of osteoblast commitment and regulating DKK1 protein levels during osteoblast differentiation provides a novel mechanism contributing to the cell-fate determination of osteochondroprogenitor cells to a committed osteoblast pathway by upregulating canonical Wnt activity.
Using in situ hybridization, we dynamically validated the in vivo biologic modulation of miR-355-5p regulation in bone tissue formation. In the perichondrium surrounding both cranial base cartilage and Meckel’s cartilage, we observed positive ALP staining in cells residing in or adjacent to the perichondrium that initially differentiate toward osteoblasts. Consistent with previous findings,(54) hypertropic chondrocytes undergoing terminal differentiation also were found to intensely express ALPase activity, reflecting the de novo bone formation. We observed that the expression pattern of miR-335-5p paralleled ALPase activity, indicating that by targeting the canonical Wnt antagonist, DKK1, miR-335-5p activates canonical Wnt signaling in both osteoblasts and hypertropic chondrocytes. Earlier findings have demonstrated that canonical Wnt signaling, characterized by cytoplasmic accumulation of β-catenin, promotes terminal differentiation of mature chondrocytes into hypertropic chondrocytes.(55) In a chick model, canonical Wnt signaling was reported to block the initiation of chondrogenesis but accelerate terminal chondrocyte differentiation.(56) In addition, Dkk1 inhibits β-catenin accumulation and β-catenin-induced Runx2 gene expression in chondrocytes, which subsequently delays hypertropic chondrocyte differentiation and endochondral bone formation.(55) Together with these previous findings, our results demonstrating upregulation of miR-335-5p in both osteoblasts and terminally differentiated hypertropic chondrocytes suggest an important regulatory role of this miRNA in promoting endochondral and intramembranous bone formation through modulating the activation level of canonical Wnt signaling.
In conclusion, our findings are consistent with a prominent role for miRNAs in the regulation of Wnt signaling and osteogenic differentiation by controlling the expression of DKK1. We are postulating that the regulatory effect of miRNAs is mandatory for the initiation and progression of osteogenic differentiation in a spatial- and temporal-specific manner. In a broader context, this mechanism may contribute to modulating the cellular levels of regulatory factors that control subsequent stages of bone formation and remodeling in a physiologically responsive manner.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DE14537 and DE16710 to JC, P01-AR46798 to LFB, GM57603 to XH, and AR039588 to GSS. We would like to thank Jean Tang for her excellent work in histology and in situ hybridization studies and Dana Murray for secretarial assistance with preparation of this manuscript.
Footnotes
Disclosures
All the authors state that they have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi-Yanaga F, Sasaguri T. The Wnt/β-catenin signaling pathway as a target in drug discovery. J Pharmacol Sci. 2007;104:293–302. doi: 10.1254/jphs.cr0070024. [DOI] [PubMed] [Google Scholar]
- 4.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 5.Behrens J, von Kries JP, Kuhl M, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 6.Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 7.Miller JR, Moon RT. Analysis of the signaling activities of localization mutants of beta-catenin during axis specification in Xenopus. J Cell Biol. 1997;139:229–243. doi: 10.1083/jcb.139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balemans W, Van Hul W. The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology. 2007;148:2622–2629. doi: 10.1210/en.2006-1352. [DOI] [PubMed] [Google Scholar]
- 9.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 10.Little RD, Recker RR, Johnson ML. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;347:943–944. doi: 10.1056/NEJM200209193471216. author reply 943-4. [DOI] [PubMed] [Google Scholar]
- 11.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 14.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 15.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–2552. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald BT, Joiner DM, Oyserman SM, et al. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology. 2008;149:1793–1801. doi: 10.1210/en.2007-0910. [DOI] [PubMed] [Google Scholar]
- 22.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 23.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 24.Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 25.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri A, Pezzetti F, Brunelli G, et al. Differences in osteoblast miRNA induced by cell binding domain of collagen and silicate-based synthetic bone. J Biomed Sci. 2007;14:777–782. doi: 10.1007/s11373-007-9193-z. [DOI] [PubMed] [Google Scholar]
- 28.Sugatani T, Hruska KA. MicroRNA-223 is a key factor in osteoclast differentiation. J Cell Biochem. 2007;101:996–999. doi: 10.1002/jcb.21335. [DOI] [PubMed] [Google Scholar]
- 29.Oskowitz AZ, Lu J, Penfornis P, et al. Human multipotent stromal cells from bone marrow and microRNA: Regulation of differentiation and leukemia inhibitory factor expression. Proc Natl Acad Sci USA. 2008;105:18372–18377. doi: 10.1073/pnas.0809807105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaur T, Hussain S, Mudhasani R, et al. Dicer inactivation in osteoprogenitor cells compromises fetal survival and bone formation, while excision in differentiated osteoblasts increases bone mass in the adult mouse. Dev Biol. 2010;340:10–21. doi: 10.1016/j.ydbio.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Hassan MQ, Volinia S, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 33.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1cells. J Bone Miner Res. 2002;17:101–110. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 34.Tu Q, Zhang J, Paz J, Wade K, Yang P, Chen J. Haploinsufficiency of Runx2 results in bone formation decrease and different BSP expression pattern changes in two transgenic mouse models. J Cell Physiol. 2008;217:40–47. doi: 10.1002/jcp.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Zeng R, Chen J, Liu X, Liao Q. Identification of conserved microRNAs and their targets from Solanum lycopersicum Mill. Gene. 2008;423:1–7. doi: 10.1016/j.gene.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 36.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20:1669–1679. doi: 10.1359/JBMR.050511. [DOI] [PubMed] [Google Scholar]
- 38.Yoshiki S, Umeda T, Kurahashi Y. An effective reactivation of alkaline phosphatase in hard tissues completely decalcified for light and electron microscopy. Histochemie. 1972;29:296–304. doi: 10.1007/BF00279812. [DOI] [PubMed] [Google Scholar]
- 39.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 40.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Shen Y, Yuan X, et al. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J Biol Chem. 2008;283:35929–35940. doi: 10.1074/jbc.M804091200. [DOI] [PubMed] [Google Scholar]
- 44.Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 2003;24:696–708. doi: 10.1016/s1044-7431(03)00232-x. [DOI] [PubMed] [Google Scholar]
- 45.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bodine PV, Zhao W, Kharode YP, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 47.Ohyama Y, Nifuji A, Maeda Y, Amagasa T, Noda M. Spaciotemporal association and bone morphogenetic protein regulation of sclerostin and osterix expression during embryonic osteogenesis. Endocrinology. 2004;145:4685–4692. doi: 10.1210/en.2003-1492. [DOI] [PubMed] [Google Scholar]
- 48.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 51.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol. 2008;19:434–443. doi: 10.1016/j.semcdb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 53.ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishizeki K, Saito H, Shinagawa T, Fujiwara N, Nawa T. Histochemical and immunohistochemical analysis of the mechanism of calcification of Meckel’s cartilage during mandible development in rodents. J Anat. 1999;194(Pt 2):265–277. doi: 10.1046/j.1469-7580.1999.19420265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Shao YY, Ballock RT. Thyroid hormone interacts with the Wnt/beta-catenin signaling pathway in the terminal differentiation of growth plate chondrocytes. J Bone Miner Res. 2007;22:1988–1995. doi: 10.1359/jbmr.070806. [DOI] [PubMed] [Google Scholar]
- 56.Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115(Pt 24):4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.