Abstract
Purpose
A number of environmental and patient-related factors contribute to implant failure. A significant fraction of these failures can be attributed to limited osseointegration resulting from poor bone healing responses. The overall goal of this study was to determine whether surface treatment of a titanium-aluminum-vanadium alloy (Ti-6Al-4V) implant material with a biomimetic protein coating could promote the differentiation of attached osteoblastic cells. The specific aims of the study were to investigate whether osteoprogenitor cells cultured on a rigorously cleaned implant specimen showed a normal pattern of differentiation and whether preadsorbed fibronectin accelerated or enhanced osteoblast differentiation.
Materials and Methods
Ti-6Al-4V disks were rigorously cleaned, passivated in nitric acid, and dry heat–sterilized; some of the disks were then coated with 1 nmol/L fibronectin. MC3T3 osteoprogenitor cells were then cultured on the pretreated disks for several weeks. Quantitative real-time polymerase chain reaction was performed to measure changes over time in the mRNA levels of osteoblast genes.
Results
Fibronectin increased the peak expression of all analyzed osteoblast gene markers. “Early” genes that normally mark the proliferative phase (0 to 10 days) of osteoblastic development showed peak expression within the first 10 days after cell attachment to the titanium alloy. In contrast, “late” genes that normally mark the differentiation (10 to 20 days) and mineralization (20 to 36 days) phases of osteoblastogenesis achieved peak expression only after approximately 3 to 4 weeks of culture.
Conclusions
Osteoprogenitors cultured on a rigorously cleaned Ti-6Al-4V alloy were found to demonstrate a normal pattern of osteoblast differentiation. Preadsorbed fibronectin was observed to stimulate osteoblast differentiation during the mineralization phase of osteoblastogenesis.
Keywords: coatings, differentiation, fibronectin, metal oxides, osteoblast, real-time polymerase chain reaction
Although the outcomes of dental and orthopedic implant therapies are usually successful, in many instances long-term stability and functionality of an implant cannot be achieved. Implant loosening and failure are still significant problems with a sizeable percentage of hip arthroplasties. In fact, 25% of hip replacement surgeries were revisions resulting from previous implant failure.1 Despite the reported long-term predictability of dental implants,2,3 failures occur in about 10% of cases within a 5-year period.4 The survival rates decrease to 71% to 83.5% over a 3.5- to 6-year period for dental implants placed in previously failed implant sites.5,6 The quality and quantity of bone at the implant-skeletal interface are generally believed to be two of the major determinants for implant success.7 Therefore, improving fixation by enhancing the attachment and function of osteoblastic cells at the implant surface is likely to substantially decrease the likelihood of implant failure, especially for implants placed in previously failed implant sites.
Commercially pure titanium and titanium alloys are widely employed in orthopedic and dental implants because of their biocompatibility.8,9 Titanium-aluminum-vanadium alloy is used in the fabrication of prosthetic joint replacements. This alloy, specifically in the form of Ti-6Al-4V, has even replaced commercially pure titanium in regions subject to high stress and dynamic loading such as hip prostheses and other articulating appendages because of the alloy’s significantly greater mechanical strength.10,11 Ti-6Al-4V has also been successfully utilized in the dental field.12 The surface oxide of Ti-6Al-4V is similar to that of pure titanium, except that it is enriched with aluminum oxide when present in air.11,13 However, the precise influence of the alloy’s surface oxide on osteoblast attachment and differentiation has yet to be fully elucidated.
A variety of methods have been explored to prevent implant failure, including the use of bioactive adhesive peptides or extracellular matrix proteins such as fibronectin to facilitate the attachment of osteogenic cells to the implant surface.14–22 Several laboratories have studied extracellular matrix proteins such as fibronectin, human bone sialoprotein (hBSP), or hBSP peptides following their noncovalent adsorption14–18 or covalent grafting19–22 to the implant surface oxide to increase surface coverage by osteoblasts. Fibronectin is one of the most intensively studied components of the extracellular matrix with respect to its structure and cellular effects. The protein is an essential component for normal development, as evidenced by the failure of fibronectin-knockout mice to develop beyond embryonic day 10 or 11.23 Fibronectin is also thought to play an important role in skeletal development by regulating osteoblast differentiation and mineralization.24 Most importantly, since it has been shown that fibronectin binds rapidly and irreversibly to titanium dioxide,17 the protein can be efficiently adsorbed to titanium materials without the use of intervening chemical coupling agents.
Previous studies suggest that modifying various surface oxide properties of Ti-6Al-4V, such as oxide chemistry or topography, will influence the attachment of osteoblasts to preadsorbed fibronectin.13,25,26 Cell adhesion to adsorbed fibronectin and other extracellular proteins is primarily mediated by integrin receptors that recognize the tripeptide arginine-glycine-aspartic acid as an important protein-binding site.22 It was previously demonstrated that when fibronectin is adsorbed to Ti-6Al-4V, the negative net charge of the alloy’s surface oxide enhanced the protein’s intrinsic capacity to bind to osteogenic cell integrin receptors and promote cell spreading.25,26 The process of cell spreading involves the recruitment and clustering of cell integrin receptors at anchorage sites called focal adhesions,27–29 which activate intracellular signaling pathways that regulate a number of cell functions, including differentiation.30 When grown on normal tissue culture plastic, osteoprogenitors have been shown to undergo several stages of development into fully mature osteoblasts, including phases of proliferation (0 to 10 days), differentiation (10 to 20 days), and mineralization (20 to 35 days).31 These phases are characterized by changes in the expression of several key proteins that play specific roles in osteogenesis, including type 1 collagen (α1), parathyroid hormone–related peptide (PTH-rp), alkaline phosphatase, osteopontin, BSP, and osteocalcin.31 However, no study has yet examined whether (1) osteoblastic cells undergo a similar pattern of altered expression of a comprehensive panel of gene markers when cultured for prolonged periods on Ti-6Al-4V and (2) whether a fibronectin coating can enhance the expression of several gene markers.
The purpose of the current study is to determine whether the surface treatment of a Ti-6Al-4V implant material in combination with a fibronectin coating could promote the differentiation of attached osteoblastic cells. This purpose was served by testing two hypotheses. This study tested the hypothesis that osteogenic cells cultured on a rigorously cleaned titanium alloy, without any further manipulation of oxide properties, show the same ability to differentiate as has been previously shown for cells grown on tissue culture plastic.31 Since it was previously shown that specific oxide properties can augment the capacity of adsorbed fibronectin to promote osteoblast spreading, which can stimulate differentiation, the authors also tested a second hypothesis: that fibronectin pre-coated on the oxide can enhance osteoblast gene expression. These two hypotheses were tested through the use of quantitative real-time polymerase chain reaction (RT-PCR) to measure changes in the mRNA levels of several osteoblast genes over time.
MATERIALS AND METHODS
Disk Preparation
Cylindric implant disks were initially prepared from Ti-6Al-4V sheets (TIMET). The metal sheets were cut into strips, polished by machining, and later punched into disks (Industrial Tool & Die Co), as previously described.25 Disks were washed successively in isopropanol, acetone, xylene, acetone, and 1 mol/L ammonium hydroxide and rinsed with deionized water according to the ASTM-F86 protocol.32 The disks were then passivated in 40% nitric acid and rinsed three times with deionized water. Disks were then dried and transferred into acid-washed scintillation vials in a high-efficiency particulate air–filtered isolation hood (USA/Scientific) and stored closed in an autodesiccator cabinet (San-platec Corp). All disks were sterilized using a rapid dry-heat oven (Alpha Medical) for 5 minutes.
Analysis of Topography and Roughness
Atomic force microscopy (AFM) was used to image Ti-6Al-4V disk surfaces to determine their surface topography. An NTEGRA Prima Scanning Probe microscope (NT-MDT) was employed in tapping mode under ambient conditions. The probes were rectangular NSG-01 silicon levers with an aluminum back coating (NT-MDT, nominal values for spring constant [k] = 5.1 N/m, radius of curvature [r] = 10 nm, and resonance frequency [f0] = 150 kHz). Several random 10- × 10-μm areas on two disks were scanned on each disk. The surface topography of prepared disks was then analyzed by AFM using a spatial high-pass filter approach to eliminate interference from larger metallic polishing grooves, as previously described.25
Cell Culture
MC3T3-E1 cells (subclone 4, American Type Culture Collection), which exhibit high levels of osteoblast differentiation,33 were cultured in alpha-minimum essential medium with 10% fetal bovine serum (Invitrogen). Ti-6Al-4V disks were placed into 24-well plates (Laboratory Disposable Products) and incubated with 1× phosphate-buffered saline (PBS) or a 1 nmol/L fibronectin (Sigma-Aldrich)/1× PBS solution overnight at room temperature under a cell culture hood. At this concentration of fibronectin solution, a surface concentration of 120 to 170 ng of adsorbed fibronectin/cm2 was obtained for untreated, heat-treated, and RFGD-treated disks, although no significant differences in fibronectin adsorption were found between the three groups.26 It has been shown that this concentration of fibronectin increased the attachment of MC3T3 cells to the titanium alloy by six- to eightfold compared to uncoated disks.26 On the following day, the PBS and fibronectin/PBS solutions were removed and each disk was plated with 500,000 cells in alpha-minimum essential medium with 10% fetal bovine serum. A confluent cell monolayer was obtained 3 days after cell plating, after which cell proliferation declined to negligible levels (unpublished observations). Because the present study focused on the effects of the alloy surface and fibronectin coating on osteoblast differentiation, rather than proliferation, 3 days following the initial cell plating was designated as day 0 of the experimental period. On day 0, the culture media was supplemented with 100 μg/mL ascorbic acid for half of the disks initially incubated in 1× PBS. The remaining half of the disks received only PBS and represented the control disks in this study. On day 0, half of the disks coated with fibronectin were also supplemented with ascorbic acid. Ascorbic acid was added as a positive control for the stimulation of osteoblast gene marker expression, since it is required for extracellular matrix synthesis and osteoblast differentiation.34 Since matrix synthesis occurs after 10 to 20 days of cell culture,31 the use of ascorbic acid is not expected to be associated with any direct effects on the initial interactions between the alloy’s surface oxide, adsorbed fibronectin, and attached osteoblastic cells. Disks receiving ascorbic acid supplementation on day 0 continued to receive fresh ascorbic acid supplements with every media change during the remaining culture period until RNA extraction. RNA was then extracted at days 3, 7, 10, 14, 21, and 28 of the experimental period.
Quantitative RT-PCR
Total RNA was isolated using RNeasy Mini Kits (Qiagen) following the direct lysis protocol. The quality and yield of recovered RNA were evaluated by absorption at 260 and 280 nm. Total RNA was reverse-transcribed into cDNA with First Strand cDNA Synthesis Kits (Fermentas, Inc). Equal amounts of RNA from each sample were reverse-transcribed, and the resultant cDNA was amplified by PCR using iQ SYBR Green Supermix (Bio-Rad). The primer pairs are shown in Table 1. Quantitative RT-PCR was performed using the My iQ Single Color Real-Time PCR Detection System (BioRad). The specificity of each primer pair for the corresponding mouse cDNA (shown by the gene accession number in Table 1) was confirmed using the NCBI BLAST program; its nucleotide database was searched for homologous sequences. The PCR program used the following parameters in sequential order: one cycle of 3 minutes at 95°C, 40 cycles of 10 seconds at 95°C followed by 45 seconds at 60°C, one cycle of 1 minute at 95°C, one cycle of 1 minute at 55°C, and one final extension of 800 s at 55°C. Beta-actin was used as an internal control for sample normalization. PCR products were analyzed within the linear range of amplification for the various genes examined. Changes in the relative levels of expression of specific genes over the time of cell culture (days 0, 3, 7, 10, 14, 21, and 28) compared to the control were monitored. Control samples received no fibronectin precoating or ascorbic acid supplementation. The expression of each specific mRNA was measured at the selected time points (relative to day 0 in the control group). For each experimental condition (either control, fibronectin, ascorbic acid, or fibronectin + ascorbic acid), experiments measuring relative mRNA expression at all of the time points were repeated at least three times (each experiment was performed with a separate independent cell culture). Data presented for each time point were averaged from multiple independent cultures so that artifacts arising from atypical single cultures (associated with passage number, differences between lots of serum, or subtle alterations in subcultivation protocol) could be minimized.
Table 1.
Primers Used for Quantitative Real-Time PCR
| Gene | Primer sequence (5′-3′) | Gene accession no. |
|---|---|---|
| Actin | F: AGATGTGGATCAGCAAGCAG R: GCGCAAGTTAGGTTTTGTCA |
NM_007393.2 |
| Alkaline phosphatase | F: AACCCAGACACAAGCATTCC R: GCCTTTGAGGTTTTTGGTCA |
NM_007431.1 |
| Bone sialoprotein | F: GAAACGGTTTCCAGTCCAG R: CTGCATCTCCAGCCTTCTT |
NM_008318.1 |
| Type 1 collagen α1 | F: AACCCGAGGTATGCTTATCT R: CCAGTTCTTCATTGCATTGC |
NM_007742.3 |
| Osteocalcin | F: CTCACAGATGCCAAGCCCA R: CCAAGGTAGCGCCGGAGTCT |
NM_007541.2 |
| Osteopontin | F: TGACCCATCTCAGAAGCAG R: GCTGACTTGACTCATGGCT |
NM_009263.1 |
| Osterix | F: TGAGGAAGAAGCCCATTCAC R: ACTTCTTCTCCCGGGTGTG |
NM_130458.2 |
| PTH-related peptide | F: CCAGAGCCAGCGAGCGGCAC R: CCAGGCAGACCGAGTCCTTC |
NM_008970.1 |
| Runx2 | F: AAATGCCTCCGCTGTTATGAA R: GCTCCGGCCCACAAATCT |
NM_009820.2 |
F = forward; R = reverse.
Statistical Analysis
Data are presented as means ± standard errors of the mean (n = total number of independent cell cultures). The data were normally distributed. The variability of data was similar for all experimental conditions at each time point and similar for all time points for each experimental condition. Statistical comparisons were performed using analysis of variance (ANOVA) with the alpha level set at .05.
RESULTS
Surface Characteristics
The surface topographies of disks were analyzed using AFM. The control disks showed typical peak-to-peak ranges of 200 nm in a 10- ×10-μm scan area. Virtually all this topography is a result of machining and polishing, which creates parallel linear grooves with micron and submicron feature sizes (unpublished observations). After a spatial high-pass filter was applied to the measured topography data, as previously described,25 the root mean square roughness values of sub–200-nm features were generally found to be in the range of 9 to 10 nm.
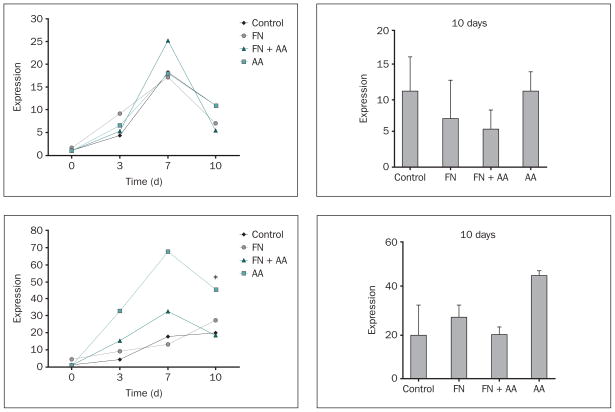
Expression of Genes for the Osteoblast Transcription Factors Runx2 and Osterix
Increases in mRNA expression were observed over time for runx235–37 and osterix38 in attached MC3T3 cells, with most experimental groups showing peak levels of expression by day 7 and declining levels of expression by day 10 (Fig 1). Notably, fibronectin appeared to decrease the expression of osterix in ascorbic acid–supplemented samples compared to ascorbic acid alone at all three time points, although only the decrease in expression promoted by fibronectin on day 10 was statistically significant (Fig 1b).
Fig 1.
a and 1b Effects of fibronectin and ascorbic acid on (top left) runx2 and (bottom left) osterix gene expression during the proliferative stage of osteoblast development on Ti-6Al-4V, as measured by quantitative RT-PCR. Changes in the relative levels of runx2 and osterix mRNA over time measured by quantitative RT-PCR are presented as relative levels of expression compared to control levels measured at day 0. Right column: Fold increase measured at day 10; data represent means ± SE. *Significantly greater than control or FN + AA groups at day 10 (P < .05) based on one-way ANOVA. Control and FN = disks were uncoated or precoated with 1 nmol/L fibronectin overnight, respectively, and plated with MC3T3 cells grown in culture media without ascorbic acid supplementation; AA and FN + AA = disks were uncoated or precoated with 1 nmol/L fibronectin overnight, respectively, and plated with cells grown in culture media supplemented with 100 μg/mL ascorbic acid with every media change.
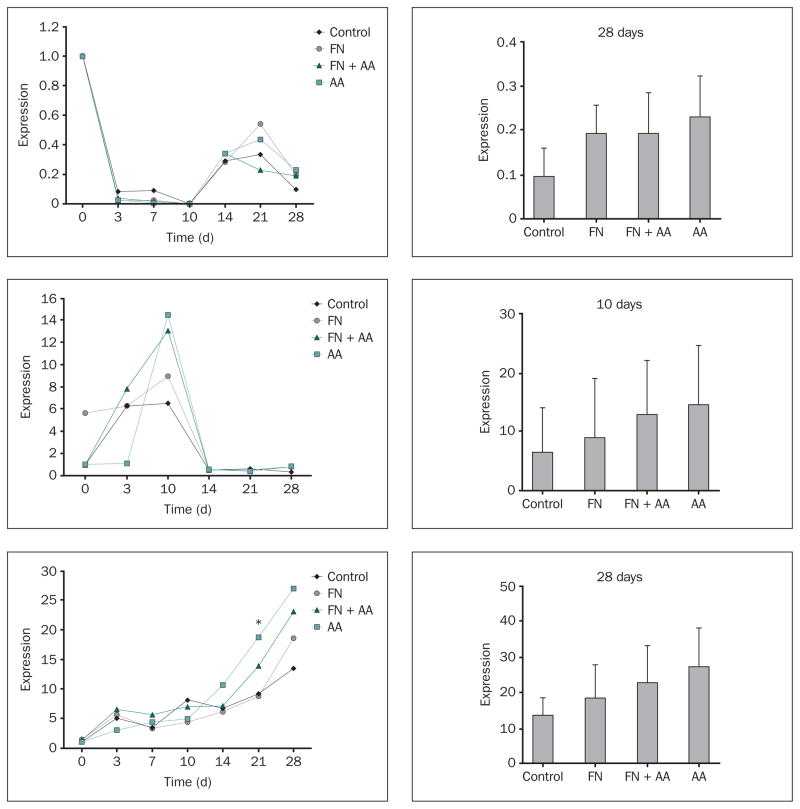
Expression of Genes for Type 1 Collagen, PTH-rp, and Alkaline Phosphatase
Type 1 collagen (α1) demonstrated its highest levels of mRNA expression in cells attached to Ti-6Al-4V disks on day 0 and displayed a dramatic down-regulation of mRNA levels between days 0 and 10 for all experimental conditions (Fig 2a). Type 1 collagen (α1) also demonstrated a second phase of up-regulation between days 14 and 28 for all of the experimental conditions. Figure 2b shows that peak levels of PTH-rp mRNA expression were observed at day 10 in all of the experimental groups. A persistent dramatic down-regulation of PTH-rp mRNA (to a level lower than that measured for the control day 0 condition) was observed for all of the experimental groups beginning on day 14 (Fig 2b). A progressive increase in the mRNA expression of alkaline phosphatase (ALP) was seen over time for all of the experimental groups (Fig 2c). However, since ALP mRNA levels had not reached a plateau, even at day 28, the findings indicate that peak levels of expression were not attained until sometime later than 28 days.
Fig 2.
a to 2c Effects of fibronectin and ascorbic acid on (top to bottom) type 1 collagen (α1), PTH-rp, and ALP gene expression. Changes in mRNA levels over the time of cell culture (relative to day 0 in control samples) were measured by quantitative RT-PCR. Right column: Levels of expression (relative to day 0 in the control) measured on days 28, 10, and 28 for type 1 collagen, PTH-rp, and ALP, respectively; data represent means ± SE. *Significantly greater than control or FN groups at day 21 (P < .05) based on one-way ANOVA.
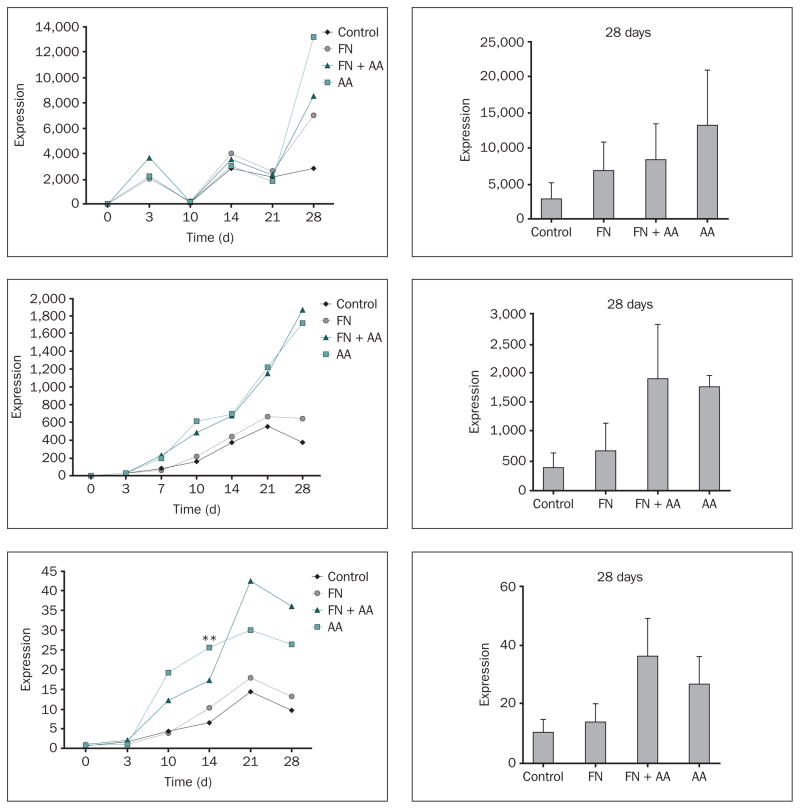
Expression of Genes for Osteopontin, BSP, and Osteocalcin
Osteopontin mRNA was poorly expressed in MC3T3 cells attached to Ti-6Al-4V disks between days 0 and 10 in the control condition, although mRNA expression in the fibronectin + ascorbic acid group was significantly greater than that in the control group at day 3 (Fig 3a). There was a dramatic increase in osteopontin transcript levels in control and all experimental groups beginning on day 14 that was sustained through day 28 (Fig 3a).
Fig 3.
a to 3c Effects of fibronectin and ascorbic acid on (top to bottom) osteopontin, bone sialoprotein, and osteocalcin gene expression. Changes in mRNA levels over the time of cell culture (relative to day 0 in control samples) were measured by quantitative RT-PCR. Right column: Levels of expression (relative to day 0 in the control) measured at day 28; data represent means ± SE. *Significantly greater than control group at day 3 for osteopontin (P < .05); **Significantly greater than FN group (P < .05) and control group (P < .01) at day 14 for osteocalcin based on one-way ANOVA.
BSP mRNA expression progressively increased over time, with peak levels of expression observed at day 21 for the control and fibronectin groups (Fig 3b). The ascorbic acid and fibronectin + ascorbic acid treatments produced a pattern of increased BSP mRNA expression compared to the control and fibronectin groups at every time point from 7 to 28 days, although the observed increases were not statistically significant (Fig 3b).
Osteocalcin exhibited a low level of mRNA expression until day 10, at which point the gene became progressively up-regulated in all of the groups until peak levels of expression were uniformly achieved by day 21 (Fig 3c). The ascorbic acid and fibronectin + ascorbic acid treatments each produced a pattern of increased osteocalcin mRNA expression compared to the control or fibronectin groups from days 10 to 28, although only the ascorbic acid–promoted increase in expression measured at day 14 was statistically significant (Fig 3c).
DISCUSSION
Characterization of Baseline Osteoblast Gene Marker Expression
For dental or orthopedic implants to be osteoinductive, the implant material must support the long-term development, maturation, and function of osteoblasts attached to the metal surface. Unfortunately, titanium and its alloys often contain significant levels of inorganic surface contaminants, including metal ions,39,40 silica and polishing agents,35 and other surface contaminants deposited by autoclaving procedures.41,42 The release of metal ions from titanium materials has been shown or suggested to diminish osteoblast gene expression, cellular function, and bone formation,39,40,43 while autoclaving can affect cellular responses to titanium materials,44 probably by introducing surface contaminants.41,42 Contaminants can also become attached to surface oxides during the sample preparation procedure and alter osteoblastic cell differentiation.45 The hypothesis that osteogenic cells cultured on a titanium alloy can have the same capacity to differentiate as cells grown on tissue culture plastic31 if the metal is first rigorously cleaned to eliminate or reduce surface contaminants has been tested. It has been found that MC3T3 cells attached to thoroughly cleaned Ti-6Al-4V disks show essentially the same temporal pattern of osteoblast gene marker expression as osteoblastic cells that differentiate on polystyrene tissue culture plastic.31,34,46 The authors have reported here precisely what these studies have also demonstrated: that type 1 collagen (α1) and PTH-rp display their greatest induction during the proliferative phase (0 to 10 days), whereas ALP, osteopontin, BSP, and osteocalcin are primarily up-regulated at a later stage of osteoblastogenesis.31,34,46 MC3T3 cells attached to Ti-6Al-4V also responded to ascorbic acid supplementation with a dramatic up-regulation of all of the genes tested. It has been shown that ascorbic acid is essential for collagen synthesis and formation of the extracellular matrix.34 The extracellular matrix is essential to the differentiation of nontransformed osteoblastlike cell lines.34 These findings indicate that osteoblastic cells cultured on carefully prepared titanium alloy material in the present study show the same capacity to differentiate as osteoblastic cells grown on polystyrene tissue culture plates.
The Importance of Specimen Preparation in the Differentiation Capacity of Attached Osteogenic Cells
A high percentage of surface impurities, including metal ions, can be removed from titanium materials through a cleaning procedure involving a series of chemical treatments followed by passivation with nitric acid, which increases the surface oxide layer that forms. The treatment of Ti-6Al-4V with high temperatures has also been shown to reduce metal ion release by increasing the thickness of the surface oxide.47,48 The present samples were highly polished, debrided of polishing residue using a trichloroethylene vapor degreaser, and treated with a rigorous cleaning procedure involving successive washes in isopropanol, acetone, xylene, acetone, and 1 mol/L ammonium hydroxide. Finally, the samples were treated for 2 minutes with 40% nitric acid, which can eliminate metallic contaminants from the surface, and then dry heat–sterilized to 191°C.25 Therefore, every effort was made to remove polishing residues, surface impurities, and other contaminants prior to the present cell culture studies.
In contrast to this study, the Ti-6Al-4V specimens in an analysis by Huang et al were not treated with nitric acid or high temperature, and the authors found that MC3T3 cells attached to the alloy did not begin to differentiate until day 28, much later than in the present study.49 Oh et al found that the mRNA levels of ALP, osteopontin, and osteocalcin in mesenchymal osteoprogenitor cells attached to titanium were only 10% of the corresponding levels in cells attached to polystyrene after 2 weeks of culture.50 The samples in the study of Oh et al were sterilized by steam autoclaving,50 which is likely to introduce surface contaminants.41,42 In contrast, the present results used specimens that were dry heat–sterilized and increases were seen in the expression of osteoblast gene markers over time that were comparable to those observed for polystyrene.31,34,46 These results, together with the present findings, suggest that a rigorous surface preparation and cleaning procedure such as that employed in the current study is essential for the optimal differentiation of attached osteogenic cells.
The Effects of the Fibronectin Coating on the Expression of Osteoblast Gene Markers
The current study also tested the hypothesis that fibronectin preadsorbed to titanium alloy can enhance the differentiation of osteoprogenitors. A previous study of fibronectin coatings on titanium disks by AFM revealed little or no evidence of cross-linking between adjacent homodimers at solution coating concentrations below 4 nmol/L.18 It has also been shown that the adsorption of fibronectin to titanium surfaces is essentially irreversible.17 These findings suggest that the incubation of disks with 1 nmol/L fibronectin as performed in the current study can produce a stable coating of fibronectin molecules on Ti-6Al-4V that are far enough apart to allow unrestricted spreading of individual molecules on the surface. It has been shown that the unimpeded spreading of adsorbed fibronectin on a surface facilitates an increase in the exposure of epitopes in the integrin-binding domain as the molecule unfolds to an extended conformation. At higher degrees of molecular packing, these epitopes are completely unavailable when the molecule assumes a more compact conformation.51 It was also found that fibronectin coated on the alloy produced a six- to eightfold stimulation of osteoblast cell attachment at the coating concentration of 1 nmol/L used in the current study.26 Therefore, the present procedure for incubating alloy disks with fibronectin in solution is likely to create a coating of fibronectin molecules capable of activating osteoblast integrin receptors.
Fibronectin exhibited a number of specific effects on osteoblast gene marker expression. Fibronectin decreased the level of ascorbic acid–induced mRNA expression of the preosteoblast marker osterix when combined with ascorbic acid. Osterix is an osteoblast transcription factor and a marker for preosteoblasts that regulates the expression of some osteogenic genes, including that for BSP.38 Therefore, the effects of fibronectin on osterix mRNA suggest that the matrix protein stimulated the maturation of preosteoblasts into osteoblasts. Also, fibronectin produced a pattern of enhanced mRNA expression for type 1 collagen (α1), ALP, osteopontin, BSP, and osteocalcin during the mineralization stage (28 days) without altering the overall temporal pattern of osteoblast gene marker expression. These findings suggest that fibronectin did not accelerate osteoblast differentiation but instead increased matrix protein expression during the mineralization stage of osteoblastogenesis.
Potential Effects of Fibronectin Coatings on the Osteoinductive Capacity of Ti-6Al-4V
A number of methods for roughening titanium surfaces have been demonstrated to stimulate osteoblast differentiation.52–57 The present results suggest that a fibronectin coating provides an alternative approach to titanium surface-roughening techniques for the purpose of promoting the maturation of attached osteoprogenitor cells. One study claimed that fibronectin coatings on titanium surfaces inhibited osteoblast differentiation, although this conclusion was based on changes in the expression of only one gene, ALP, on days 14 and 28.58 In contrast, in the current study it was observed that adsorbed fibronectin appeared to increase the expression of genes for type 1 collagen, ALP, osteopontin, BSP, and osteocalcin at 28 days after cell confluence (31 days after cell plating). Studies in other laboratories confirm the finding that the fibronectin coating of titanium materials stimulates osteoblastic cell differentiation.24,59,60 Moursi et al reported that anti-fibronectin antibodies selectively inhibited steady-state expression of mRNA for genes associated with osteoblast differentiation, including ALP and osteocalcin, in rat calvarial osteoblast cultures.24 Another study demonstrated that a fibronectin fragment, when precoated on titanium disks, increased the ALP activity of MC3T3 cells by threefold after 7 days of culture.59 Pugdee and coworkers reported that fibronectin immobilized on commercially pure titanium significantly up-regulated BSP mRNA expression in MC3T3-E1 cells after 14 days of culture.60 It should be noted that conflicting reports in the literature on whether fibronectin coatings of titanium materials stimulate or inhibit osteoblast differentiation are likely to arise when only one or two genes are studied at limited time points during osteoblastogenesis. The current study has overcome such limitations by showing that a fibronectin coating of Ti-6Al-4V increased the expression of five different osteoblast gene markers over a prolonged incubation period.
Fibronectin coatings of titanium materials exert additional cellular actions that may increase the capacity of the implant material to support osteogenesis. It was previously reported that coating a titanium alloy with 1 nmol/L fibronectin could produce a six- to eightfold increase in the number of attached cells from three different osteogenic cell lines, including the MC3T3 line.26 Importantly, the stimulatory effects of fibronectin on osteoblast attachment provide an advantage over surface-roughening procedures, which have also been shown to inhibit osteogenic cell attachment.49,61 Preadsorbed fibronectin may improve the osteoinductive capacity of titanium implant materials through its combined effects on osteoblast attachment and differentiation.
CONCLUSIONS
Osteoprogenitors cultured on a rigorously cleaned titanium alloy (Ti-6Al-4V) were found to demonstrate a pattern of osteoblast gene marker expression resembling that shown in studies performed on polystyrene tissue culture plates. The expression of osteoblast markers during the mineralization phase of osteoblastogenesis was increased by preadsorbed fibronectin. This and other studies suggest that, by combining careful specimen preparation with biointerfaces consisting of osteogenic matrix protein coatings, an implant surface can be developed that will more effectively support osteoblast differentiation. Such a strategy may ultimately prove to be effective in improving fixation during the early implant healing phase than existing surface engineering methods. Future studies will examine the effects of modifying Ti-6Al-4V surface oxide properties, such as charge or topography, on the capacity of adsorbed fibronectin to increase the expression of osteoblast gene markers.
Acknowledgments
The project described was supported by a grant from the U.S. National Institutes of Health (#RO1 DE017695, awarded to DEM). The sponsor did not have any role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. This material is also the result of work supported with resources and the use of facilities at the James J. Peters VA Medical Center, Bronx, New York. This investigation was also conducted at the Hospital for Special Services research facility constructed with support of Grant C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health. The authors would like to acknowledge Dr Xiaoyu Hu for technical advice with the qRT-PCR analyses. Special thanks goes to Christine Marsh for her technical assistance with cell culture and analysis of gene expression using qRT-PCR.
References
- 1.Webster TJ. Nanophase ceramics as improved bone tissue engineering materials. Am Ceram Soc Bull. 2003;82:1–8. [Google Scholar]
- 2.Adell R, Eriksen B, Lekholm U, Brånemark PI, Jemt T. A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. [PubMed] [Google Scholar]
- 3.Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 4.Hardt CR, Grondahl K, Lekholm U, Wennstrom JL. Outcome of implant therapy in relation to experienced loss of periodontal bone support: A retrospective 5-year study. Clin Oral Implants Res. 2002;13:488–494. doi: 10.1034/j.1600-0501.2002.130507.x. [DOI] [PubMed] [Google Scholar]
- 5.Grossmann Y, Levin L. Success and survival of single dental implants placed in sites of previously failed implants. J Periodontol. 2007;78:1670–1674. doi: 10.1902/jop.2007.060516. [DOI] [PubMed] [Google Scholar]
- 6.Machtei EE, Mahler D, Oettinger-Barak O, Zuabi O, Horwitz J. Dental implants placed in previously failed sites: Survival rate and factors affecting the outcome. Clin Oral Implants Res. 2008;19:259–264. doi: 10.1111/j.1600-0501.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 7.Johansson P, Strid KG. Assessment of bone quality from cutting resistance during implant surgery. Int J Oral Maxillofac Implants. 1994;9:279–288. [Google Scholar]
- 8.Larsson G, Thomsen P, Aronsson BO, et al. Bone response to surface-modified titanium implants: Studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996;17:605–616. doi: 10.1016/0142-9612(96)88711-4. [DOI] [PubMed] [Google Scholar]
- 9.Tengvall P, Lundström I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992;9:115–134. doi: 10.1016/0267-6605(92)90056-y. [DOI] [PubMed] [Google Scholar]
- 10.Lausmaa J. Mechanical, thermal, chemical and electrochemical surface treatment of titanium. In: Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. New York: Springer; 2001. pp. 232–266. [Google Scholar]
- 11.Ask M, Lausmaa J, Kasemo B. Preparation and surface spectroscopic characterization of oxide films on Ti6Al4V. Appl Surf Sci. 1988;35:283–301. [Google Scholar]
- 12.Morris HF, Winkler S, Ochi S. A 48-month multicenter clinical investigation: Implant design and survival. J Oral Implantol. 2001;27:180–186. doi: 10.1563/1548-1336(2001)027<0180:AMCIID>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald DE, Rapuano BE, Deo N, Stranick M, Somasundaran P, Boskey AL. Thermal and chemical modification of titanium-aluminum-vanadium implant materials: Effects on surface properties, glycoprotein adsorption, and MG63 cell attachment. Biomaterials. 2004;25:3135–3146. doi: 10.1016/j.biomaterials.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J Biomed Mater Res. 2003;64A:457–464. doi: 10.1002/jbm.a.10368. [DOI] [PubMed] [Google Scholar]
- 15.Rapuano BE, Wu C, MacDonald DE. Osteoblast-like cell adhesion to bone sialoprotein peptides. J Orthop Res. 2004;22:353–361. doi: 10.1016/S0736-0266(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 16.Sauberlich S, Klee D, Richter EJ, Hocker H, Spiekermann H. Cell culture tests for assessing the tolerance of soft tissue to variously modified titanium surfaces. Clin Oral Implants Res. 1999;10:379–393. doi: 10.1034/j.1600-0501.1999.100505.x. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials. 2002;23:1269–1279. doi: 10.1016/s0142-9612(01)00317-9. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald DE, Markovic B, Allen M, Somasundaran P, Boskey AL. Surface analysis of human plasma fibronectin adsorbed to commercially pure titanium materials. J Biomed Mater Res. 1998;41:120–130. doi: 10.1002/(sici)1097-4636(199807)41:1<120::aid-jbm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res. 2005;75:855–869. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 20.Barber TA, Golledge SI, Castner DG, Healy KE. Peptide-modified p(AAm-co- EG/AAc) IPN’s grafted to bulk titanium modulate osteo-blast behavior in vitro. J Biomed Mater Res. 2003;64:38–47. doi: 10.1002/jbm.a.10321. [DOI] [PubMed] [Google Scholar]
- 21.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. The detachment strength and morphology of bone cells contacting materials modified with a peptide sequence found within bone sialoprotein. J Biomed Mater Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 23.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 24.Moursi AM, Damsky CH, Lutt J, et al. Fibronectin regulates calvarial osteoblast differentiation. J Cell Sci. 1996;109:1369–1390. doi: 10.1242/jcs.109.6.1369. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald DE, Rapuano BE, Schniepp HC. Surface oxide net charge of a titanium alloy: Comparison between effects of treatment with heat or radiofrequency plasma glow discharge. Colloids Surf B Biointerfaces. 2011;82:173–181. doi: 10.1016/j.colsurfb.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapuano BE, MacDonald DE. Surface oxide net charge of a titanium alloy: Modulation of fibronectin-activated attachment and spreading of osteogenic cells. Colloids Surf B Biointerfaces. 2011;82:95–103. doi: 10.1016/j.colsurfb.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss G. The adhesion of cells. In: Bourne GH, Danielli JF, editors. International Review of Cytology. New York: Academic Press Inc; 1960. pp. 187–225. [DOI] [PubMed] [Google Scholar]
- 28.Vogler EA. Interfacial chemistry in biomaterials science. In: Berg J, editor. Wettability. New York: Marcel Dekker; 1993. pp. 184–250. [Google Scholar]
- 29.Grinnell F. Cellular adhesiveness and extracellular substrata. In: Bourne GH, Danielli JF, Jeon RW, editors. International Review of Cytology. New York: Academic Press; 1978. pp. 67–145. [DOI] [PubMed] [Google Scholar]
- 30.Docheva D, Popov C, Mutschier W, Schieker M. Human mesenchymal stem cells in contact with their environment surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raouff A, Seth A. Ets transcription factors and targets in osteogenesis. Oncogene. 2000;19:6455–6463. doi: 10.1038/sj.onc.1204037. [DOI] [PubMed] [Google Scholar]
- 32.F-86 AS. Annual Book of ASTM Standards, v 13.01. Philadelphia, PA: American Society for Testing and Materials; 1996. Standard practice for surface preparation and marking of metallical surgical implants; pp. 6–8. [Google Scholar]
- 33.Wang D, Christensen R, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast sub-clones with distinct in vitro differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 34.Franchesi RT. The developmental control of osteoblast-specific gene expression: Role of specific transcription. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- 35.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 36.Ducy P, Starbuck M, Priemel M, et al. A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada H, Tagashira S, Fujiwara M, et al. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J Biol Chem. 1999;274:6972–6978. doi: 10.1074/jbc.274.11.6972. [DOI] [PubMed] [Google Scholar]
- 38.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 39.Zreiqat H, Howlett CR. Titanium substrata composition influences osteoblastic phenotype: In vitro study. J Biomed Mater Res. 1999;47:360–366. doi: 10.1002/(sici)1097-4636(19991205)47:3<360::aid-jbm10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal NC, Posner AS. In vitro model of aluminum-induced osteomalacia: Inhibition of hydroxyapatite formation by formation and growth. Calcif Tissue Int. 1984;36:439–441. doi: 10.1007/BF02405357. [DOI] [PubMed] [Google Scholar]
- 41.Oya K, Tanaka Y, Moriyama Y, et al. Differences in the bone differentiation properties of MC3T3-E1 cells on polished bulk and sputter-deposited titanium specimens. J Bomed Mater Res A. 2010;94:611–618. doi: 10.1002/jbm.a.32751. [DOI] [PubMed] [Google Scholar]
- 42.Kilpadi DV, Lemons JE, Liu J, Raikar GN, Weimer JJ, Vohra Y. Cleaning and heat- treatment effects on unalloyed titanium implant surfaces. Int J Oral Maxillofac Implants. 2000;15:219–230. [PubMed] [Google Scholar]
- 43.Mine Y, Makihira S, Nikawa H, et al. Impact of titanium ions on osteoblast-, osteoclast- and gingival epithelial-like cells. J Prosthodont Res. 2010;54:1–6. doi: 10.1016/j.jpor.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Vezeau PJ, Koorbusch GF, Draughn RA, Keller JC. Effects of multiple sterilization of surface characteristics in vitro biologic responses to titanium. J Oral Maxillofac Surg. 1996;54:738–746. doi: 10.1016/s0278-2391(96)90694-1. [DOI] [PubMed] [Google Scholar]
- 45.Hiromoto S, Hanawa T, Asami K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials. 2004;25:979–986. doi: 10.1016/s0142-9612(03)00620-3. [DOI] [PubMed] [Google Scholar]
- 46.Quarles DL, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683–691. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 47.Lee TM, Chang E, Yang CY. Surface characteristics of Ti6Al4V alloy: Effect of materials, passivation and autoclaving. J Mater Sci Mater Med. 1998;9:439–448. doi: 10.1023/a:1008815316564. [DOI] [PubMed] [Google Scholar]
- 48.Lee TM, Chang E, Yang CY. Effect of passivation on the dissolution behavior of Ti6Al4V and vacuum-brazed Ti6Al4V in hank’s ethylene diamine tetra-acetic acid solution. Part I: Ion release. J Mater Sci Mater Med. 1999;10:541–548. doi: 10.1023/a:1008916314329. [DOI] [PubMed] [Google Scholar]
- 49.Huang Z, Daniels H, Enzerink RJ, Hardev V, Sahi V, Goodman SB. Effect of nanofiber-coated surfaces on the proliferation and differentiation of osteoprogenitors in vitro. Tissue Eng. 2008;14:1853–1859. doi: 10.1089/ten.tea.2007.0399. [DOI] [PubMed] [Google Scholar]
- 50.Oh S, Brammer KS, Li YS, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ugarova TP, Zamarron C, Veklich Y, et al. Conformational transitions in the cell binding domain of fibronectin. Biochem. 1995;34:4457–4466. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- 52.Boyan BD, Lossdörfer S, Wang L, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cells Mater. 2003;6:22–27. doi: 10.22203/ecm.v006a03. [DOI] [PubMed] [Google Scholar]
- 53.Links J, Boyan BD, Blanchard CR, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19:2219–2232. doi: 10.1016/s0142-9612(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 54.Martin JY, Schwartz Z, Hummert TW, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 55.Silva TSN, Machado DC, Viezzer C, Silva AN, Jr, de Oliveira MG. Effect of titanium surface roughness on human bone marrow cell proliferation and differentiation. An experimental study. Acta Cir Bras. 2009;24:200–205. doi: 10.1590/s0102-86502009000300007. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz Z, Raz P, Zhao G, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90:2485–2498. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosa AL, Beloti MM. Rat bone marrow cell response to titanium and titanium alloy with different surface roughness. Clin Oral Implants Res. 2003;14:43. doi: 10.1034/j.1600-0501.2003.140106.x. [DOI] [PubMed] [Google Scholar]
- 58.Pequeroles M, Aguirre A, Engel E, et al. Effect of blasting treatment and Fn coating on MG63 adhesion and differentiation on titanium: a gene expression study using real-time RT-PCR. J Mater Sci Mater Med. 2011;22:617–27. doi: 10.1007/s10856-011-4229-3. [DOI] [PubMed] [Google Scholar]
- 59.Ku Y, Chung CP, Jang JH. The effect of the surface modification of titanium using a recombinant fragment of fibronectin and vitronectin on cell behavior. Biomaterials. 2005;26:5153–5157. doi: 10.1016/j.biomaterials.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 60.Pugdee K, Shibata Y, Yamamichi N, et al. Gene expression of MC3T3-E1 cells on fibronectin-immobilized titanium using tresyl chloride activation technique. Dent Mater J. 2007;26:647–655. doi: 10.4012/dmj.26.647. [DOI] [PubMed] [Google Scholar]
- 61.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258–264. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]