Abstract
This study investigates the effect of involuntary motor activity of paretic-spastic muscles on classification of surface electromyography (EMG) signals. Two data collection sessions were designed for 8 stroke subjects to voluntarily perform 11 functional movements using their affected forearm and hand at a relatively slow and fast speed. For each stroke subject, the degree of involuntary motor activity present in voluntary surface EMG recordings was qualitatively described from such slow and fast experimental protocols. Myoelectric pattern recognition analysis was performed using different combinations of voluntary surface EMG data recorded from slow and fast sessions. Across all tested stroke subjects, our results revealed that when involuntary surface EMG was absent or present in both training and testing datasets, high accuracies (> 96%, > 98%, respectively, averaged over all the subjects) can be achieved in classification of different movements using surface EMG signals from paretic muscles. When involuntary surface EMG was solely involved in either training or testing datasets, the classification accuracies were dramatically reduced (< 89%, < 85%, respectively). However, if both training and testing datasets contained EMG signals with presence and absence of involuntary EMG interference, high accuracies were still achieved (> 97%). The findings of this study can be used to guide appropriate design and implementation of myoelectric pattern recognition based systems or devices toward promoting robot-aided therapy for stroke rehabilitation.
INTRODUCTION
Myoelectric signals have been used for over 40 years in prosthesis control for amputees [1]. Myoelectric control of robotic systems has also been used to aid rehabilitation of motor skills for individuals with neurological injuries, such as stroke and spinal cord injury [2]–[7]. In the latter case, users’ intention of moving can be detected by measurement of surface electromyography (EMG) signals from paretic or impaired muscles, even though no actual or sufficient movement is produced. An EMG-controlled robotic system is able to provide interactive user interface for the system to act according to user’s intention of movement. The involvement of user’s voluntary input in rehabilitation training can enhance motor recovery or promote functional restoration [7]–[10].
Recent development of myoelectric control has been oriented toward accurate decoding of muscle co-activation patterns, continuous identification of various movements and simultaneous control of multiple degrees of freedom (DOFs) [11]–[13], [15], [17], [33]–[35], among which a novel pattern-recognition based control strategy uses a variety of features derived from EMG signals as control input via surface electrodes placed over a group of functional muscles. Its effectiveness has been demonstrated in prosthetic control for upper limb amputees [11] [13] [15]. In previous studies we also found that substantial motor control information can be extracted from paretic arm or hand muscles of chronic stroke [18] and spinal cord injury [19] subjects through classification of surface EMG signals. This demonstrates a possible approach of using the myoelectric pattern recognition strategy for controlling multiple DOFs, which is expected to facilitate restoration of upper-limb function for hemiplegic or quadriplegic patients [4]–[7].
EMG signals used for myoelectric prosthesis control are derived from an amputee’s residual muscles which are neurologically largely intact. In contrast, neurological injuries may induce changes in intrinsic motoneuron, motor control and muscle properties, giving rise to muscle spasticity, contracture and associated alterations in muscle internal structure [20]. Such neuromuscular property changes should be considered when implementing a myoelectric control system. For example, paretic muscles may present a large amount of involuntary motor activity [23], [24], [25], as a result of spasticity, a common impairment that interferes with motor function in stroke [20], [21] and SCI [22]. It is very likely that voluntary EMG from paretic muscles may be contaminated by such involuntary motor activity.
Involuntary muscle activity may interfere with implementation of a myoelectric control system. One difficulty imposed by such involuntary surface EMG spikes is onset/offset detection of voluntary muscle activation. We have recently developed a novel method for onset/offset detection of voluntary muscle activity using sample entropy (SampEn) analysis of surface EMG signals [26], taking advantage of the distinct difference in the signal complexity domain between voluntary and involuntary EMG activity.
It is presently unknown how involuntary motor activity of paretic-spastic muscles may affect classification of different movement intentions of neurologically impaired individuals. In the current study, we seek to assess the effect of such involuntary motor activity on surface EMG classification performance of hemiparetic stroke subjects. The analyses of this study reveal the classification performance when involuntary EMG signals induced from paretic-spastic muscles are present under different situations. The findings can help design and implementation of a pattern recognition based myoelectric control system toward stroke rehabilitation.
METHODS
A. Subjects
Eight choric stroke subjects participated in this study. The subjects were recruited from the Clinical Neuroscience Research Registry at the Rehabilitation Institute of Chicago (Chicago, IL). The study was approved by the Institutional Review Board of Northwestern University (Chicago, IL). All stroke subjects gave their written consent before the experiment.
For each stroke subject, a screening examination and clinical assessment were performed by a physical therapist. These scales included the upper-extremity component of the Fugl-Meyer scale [27] (denoted as UEFM), the hand impairment part of the Chedoke-McMaster stroke assessment scale [28] (denoted as Ch-M Hand) for the evaluation of arm and hand motor function, and the modified Ashworth scale [29] (denoted as MAS) for the evaluation of muscle tone/spasticity at the wrist joint. The UEFM scale uses ordinal 3-point score (0 = cannot perform, 1 = can partially perform, and 2 = can fully perform) to quantitatively assess the movement, sensation and balance functions of a patient’s shoulder, elbow, forearm, wrist, and hand after a stroke, producing a possible total score of 66 for the upper-extremity component [27]. The Ch-M Hand is used to determine the presence and severity of common physical impairments of the hand of a stroke patient, with a 7-grade scale, where grade 1 indicates the most severe impairment and grade 7 indicates an ability to perform all of the tested tasks [28]. The MAS is used to score the average resistance to passive movement for the wrist joint, using 6-grade scale ranging from grade 0 (no increase in muscle tone) through grade 1, 1+, 2 and 3 to grade 4 (affected part rigid in flexion or extension) [29]. Demographic and clinical measures for the stroke subjects of this study are detailed in Table I.
Table I.
Physical characteristic of stroke subjects
| Subject # | Gender | Age | Duration | Handed | Paretic Side | UEFM | Ch-M Hand | MAS
|
|
|---|---|---|---|---|---|---|---|---|---|
| Flexors | Extensors | ||||||||
| 1 | M | 56 | 8 | L | R | 52 | 5 | 2 | 2 |
| 2 | M | 67 | 9 | R | L | 16 | 4 | 3 | 0 |
| 3 | M | 52 | 4 | R | L | 21 | 3 | 1+ | 0 |
| 4 | M | 56 | 6 | R | R | 19 | 3 | 2 | 0 |
| 5 | F | 54 | 7 | R | L | 19 | 3 | 3 | 0 |
| 6 | M | 62 | 19 | R | R | 10 | 2 | 4 | 4 |
| 7 | F | 63 | 16 | R | L | 17 | 2 | 3 | 0 |
| 8 | M | 66 | 18 | L | R | 10 | 2 | 0 | 2 |
B. Data Acquisition
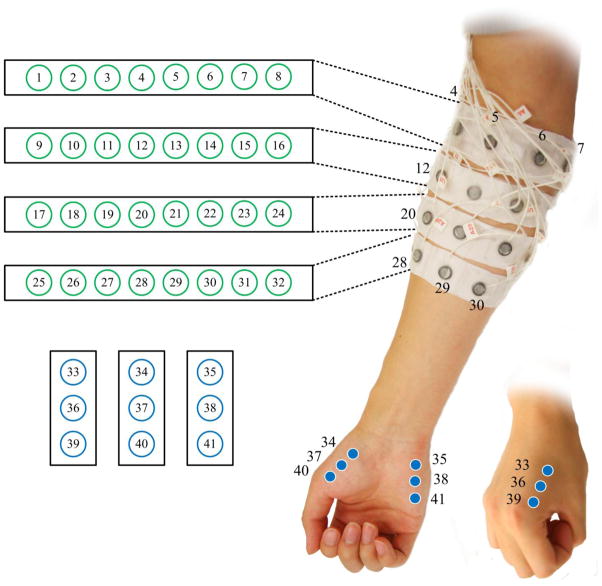
A Refa128 EMG system (TMS International B.V., The Netherlands) was used to record multi-channel surface EMG signals from the forearm and hand muscles in the paretic side of each stroke subject. In total 41 surface electrodes were used as demonstrated in Fig. 1, with a reference electrode located on the olecranon. From each of the electrodes EMG signals were recorded with respect to the reference, while each electrode also had a common feedback subtraction (equivalent to signal average of all the electrodes). The size of each individual electrode is 10 mm in diameter while the recording surface has a diameter of 5 mm. Among all the 41 electrodes, 32 were placed in a 4 × 8 grid formation over the forearm. Eight electrodes in each row were equally spaced and embedded in the inner side of a stretchable arm-band. Four such arm-bands were attached parallel around forearm at different locations from approximately 15% to 60% (with 15% increments) of the entire distance from the medial epicondyle of the humerus to the styloid process of the ulna. The stretch of the arm-bands can facilitate electrode placement around forearms with different sizes and ensure solid electrode-skin contact. In addition, 9 remaining electrodes were placed on hand muscles, with every 3 electrodes targeting the first dorsal interosseous (FDI), the thenar group and the hypothenar group muscles, respectively. The inter-electrode-distance depends on the size of the arm. Generally, the center-to-center distance between two consecutive electrodes was approximately 8–15 mm. All surface electrodes produced 41-channel surface EMG signals that were band-pass filtered between 20 and 500 Hz, amplified with a gain of 60 dB and sampled at 2 kHz per channel.
Figure 1.
Schematic description of the electrode placement for 41-channel surface EMG recordings.
C. Experimental Protocol
Each stroke subject was comfortably seated upright on a chair and relaxed the affected upper limb on a height-adjustable side table. The subject was instructed to perform 11 different functional movements as shown in Fig. 2, including 6 fine hand tasks (cylindrical grip, light tool grip, power grip, lateral pinch, open pinch and fine pinch), 4 wrist movements (wrist flexion/extension and wrist pronation/supination) and hand opening. The experiment protocol comprised of 11 trials, each trial performing 6 repetitions of the same movement pattern. For each trial, an experimenter first demonstrated the movement pattern to the subject, and the subject received auditory cues from the experimenter indicating when to start or terminate each repetition of contraction. For each repetition, the subject was required to perform (or intent to perform) an isometric muscle contraction for the movement with a natural or comfortable force, hold the task for approximately 3 seconds and then relax between repetitions. Additional rest periods were allowed between trials to avoid muscular and mental fatigue of the subject. To examine the effect of involuntary motor activity on surface EMG classification performance, two sessions were performed for each trial.
Figure 2.
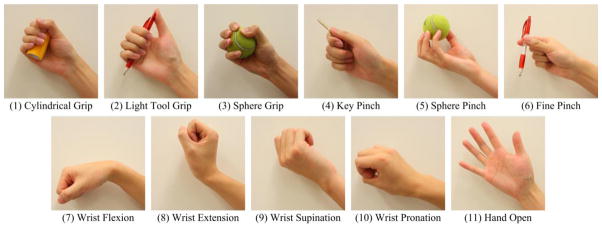
Illustration of the 11 different wrist and hand functional movements used in this study.
The first session was considered as a slow session, during which the subject was allowed to have sufficient relaxation time for duration of approximate 10 s between repetitions of each movement pattern. For some severely impaired subjects, such relaxation periods were extended up to 20 s. The long relaxation periods in the slow session were expected to help the stroke subject decrease muscle spasticity or involuntary EMG activity before performing the next repetition. Such a protocol was consistent with previous studies [4], [18], [19]. The second session was considered as a fast session, during which only very brief relaxation periods of no more than 3 s were allowed between repetitions, thus involuntary motor activity was routinely present in the surface EMG recordings.
D. Data Processing
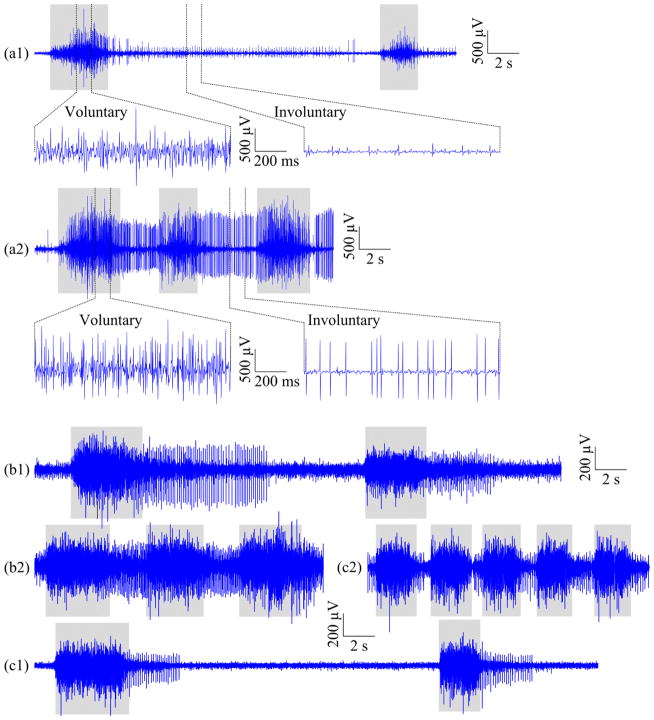
For surface EMG signals recorded from each trial, the onset and offset of each repetition of voluntary muscle contraction were first determined. To overcome the difficulty induced by involuntary muscle activity (usually demonstrated as continuously firing spikes, Figure 3) in determining onset and offset of voluntary muscle activity, the SampEn analysis of surface EMG signal developed in [26] was used to distinguish voluntary muscle activity from the involuntary background interference. This was based on the fact that the EMG burst induced from voluntary muscle contraction is prone to have larger SampEn values compared with involuntary spikes or background baseline. For each movement, the SampEn analysis was applied on a reference surface EMG signal averaged from a limited number (8–10) of channels that were examined and selected with relatively distinct voluntary surface EMG activity through a brief visual inspection. The SampEn values were calculated over a 128-ms analysis window sliding along the reference signal with a window increment of 32 ms. The voluntary muscle activity was determined when the SampEn value of the reference signal was detected above the preset threshold in the SampEn domain. The sensitivity analysis for choosing the threshold was performed in our previous study [26]. The pretests with the current dataset confirmed that the threshold of 0.4 was appropriate for detecting the onset and offset of each voluntary muscle contraction in the signal complexity domain.
Figure 3.
Illustration of representative signal segments of a single surface EMG channel recorded from (a) Subject 3, (b) Subject 6, and (c) Subject 8, within (1) the slow session and (2) the fast session, respectively, when the subject was performing cylindrical grip. The gray rectangles under every signal segment mark voluntary muscle contractions. Each of the top two signal segments, (a1) and (a2), is also shown with an overview (top) and two expanded views (bottom) of voluntary and involuntary EMG activity, respectively.
After onset and offset identification of voluntary surface EMG, the original 41-channel EMG data within each repetition of voluntary muscle contraction were further segmented into a series of overlapping analysis windows with a window length of 256 ms and a window increment of 64 ms. These windows were considered to be elementary units on which the following feature extraction and pattern recognition analyses were performed.
For each analysis window, the four time-domain (TD) features (known as the TD feature set) [11–14] and the sixth-order autoregressive (AR) model coefficients combined with the root mean square (RMS) amplitude of the signal (known as the AR+RMS feature set) [14], [15] were used to characterize different EMG patterns, respectively. After concatenating the feature sets of all the channels, the final feature set vector was provided to the classifier. A linear discriminant analysis (LDA) classifier [30] was used for automatic classification of different movements, which is simple to implement and fast to train and does not compromise classification accuracy compared with more complex and potentially more powerful classifiers [14].
Pattern recognition procedure was performed for each stroke subject. The voluntary EMG data in the slow session can be considered to be approximately free of involuntary EMG interference or to be contaminated by a relatively small amount of involuntary EMG interference, while the voluntary EMG data from the fast session can be considered as being contaminated by a sufficiently large amount of involuntary EMG interference. The effect of such involuntary muscle activity on classification performance was investigated using the following testing schemes:
Slow-Fast scheme
The EMG data from the slow session for each movement were used as training dataset, whereas those from the fast session were used as testing dataset. This scheme was designed to evaluate the classification performance when involuntary EMG interference was primarily present in the testing dataset. Both training and testing datasets consisted of EMG data within all 6 repetitions in their corresponding session.
Fast-Slow scheme
The EMG data from the fast session for each movement were used as training dataset, whereas those from the slow session were used as testing dataset. With such a scheme, the classification performance was evaluated when involuntary EMG interference was primarily present in the training dataset. The EMG data within all 6 repetitions in the fast and slow sessions were assigned as training and testing datasets, respectively.
Slow-Slow scheme
Both training and testing datasets were selected from the slow session for each movement. In contrast to the previous two schemes, this scheme was consistent to our previous studies [18], [19], where the classification performance was evaluated using an experimental to reduce the involuntary EMG interface. To make efficient use of the collected data, the six-fold cross-validation tests were performed. The EMG data within any five repetitions in the slow session were selected as training dataset, while the EMG data of the remaining repetition were used as the testing dataset.
Fast-Fast scheme
Both training and testing datasets were selected from the fast session for each movement. We used this scheme to evaluate the classification performance when the involuntary EMG interference was always present in both training and testing datasets. Similar to the Slow-Slow scheme, the six-fold cross-validation tests were performed with the six repetitions in the fast session.
Mixture-Mixture scheme
The EMG data from both slow and fast sessions for each movement were mixed together. Training and testing datasets were selected from the mixed data pool to evaluate the classification performance when involuntary EMG activity was randomly present in both training and testing datasets. The twelve-fold cross-validation tests were performed. The EMG data within any eleven repetitions in both sessions were selected as training dataset, while the EMG data of the remaining repetition were used as the testing dataset.
To evaluate the performance of automatic pattern classification, four different statistical indexes, namely sensitivity (Sen), specificity (Spe), precision (Pre) and accuracy (Acc) for each pattern defined as following, have been employed by previous studies [36], [37].
where TP represents the number of true positives that are testing windows of pattern x classified as belonging to pattern x; FP represents the number of false positives that are windows belonging to any other pattern classified as pattern x; FN is the number of false negatives that are windows of pattern x classified as belonging to any other pattern; and TN is the number of true negatives that are windows of any other patterns that are not classified as belonging to pattern x. Note that a classification error may be even considered as a true negative [36]. For example, if a window belonging to pattern x is incorrectly classified into pattern y (y ≠ x), it is obviously a false negative to pattern x, a false positive to pattern y, but a true negative to any other pattern z (z ≠ y and z ≠ x). Because of this, Rojas-Martinez et al. [37] reported that Spe and Acc defined above are not appropriate to be considered in the classification of multiple patterns since they involve TN which is usually a very high number and lead to biased estimate of the performance. Thus, the use of Pre and Sen, which mainly take into account the type I (false positive, false alarm) and type II (false negative, miss) errors for each pattern, respectively, was suggested [37]. With this regard, another form of accuracy, an overall accuracy for each test, rather than for each pattern, was defined as the percentage of correctly classified windows to the number of all the testing windows including all the investigated movement patterns. With respect to its definition, the overall accuracy is equivalent to the ratio of the summation of TP for all patterns to the number of total testing windows covering TP, Fp and FN, without taking into account TN. It was also found that for classification of multiple patterns, the overall accuracy was just slightly different from the averaged Pre or Spe over all movement patterns, because of slight variation in the number of testing windows among movement patterns [17]. This was confirmed by pretests (see Fig. 5) in our study. Actually, the overall accuracy as well as its complementary measure, i.e. error rate (1 minus the accuracy), has been widely used as a standard performance index in a variety of pattern recognition based myoelectric control studies [4], [11]–[18]. Consequently, the overall accuracy for each test was employed in this study for evaluating classification performance under different testing schemes. For the schemes using multiple cross-validation tests (Slow-Slow, Fast-Fast and Mixture-Mixture), the number of testing windows was summed up over all cross-validation tests to obtain a global accuracy.
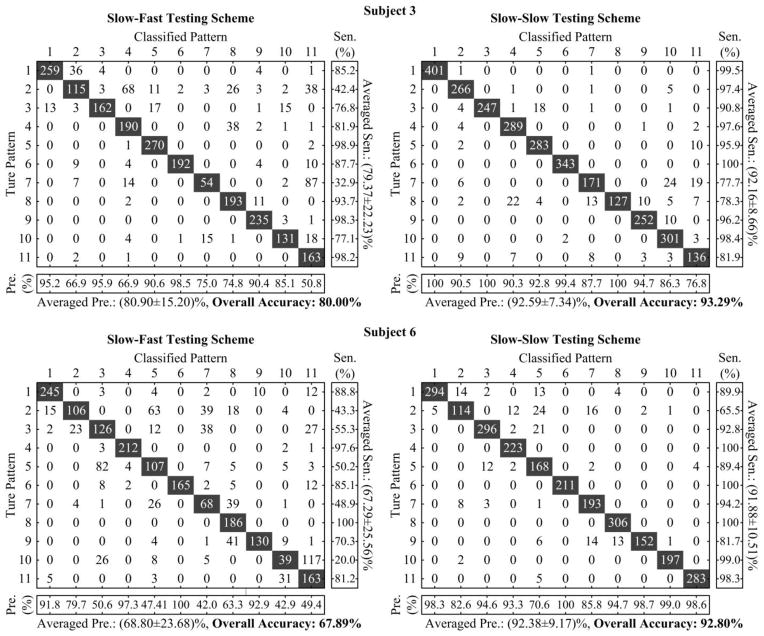
Figure 5.
Pattern-to-pattern confusion matrices derived from both two subjects using TD feature set under the Slow-Fast and Slow-Slow testing schemes, respectively. In each confusion matrix, an element at the x-th row and y-th column represents the number of testing windows for pattern x classified to pattern y. The main diagonal elements (shaded in dark) correspond to the numbers of correctly classified windows (i.e., TP) for each pattern, whereas other non-zero elements off the main diagonal are errors (FP or FN). Based on a confusion matrix, the Pre for each pattern is computed as the ratio of the diagonal element to the summation of all elements in the corresponding column, the Sen is computed as the ratio of diagonal element to the summation of all elements in the corresponding row, and the overall accuracy is computed as the ratio of the summation of all diagonal elements to the summation of all elements in the entire matrix.
E. Statistical Analysis
The two-way repeated-measure analysis of variance (ANOVA) was applied on the overall accuracy, with the feature set (TD and AR+RMS) and the testing scheme (Fast-Slow, Slow-Fast, Slow-Slow, Fast-Fast and Mixture-Mixture) considered as both within-subject factors. Considering that the overall accuracy was measured as percentage that was upper bounded to 1 (100%) and thus non-normally distributed, the arcsine transformation was used to normalize the accuracy prior to the ANOVA. The level of statistical significance was set to p < 0.05 for all analyses. When necessary, post hoc pairwise multiple comparisons with Bonferroni correction were used. All statistical analyses were completed using SPSS software (ver. 16.0, SPSS Inc. Chicago, IL).
RESULTS
Fig. 4 shows the efficiency of performing SampEn analysis of surface EMG signal for detection of voluntary muscle activation when involuntary EMG interference was present. The root mean square amplitude of the EMG signal was also calculated for comparison. Both surface EMG amplitude and SampEn measurements were calculated over 128-ms sliding windows. It was observed from Fig. 4 that the resultant SampEn curve was able to highlight voluntary muscle contractions, whereas the filtered amplitude curve did not reflect such a distinct separation between the voluntary EMG activity and the involuntary EMG interference.
Figure 4.
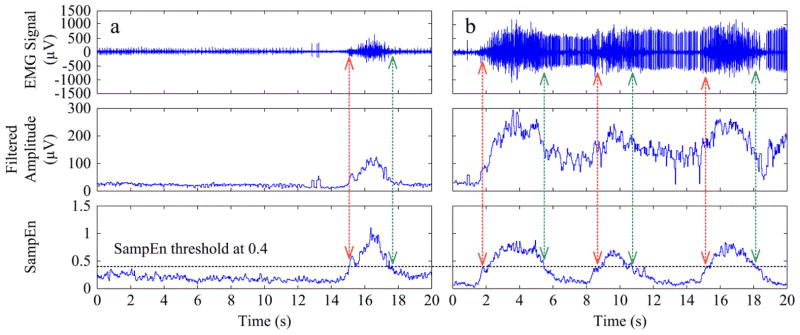
Illustration of surface EMG onset/offset detection using sample entropy analysis, with signal segments recorded within (a) the slow session and (b) the fast session, respectively (the same as shown in Fig. 3a). The vertical arrows represent the detected onset/offset timing of voluntary contractions based on the sample entropy results in the bottom panel. The filtered EMG signal amplitude is also shown in the middle panel for comparison purpose.
For every stroke subject, surface EMG pattern recognition analysis was performed for each of the 5 different testing schemes, using the TD and the AR+RMS feature sets respectively. Fig. 5 exhibits patter-to-pattern classification results in the form of confusion matrices derived from two specific subjects (Subjects 3 and 6) under Slow-Fast and Slow-Slow testing schemes, respectively, when the TD feature set was used. This figure also illustrates the computational principle of three performance indexes, namely Pre, Sen and overall accuracy, based on the confusion matrix for a test. From these representative examples shown in Fig. 5, we found that the presence of sufficient involuntary motor activity degraded the classification performance by comparing the performance indexes under the Slow-Fast scheme with those under the Slow-Slow scheme. In addition, the resulting overall accuracy for all movement patterns appeared to be very close to the mean of the Pre or Sen measures for all individual movement patterns, indicating that they were equivalent performance indexes in classification of multiple patterns. Consequently, Table II displays the performance for classification of 11 functional movements under different situations, as evaluated by the overall accuracy for each situation. The ANOVA showed an overall significant effect of both feature set (F = 16.640, p = 0.005) and testing scheme (F = 26.632, p < 0.001) on the classification performance. However, no significant interaction (F = 0.161, p = 0.956) was observed between both within-subject factors. When the data derived from the slow session and the fast session were separately used to form the training and testing datasets (i.e., Slow-Fast or Fast-Slow testing scheme), the average overall classification accuracies across all subjects (ranging from 83.15% to 88.43%) were much lower than those under the Slow-Slow testing scheme (above 96%). This was true for both the TD and AR+RMS feature sets. Among all the subjects, we observed that three subjects (Subjects 3, 5 and 6) yielded classification accuracies of less than 85%, and Subject 6 had the lowest classification accuracy under either Slow-Fast or Fast-Slow scheme, regardless of the feature set. By contrast, with the Fast-Fast scheme where the involuntary EMG interference was consistently involved in both training and testing datasets, high overall classification accuracies of above 98% were achieved across all tested subjects, which were comparable to the performance under the Slow-Slow scheme.
Table II.
Overall classification accuracies (%) for each stroke subject with two feature sets under five different testing schemes
| Feature Set | Subject # | Testing Schemes
|
||||
|---|---|---|---|---|---|---|
| Slow-Fast | Fast-Slow | Slow-Slow | Fast-Fast | Mix-Mix | ||
| TD | 1 | 99.34 | 95.88 | 99.26 | 99.63 | 99.10 |
| 2 | 81.54 | 96.07 | 99.36 | 97.12 | 97.97 | |
| 3 | 80.00 | 72.32 | 93.29 | 97.43 | 94.56 | |
| 4 | 91.41 | 84.81 | 96.57 | 99.72 | 98.12 | |
| 5 | 77.09 | 73.94 | 92.42 | 98.51 | 95.71 | |
| 6 | 67.89 | 62.22 | 92.80 | 93.26 | 92.18 | |
| 7 | 92.03 | 94.33 | 99.77 | 99.37 | 99.79 | |
| 8 | 96.36 | 85.66 | 98.63 | 99.21 | 98.96 | |
| Averaged (Mean±SD) | 85.71±10.78 | 83.15±12.55 | 96.51±3.20 | 98.03±2.16 | 97.05±2.65 | |
|
| ||||||
| AR+RMS | 1 | 99.12 | 96.68 | 99.63 | 99.78 | 99.60 |
| 2 | 83.61 | 93.53 | 99.42 | 97.82 | 98.45 | |
| 3 | 83.22 | 76.67 | 96.07 | 99.27 | 95.99 | |
| 4 | 93.63 | 89.24 | 99.37 | 99.82 | 99.42 | |
| 5 | 83.54 | 74.70 | 92.92 | 98.96 | 96.57 | |
| 6 | 72.53 | 63.98 | 93.07 | 93.96 | 94.48 | |
| 7 | 95.77 | 97.76 | 100.0 | 99.56 | 99.95 | |
| 8 | 96.04 | 86.84 | 98.29 | 99.79 | 99.07 | |
| Averaged (Mean±SD) | 88.43±9.10 | 84.92±12.02 | 97.35±2.95 | 98.62±2.00 | 97.94±2.01 | |
Our experimental results also indicated that when the pooled data from both slow and fast sessions were used to form the training and testing datasets under the Mixture-Mixture scheme, high classification accuracies of above 97% was still able to be achieved. Across all tested subjects, the average overall classification accuracy was 97.05 ± 2.65% and 97.94 ± 2.01% using TD and AR+RMS feature sets, respectively.
Pairwise comparisons in the ANOVA indicated that there was a significant difference (p < 0.02) in overall classification accuracy when either the Fast-Slow or Slow-Fast scheme was compared with any of the Slow-Slow, Fast-Fast and Mixture-Mixture schemes. However, no significant difference was observed for all the other possible pairs or comparisons between two testing schemes (p values close to 1). In examination of feature set, it was found that the AR+RMS feature set yielded a slight improvement in the overall classification accuracy over the TD feature set (p = 0.005). Such an improvement was most evident (approximately 2.7%, p = 0.002) under the Slow-Fast scheme.
DISCUSSION
Stroke has a detrimental effect on health-related quality of life. Following a hemispheric stroke, many patients suffer a variety of disabling physical symptoms on the contralesional side of the body. Spasticity is considered to be a major determinant of motor impairments after stroke, which is measured as an excessive response to passive muscle stretch [20]. It is believed that the mechanisms underlying spasticity vary in different types of neurological disorders, including alterations in both motoneuron and muscle contractile properties [20]–[22]. As a result of spasticity, involuntary motor activity can be seen in paretic-spastic muscles when a stroke subject is instructed to fully relax [23], [24]. Such involuntary activity is more often observed to accompany active muscle contractions and likely presents in voluntary surface EMG signals.
The aim of current study was to examine the influence of involuntary motor activity of paretic-spastic muscles on the classification of surface EMG signals recorded from stroke subjects. This was examined by designing different experimental protocols for performance of the selected movements. In particular, a fast session was used during which the stroke subject was asked to repeat each movement in a relatively fast speed. Thus distinct involuntary motor activity was induced and may present within voluntary surface EMG recordings. In contrast, a slow session was designed to allow sufficient relaxation time between repetitions of each movement to reduce involuntary motor activity. The classification performance was then assessed using different combinations of training and testing datasets selected from slow and fast sessions. High accuracy in classification of different movements is a prerequisite for implementation of a myoelectric pattern recognition control system. Thus, understanding the effects of involuntary motor activity on surface EMG classification is important to implement such a system or device for stroke rehabilitation.
Across all the tested stroke subjects, high classification accuracies were achieved under the Slow-Slow scheme. This was consistent to our previous studies [18], [19] where the protocol was designed to allow sufficient relaxation time for subjects to reduce involuntary EMG activity before performing the next task. The classification performance under the Slow-Slow scheme can be used as a reference for evaluating different schemes. Under the Slow-Fast or the Fast-Slow scheme, the significant decrease in classification accuracy was observed in comparison with the Slow-Slow scheme. This suggests that involuntary EMG interference solely involved in either training or testing datasets does contribute to compromise the classification of surface EMG signals.
Furthermore, under the Slow-Fast and Fast-Slow schemes, the decrease of classification performance was correlated to clinical assessment of muscle spasticity. Among all stroke subjects, the lowest classification accuracy was found in Subject 6 (with TD feature set: 67.89% for the Slow-Fast scheme and 62.22% for the Fast-Slow scheme; with AR+RMS feature set: 72.53% for the Slow-Fast scheme and 63.98% for the Fast-Slow scheme), who demonstrated severe spasticity in hand muscles (MAS=4 for both wrist flexors and extensors). Thus a large amount of involuntary motor activity was likely to present in voluntary surface EMG signals recorded from the fast session (See Fig. 3b), which may compromise the classification performance.
It is noted that under the Fast-Fast testing scheme, sufficiently high classification accuracies were achieved comparable to those under the Slow-Slow scheme. This suggests that involuntary EMG interference, if present in both training and testing datasets, has little effect on the classification performance. Such an observation is consistent to previous findings that the classification performance might not be compromised as long as the interference was consistently present through the entire dataset [16]. It is worth noting that the interference investigated in [16] was electrocardiography (ECG) artifacts contaminating EMG signals. ECG artifacts have relatively low frequency components, stable firing patterns and constant action potential waveforms. Taking this advantage, different methods have been developed to remove ECG artifacts from EMG signals for myoelectric control [38]. In contrast, the involuntary motor activity examined in this study may be originated from multiple motor units with different firing patterns and action potentials waveforms. Their power spectrum of involuntary and voluntary EMG can overlap. As a result, compared with ECG artifacts it is more challenging to remove such involuntary motor activity from voluntary EMG signals.
Another interesting finding of the present study is that under the Mixture-Mixture testing scheme, the classification accuracies across all subjects tended to maintain similarly high values, as compared with those achieved under the Slow-Slow scheme. This indicates that if involuntary EMG interference is occasionally distributed in both training and testing datasets, its influence on the classification performance can be neglected, due to some certain degree of adaptability of the used statistical classifier (i.e., LDA) to data variation.
Across all subjects, we observed the AR+RMS feature set achieved slightly superior classification performance to the TD feature set under all the testing schemes. Statistical analysis demonstrated significant difference (p = 0.005) in average classification accuracy between both feature sets, especially under the Slow-Fast scheme. The slight performance improvement of the AR+RMS feature set in this study may be due to the less sensitive character of the AR coefficients to addictive involuntary interference compared with the TD feature set.
One limitation of the study is that it is difficult to quantitatively describe or predict the amount of involuntary motor activity in voluntary surface EMG signals. It is not clear whether or how the involuntary muscle activity that occurs after stroke is correlated to the assessment of spasticity using different methods (such as clinical examination scales, manual and isokinetic dynamometry and pendulum-test methods, or the documentation of spasm frequency [20], [21]). Thus the design of our study was oriented toward qualitative differentiation of voluntary surface EMG with relative presence or absence of involuntary motor activity.
In this study, up to 41 EMG channels were used for classification analysis. Our previous studies have demonstrated that it is possible to dramatically reduce the number of electrodes while maintaining the similar level of high classification accuracies. Thus it is important to determine the most appropriate channels for implementation of a practical myoelectric control system. Furthermore, multiple features extracted from each channel or analysis window have been used for the classification. It is also necessary to perform statistical feature selection to determine the most appropriate features for classification. Feature dependent channel selection will further reduce the complexity of signal processing and make the method suitable for online application.
This study examined the influence of involuntary EMG activity on myoelectric pattern recognition performance of hemiparetic stroke patients. Recently, attempts have been made toward simultaneous and proportional control of multi-DOFs, using artificial neural networks to learn and predict associations between surface EMG features and force levels produced by individual patterns and their combinations [33]–[35]. Such an approach, if applied in stroke patients, may be similarly affected by involuntary motor activity present in surface EMG signals. Further studies are needed to quantitatively assess how involuntary motor activity may affect control performance.
Finally, this study focused on the effect of involuntary motor activity on surface EMG classification performance by testing a group of stroke subjects with mild to severe spasticity in wrist and hand muscles. It is acknowledged that the involuntary motor activity may be present in different degrees for each specific subject. The variability of the recorded involuntary motor activity across subjects can be illustrated by examples in Fig. 3, where three subjects exhibited involuntary motor activity presented at different timings and intensity levels with respect to the EMG bursts during voluntary muscle contractions. Thus the design of myoelectric control system should be subject-specific according to each subject’s impairment characteristics. Furthermore, involuntary motor activity was also reported in other neurological disorders such as spinal cord injury [31] and cereal palsy [32]. The findings of the current study can provide helpful reference to predict or assess the influence of involuntary motor activity from other neurologic disorders on surface EMG classification performance.
In summary, this study for the first time investigates the effect of involuntary motor activity of paretic-spastic muscles on classification of surface EMG signals. For each stroke subject, the degree of involuntary motor activity present in voluntary surface EMG recordings was qualitatively described from different experimental protocols for performing the movements (i.e. a slow session and a fast session). Across all tested stroke subjects, our results revealed that when involuntary surface EMG was absent or present in both training and testing datasets, high accuracies can be achieved in classification of different movements using surface EMG signals from paretic muscles. When involuntary surface EMG was solely involved in either training or testing datasets, the classification accuracies would be dramatically reduced. However, if both training and testing datasets contained EMG signals with presence and absence of involuntary EMG interference, high accuracies can still be achieved. The findings of this study can be used to guide appropriate design and implementation of myoelectric pattern recognition based systems or devices toward promoting robot-aided therapy for stroke survivors.
Acknowledgments
This work was supported in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grant 2R24HD050821, and in part by the 1000 Talent Plan Special Program of China (Recruitment Program of Global Experts).
References
- 1.Oskoei MA, Hu H. Myoelectric control systems—A survey. Biomedical Signal Processing and Control. 2007;2:275–94. [Google Scholar]
- 2.Dipietro L, Ferraro M, Palazzolo JJ, Krebs HI, Volpe BT, Hogan N. Customized interactive robotic treatment for stroke: EMG-triggered therapy. IEEE Trans Neural Syst Rehabil Eng. 2005;13:32–34. doi: 10.1109/TNSRE.2005.850423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein J, Narendran K, McBean J, Krebs K, Hughes R. Electromyography-controlled exoskeletal upper-limb-powered orthosis for exercise training after stroke. Am J Phys Med Rehabil. 2007;86:25–61. doi: 10.1097/PHM.0b013e3180383cc5. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Wilson KM, Lock BA, Kamper DG. Subject-specific myoelectric pattern classification of functional hand movements for stroke survivors. IEEE Trans Neural Syst Rehabil Eng. 2011;19:55–66. doi: 10.1109/TNSRE.2010.2079334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song R, Tong K, Hu X, Li L. Assistive control system using continuous myoelectric signal in robot-aided arm training for patients after stroke. IEEE Trans Neural Syst Rehabil Eng. 2008;16:371–79. doi: 10.1109/TNSRE.2008.926707. [DOI] [PubMed] [Google Scholar]
- 6.Hu XL, Tong KY, Song R, Zheng XJ, Lui KH, Leung WW, Ng S, Au-Yeung SS. Quantitative evaluation of motor functional recovery process in chronic stroke patients during robot-assisted wrist training. J Electromyogr Kinesiol. 2009a;19:639–50. doi: 10.1016/j.jelekin.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil Neural Repair. 2009b;23:837–46. doi: 10.1177/1545968309338191. [DOI] [PubMed] [Google Scholar]
- 8.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: A rat brain ischemia model. PLoS One. 2011;6:e16643, 1–8. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lum PS, Burgar CG, Kenney DE, Vander Loos HF. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans Biomed Eng. 1999;46:652–62. doi: 10.1109/10.764942. [DOI] [PubMed] [Google Scholar]
- 10.Krakauer JW. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 11.Hudgins B, Parker PA, Scott R. A new strategy for multifunction myoelectric control. IEEE Trans Biomed Eng. 1993;40:82–94. doi: 10.1109/10.204774. [DOI] [PubMed] [Google Scholar]
- 12.Englehart K, Hudgins B, Parker PA, Stevenson M. Classification of the myoelectric signal using time-frequency based representations. Med Eng Phys. 1999;21:431–8. doi: 10.1016/s1350-4533(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 13.Englehart K, Hudgins B. A Robust, Real-Time Control Scheme for Multifunction Myoelectric Control. IEEE Trans Biomed Eng. 2003;50:848–54. doi: 10.1109/TBME.2003.813539. [DOI] [PubMed] [Google Scholar]
- 14.Hargrove LJ, Englehart K, Hudgins B. A comparison of surface and intramuscular myoelectric signal classification. IEEE Trans Biomed Eng. 2007;54:847–53. doi: 10.1109/TBME.2006.889192. [DOI] [PubMed] [Google Scholar]
- 15.Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, Dewald JP, Kuiken TA. Decoding a new neural machine interface for control of artificial limbs. J Neurophysiol. 2007;98:2974–82. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hargrove L, Zhou P, Englehart K, Kuiken TA. The effect of ECG interference on pattern-recognition-based myoelectric control for targeted muscle reinnervated patients. IEEE Trans Biomed Eng. 2009;56:2197–201. doi: 10.1109/TBME.2008.2010392. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Kuiken TA, Lipschutz RD. A strategy for identifying locomotion modes using surface electromyography. IEEE Trans Biomed Eng. 2009;56:65–73. doi: 10.1109/TBME.2008.2003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Zhou P. High density myoelectric pattern recognition towards improved stroke rehabilitation. IEEE Trans Biomed Eng. 2012;59:1649–57. doi: 10.1109/TBME.2012.2191551. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Zhou P. A novel myoelectric pattern recognition strategy for hand function restoration after incomplete cervical spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2013;21:96–103. doi: 10.1109/TNSRE.2012.2218832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil. 1989;70:144–55. [PubMed] [Google Scholar]
- 21.Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. 2004;35:134–9. doi: 10.1161/01.STR.0000105386.05173.5E. [DOI] [PubMed] [Google Scholar]
- 22.Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair. 2010;24:23–33. doi: 10.1177/1545968309343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Long-lasting involuntary motor activity after spinal cord injury. Spinal Cord. 2011;9:87–93. doi: 10.1038/sc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawes H, Bateman A, Wade D, Scott OM. High-intensity cycling exercise after a stroke: a single case study. Clin Rehabil. 2000;14:570–3. doi: 10.1191/0269215500cr363oa. [DOI] [PubMed] [Google Scholar]
- 25.Sorinola IO, White CM, Rushton DN, Newham DJ. Electromyographic response to manual passive stretch of the hemiplegic wrist: accuracy, reliability, and correlation with clinical spasticity assessment and function. Neurorehabil Neural Repair. 2009;23:287–94. doi: 10.1177/1545968308321778. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Zhou P. Sample entropy analysis of surface EMG for improved muscle activity onset detection against spurious background spikes. J Electromyogr Kinesiol. 2012;22:901–7. doi: 10.1016/j.jelekin.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient—1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 28.Gowland CA. Staging motor impairment after stroke. Stroke. 1990;21(Suppl II):19–21. [PubMed] [Google Scholar]
- 29.Bohannon R, Smith M. Interrater reliability of a modified Ashworth scale of muscle spasticity. Physical Therapy. 1987;67:206. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Wechsler H. Robust coding schemes for indexing and retrieval from large face databases. IEEE Trans Image Processing. 2000;9:132–37. doi: 10.1109/83.817604. [DOI] [PubMed] [Google Scholar]
- 31.Kinnaird CR, Ferris DP. Medial gastrocnemius myoelectric control of a robotic ankle exoskeleton. IEEE Trans Neural Syst Rehabil Eng. 2009;17:31–7. doi: 10.1109/TNSRE.2008.2008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YN, Ren Y, Goldsmith A, Gaebler D, Liu SQ, Zhang LQ. Characterization of spasticity in cerebral palsy: dependence of catch angle on velocity. Dev Med Child Neurol. 2010;52:563–9. doi: 10.1111/j.1469-8749.2009.03602.x. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen JL, Holmgaard S, Jiang N, Englehart KB, Farina D, Parker PA. Simultaneous and proportional force estimation for multifunction myoelectric prostheses using mirrored bilateral training. IEEE Trans Biomed Eng. 2011;58:681–8. doi: 10.1109/TBME.2010.2068298. [DOI] [PubMed] [Google Scholar]
- 34.Kamavuako EN, Englehart KB, Jensen W, Farina D. Simultaneous and proportional force estimation in multiple degrees of freedom from intramuscular EMG. IEEE Trans Biomed Eng. 2012;59:1804–7. doi: 10.1109/TBME.2012.2197210. [DOI] [PubMed] [Google Scholar]
- 35.Jiang N, Vest-Nielsen JL, Muceli S, Farina D. EMG-based simultaneous and proportional estimation of wrist/hand kinematics in uni-lateral trans-radial amputees. J Neuroeng Rehabil. 2012;9:42. doi: 10.1186/1743-0003-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farina D, Colombo R, Merletti R, Olsen HB. Evaluation of intra-muscular EMG signal decomposition algorithms. J Electromyogr Kinesiol. 2001;11:175–87. doi: 10.1016/s1050-6411(00)00051-1. [DOI] [PubMed] [Google Scholar]
- 37.Rojas-Martínez M, Mañanas MA, Alonso JF, Merletti R. Identification of isometric contractions based on High Density EMG maps. J Electromyogr Kinesiol. 2013;23:33–42. doi: 10.1016/j.jelekin.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhou P, Lock B, Kuiken TA. Real time ECG artifact removal for myoelectric prosthesis control. Physiol Meas. 2007;28:397–413. doi: 10.1088/0967-3334/28/4/006. [DOI] [PubMed] [Google Scholar]