Abstract
Objective To measure the efficacy of sulfadoxine-pyrimethamine treatment of falciparum malaria in Malawi from 1998 to 2002, after a change from chloroquine to sulfadoxine-pyrimethamine as first line treatment in that country in 1993.
Design Prospective open label drug efficacy study.
Setting Health centre in large peri-urban township adjacent to Blantyre, Malawi.
Participants People presenting to a health centre with uncomplicated Plasmodium falciparum malaria.
Main outcome measures Therapeutic efficacy and parasitological resistance to standard sulfadoxine-pyrimethamine treatment at 14 days and 28 days of follow up.
Results Therapeutic efficacy remained stable, with adequate clinical response rates of 80% or higher throughout the five years of the study. Analysis of follow up to 28 days showed modest but significant trends towards diminishing clinical and parasitological efficacy over time within the study period.
Conclusion Contrary to expectations, sulfadoxine-pyrimethamine has retained good efficacy after 10 years as the first line antimalarial drug in Malawi. African countries with very low chloroquine efficacy, high sulfadoxine-pyrimethamine efficacy, and no other immediately available alternatives may benefit from interim use of sulfadoxine-pyrimethamine while awaiting implementation of combination antimalarial treatments.
Introduction
In 1993 Malawi became the first African country to change its first line antimalarial drug from chloroquine to sulfadoxine-pyrimethamine on a nationwide basis in the face of rising rates of resistance to chloroquine.1 At the time, this was a controversial decision. On the basis of the rapid selection for mutant Plasmodium falciparum parasites resistant to these antifolate drugs and the precipitous decline in efficacy of sulfadoxine-pyrimethamine after it was introduced in South America and Southeast Asia, experts predicted that sulfadoxine-pyrimethamine would have a useful therapeutic life of only about five years in Africa and might fail even more quickly because of higher rates of transmission of malaria and of use of the drug.2 Neighbouring countries were concerned that resistance to sulfadoxine-pyrimethamine would develop in Malawi and spread to their countries, so that when they were eventually forced to drop chloroquine, sulfadoxine-pyrimethamine would already be compromised. Because other countries in Africa continued to rely on chloroquine and only a few have begun to change their policies within the past few years, Malawi serves as a sentinel site for failure of sulfadoxine-pyrimethamine for the rest of the continent.
We began monitoring the efficacy of sulfadoxine-pyrimethamine at one site in Malawi in 1998. We treated patients (mostly children) with uncomplicated falciparum malaria and measured their parasitological and therapeutic responses to the drug.
Methods
Study site and participants
We monitored the efficacy of sulfadoxine-pyrimethamine from February 1998 to June 2002 at the government health centre in Ndirande, an urban township in Blantyre, Malawi. Ndirande has approximately 100 000 inhabitants and year round transmission of P falciparum, peaking in the December-March rainy season. Sulfadoxine-pyrimethamine has been the standard treatment for uncomplicated malaria and the presumptive treatment for most fevers since 1993 at all health facilities and in many shops in the township. Alternative oral antimalarial drugs are seldom used, and chloroquine has been almost unobtainable locally for the past 10 years.
Patients were eligible for the study if they were aged 3 months or over and presented to the health centre with signs or symptoms consistent with malaria, had positive smears for P falciparum mono-infection, and had none of the following exclusion criteria: history of allergy or adverse reaction to sulfadoxine-pyrimethamine or sulfa drugs; known pregnancy; haematocrit < 15%; parasitaemia > 10%; prostration; respiratory distress; bleeding; recent seizures, coma, or obtundation; inability to drink; or persistent vomiting. Documented fever was not a requirement for eligibility. We obtained informed consent from participants or their guardians.
Definitions of resistance and treatment failure
We stained thin and thick blood smears with Field's stain and counted asexual parasites against 200 leucocytes. For the final calculation of the number of parasites per microlitre we assumed an average leucocyte count of 10 000 leucocytes/μl. A microscopist blinded to the first results read the slides a second time, and a third microscopist adjudicated discrepancies of > 10%. When parasitaemia was too high to count on thick smear, we counted the percentage of parasitised erythrocytes on the thin smear.
We used current World Health Organization definitions of therapeutic efficacy and, because the newer definitions do not take into account persistent infection in the absence of fever, older WHO definitions of parasitological resistance.3,4 Both definitions were modified slightly as described elsewhere.5
We defined therapeutic efficacy as follows. Early treatment failure: danger signs (not able to drink or breast feed, vomiting everything, recent history of convulsions, lethargic or unconscious state, unable to sit or stand up) or severe malaria on days 1, 2, or 3, with parasitaemia; axillary temperature ≥ 37.5°C on day 3 in the presence of parasitaemia; or parasitaemia on day 3 ≥ 25% of day 0 level. Late treatment failure: development of danger signs or severe malaria in the presence of parasitaemia during days 4-14, or axillary temperature ≥ 37.5°C in the presence of parasitaemia during days 4-14, and no criteria for early treatment failure. Adequate clinical response: absence of parasitaemia on day 14 irrespective of temperature, or axillary temperature < 37.5°C irrespective of the presence of parasitaemia, without previously having met any of the criteria for early or late treatment failure.
We defined parasitological resistance as follows. RIII: no reduction in parasitaemia, or reduction to ≥ 25% of day 0 level, by day 3. RII: reduction in parasitaemia to < 25% of day 0 level without clearance leading to retreatment or followed by persistent parasitaemia. RI: initial clearance of parasites indicated by negative thick smear after day 0, with subsequent positive thick smear by day 14. Sensitive: clearance of parasites by day 14 with no recurrence of parasitaemia.
Whenever possible, we did additional follow up through day 28 to permit longer term measurements of efficacy and haematological recovery.
Treatment
We gave standard treatment doses of sulfadoxine-pyrimethamine (1/4 tablet per 5 kg weight for age ≤ 12 years, three tablets for age > 12 years; one tablet = pyrimethamine 25 mg + sulfadoxine 500 mg) under direct observation, and we observed participants for at least 60 minutes to monitor for adverse reactions and to make sure the medicine was not vomited. If vomiting occurred within 30 minutes, we repeated the full dose; if it occurred within 60 minutes we repeated half of the dose. We treated treatment failures with halofantrine at standard doses of 8 mg/kg every eight hours for a total of three doses.
Statistical analysis
We assessed trends over time in rates of adequate clinical response versus early or late treatment failure and rates of sensitive or RI parasitological response versus RII or RIII resistance by using logistic regression with two sided significance set at P = 0.05, with odds ratios calculated to represent the average odds of treatment success in successive years (SAS 8.02 software). We used SPSS 10.1 to do univariate analyses and multiple logistic regression to test for associations with post-treatment anaemia.
Results
We enrolled 1377 patients into the study. Characteristics on enrolment were similar over the five years of study (table 1), although parasite density on presentation apparently increased in 2001 and 2002. One thousand and eighteen (73.9%) of the enrolled participants completed 14 days of follow up; 246 (17.9%) were lost to follow up before day 14, 30 (2.2%) withdrew consent to continue in the study, 40 (2.9%) were withdrawn owing to protocol violations, and 43 (3.1%) had incomplete follow up for unspecified reasons. Most protocol violations were receipt of curative treatment for malaria from sources other than study investigators. We could not determine therapeutic efficacy for 95 (9%) of 1054 participants for whom parasitological outcomes could be determined. This was because of clinical judgments made to treat persistent or recurrent malaria infections that did not meet criteria for treatment failures—that is, treatment of symptomatic but afebrile parasitaemias between day 4 and day 14. No trends towards increasing rates of withdrawal or loss to follow up occurred throughout the course of the study.
Table 1.
Characteristics at time of treatment for uncomplicated falciparum malaria
|
Study year
|
||||||
|---|---|---|---|---|---|---|
| Characteristic | 1998 (n=124) | 1999 (n=210) | 2000 (n=289) | 2001 (n=544) | 2002 (n=210) | Total (n=1377) |
| Median (range) age (years) | 3.3 (0.3-35.5) | 2.7 (0.3-36.3) | 2.7 (0.4-68.2) | 2.0 (0.3-39.3) | 2.5 (0.4-36.8) | 2.4 (0.3-68.3) |
| No (%) male | 64 (52) | 105 (50) | 130 (45) | 261 (48) | 95 (45) | 655 (48) |
| Geometric mean (95% confidence interval) parasite density (No/μl) | 11 103 (6701 to 18 069) | 9839 (6809 to 14 216) | 8719 (6621 to 11 480) | 15 179 (12 539 to 18 376) | 20 468 (14 936 to 28 050) | 12 967 (11 409 to 14 739) |
| Mean (SE) haemoglobin (g/dl) | 10.8 (0.2) | 10.5 (0.1) | 10.0 (0.1) | 8.8 (0.1) | 9.5 (0.1) | 9.6 (0.1) |
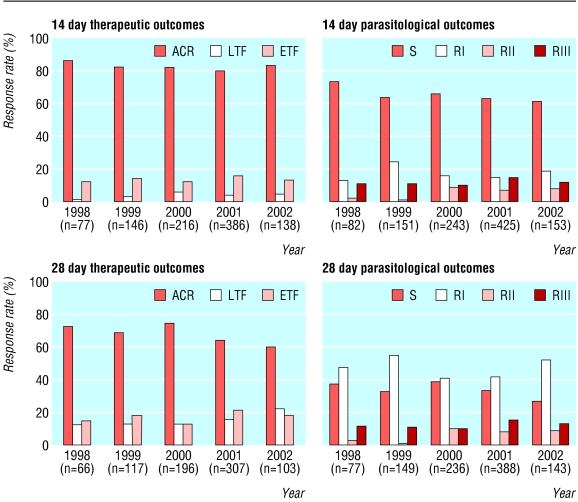
As shown in the figure and table 2, the 14 day efficacy remained stable over the five year study period, with adequate clinical response rates remaining above 80% (top left of figure: P = 0.44, odds ratio 0.95, 95% confidence interval 0.82 to 1.09). Among participants followed for 28 days, the rate of adequate clinical response decreased from 73% in 1998 to 60% in 2002 (bottom left: P = 0.02, odds ratio 0.86, 0.75 to 0.98). Rates of sensitive or RI parasitological responses at both 14 and 28 days also decreased significantly over the five year study period (top right: P = 0.015, odds ratio 0.84, 0.73 to 0.97; bottom right: P = 0.004, odds ratio 0.81, 0.71 to 0.94). Neither parasitological resistance nor therapeutic failure was associated with age in this population with a median age of 2.4 years.
Figure 1.
Sulfadoxine-pyrimethamine treatment outcomes, 1998-2002. Top left: therapeutic efficacy at 14 days; bottom left: therapeutic efficacy at 28 days; top right: parasitological resistance at 14 days; bottom right: parasitological resistance at 28 days. ACR=adequate clinical response; LTF=late treatment failure; ETF=early treatment failure; S=sensitive; RI-RIII=parasitological resistance at the RI-RIII levels
Table 2.
Sulfadoxine-pyrimethamine treatment outcomes, 1998-2002. Values are numbers (percentages)
|
Study year
|
||||||
|---|---|---|---|---|---|---|
| Outcomes | 1998 | 1999 | 2000 | 2001 | 2002 | Total |
| Therapeutic outcomes at 14 days | (n=77) | (n=146) | (n=216) | (n=386) | (n=138) | (n=963) |
| Adequate clinical response | 66 (86) | 120 (82) | 177 (82) | 308 (80) | 114 (83) | 785 (82) |
| Late treatment failure | 1 (1) | 5 (3) | 13 (6) | 15 (4) | 6 (4) | 40 (4) |
| Early treatment failure | 10 (13) | 21 (14) | 26 (12) | 63 (16) | 18 (13) | 138 (14) |
| Therapeutic outcomes at 28 days | (n=66) | (n=117) | (n=196) | (n=307) | (n=103) | (n=789) |
| Adequate clinical response | 48 (73) | 81 (69) | 144 (74) | 196 (64) | 62 (60) | 531 (67) |
| Late treatment failure | 8 (12) | 15 (13) | 26 (13) | 48 (16) | 23 (22) | 120 (15) |
| Early treatment failure | 10 (15) | 21 (18) | 26 (13) | 63 (21) | 18 (18) | 138 (18) |
| Parasitological outcomes at 14 days | (n=82) | (n=151) | (n=243) | (n=425) | (n=153) | (n=1054) |
| Sensitive | 60 (73) | 96 (64) | 159 (65) | 267 (63) | 94 (61) | 676 (64) |
| Resistant at the RI level | 11 (13) | 37 (25) | 38 (16) | 65 (15) | 29 (19) | 180 (17) |
| Resistant at the RII level | 2 (2) | 1 (1) | 22 (9) | 30 (7) | 12 (8) | 67 (6) |
| Resistant at the RIII level | 9 (11) | 17 (11) | 24 (10) | 63 (15) | 18 (12) | 131 (12) |
| Parasitological outcomes at 28 days | (n=77) | (n=149) | (n=236) | (n=388) | (n=143) | (n=993) |
| Sensitive | 29 (38) | 49 (33) | 93 (39) | 130 (34) | 38 (27) | 339 (34) |
| Resistant at the RI level | 37 (48) | 82 (55) | 96 (41) | 164 (42) | 74 (52) | 453 (46) |
| Resistant at the RII level | 2 (3) | 1 (1) | 23 (10) | 31 (8) | 13 (9) | 70 (7) |
| Resistant at the RIII level | 9 (12) | 17 (11) | 24 (10) | 63 (16) | 18 (13) | 131 (13) |
RI-RIII: see text for definitions.
Haematological recovery rates between days 0 and 14 did not differ between cases of adequate clinical response and those with treatment failure or between sensitive cases and those with RI-RIII resistance (data not shown). In univariate analyses, anaemia (haemoglobin < 10 g/dl) at day 14 was associated with both treatment failure (odds ratio 2.05, 1.15 to 3.65, P = 0.009) and RI-RIII resistance (odds ratio 1.46, 1.01 to 2.10, P = 0.034). However, when we included treatment failure and parasitological resistance separately in a logistic regression model with parasite density at day 0 and age as covariates, only the association between age and anaemia at day 14 remained significant (P < 0.001). Anaemia was more common at day 28 among children with adequate clinical response and asymptomatic parasitaemia than in children with adequate clinical response and no parasites (42.4% v 26.7%; relative risk 1.59, 1.23 to 2.05, P < 0.001). This association remained significant after we controlled for age and initial parasitaemia in a regression model (P = 0.009).
We found excellent agreement between measures of therapeutic efficacy and parasitological resistance. With adequate clinical response corresponding to sensitivity or RI resistance, late treatment failure corresponding to RI or RII resistance, and early treatment failure corresponding to RIII resistance, we found only 10/959 (1%) cases of discordance among cases for which both efficacy and resistance could be determined. One sensitive case and two RI cases met non-parasitological criteria for early treatment failure, and seven cases met criteria for RII resistance but had adequate clinical responses (one case) or were late treatment failures (six cases) because fever did not always accompany persistent parasitaemia.
Discussion
A very short useful therapeutic life was expected after sulfadoxine-pyrimethamine became first line treatment for malaria in sub-Saharan Africa, with some experts predicting that this drug would fail within five years of introduction. Our data from one site in Malawi show that rates of adequate clinical response at 14 days, the standard measure of efficacy of antimalarial drugs, have remained stable at over 80% for five years, starting five years after sulfadoxine-pyrimethamine became the standard treatment for uncomplicated malaria in this country. Rates of early treatment failure also remained stable, at 13% in both 1998 and 2002. Although early treatment failure rates over 10% are of concern, most of these treatment failures were so designated on the basis of the rate of parasite clearance and not of clinical deterioration of study participants. We have previously shown that the standard definition of therapeutic efficacy may be too sensitive, in that cases of early treatment failure or RIII resistance that are detected retrospectively often go on to cure with no further treatment.5 Thus we can conclude that under circumstances in which retreatment is not administered on day 3 solely on the basis of measurements of parasite density, treatment success rates at this site in Malawi are at least 80% and possibly higher.
Losses to follow up were relatively high at nearly 18%, and treatment failure rates may have been higher in this group. However, nearly all of the losses to follow up were attributable to migration from the study area. Participants with unknown parasitological or therapeutic outcomes at day 14 or day 28 did not differ from those with known outcomes with respect to age, parasite density, or haemoglobin concentration at the time of treatment (data not shown).
Why has loss of efficacy been slower than expected?
We hypothesise that a combination of epidemiological and molecular factors may explain the surprisingly durable efficacy of sulfadoxine-pyrimethamine in this setting. Mutations in parasite dihydrofolate reductase (DHFR) confer resistance to pyrimethamine, and mutations to dihydropteroate synthase (DHPS) confer resistance to sulfadoxine.6,7 Resistance to sulfadoxine-pyrimethamine in Africa is associated with three mutations in DHFR and two in DHPS that became highly prevalent in Malawi in 1998, just as these studies began.8,9 Despite the near ubiquity of these mutations, sulfadoxine-pyrimethamine resistance may have peaked and stabilised in Malawi.
The DHFR mutation from isoleucine to leucine at codon 164 is common in parts of Southeast Asia and South America where sulfadoxine-pyrimethamine resistance is high,10 but it has not been confirmed to be present in Africa. The DHPS ala-581-gly mutation is also common in areas with high rates of sulfadoxine-pyrimethamine resistance but is very rare in Africa.
The ile-164-leu mutation in DHFR is deleterious to enzyme function, so, in the absence of drug pressure, parasites containing this mutation are at a disadvantage and would be selected against.11,12 In Africa, where transmission is usually intense, the proportion of parasites under drug pressure is much lower than in areas of low transmission such as South America and Southeast Asia, where sulfadoxine-pyrimethamine failed quickly. This is because where transmission is high, semi-immunity is common and most infections are asymptomatic and untreated. Where transmission and immunity are low, most infections are symptomatic and come under drug pressure. The amount of drug pressure relative to the total parasite population may be insufficient to permit DHFR ile-164-leu to arise or persist in Africa. Although no data are available on DHPS ala-581-gly affecting parasite fitness, it too might be selected against in the absence of drug pressure. If this hypothesis is correct, the safe and inexpensive antifolate class of antimalarial drugs may have a longer useful life in high endemicity areas of Africa than they have elsewhere.
Ten years: approaching the limit?
Increases in both treatment failures and parasitological resistance occurred in this setting over five years when follow up was extended to 28 days. These trends suggest that sulfadoxine-pyrimethamine efficacy may before long fall to unacceptable levels in Malawi, and the impact of increasing parasitological failure rates on anaemia is also of concern.
The stable clinical efficacy of sulfadoxine-pyrimethamine in Malawi does not warrant complacency about seeking alternative treatments for its replacement. Several antimalarial combination drug regimens are being evaluated, but obstacles to implementing these regimens13,14 include cost, toxicity, and compliance problems. We have recently found that after cessation of use of chloroquine in Malawi, the prevalence of P falciparum isolates bearing mutations that cause chloroquine resistance has decreased to undetectable levels in this country while remaining over 90% in nearby countries.9 Studies to evaluate the efficacy of chloroquine alone and in combination with other drugs in this setting are planned.
What is already known on this topic
Sulfadoxine-pyrimethamine has had a short useful therapeutic life where it has been used as the first line treatment for malaria in South America and Southeast Asia
Malawi was the first African country to use sulfadoxine-pyrimethamine on a national basis in 1993
Experts predicted that sulfadoxine-pyrimethamine would fail in Africa in as little as five years
What this study adds
The therapeutic efficacy of sulfadoxine-pyrimethamine was stable from 1998 through 2002, indicating a longer than predicted useful therapeutic life
Rates of parasite clearance declined during the study period, presaging a decline in efficacy and highlighting the need for new, effective treatments for malaria
Countries still using chloroquine despite high resistance but where sulfadoxine-pyrimethamine remains efficacious should consider using sulfadoxine-pyrimethamine as an interim measure
Sulfadoxine-pyrimethamine while awaiting combination therapies
Several African countries continue to use chloroquine despite very high rates of resistance, fearing that sulfadoxine-pyrimethamine would fail quickly. On the basis of Malawi's experience, countries with similar levels of malaria endemicity where this drug is currently efficacious could consider replacing chloroquine with sulfadoxine-pyrimethamine as an interim measure. Sulfadoxine-pyrimethamine as first line treatment could be implemented immediately and would provide effective treatment while better alternatives (for example, combination chemotherapy) are being developed and evaluated. Parts of Africa where rates of transmission and immunity are lower, such as the KwaZulu-Natal region of South Africa, where sulfadoxine-pyrimethamine was first instituted in 1998 and where resistance arose quickly,15,16 may be more similar to Southeast Asia and South America with regard to patterns of development of antifolate resistance. Regional studies of drug efficacy, and not predictions based on experiences in very different epidemiological settings, should form the basis of rational antimalarial drug policy.
We thank the clinical officers and district health officers of the Ndirande Health Centre for sharing their facilities and allowing us to recruit participants from their patient population; the clinical and laboratory staff of the Blantyre Malaria Project and Malawi-Liverpool Wellcome Trust Programme for assisting with the study; Steven Wasserman for statistical assistance; and Alassane Dicko for critical reading of the manuscript.
Contributors: CVP designed the study and supervised its conduct, cleaned and analysed the data, and drafted the manuscript. JGK supervised the study onsite for part of the study period and assisted with data analysis and manuscript preparation. FKD oversaw the study in Malawi and assisted with data cleaning and analysis and manuscript preparation. DSK and RAGM helped to supervise the study in Malawi and assisted with data analysis and manuscript preparation. PC assisted with study design and led the clinical team throughout the course of the study. MEM assisted with supervision and oversight of the study, supervised medical care of study participants, and contributed to preparation of the manuscript. TET assisted with designing the study, supervised and oversaw the study, supervised medical care of study participants, and participated in data analysis and manuscript preparation. CVP is the guarantor.
Funding: This study was supported by the National Institute of Allergy and Infectious Diseases, USA (grants no. R29 AI-40539 and R01-AI-44824).
Competing interests: None declared.
Ethical approval: The study protocol was reviewed and approved by institutional review boards at the College of Medicine, University of Malawi, and the University of Maryland, Baltimore.
References
- 1.Bloland PB, Lackritz EM, Kazembe PN, Were JB, Steketee R, Campbell CC. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J Infect Dis 1993;167: 932-7. [DOI] [PubMed] [Google Scholar]
- 2.Nzila AM, Nduati E, Mberu EK, Hopkins SC, Monks SA, Winstanley PA, et al. Molecular evidence of greater selective pressure for drug resistance exerted by the long-acting antifolate pyrimethamine/sulfadoxine compared with the shorter-acting chlorproguanil/dapsone on Kenyan Plasmodium falciparum. J Infect Dis 2000;181: 2023-8. [DOI] [PubMed] [Google Scholar]
- 3.Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria in areas with intense transmission. Geneva: World Health Organization, Division of Control of Tropical Diseases, 1996.
- 4.Chemotherapy of malaria and resistance to antimalarials. Geneva: World Health Organization, 1973. (WHO technical report series 529.) [PubMed]
- 5.Plowe CV, Doumbo OK, Djimde A, Kayentao K, Diourte Y, Doumbo SN, et al. Chloroquine treatment of uncomplicated Plasmodium falciparum malaria in Mali: parasitologic resistance versus therapeutic efficacy. Am J Trop Med Hyg 2001;64: 242-6. [DOI] [PubMed] [Google Scholar]
- 6.Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA 1990;87: 3018-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triglia T, Menting JGT, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci USA 1997;94: 13944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis 2002;185: 380-8. [DOI] [PubMed] [Google Scholar]
- 9.Kublin JG, Cortese JF, Njunju EM, Mukadam RAG, Wirima JJ, Kazembe PN, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria following cessation of chloroquine use in Malawi. J Infect Dis 2003;187: 1870-5. [DOI] [PubMed] [Google Scholar]
- 10.Plowe CV, Kublin JG, Doumbo OK. P. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: epidemiology and role in clinical resistance to antifolates. Drug Resist Update 1998;1: 389-96. [DOI] [PubMed] [Google Scholar]
- 11.Cortese JF, Plowe CV. Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutants expressed in yeast. Mol Biochem Parasitol 1998;94: 205-14. [DOI] [PubMed] [Google Scholar]
- 12.Sirawaraporn W, Sathitkul T, Sirawaraporn R, Yuthavong Y, Santi DV. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc Natl Acad Sci USA 1997;94: 1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloland PB, Ettling M, Meek S. Combination therapy for malaria in Africa: hype or hope? Bull World Health Organ 2000;78: 1378-88. [PMC free article] [PubMed] [Google Scholar]
- 14.Bloland PB. A contrarian view of malaria therapy policy in Africa. Am J Trop Med Hyg 2003;68: 125-6. [PubMed] [Google Scholar]
- 15.Bredenkamp BL, Sharp BL, Mthembu SD, Durrheim DN, Barnes KI. Failure of sulphadoxine-pyrimethamine in treating Plasmodium falciparum malaria in KwaZulu-Natal. S Afr Med J 2001;91: 970-2. [PubMed] [Google Scholar]
- 16.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, et al. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet 2003;361: 1174-81. [DOI] [PubMed] [Google Scholar]