Abstract
Objective
To investigate the long-term patient outcomes following tumor debulking for internal auditory canal facial schwannoma (FNS).
Study Design
retrospective case review
Setting
Tertiary referral center
Patients
Patients operated on between 1998–2010 for a preoperative diagnosis of vestibular schwannoma with the intraoperative identification FNS instead.
Intervention
diagnostic and therapeutic
Main Outcome Measures
House-Brackmann facial nerve score immediately and at long term follow up (>1 yr); recurrence of tumor.
Results
16 patients were identified who were presumed to have vestibular schwannoma but intraoperatively were diagnosed with facial nerve schwannoma. Eleven underwent debulking surgery (67%–99% tumor removal), 2 underwent decompression only, 2 were diagnosed with nervus intermedius tumors and had total tumor removal with preservation of the motor branch of CN VII, 1 had complete tumor removal with facial nerve grafting. Five of 11 debulking patients underwent the MCF approach for tumor removal; the remainder had translabyrinthine resections. One debulking patient was lost to follow-up. Nine of 10 patients with long term follow up had H/B grade I or II facial function. One patient had recurrence of the tumor that required revision surgery with total removal and facial nerve grafting.
Conclusions
Tumor debulking for FNS provides an opportunity for tumor removal and excellent facial nerve function. Continuous facial nerve monitoring is vital for successful debulking surgery. FNS debulking is feasible via the MCF approach. Serial postoperative imaging is warranted to monitor for recurrence.
Keywords: facial nerve schwannoma, internal auditory canal, middle cranial fossa, translabyrinth, debulking, surgical decision making
Introduction
Facial nerve schwannoma (FNS) of the internal auditory canal (IAC) and cerebropontine angle (CPA) is reported in the literature to represent <2% of all FNS. When isolated to this region of the facial nerve, patients present clinically and radiographically with a tumor that mimics a vestibular schwannoma (VS). The classic symptom cluster of VS, unilateral tinnitus, unilateral sensorineural hearing loss and occasional imbalance, is also seen in IAC/CPA FNS patients. Facial nerve symptoms of weakness or twitching are unusual in VS. Intratemporal FNS most commonly involve multiple segments of the facial nerve and appear as “beads on a string” on MRI imaging. Many patients present with facial nerve symptoms of twitching or weakness. Patients with these symptoms and signs are presumed to have FNS and are generally observed until their facial nerve function declines to or beyond a House-Brackmann grade III1. Without facial nerve symptoms or radiographic involvement of the labyrinthine, geniculate ganglion or tympanic segments, differentiating FNS from VS on imaging is difficult. Thus, patients are not typically diagnosed until the tumor is identified arising from the facial nerve at the time of operation.
FNS that mimic VS present a unique therapeutic challenge. When they are encountered unexpectedly the surgeon can choose to do nothing further, widely decompress the facial nerve, debulk the tumor, or resect the facial nerve and reconstruct with a nerve graft. Nerve resection is particularly undesirable to a patient who presents with completely normal facial function preoperatively. Facial nerve decompression has also been advocated for patients with good (>HB III) function as a way to preserve function1. A small number of patients reported in the literature have undergone tumor debulking with anatomic preservation of the main trunk of the facial nerve2,3. In most cases this management strategy allows patients the benefit of near-total tumor removal with excellent postoperative facial nerve function.
This retrospective case series reviews the University of Iowa experience with VS-like FNS with regard to intraoperative management and long-term outcomes of these rare tumors.
Material and Methods
This review was conducted with approval from the University of Iowa Institutional Review Board (#201107762). All patients undergoing surgery with a diagnosis of benign neoplasm of the cranial nerves (ICD-9 225.1), intradural tumor removal (CPT 61616) and/or facial nerve surgery (CPT 69955) between 1998 and 2010 were reviewed. This included patients undergoing surgery for VS, known FNS and FN decompression surgery. This does not include patients with VS or FNS who elected for observation of their tumor with serial imaging.
Patients eligible for inclusion in the study presented with an IAC lesion presumed to be VS but subsequently identified as FNS at the time of operation. Patients were excluded if they had a preoperative diagnosis of FNS, facial nerve hemangioma, or intraoperative diagnosis was other than that of VS or FNS (i.e., lipoma). Patients were excluded if they had a diagnosis of neurofibromatosis type 2. Records were reviewed for the following: presenting symptoms, side of the lesion, preoperative facial function, radiographic tumor dimensions, type of procedure performed, intraoperative findings, immediate postoperative facial function, long term facial nerve function, tumor recurrence, and hearing outcomes for those undergoing MCF approach. Facial movement was assessed using the House-Brackmann (HB) grading scale 4.
Results
During the 12 year study period a total of 543 patients underwent surgery for VS via any approach. Of those, 352 records included coding for intradural tumor removal and facial nerve surgery; these records were reviewed, and 16 patients were identified who fulfilled the inclusion criteria. Thus, VS-like FNS presented intraoperatively in 2.9% of patients who underwent surgery for their IAC/CPA lesion. There were 10 females (63%) and the average age at presentation was 50.1 years of age (range 36–59 years old). The right side was involved in 11/16 patients (69%).
Presenting Symptoms
The primary symptoms for patients were audiologic: 15 reported unilateral hearing loss, 11 reported unilateral tinnitus, 11 reported vertigo or imbalance, and 1 reported hemifacial paresthesias. Only one patient reported slight blepharospasm as part of their symptoms cluster (Table I). One patient had long standing hearing loss; he lost hearing in the ipsilateral ear following a motor vehicle collision as a teen.
Table I.
Primary complaints of Patients
| Complaint | No. of Patients (%) |
|---|---|
| Unilateral hearing loss | 15 (94%) |
| Unilateral tinnitus | 11 (69%) |
| Imbalance/vertigo | 11 (69%) |
| Facial paresthesia/anesthesia | 1 (6%) |
| Facial twitching/weakness | 1 (6%) |
Radiographic Findings
No patient had radiographic evidence of tumor involvement outside of the posterior fossa. Eight patients (50%) had tumor limited to the internal auditory canal (Figure 1). These IAC lesions ranged in size from 3 by 5 mm up to 11 by 12 mm. The remaining 9 tumors all extended into the CPA (1 mm to 4.3 cm in largest dimension).
Figure 1.
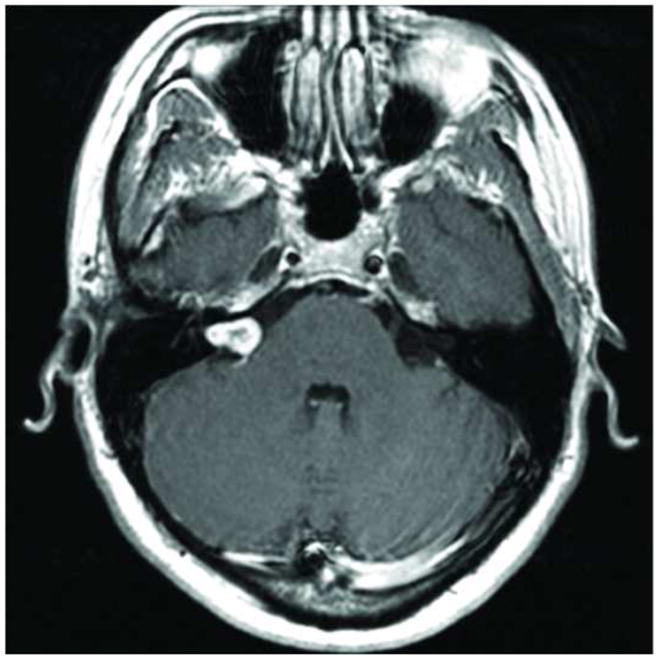
Axial MRI post contrast demonstrating intracanalicular facial nerve schwannoma. Note that no other segment of the facial nerve is radiographically involved with tumor.
Surgical Findings
Patients underwent either a translabyrinthine (TL) or middle cranial fossa (MCF) approach to the internal auditory canal. Patients were offered a MCF approach if the tumor did not impact the brainstem and they had serviceable hearing (AAO-HNS class A-C). Eight patients underwent a MCF approach, 7 underwent a translabyrinthine approach and one patient had a transcochlear approach.
At the time of operation 3 patients (19%) were found to have tumor involvement of multiple segments of the facial nerve. The remaining 13 patients had involvement limited to the IAC/CPA segment of the nerve (Table II). In 2 (12%) of these 13 patients, the tumor was felt to arise from the nervus intermedius.
Table II.
Summary table of patient demographic data, surgical information and follow up data.
| Patient | Age at Surgery/Gender | Symptoms | Side | HB Pre op | Dimensions | Procedure | Operative Findings | HB Immediate Post op | HB Last exam (duration) | Pre op PTA (dB)/WRS(%) | Post op PTA (dB)/WRS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 36/M | HL | R | I | 21 mm CPA | TL | Multisegment involvement; DB | III | II (8 yrs) | ||
| 2 | 53/F | T, HL, hyperacucsis, IB | R | I | 10×4×4 mm IAC | MCF | DB | III–IV | II(2 mo) | 10/92 | 25/100 |
| 3 | 50/F | HL, T, V | L | I | 16×18 mm IAC/CPA | TL | DB | VI | II(4 yrs) | ||
| 4 | 42/F | T, fluctuating HL, FT | R | I | 7×11 mm IAC | MCF | DB | IV | Lost to F/U | 43/60 | NA |
| 5 | 46/M | IB | R | I | 6×4 mm IAC | TL | DB (80%) | II | I (1 yr) | ||
| 6 | 59/M | IB | R | I | 13×7 mm IAC | MCF | DB | I | I (1 yr) | 14/96 | 40/100 |
| 7 | 50/M | HL, T | R | I | 4×5×3 mm IAC | MCF | NI; CR | III | II (3 mo) | 26/92 | 26/96 |
| 8 | 53/F | HL, T, IB | R | I | 5 mm IAC | MCF | NI; CR | II | I (2 yrs) | 13/96 | 16/92 |
| 9 | 54M | HL | L | I | 22×20 mm CPA | TL | DB | VI | III–IV (2 yrs) | ||
| 10 | 52/F | HL, T, IB | R | I | 11.5×12.3 mm IAC | MCF | Mulitsegment involvement; decompression | I | I (2 yrs) | ||
| 11 | 52/F | V, T, HL | L | I | 14.8×9.2×7.7 mm IAC/CPA | TL | DB | II | I (2 yrs) | ||
| 12 | 57/F | Facial numbness, IB, HL | L | I | 43 mm cystic CPA | TC, NG | Multisegment involvement; CR incld FN | VI | Grade C (2 yrs) | ||
| 13 | 41/M | HL, T | L | I | 8×6×5 mm IAC | MCF | DB | II | I (2 yrs) | 36/88 | 33/80 |
| 14 | 58/M | HL | R | I | 17 mm CPA | TL | Decompression | I | I (2 yrs) | ||
| 15 | 48/F | Aural fullness, HL, V, T | R | I | 10×16 mm IAC | MCF | DB | III | I (1 yr) | 15/96 | 35/100 |
| 16* | 47;54/F | IB, HL, T, facial pain; facial weakness | R | I;II | 23 mm CPA; 22×35×19 mm CPA | TL; TL, NG | DB; CR incld FN | I;VI | I (8 yrs); Grade C (2 yrs) |
- Pt 16 experienced recurrence of her tumor. Data for the initial presentation is separated from the recurrence data by a “;”.
HL = hearing loss, T= tinnitus, IB=imbalance, V=vertigo, FT=facial twitch, DB=debulking, NI=nervus intermedius, CR=complete resection, NG=nerve graft
Decompression
Two patients had wide decompression of the facial nerve and tumor (1 MCF, 1 TL), including the IAC, labyrinthine, geniculate and the proximal tympanic segments. Both of these patients had normal facial nerve function immediately postoperatively and at 2 year follow up.
Complete resection
One patient underwent complete resection of the tumor and facial nerve with cable grafting. She presented with a 4.3 cm cystic mass in the CPA. During the tumor dissection the 8th nerve complex could be readily removed from the surface of the tumor but the 7th nerve could not be located along the surface of the tumor. During dissection facial nerve stimulation was lost and the decision was made to graft the nerve from near the root entry zone to the tympanic segment. Following grafting she achieved eye closure and some movement of the lower division (Grade C5).
Nervus Intermedius
Two patients who were felt to have nervus intermedius tumors had complete tumor excision with preservation of the motor branch of the facial nerve. Both of these patients underwent MCF approaches. Post operatively, one patient developed a delayed facial paresis (HB III) that improved to HB II by 3 months; the patient was then lost to follow up. The other patient was mildly paretic (HB II) immediately postoperatively but had regained normal facial function on long term follow up (2 years). Both patients retained their hearing at their preoperative levels.
Tumor Debulking
Eleven patients underwent tumor debulking with preservation of the main trunk of the facial nerve. The debulking procedure involves meticulous microdissection between the nerve fibers and the tumor under high magnification. Continuous facial nerve monitoring is imperative. Short bursts of activity may occur but dissection is halted if fibrillation potentials are produced. The amount of tumor removed can then be estimated. Nine of the 11 patients had >95% of the tumor removed in this fashion. One patient had 80% removed and the remaining patient had 66% removed. Five underwent MCF debulking and 6 underwent translabyrinthine surgery.
MCF Debulking
Of those 5 patients undergoing MCF debulking, 1 patient was HB III, 3 were HB grade II and 1 was HB grade I in the immediate postoperative period. One patient did progress to HB IV at the one month follow up visit. One patient was lost to long term follow up. One patient was HB II with follow up for 4 years. The other 3 patients were all HB I with at least one year follow up. With regard to hearing preservation, all patients undergoing MCF maintained their word recognition scores (80–100% WRS scores) (one patient was lost to follow up for audiometric data). One patient also maintained his pure tone averages. The remaining 3 patients demonstrated mild to moderate declines in the PTA on long term follow up, raging from −10 to −25 dB.
Translabyrinthine Debulking
Debulking proceeded via the translabyrinthine approach for 6 patients. In the immediate postoperative period, 2 patients developed complete paralysis, one patient had grade III, 2 patients were grade II and one had normal facial function. At long term follow up, no patient was worse than grade III (one patient). Three patients had normal facial function and 2 had mild asymmetry with maximal exertion (grade II). Table III summarizes the data for patients who underwent debulking operations.
Table III.
Summary of facial nerve outcomes for immediate postoperative and function at last visit for patients undergoing debulking operations.
| HB Grade | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Immediate | MCF - 1 TL - 1 |
MCF - 1 TL – 2 |
MCF – 2 TL - 1 |
MCF – 1 TL - 0 |
MCF – 0 TL - 2 |
|
| Long-term | MCF - 3 TL - 3 |
MCF - 1 TL - 2 |
MCF – 0 TL – 1 |
Tumor Recurrence
One patient developed a recurrence of the tumor 8 years after the initial translabyrinthine resection. At the initial operation, she had a 23 mm tumor in the CPA and postoperatively she had normal facial function (grade I). At the time of recurrence she noted mild weakness around the eye and on imaging was noted to have a 22 mm × 35 mm lesion in the CPA. She underwent revision TL with complete tumor removal and greater auricular nerve grafting. Two years after the graft she had movement in the obicularis oculi and obicularis oris and eye closure (grade C5). Patient data are summarized in Table II.
Discussion
Facial nerve schwannoma FNS mimicking VS are rare tumors. The goals of debulking surgery are to remove tumor and preserve excellent facial nerve function. These objectives are feasible with meticulous microsurgical technique assisted by continuous electromyographic facial nerve monitoring. Debulking can successfully proceed via either a MCF or TL approach.
This series presents sixteen new patients with FNS isolated to the IAC and CPA. These 16 patients represent 2.9% of IAC lesions undergoing surgery at a high volume tertiary referral center. While uncommon, FNS must be considered in the differential diagnosis for any isolated IAC mass. As with VS, these patients had audiologic and vestibular symptoms. Mild facial nerve symptoms occurred in only one patient. Occasional facial nerve symptoms are seen with VS as well and as such facial nerve symptoms are not necessarily diagnostic of FNS.
Patients should be counseled regarding the possibility of finding a facial nerve tumor and the surgeon’s preference for intraoperative management in such a case. The authors currently counsel patients that should a FNS be identified at the time of operation, tumor debulking proceeds until any fibrillation potentials are encountered. The authors’ preference is to leave tumor on a functional facial nerve.
The Nashville group reported another large series of patients with VS-like FNS2. In their series of 12 patients, all presented with sensorineural hearing loss and half had unilateral tinnitus. A minority had vestibular complaints (33%). Preoperatively, none of the patients had any facial nerve motor symptoms but a third reported taste change. Five of the 12 patients had an anatomically intact facial nerve following surgery. One patient achieved a HB grade II, 3 patients were HB grade III and 1 was HB grade V.
Pulec reported 2 facial nerve tumors on which he performed partial nerve resection in 19696. This was followed by a larger series of FNS patients in 1972. Two patients in the later series also underwent partial resection. One of these patients had good facial nerve function post operatively, but the other required subsequent hypoglossal – facial anastamosis 7. Neither of these patients had tumor limited to the IAC.
Several other authors have presented cases series of patients undergoing FNS debulking. Perez et al presented 7 patients who had undergone FNS debulking. All of the patients in that series had multisegment involvement. All patients maintained or improved facial nerve function following debulking 8. Nadeau et al presented 3 patients who underwent debulking operations for FNS isolated to the posterior cranial fossa. Two of these had HB grade II function at follow up and one had a grade V facial palsy. There are scattered other reports involving single patient reports, generally with good facial nerve outcomes (better or equal to HB III) (Table IV2,3,8–16).
Table IV.
Literature Assessment of FNS Debulking outcomes as assessed by the House-Brackmann Facial Nerve Grading Scale at follow-up.
| Authors (no. of patients)ref# | HB I | HB II | HB III | HB IV | HB V | HB VI |
|---|---|---|---|---|---|---|
| Present series (10) | 6 | 3 | 1 | |||
| McMenomey et al. (5)2 | 1 | 3 | 1 | |||
| Sherman et al. (5)3 | 4 | 1 | ||||
| Perez et al. (7)8 | 2 | 1 | 4* | |||
| Nadeau et al. (3)9 | 2 | 1 | ||||
| Lee et al. (1)10 | 1 | |||||
| Saada et al. (1)11 | 1 | |||||
| Fenton (1)†12 | 1 | 1 | ||||
| Park (1)13 | 1 | |||||
| Bian (1)14 | 1 | |||||
| Kubota (2)15 | 1 | 1 | ||||
| Kohmura (1)16 | 1 | |||||
| Total (39) | 14 | 12 | 10 | 0 | 2 | 1 |
Two pt presented with grade III paresis and two improved from grade IV paresis
One patient who had bilateral facial nerve schwannoma thus 2 outcomes are reported.
Use of the MCF approach to the IAC segment of the facial nerve was first described by House and Crabtree in 1965 17. Use of the approach for removal of FNS was suggested by Pulec 7. He used the MCF approach combined with the transmastoid approach for many of the patients in his original series. Other series have reported the combined approach as well 3,8,12. However, MCF alone for tumor debulking has been sparsely reported in the literature 3,12. Sherman et al reported on a series of patients in which only one patient had a subtemporal approach for tumor debulking. Postoperatively the patient had HB grade I facial function. Unfortunately, the tumor demonstrated regrowth at 37 months 3. The relative rarity in the literature of the MCF procedure for FNS is not surprising as many clinicians recommend observation or radiation for intracanalicular VS.
The authors advocate for early surgical intervention for small VS as a means for possible hearing preservation. At our institution, hearing preservation surgery is preformed primarily via the MCF approach. It is possible some of these patients would go on to develop clinical or radiographic signs of FNS if they were observed, as is the practice for many intracanalicular VS at other institutions. However five of the tumors in this series had CPA components that were greater than 20 mm in largest dimension. These particular tumors demonstrate that FNS can grow to large dimensions without involvement of other segments of the facial nerve and without any facial nerve symptoms.
Identification of the nerve of origin for a schwannoma in the IAC/CPA intraoperatively requires a high index of suspicion. In all of the tumors in this report the facial nerve could not be separated from the tumor. Furthermore the superior and inferior vestibular nerves (in the case of small tumors) or the 8th nerve complex (for larger tumors) could be readily separated from the surface of the tumor. However, it is possible that several of the tumors presented here were VSs with significant adhesion to the facial nerve and thus true VSs.
The MCF approach has the benefit of possible hearing preservation as well as tumor debulking. In this series, all patients undergoing this procedure maintained their preoperative word recognition scores of between 80–100%. The pure tone averages did decline for 3 of 4 patients; however, all patients retained serviceable hearing. By comparison, for patients with >70% word recognition and intracanalicular VS of less than 1 cm in greatest dimension hearing preservation rates are 75% at our institution 18.
If the patient has opted for an operation and a FNS is identified the surgeon has several options: decompression, debulking or facial nerve resection with nerve grafting. Decompression is a reasonable option in the setting of normal preoperative facial function. Two patients in this series underwent decompression and retained normal facial nerve function (Table II). A recent review of FNS management by Wilkinson et. al. compared decompression to observation (all patients with preoperatively identified FNS). The authors did not find any difference in long term facial nerve function between the two groups 1. Certainly, FN decompression is a viable option when a FNS is identified preoperatively. The benefit of this approach is that the patient will most likely retain their preoperative facial nerve functional level. Conversely, decompression alone does not treat the underlying tumor.
Debulking is another option for IAC FNS. In approximately 75% of cases the tumor pushes the nerve trunk to the periphery 19. In such cases the majority of the tumor may be removed without disrupting the nerve fibers. However, in nearly a quarter of tumors, the nerve fibers are found throughout the substance of the tumor. Understanding the various microstructures of FNS and use of the intraoperative facial nerve monitor can assist the surgeon in intraoperative decision-making. Early defibrillation activity on the facial nerve monitor may suggest that the nerve fibers that are scattered throughout the tumor and thus not suitable for debulking. Assuming the debulking begins away from the nerve fibers in tumors with peripherally located nerve fibers, this anatomic situation is more likely to be amenable to debulking.
Debulking does risk the possibility of post-operative facial nerve weakness. However, nine of the eleven patients who underwent debulking surgery had HB grade I or II facial function at follow up. Of these nine, six were grade I. In VS surgery, House-Brackmann grade I or II is considered an acceptable outcome. Patients should be counseled that if a FNS is identified, debulking does risk some decline in function; however a high percentage of patients undergoing debulking can expect excellent facial nerve function in the long term.
By its very nature debulking surgery leaves tumor on the facial nerve. Serial post-operative MRI is therefore warranted to assess for tumor regrowth. One patient in the present series developed tumor regrowth after 8 years. Her only symptom was mild weakness of the ipsilateral obicularis oculi. She had a large recurrence in the CPA and underwent complete facial nerve excision with grafting. Sherman et al also reported one patient who developed tumor regrowth 37 months after debulking surgery. Due the possibility of recurrence, imaging is recommended at least every other year.
Conclusion
The surgeon may be faced with a difficult management decision when a FNS is encountered during surgery for a presumed VS. Debulking of the tumor with guidance from intraoperative nerve monitoring is a treatment option that provides patients with the opportunity for tumor removal while maintaining good facial nerve function. As in VSs, hearing preservation is possible for those patients who undergo the MCF approach to the FNS. As with any rare lesion, a high index of suspicion is required in order to appropriately diagnose and manage these lesions.
Footnotes
Presented at the American Neurotology Society, Facial Nerve Study Section, September 10, 2011, San Francisco, California, USA.
The Authors have no disclosures or funding relevant to this project.
References
- 1.Wilkinson EP, Hoa M, Slattery WH, et al. Evolution in the managment of facial nerve schwannoma. The Laryngoscope. 2011;121:2065–2074. doi: 10.1002/lary.22141. [DOI] [PubMed] [Google Scholar]
- 2.McMenomey SO, Glasscock ME, Minor LB, Jackson CG, Strasnick B. Facial nerve neuromas presenting as acoustic tumors. Am J Otol. 1994;15(3):307–312. [PubMed] [Google Scholar]
- 3.Sherman JD, Dagnew E, Pensak M, Loveren HRv, Tew JM. Facial nerve neuromas: report of 10 cases and review of the literature. Neurosurgery. 2002;50(3):450–456. doi: 10.1097/00006123-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 4.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93(2):146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 5.Gidley P, Gantz BJ, Rubinstein JT. Facial nerve grafts: from the cerebropontine angle and beyond. Am J Otol. 1999;20(6):781–788. [PubMed] [Google Scholar]
- 6.Pulec JL. Facial Nerve Tumors. Ann Otol Rhinol Laryngol. 1969;78(5):962–982. doi: 10.1177/000348946907800505. [DOI] [PubMed] [Google Scholar]
- 7.Pulec JL. Symposium on Ear Surgery. II. Facial Nerve Neuroma. The Laryngoscope. 1972;82(7):1160–1176. doi: 10.1288/00005537-197207000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Perez R, Chen JM, Nedzelski JM. Intratemporal facial nerve schwannoma: a managment dilemma. Otology & Neurotology. 2005;26:121–126. doi: 10.1097/00129492-200501000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Nadeau DP, sataloff RT. Fascicle preservation surgery for facial nerve neuromas involving the posterior cranial fossa. Otology & Neurotology. 2003;24:317–325. doi: 10.1097/00129492-200303000-00031. [DOI] [PubMed] [Google Scholar]
- 10.Lee JD, Kim SH, Song MH, Lee H-K, Lee W-S. Managment of facial nerve schwannoma in patiens with favorable facial function. The Laryngoscope. 2007;117:1063–1068. doi: 10.1097/MLG.0b013e31804b1a51. [DOI] [PubMed] [Google Scholar]
- 11.Saada AA, Limb CJ, Long DM, Niparko JK. Intracanalicular schwannoma of the facial nerve. Arch Otolaryngol Head Neck Surg. 2000;126:547–549. doi: 10.1001/archotol.126.4.547. [DOI] [PubMed] [Google Scholar]
- 12.Fenton JE, Morrin MM, Smail M, Sterkers O, Sterkers JM. Bilateral facial nerve schwannomas. Eur Arch Otorhinolaryngol. 1999;256:133–135. doi: 10.1007/s004050050125. [DOI] [PubMed] [Google Scholar]
- 13.Park HY, Kim SH, Son EJ, Lee H-K, Lee W-S. Intracanilicular facial nerve schwannoma. Otology & Neurotology. 2007;28(3):376–380. doi: 10.1097/01.mao.0000265191.24131.ed. [DOI] [PubMed] [Google Scholar]
- 14.Bian LG, Sun QF, Tirakotai W, Zhao WG, Bertalanffy H, Shen JK. Surgical management of a PICA aneurysm and incidental facial nerve schwannoma: case report. Skull Base. 2007;17(2):145–151. doi: 10.1055/s-2006-953515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota Y, Kawanata T, Kubo O, Kasuya H, Muragaki Y, Hori T. Large facial nerve schwannomas without facial palsy: case reports and review of the literature. Neurosurg Rev. 2005;28(3):234–238. doi: 10.1007/s10143-005-0381-x. [DOI] [PubMed] [Google Scholar]
- 16.Kohmura E, Aihara H, Miyake S, Fujita A. Intradural facial nerve schwannoma: diagnostic and therapeutic problems. Skull Base. 2007;17(3):215–222. doi: 10.1055/s-2007-977463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.House W, Crabtree JA. Surgical exposure of the petrous portion of the 7th cranial nerve. Arch Otolaryngol. 1965;81:506–507. doi: 10.1001/archotol.1965.00750050519016. [DOI] [PubMed] [Google Scholar]
- 18.Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otology & Neurotology. 2006;27:380–392. doi: 10.1097/00129492-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Hajjaj M, FHL Facial nerve schwannoma: nerve fibre dissemination. J Laryngol Otol. 1996;110(7):632–633. doi: 10.1017/s0022215100134474. [DOI] [PubMed] [Google Scholar]


