Abstract
Aims
Cross-sectional evidence indicates that abdominal adiposity, hypertension, dyslipidemia and glycemia are associated with reduced metabolic clearance of insulin (MCRI). Little is known about the progression of MCRI and whether components of metabolic syndrome are associated with the change in MCRI. In this study, we examined the association between components of metabolic syndrome and the 5-year change of MCRI.
Methods and Materials
At baseline and 5-year follow-up, we measured fasting plasma triglycerides (TG), high density lipoprotein (HDL)-cholesterol, blood pressure (BP), waist circumference (WC) and fasting blood glucose (FBG) in 784 non-diabetic participants in the Insulin Resistance Atherosclerosis Study. MCRI, insulin sensitivity (SI) and acute insulin response (AIR) were determined from frequently sampled intravenous glucose tolerance tests.
Results
We observed a 29% decline of MCRI at follow-up. TG, systolic BP and WC at baseline were inversely associated with a decline of MCRI regression models adjusted for age, sex, ethnicity, smoking, alcohol consumption, energy expenditure, family history of diabetes, BMI, SI and AIR (β= −0.057 [95% CI −0.11, −0.0084] for TG, β= −0.0019 [95% CI −0.0035, −0.00023] for systolic BP, β= −0.0084 [95% CI −0.013, −0.0039] for WC; all p<0.05). Higher HDL-cholesterol at baseline was associated with an increase in MCRI (multivariable-adjusted β= 0.0029 [95% CI 0.0010, 0.0048], p=0.002). FBG at baseline was not associated with MCRI at follow-up (multivariable-adjusted β= 0.0014 [95% CI −0.0026, 0.0029]).
Conclusions
MCRI declined progressively over 5 years in a non-diabetic cohort. Components of metabolic syndrome at baseline were associated with a significant change in MCRI.
Introduction
Type 2 diabetes is reaching epidemic proportions, thus there is an urgent need for a better understanding of pathophysiological mechanisms underlying diabetes to prevent its onset and slow its progression. It is well-established that insulin resistance and pancreatic beta-cell dysfunction are critical metabolic disorders underlying the development of type 2 diabetes [1], although less is known regarding insulin clearance. Insulin clearance is a dynamic physiological process in insulin metabolism in which a large proportion of insulin is removed from the circulation after secretion, mostly by the liver and the reminder by the kidney and other tissues [2–3]. It is reduced during the emergence of insulin resistance as a compensatory mechanism to preserve beta-cell function in pre-diabetes states [4–6]. Despite its importance in the metabolism of insulin and its potential role in the etiology of diabetes, few studies had examined the dynamic change of insulin clearance over time.
Diabetes is closely related to a constellation of metabolic factors, including abdominal adiposity, hypertension, hyperglycemia and dyslipidemia. The clustering of these risk factors, a phenomenon referred to as metabolic syndrome, raises the risk of cardiovascular disease [7–8]. Clinically, metabolic syndrome is manifested as high blood pressure (BP), large waist circumference (WC), low plasma high-density-lipoprotein (HDL) cholesterol, high plasma triglycerides (TG) and high fasting blood glucose (FBG) [9]. Previous cross-sectional studies showed that insulin clearance is decreased in individuals with abdominal adiposity [10–11] or hypertension [12–13]. However, to our knowledge, no studies have investigated the prospective association between components of metabolic syndrome and change in metabolic clearance rate of insulin (MCRI). In this study, we estimated insulin clearance using MCRI which is derived from the frequently sampled intravenous glucose tolerance tests (FSIGTT) [14], and aimed to describe the longitudinal progression of MCRI, as well as to explore the association between components of metabolic syndrome (systolic BP, WC, plasma HDL-cholesterol, plasma TG, FBG) at baseline and the difference in MCRI between baseline and 5-year follow-up examinations in the Insulin Resistance Atherosclerosis Study (IRAS).
Methods and Materials
The IRAS is a multicenter epidemiologic study designed to explore the relationships between insulin resistance, cardiovascular disease and its known risk factors among non-Hispanic whites, Hispanics and African-Americans with varying states of glucose tolerance. Details of the study population and methods have been described [15]. The institutional review boards at each study site approved the study protocol and all participants provided written informed consent. The IRAS recruited 1,625 participants from four clinical centers located in San Antonio, TX, San Luis Valley, CO, Oakland, CA and Los Angeles, CA between October 1992 and April 1994. Participants were followed for an average of 5.2 years (range 4.5–6.6 years). After excluding individuals with prevalent diabetes (n=537) and those with missing data on MCRI from either baseline or follow-up examinations due to loss to follow-up (n=191) or technical issues (n=113), we included 784 non-diabetic participants in the current report.
Examinations at both baseline and follow-up each constituted two visits, separated by one week. Participants were asked before each visit to fast for 12 hours, to abstain from alcohol and heavy exercise for 24 hours, and to abstain from smoking the morning of the examination. During the first visit, a 75-gram oral glucose tolerance test was administered. During the second visit, insulin sensitivity (SI), acute insulin response (AIR) and MCRI were determined from a FSIGTT, with two modifications to the original protocol. First, insulin, instead of tolbutamide, was injected to ensure adequate levels of plasma insulin to calculate insulin sensitivity accurately across a broad range of glucose tolerance. Second, a reduced sampling protocol, using 12 instead of 30 samples, was used because of the large number of participants. Insulin resistance, expressed as SI, was calculated using minimal model analysis. Insulin secretion was measured by AIR, defined as the average increase in plasma insulin at time points 2 and 4 minutes after infusing glucose [16]. MCRI was calculated as the ratio of the insulin dose over the incremental area under the curve of insulin from 20 minutes to infinity [14] using the following equation:
where Dose represents the amount of insulin injected at 20 min, Ins(t) the plasma insulin concentration in standard units at each FSIGTT sampling point and Ins(0) the fasting plasma insulin concentration determined before injecting glucose in the FSIGTT.
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as weight in kg divided by height in m2. WC was measured to the nearest 0.5 cm using a steel tape. All anthropometric measurements were taken in duplicate following standardized procedures and the averages of these measurements were used in the analyses. Resting systolic and diastolic BP was recorded using a standard mercury sphygmomanometer after a 5-minute rest. The average of the second and the third readings were used in the analyses. Information on demographics (age, sex, ethnicity), lifestyle factors (smoking, alcohol consumption, energy expenditure) and family history of diabetes were collected in validated questionnaires by self-report [15].
Participants provided a fasting blood draw at each examination. Plasma glucose was measured using the glucose oxidase technique on an auto-analyzer. Plasma lipids and lipoproteins, including TG and HDL-cholesterol were determined at the central IRAS laboratory using methods from the Lipid Research Clinics [17]. We defined metabolic syndrome using the IDF/AHA harmonized criteria [18].
Statistical analysis
We summarized the characteristics of participants at baseline and at 5-year follow-up using median and interquartile range for continuous variables and percentages for categorical variables. We tested the difference in MCRI between baseline to follow-up examinations using McNemar tests for categorical variables, paired t-tests for normally distributed continuous variables and Wilcoxon signed rank tests for non-normally distributed continuous variables. We stratified these descriptive statistics by sex, ethnicity, family history of diabetes and glycemic status at baseline, and tested the difference in the percentage of change in these subgroups using t-tests. We described the relationship between each component of metabolic syndrome and MCRI at baseline using Spearman’s rank correlation coefficient. We plotted the means and standard errors of MCRI at baseline by the number of the components of metabolic syndrome at baseline.
Since the distribution of TG, MCRI and AIR were skewed, we natural log-transformed them to achieve normality for subsequent analyses. Because of the presence of 0 values for SI, we added a constant of 1 to all values of SI before the log-transformation. We used unadjusted and multivariable-adjusted linear regression to explore the association between components of metabolic syndrome at baseline and change in MCRI over time. We modeled TG, HDL-cholesterol, FBG, systolic BP and WC at baseline as continuous exposures. In regression models, we modeled MCRI at follow-up as the continuous outcome, and then adjusted for MCRI at baseline to obtain the estimates for the difference of MCRI between two examinations [19]. We presented the regression coefficients (β) with their 95% confidence intervals (CI).
We included covariates in multivariable-adjusted models if they were associated with both the exposure and the outcome, or if they were of a priori clinical relevance. Potential confounders included age, sex, ethnicity, smoking, alcohol consumption, energy expenditure, family history of diabetes, BMI, SI, AIR and FBG (except in the model where FBG was the exposure). We examined the interaction of each of the components of metabolic syndrome at baseline with age, sex, ethnicity, BMI, glycemic status on the change in MCRI over time. In sensitivity analysis, we performed a backward stepwise regression analysis which included all components of metabolic syndrome in the model to determine which of these components was independently associated with the change in MCRI over time when modeled together. Statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX, USA).
Results
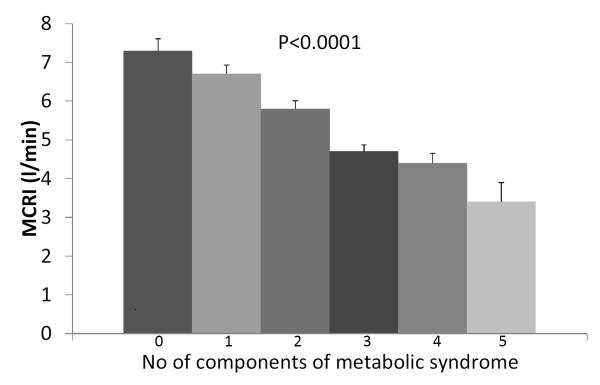
Of the 784 non-diabetic participants with data on MCRI at baseline and follow-up examinations, 315 (40%) were non-Hispanic whites, 199 (25%) were African-Americans and 270 (35%) were Hispanics. Among these participants, 45% were women and 60% reported to have family history of diabetes. At baseline, the median age of the study population was 54 years (range 40–69 years) and 33% had impaired glucose tolerance. Using the IDF/AHA harmonized criteria, 86% of the study population has at least one component of metabolic syndrome. The prevalence of metabolic syndrome was 29.6% for one component, 26.3% for two components, 18.0% for three components, 10.1% for four components and 1.7% for five components. The IRAS participants had lower MCRI at baseline as the number of components of metabolic syndrome increased (p<0.0001, Figure 1). Systolic BP, WC, FBG and TG were inversely correlated with MCRI, whereas HDL was positively correlated (all p<0.001) (Table 1).
Figure 1.
Means (standard errors) of MCRI, stratified by the number of components of metabolic syndrome at baseline
Table 1.
Spearman correlation analysis between components of metabolic syndrome and MCRI at baseline
| MCRI | p-value | |
|---|---|---|
| Systolic BP | −0.138 | 0.0001 |
| Waist circumference | −0.528 | <0.0001 |
| HDL-cholesterol | 0.305 | <0.0001 |
| Plasma triglycerides | −0.234 | <0.0001 |
| Fasting blood glucose | −0.214 | <0.0001 |
Participants had a significant decline in MCRI, SI, systolic BP and smoking, but an increase in BMI, WC, HDL-cholesterol, FBG and AIR at the 5-year follow-up examination (all p<0.0001). Plasma TG was not significantly different between baseline and follow-up examinations (Table 2). The magnitude of the decline in MCRI was similar between categories of sex, ethnicity and family history of diabetes. Despite having higher values of MCRI at baseline and at follow-up, participants with normal glucose tolerance had greater decline of MCRI over time than those with impaired glucose tolerance at baseline (Table 3).
Table 2.
Characteristics of 784 non-diabetic participants at baseline and at 5-year follow-up in the IRAS
| Baseline | Follow-up | Change | % change |
p-value | |
|---|---|---|---|---|---|
| MCRI (l/min) | 5.28 (3.92, 7.10) | 3.67 (2.87, 4.80) | −1.47 (−2.91, −0.34) | −29.4 | <0.0001 |
| BMI (kg/m2) | 27.3 (24.9, 30.5) | 28.1 (25.3, 31.5) | 0.72 (−0.36, 1.74) | 2.67 | <0.0001 |
| WC (cm) | 90.0 (81.3, 97.8) | 92.3 (83.9, 99.9) | 2.7 (−0.5, 5.9) | 3.10 | <0.0001 |
| Systolic BP (mmHg) | 121 (110, 133) | 118 (107, 130) | −2 (−8, 3) | −1.85 | <0.0001 |
| TG (mg/dl) | 111 (79, 159) | 112 (79, 162) | −1 (−26, 29) | −1.12 | 0.98 |
| HDL (mg/dl) | 44 (36, 55) | 46 (38, 58) | 2 (−3, 7) | 4.50 | <0.0001 |
| FBG (mg/dl) | 97 (91, 105) | 98 (91, 109) | 2.5 (−3.5, 8.8) | 2.35 | <0.0001 |
| AIR (μU/ml) | 51 (29, 86) | 65 (35, 105) | 10 (−8, 36) | 22.7 | <0.0001 |
| SI (×10−4 min−1 [μU/l]−1) | 1.64 (0.94, 2.94) | 1.02 (0.52, 1.73) | −0.6 (−1.43, 0) | −43.6 | <0.0001 |
| Current smoker (%) | 126 (14.0) | 91 (10.1) | −35 (−3.9) | −27.9 | <0.0001 |
| Energy expenditure (kcal/kg/day) |
38.5 (35.6, 43.3) | 37.1 (34.8, 41.8) | −0.68 (−3.96, 2.62) | −1.78 | 0.0006 |
Data presented are medians (interquartile range) or n (%)
Table 3.
Values of MCRI at baseline and at 5-year follow-up of 784 non-diabetic participants in the IRAS, stratified by sex, ethnicity, family history of diabetes and glycemic status
| MCRI (l/min) | Baseline | Follow-up | Change | %change | p-value |
|---|---|---|---|---|---|
| By sex | |||||
| Men | 5.16 (3.92, 7.11) | 3.52 (2.79, 4.60) | −1.52 (−3.17, −0.43) | −31.4 | <0.0001 |
| Women | 5.32 (3.92, 7.06) | 3.84 (2.95, 5.00) | −1.37 (−2.70, −0.31) | −27.7 | <0.0001 |
| *p=0.1 between groups | |||||
| By ethnicity | |||||
| Non-Hispanic whites |
5.73 (4.37, 7.61) | 4.05 (3.10, 5.32) | −1.55 (−3.21, −0.44) | −28.9 | <0.0001 |
| African-Americans | 5.20 (3.97, 6.51) | 3.39 (2.60, 4.26) | −1.61 (−2.98, −0.83) | −34.5 | <0.0001 |
| Hispanics | 4.81 (3.62, 6.56) | 3.63 (2.82, 4.66) | −1.11 (−2.49, −0.014) | −24.1 | <0.0001 |
| *p=0.07 between groups | |||||
| By family history of diabetes | |||||
| Yes | 4.84 (3.70, 6.46) | 3.50 (2.61, 4.51) | −1.26 (−2.63, −0.33) | −27.3 | <0.0001 |
| No | 5.55 (4.05, 7.37) | 3.81 (3.02, 4.97) | −1.55 (−3.02, −0.36) | −30.1 | <0.0001 |
| *p=0.4 between groups | |||||
| By glycemic status at baseline | |||||
| NGT | 5.54 (4.24, 7.41) | 3.81 (3.03, 4.96) | −1.58 (−3.22, −0.44) | −31.3 | <0.0001 |
| IGT | 4.71 (3.47, 6.10) | 3.48 (2.61, 4.43) | −1.15 (−2.38, −0.079) | −26.8 | <0.0001 |
| *p=0.02 between groups | |||||
Data presented are medians (interquartile range) or n (%)
the p-value refers to the % change between groups
In unadjusted linear regression models, higher systolic BP at baseline was associated with a decline of MCRI over time. The significant association remained after adjusting for age, sex, ethnicity, smoking, alcohol consumption, energy expenditure, family history of diabetes, BMI, FBG, SI and AIR. Plasma TG at baseline was inversely associated with MCRI at follow-up in unadjusted regression models. Adjusting for demographic and lifestyle factors, as well as known risk factors of diabetes did not change the association materially. Similarly, larger WC at baseline was associated with a lower MCRI at follow-up in both unadjusted and multivariable-adjusted regression models. Higher HDL-cholesterol at baseline was associated with an increase in MCRI over time in unadjusted regression models and after multivariable adjustment. However, there was no association between FBG at baseline and the change in MCRI in both unadjusted and multivariable-adjusted regression models (Table 4). We did not find significant interactions of any of the components of metabolic syndrome with age, sex, ethnicity, BMI, glycemic status at baseline on the change in MCRI over time. In sensitivity analysis, when including all components of metabolic syndrome in a backward stepwise regression model, we observed that WC, HDL-cholesterol and systolic blood pressure, but not triglyceride and fasting blood glucose, were independently associated with the change in MCRI over time.
Table 4.
Estimated regression coefficients (95% confidence intervals) on the association between components of metabolic syndrome at baseline and change in MCRI in non-diabetic participants of the IRAS
| Outcome: follow-up ln(MCRI) (l/min) | Beta-coefficients (95% CI) | p-value |
|---|---|---|
| Systolic blood pressure (mmHg) | ||
| Unadjusted* | −0.0029 (−0.0044, −0.0013) | <0.001 |
| adjusted for age, sex, ethnicity | −0.0028 (−0.0044, −0.0013) | <0.001 |
| + smoking, alcohol consumption, energy expenditure, family history of diabetes |
−0.0029 (−0.0045, −0.0012) | <0.001 |
| + BMI, fasting blood glucose | −0.0020 (−0.0036, −0.0038) | 0.016 |
| + ln(SI), ln(AIR) | −0.0019 (−0.0035, −0.00023) | 0.025 |
| Waist circumference (cm) | ||
| Unadjusted* | −0.011 (−0.013, −0.0087) | <0.001 |
| adjusted for age, sex, ethnicity | −0.012 (−0.014, −0.0092) | <0.001 |
| + smoking, alcohol consumption, energy expenditure, family history of diabetes |
−0.011 (−0.014, −0.0089) | <0.001 |
| + BMI, fasting blood glucose | −0.0099 (−0.014, −0.0055) | <0.001 |
| + ln(SI), ln(AIR) | −0.0084 (−0.013, −0.0039) | <0.001 |
| Ln(plasma triglyceride) (mg/dl) | ||
| Unadjusted* | −0.057 (−0.11, −0.0098) | 0.018 |
| adjusted for age, sex, ethnicity | −0.088 (−0.14, −0.038) | 0.001 |
| + smoking, alcohol consumption, energy expenditure, family history of diabetes |
−0.087 (−0.14, −0.037) | 0.001 |
| + BMI, fasting blood glucose | −0.070 (−0.12, −0.022) | 0.005 |
| + ln(SI), ln(AIR) | −0.057 (−0.11, −0.0084) | 0.022 |
| HDL-cholesterol (mg/dl) | ||
| Unadjusted* | 0.0038 (0.0021, 0.0056) | <0.001 |
| adjusted for age, sex, ethnicity | 0.0046 (0.0027, 0.0065) | <0.001 |
| + smoking, alcohol consumption, energy expenditure, family history of diabetes |
0.0045 (0.0025, 0.0064) | <0.001 |
| + BMI, fasting blood glucose | 0.0036 (0.0017, 0.0055) | <0.001 |
| + ln(SI), ln(AIR) | 0.0029 (0.0010, 0.0048) | 0.002 |
| Fasting blood glucose (mg/dl) | ||
| Unadjusted* | −0.0019 (−0.0043, 0.00052) | 0.125 |
| adjusted for age, sex, ethnicity | −0.00089 (−0.0033, 0.0016) | 0.476 |
| + smoking, alcohol consumption, energy expenditure, family history of diabetes |
−0.00082 (−0.0033, 0.0017) | 0.516 |
| + BMI | 0.0016 (−0.00086, 0.0040) | 0.205 |
| + ln(SI), ln(AIR) | 0.0014 (−0.0026, 0.0029) | 0.921 |
adjusted for baseline ln(MCRI) in all models
Discussion
In this multi-ethnic cohort of individuals with high prevalence of metabolic syndrome, we observed a 29% decline of MCRI in a 5-year follow-up period. Overall, the IRAS participants deteriorated metabolically between baseline and follow-up examinations, with slight improvements in systolic blood pressure and HDL-cholesterol. This progressive nature of metabolic abnormalities in our study population may explain the large decline of MCRI over time. Compared to individuals with normal glycemia, those with impaired glucose tolerance had lower MCRI at baseline, but a slower decline in MCRI over time. Higher plasma TG and larger WC at baseline were associated with a decline in MCRI. In contrast, higher HDL-cholesterol at baseline was associated with an increase in MCRI. Higher systolic BP at baseline was associated with a lower MCRI at follow-up. When considering all components of metabolic syndrome as a whole, WC, systolic BP and HDL-cholesterol were independently associated with the change in MCRI over time. Our study reports several novel findings on insulin clearance. First, we document for the first time the progressive decline in MCRI. Second, we describe the longitudinal association between components of metabolic syndrome at baseline and change in MCRI; these findings extend the existing literature on this topic, which had consisted of mostly cross-sectional studies.
In our study population, individuals with impaired glucose tolerance had lower MCRI, lower SI and lower AIR than those with normal glycemia. These metabolic traits correspond to insulin resistance and beta-cell dysfunction which predispose to type 2 diabetes [20]. However, these individuals also had a relatively slower rate of decline in MCRI. Although previous animal models have suggested that the decline in MCRI may represent an adaptive mechanism during the evolution of insulin resistance to preserve β-cell function in pre-diabetes states [4], further experimental studies have yet to determine whether the rate of decline slows later in the pathogenesis of diabetes.
Individuals with essential hypertension have a high prevalence of hyperinsulinemia [21]. Previous cross-sectional studies demonstrated an association between essential hypertension and impaired insulin clearance in various populations [12–13]. Elevated fasting insulin is associated with reduced hepatic insulin clearance to a greater extent than with increased pancreatic insulin secretion in hypertensive individuals [22]. However, the temporality of this association is unclear. In our study, we observed that higher systolic BP at baseline was significantly associated with a decline of MCRI over time. Hypertension may decrease MCRI through mechanisms involving capillary permeability. There is evidence suggesting that elevated BP is associated with abnormal capillary permeability [23], and impaired capillary transport from decreased blood flow or permeability could decrease the efflux of insulin from the intravascular space, hence decreasing insulin clearance and resulting in an increase in plasma insulin if this occurs in tissues that are targeted for insulin degradation [13]. Several clinical studies supported that certain classes of anti-hypertensive medication, such as calcium channel blockers or alpha-adrenergic blockers, may augment insulin clearance [24–25]. Nonetheless, long-term randomized controlled trials are needed to determine the clinical significance of therapeutic intervention in lowering blood pressure on insulin clearance.
Larger WC at baseline was associated with a significant decline in MCRI over time. Consistent with our findings, previous cross-sectional studies have demonstrated that insulin clearance is decreased in individuals with abdominal adiposity [10–11]. These associations are biologically plausible, as abdominal adiposity increases the release of free fatty acids from adipose tissues, resulting in hyperinsulinemia and leading to a decline in insulin clearance [26–27]. Given its significance in the decline of insulin clearance, decreasing abdominal adiposity could be used as a therapeutic target. To support this notion, restricting caloric intake of diabetic patients has been shown to result in weight loss, decrease insulin resistance and increase insulin clearance [28].
Our study showed that higher plasma TG at baseline was associated with the decline of MCRI over time. With respect to HDL-cholesterol, we observed a positive association with the change in MCRI. In stepwise regression models, HDL-cholesterol, but not plasma TG, independently associated with the change in MCRI which may be related to their strong inverse correlations. Insulin resistance is associated with abnormal lipid metabolism, including elevated plasma TG and decreased HDL-cholesterol [29]. In individuals with impaired glucose tolerance, elevated plasma TG is significantly associated with increased HDL-cholesterol catabolism [30]. Mechanistically, insulin resistance and/or hyperinsulinemia could increase serum free fatty acids or divert dietary carbohydrates to become substrates for de novo lipogenesis, resulting in an increase in hepatic synthesis of TG [8, 31]. Insulin resistance could also decrease HDL-cholesterol by blunting the stimulation of lipoprotein lipase activity, increasing triglyceride-enrichment of HDL and increasing hepatic lipase activity [32–33]. Since elevated TG and decreased HDL-cholesterol are associated with insulin resistance, and insulin resistance precedes the reduction of insulin clearance [4], it is plausible that abnormal lipids are associated with impaired insulin clearance, as we observed in the present study. Alternatively, dyslipidemia in metabolic syndrome is closely related to non-alcoholic fatty liver disease [34]. Insulin clearance was reduced in individuals with increased liver fat [35], whereas therapeutic intervention with rosiglitazone reduced liver fat and increased insulin clearance [36].
Higher FBG at baseline was not associated with MCRI at follow-up. Our participants were diabetes-free at baseline and their median FBG was in the normal range. This may explain the lack of association between FBG at baseline and MCRI at follow-up, as elevated FBG may correspond to the decline of insulin sensitivity and insulin clearance, thus emerge in the later stages of the etiology of type 2 diabetes.
The strengths of our study include the well-characterized multi-ethnic cohort, detailed and direct measurements of insulin clearance, insulin sensitivity and β-cell function, and the prospective design which allows us to explore the temporal relationship of the exposure variables with the change of MCRI over time. However, our findings may apply only to individuals with similar demographic characteristics and may not be generalized to other ethnic populations.
In conclusion, we demonstrated a progressive decline of MCRI in the non-diabetic participants of the IRAS. Components of metabolic syndrome at baseline, including dyslipidemia, hypertension and abdominal adiposity, were associated with a decline of MCRI over time. These findings provide a basis for long-term randomized controlled trials to confirm that reducing weight, improving lipids and blood pressure through pharmaceutical or lifestyle intervention could improve insulin clearance and hence slow the onset of diabetes in high risk populations.
Acknowledgement
C. Lee is supported by a postdoctoral research fellowship from the Banting & Best Diabetes Centre, University of Toronto, Canada. A. Hanley holds a Tier II Canada Research Chair in Diabetes Epidemiology. IRAS was supported by grants U01-HL47892, U01-HL47902, DK-29867, R01-58329, DK-079888 from National Heart, Lung and Blood Institute, and grant M01-RR-43 from the National Institutes of Health.
Footnotes
Declaration of interests The authors have no conflict of interests to disclose.
References
- 1.Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3):35–45. doi: 10.1046/j.1365-2362.32.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 2.Duckworth WC, Bennett RG, Hamel FG. The significant of intracellular insulin to insulin action. J Invest Med. 1997;45:20–27. [PubMed] [Google Scholar]
- 3.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 4.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes. 2000;49:2116–2125. doi: 10.2337/diabetes.49.12.2116. [DOI] [PubMed] [Google Scholar]
- 5.Flier JS, Minaker KL, Landsberg L, et al. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes. 1982;31:132–135. doi: 10.2337/diab.31.2.132. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Stern MP, Watanabe RM, Bergman RN. Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest. 1992;22:147–153. doi: 10.1111/j.1365-2362.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith JC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 10.Peiris AN, Mueller RA, Smith GA, Struve MF, Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest. 1986;78:1648–1657. doi: 10.1172/JCI112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg GE, Hoffman RG, Mueller RA, Kissebah AH. Splanchnic insulin dynamics and secretion pulsatilities in abdominal obesity. Diabetes. 1994;43:468–477. doi: 10.2337/diab.43.3.468. [DOI] [PubMed] [Google Scholar]
- 12.Salvatore T, Cozzolino D, Giunta R, et al. Decreased insulin clearance as a feature of essential hypertension. J Clin Endocrinol Metab. 1992;74:144–149. doi: 10.1210/jcem.74.1.1727814. [DOI] [PubMed] [Google Scholar]
- 13.Lender D, Arauz-Pacheco C, Adams-Huet B, Raskin P. Essential hypertension is associated with decreased insulin clearance and insulin resistance. Hypertension. 1997;29:111–114. doi: 10.1161/01.hyp.29.1.111. [DOI] [PubMed] [Google Scholar]
- 14.Polonsky KS, Pugh W, Jaspan JB, et al. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest. 1984;74:1821–1829. doi: 10.1172/JCI111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagenknecht LE, Mayer EJ, Rewers M, et al. The insulin resistance atherosclerosis study (IRAS) objectives, design, and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 16.Lorenzo C, Wagenknecht LE, D’Agostino RB, Jr, et al. Insulin resistance, beta-cell dysfunction, and conversion to type 2 diabetes in multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2010;33:67–72. doi: 10.2337/dc09-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard BV, Mayer-Davis EJ, Goff D, et al. Relationships between insulin resistance and lipoproteins in nondiabetic African Americans, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Metabolism. 1998;47:1174–1179. doi: 10.1016/s0026-0495(98)90319-5. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. The Journal of clinical investigation. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano D, Quatraro A, Minei A, De Rosa N, Coppola L, D’Onofrio F. Hyperinsulinemia in hypertension: increased secretion, reduced clearance or both? J Endocrinol Invest. 1993;16:315–321. doi: 10.1007/BF03348843. [DOI] [PubMed] [Google Scholar]
- 23.Dell’Omo G, Penno G, Pucci L, Mariani M, Del Prato S, Pedrinelli R. Abnormal capillary permeability and endothelial dysfunction in hypertension with comorbid metabolic syndrome. Atherosclerosis. 2004;172:383–389. doi: 10.1016/j.atherosclerosis.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Katzman PL, Hulthén UL, Hökfelt B. Unchanged insulin secretion and glucose tolerance but increased insulin clearance during long-term calcium antagonism with felodipine in essential hypertension. Horm Metab Res. 1987;19:426–429. doi: 10.1055/s-2007-1011843. [DOI] [PubMed] [Google Scholar]
- 25.O’Callaghan CJ, Komersova K, Louis WJ. Acute effects of blood pressure elevation on insulin clearance in normotensive healthy subjects. Hypertension. 1998;31:104–109. doi: 10.1161/01.hyp.31.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes. 1999;48:766–774. doi: 10.2337/diabetes.48.4.766. [DOI] [PubMed] [Google Scholar]
- 27.Balent B, Goswami G, Goodloe G, et al. Acute elevation of NEFA causes hyperinsulinemia without effect on insulin secretion rate in healthy human subjects. Ann NY Acad Sci. 2002;967:535–543. doi: 10.1111/j.1749-6632.2002.tb04313.x. [DOI] [PubMed] [Google Scholar]
- 28.Polonsky KS, Gumbiner B, Ostrega D, Griver K, Tager H, Henry RR. Alternations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes. 1994;43:871–877. doi: 10.2337/diab.43.7.871. [DOI] [PubMed] [Google Scholar]
- 29.Laakso M, Sarlund H, Mykkänen L. Insulin resistance is associated with lipid and lipoprotein abnormalities in subjects with varying degrees of glucose tolerance. Arteriosclerosis. 1990;10:223–231. doi: 10.1161/01.atv.10.2.223. [DOI] [PubMed] [Google Scholar]
- 30.Pietzsch J, Julius U, Nitzsche S, Hanefeld M. In vivo evidence for increased apolipoprotein A-1 catabolism in subjects with impaired glucose tolerance. Diabetes. 1998;47:1928–1934. doi: 10.2337/diabetes.47.12.1928. [DOI] [PubMed] [Google Scholar]
- 31.Reaven GM, Chen YD. Role of insulin in regulation of lipoprotein metabolism in diabetes. Diabetes Metab Rev. 1998;4:639–652. doi: 10.1002/dmr.5610040703. [DOI] [PubMed] [Google Scholar]
- 32.Eckel RH, Yost TJ, Jensen DR. Alternations in lipoprotein lipase in insulin resistance. Int J Obes Relat Metab Disord. 1995;19(Suppl 1):S16–21. [PubMed] [Google Scholar]
- 33.Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. 2003;36:421–429. doi: 10.1016/s0009-9120(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 34.Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 2010;30:273–290. doi: 10.1146/annurev.nutr.012809.104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotronen A, Vehkavaara S. Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–1705. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 36.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]