Abstract
The crude extracts of plants from Asteraceae and Lamiaceae family and essential oils from Salvia officinalis and Salvia sclarea were studied for their antibacterial as well as antibiotic resistance modifying activity. Using disc diffusion and broth microdilution assays we determined higher antibacterial effect of three Salvia spp. and by evaluating the leakage of 260 nm absorbing material we detected effect of extracts and, namely, of essential oils on the disruption of cytoplasmic membrane. The evaluation of in vitro interactions between plant extracts and oxacillin described in terms of fractional inhibitory concentration (FIC) indices revealed synergistic or additive effects of plant extracts and clearly synergistic effects of essential oil from Salvia officinalis with oxacillin in methicillin-resistant Staphylococcus epidermidis.
1. Introduction
Staphylococcus epidermidis belongs to the group of coagulase negative staphylococci (CoNS). It is commensal microorganism that constitutes a major component of the normal skin and mucosal microflora of humans [1]. However, in recent years these bacteria have emerged as an opportunistic pathogen, important causative agents of bacteremia, and the leading cause of nosocomial infections particularly associated with indwelling medical devices (e.g., prosthetic joints and heart valves) and in individuals with a compromised immune system (e.g., cancer patients and neonates) [2, 3]. Staphylococcus epidermidis pathogenesis relies on the ability to adhere and form biofilms on the surfaces of the medical devices mentioned earlier [4].
Staphylococci are considered as naturally susceptible to almost all antimicrobial agents developed but at the same time have a reputation of rapidly developing resistance [5]. CoNS, especially S. epidermidis, are often multiresistant, including resistance to methicillin. Resistance to methicillin is at 75–90% among hospital isolates of S. epidermidis, which is even higher than the corresponding rate for S. aureus (40–60%) [6]. In addition to methicillin resistance, S. epidermidis strains have acquired resistance to several other antibiotics. Most antibiotic resistance genes are plasmid-encoded and are more often found in methicillin-resistant than methicillin-susceptible strains [7]. These facts together with the ubiquity of S. epidermidis as a human commensal microorganism render this bacterium an optimal carrier and reservoir for antibiotic resistance genes and for the transfer of genetic elements to pathogenic bacteria.
The increasingly growing rate of antibiotic resistance of microorganisms necessitates the development and research of new antimicrobial agents or resistance modifiers. Medicinal plant-derived compounds have increased widespread interest in the search of alternative antibacterial agents because of the perception that they are safe and have a long history of use in folk medicine for the treatment of infectious diseases [8]. Natural products of higher plants may possess a new source of antimicrobial agents with possibly novel mechanisms of action [9, 10]. They are effective in the treatment of infectious diseases while simultaneously mitigating many of the side effects that are often associated with conventional antimicrobials [11]. Systematic and methodical screening of them may result in the discovery of novel active compounds [12].
Reversal of multidrug resistance may be another attempt to mitigate the spread of resistance. One of the promising methods in coping with bacterial resistance is, along the use of alternative classes of antimicrobial agents, also the application of synergistic activity between antibiotics and nonantibiotics. Many plants have direct antimicrobial activity but also resistance modifying/modulating activities [13]. Resistance modifying agents may inhibit multidrug resistance mechanism. This ability of plant extracts to enhance antibiotics has not been well defined.
It is well documented that some plants belonging to Asteraceae and Lamiaceae families possess suitable medicinal properties, which are based mainly on the presence of essential oils [14]. Antimicrobial activity of many species of Salvia plants against several microorganisms has been recognized for decades and has been attributed to the presence of 1,8-cineole, β-thujone, camphor, borneol, and p-cymene, among others [15]. Terpenes of essential oils extracted from different herbs are proved to have antimicrobial activity and some of them may act as resistance modifiers.
Researchers studied antibacterial properties of various plants against Gram-negative as well as Gram-positive bacterial strains including Staphylococcus aureus [16–18], but there are only few reports on antibacterial activity against Staphylococcus epidermidis. Moreover, the interactions between plant extracts or essential oils and antibiotic in methicillin-resistant S. epidermidis have not been documented earlier.
The principal objective of the present study was to evaluate in vitro antibacterial activities of selected plant extracts from Asteraceae and Lamiaceae family against clinical isolates of S. epidermidis and to evaluate interactions between antibiotic and plant extracts or essential oils in coping with methicillin resistance.
2. Materials and Methods
2.1. Plant Extracts
In this work crude extracts of plants from Asteraceae (Anthemis tinctorium, Chamaemelum nobile, Matricaria recutita, Tanacetum argyrophyllum, and Tanacetum parthenium) and Lamiaceae (Salvia fruticosa, Salvia officinalis, and Salvia sclarea) family were used. The plants were harvested at the optimal growing and development stage. Essential oils from Salvia officinalis and Salvia sclarea were prepared in accordance with the European Pharmacopoeia [19]. Air-dried aerial parts were subject to hydrodistillation for 4 h, and isolated oil was diluted in n-hexane and dried over anhydrous sodium sulphate.
2.2. Bacterial Strains
Methicillin-resistant and methicillin-susceptible Staphylococcus epidermidis strains were isolated from patients with positive haemocultures from University Teaching Hospital Old Town, Bratislava, Slovak Republic, and were kindly provided by Dr. Slobodníková from Institute of Microbiology, Faculty of Medicine, Comenius University in Bratislava, Slovak Republic. Strains are marked as Sep1 to Sep7. All strains were routinely grown aerobically in brain-heart infusion medium (Biomark, India) with shaking for 24 h at 37°C.
2.3. Disc Diffusion Assay
The plant extracts were tested for antimicrobial activity by disc diffusion assay on Mueller-Hinton agar (Himedia, India). Suspension of the tested bacteria (0.1 mL of 108 cells/mL) was spread onto solid media plates. The sterile paper discs (6 mm in diameter), which were impregnated with 10 μL of individual extract, were placed on the incubated plates. These plates after 2 h of maintenance at 4°C were incubated for 24 h at 37°C and the diameters of the resulting zones of inhibition were measured in millimeters.
2.4. Determination of Minimum Inhibitory Concentration (MIC)
The MIC values of plant extracts were determined by broth microdilution method using 96-well microtiter plates in accordance with CLSI (2011) guidelines [20]. Serial twofold dilutions of the plant extracts were prepared by vortexing the extracts in Millipore water. Inoculum of microorganism was prepared in Mueller-Hinton Broth (Himedia, India), and the turbidity was adjusted to 0.5 McFarland and diluted to obtain a final turbidity in wells approximately 1 × 106 CFU/mL. Twenty μL of solution of plant extract and 180 μL of bacterial inoculum were placed into wells of microtiter plate and incubated at 37°C for 24 h. Growth of the bacteria was examined as a function of turbidity (optical density (OD) at 600 nm) using Varioskan Flash (Thermo Fisher Scientific, Finland). The MIC is defined as the lowest concentration of antimicrobial agent that completely inhibits growth of the organism.
2.5. PCR for mecA Gene
Detection of the mecA gene in Staphylococcus epidermidis strains was accomplished using polymerase chain reaction (PCR) amplification. Cells were suspended in a lysis buffer containing 1 M Tris HCl, 5 M NaCl, and 0.1 M EDTA, which was incubated at 95°C for 10 minutes. After incubation, the suspension was centrifugated at 23 000 ×g for 5 min. The supernatant was used as a template in PCR. PCR assay was carried out as described by Zhang et al. [21] using primers MecA147-F (GTGAAGATATACCAAGTGATT) and MecA147-F (GTGAAGATATACCAAGTGATT). The final PCR products were visualized using UV-transilluminator after electrophoresis on 1.5% agarose gel containing 50 mg/mL EtBr.
2.6. Checkerboard Method
The initial inoculum was prepared as described above. The 96-well microtiter plates were inoculated with test organism and serial dilutions of two antimicrobial agents—antibiotic and plant extract. Each well contained unique combination of plant extract/antibiotic concentrations. The plates were incubated for 24 h at 37°C. The absorbance of the plates was recorded at 600 nm using Varioskan Flash (Thermo Fisher Scientific, Finland).
Interactions between antimicrobial agents were determined by calculating the fractional inhibitory concentration (FIC) indices. The FIC is defined as follows: MIC of substance A tested in combination/MIC of substance A tested alone + MIC of substance B tested in combination/MIC of substance B tested alone. The FIC index is interpreted as FIC < 0.5—synergistic effect, 0.5 < FIC < 1—additive effect, 1 < FIC < 4—indifferent effect, and FIC > 4.0—antagonistic effect [22].
2.7. Time-Kill Assay
The effect of combinations of plant extract and oxacillin against methicillin-resistant S. epidermidis was evaluated using the time-kill assay method. All antimicrobial agents alone and in combination were tested against six strains of methicillin-resistant S. epidermidis. The concentration of extracts and oxacillin alone or in combination was 1/2 MIC. Time-kill curves were performed in tube containing nutrient broth, using inoculum density of approximately (107 CFU/mL) in the presence of a single agent or a combination of antimicrobial agents. The tubes were continuously shaken and incubated at 37°C. Samples were obtained at 0, 6, 10, and 24 h. At each sample time, aliquots were taken and serially diluted. Fifty microliters of undiluted and diluted samples were plated on nutrient agar. The plates were incubated at 37°C for 24 h. After incubation, the numbers of colonies were enumerated and the mean counts (CFU/mL) for each test and controls were determined and expressed as log10. The effect of the antimicrobial combinations was interpreted as follows: synergy was defined as a decrease of ≥2 log10 CFU/mL in colony counts after 24 h by the combination compared to the most active single agent. Additivity or indifference was defined as a <2 log10 CFU/mL change in the average viable counts after 24 h for the combination, compared with the most active single agent. Antagonism was described as a ≥2 log10 CFU/mL increase in colony counts after 24 h by the combination compared to that by the most active agent alone [23].
2.8. Loss of 260 nm Absorbing Material
Loss of 260 nm absorbing material released from bacteria was measured by the technique of Devi et al. [24]. Bacterial suspension was prepared from overnight culture (OD600 2.0). Cells were separated from medium by centrifugation at 400 ×g, for 15 min, washed twice in phosphate-buffered-saline (pH 7.4), and resuspended in the same buffer. Different concentrations of plant extracts 10%–0.03% (v/v) were added to the cell suspension. Ciprofloxacin (500 mg/L) and cell suspension without plant extract were used as controls. The samples were incubated at 37°C for 60 min with shaking. Samples from 0 and 60 min of the experiment were centrifuged at 13 400 ×g, for 15 min. For each time point and treatment agent optical density was measured at 260 nm with spectrophotometer (NanoDrop, Thermoscientific, USA).
3. Results and Discussion
3.1. Antibacterial Effect
Six clinical isolates of methicillin-resistant Staphylococcus epidermidis (Sep1–Sep6) and one methicillin-susceptible strain (Sep7) were used to evaluate the possible antistaphylococcal activity of selected plant extracts. Our results, determined using disc diffusion and broth microdilution methods, are presented as average values in Tables 1 and 2. According to inhibition zone diameters, most effective were extracts from three species of Salvia (inhibition zone from 12.4 mm to 12.7 mm) followed by extract from Matricaria recutita. Extracts from the rest of Asteraceae family plants showed smaller inhibition zones, from 7.0 mm to 10.4 mm. There was no difference between inhibition zones of methicillin-resistant and methicillin-susceptible strains.
Table 1.
Inhibition zone diameter in mm as established by disc diffusion method.
| Plant extract | Zone of inhibition (mm)a | Mean | ||||||
|---|---|---|---|---|---|---|---|---|
| Sep1 | Sep2 | Sep3 | Sep4 | Sep5 | Sep6 | Sep7 | ||
| Salvia fruticosa | 13 | 10 | 13 | 10 | 13 | 16 | 14 | 12.7 |
| Salvia officinalis | 12 | 11 | 14 | 10 | 14 | 15 | 13 | 12.7 |
| Salvia sclarea | 12 | 9 | 11 | 12 | 13 | 15 | 15 | 12.4 |
| Anthemis tinctoria | 7 | 7 | 6 | 6 | 8 | 7 | 8 | 7.0 |
| Chamaemelum nobile | 10 | 9 | 10 | 10 | 9 | 11 | 10 | 9.8 |
| Matricaria recutita | 10 | 8 | 8 | 9 | 15 | 15 | 14 | 11.3 |
| Tanacetum argyrophyllum | 9 | 8 | 7 | 8 | 13 | 12 | 8 | 9.3 |
| Tanacetum parthenicum | 12 | 9 | 12 | 10 | 10 | 12 | 8 | 10.4 |
aDiameter includes 6 mm disc.
Table 2.
Minimum inhibition concentration (MIC) of plant extracts and oxacillin established by both microdilution method. MIC is given as % (v/v) for plant extracts and mg/L for oxacillin.
| Plant extract | Minimum inhibitory concentration | ||||||
|---|---|---|---|---|---|---|---|
| Sep1 | Sep2 | Sep3 | Sep4 | Sep5 | Sep6 | Sep7 | |
| Salvia fruticosa | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Salvia officinalis | 2.5 | 2.5 | 5 | 10 | 1.25 | 5 | 5 |
| Salvia sclarea | 10 | 1.25 | 10 | 10 | 5 | 10 | 10 |
| Anthemis tinctoria | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Chamaemelum nobile | ≥10 | >10 | ≥10 | ≥10 | >10 | 10 | >10 |
| Matricaria recutita | ≥10 | >10 | >10 | >10 | 5 | 5 | 10 |
| Tanacetum argyrophyllum | >10 | >10 | >10 | >10 | 5 | 10 | >10 |
| Tanacetum parthenicum | 10 | >10 | 10 | ≥10 | ≥10 | 10 | >10 |
| Oxacillin | 128 | 32 | 16 | 256 | 32 | 32 | 0.125 |
More precise data on the antimicrobial properties were obtained through the determination of minimum inhibitory concentration and the results from microdilution method confirmed the previous results. Extracts from S. officinalis and S. sclarea showed higher inhibitory properties with MIC values in the range of 1.25% to 10% (v/v) than those of Asteraceae extracts, most of which had MIC 10% or more than 10% (v/v).
Antibacterial effects of crude extracts and, namely, of essential oils from different species of Salvia on the growth of Gram-positive and Gram-negative bacteria were evaluated by many authors. Some of them confirmed antibacterial effect of different Salvia species also on staphylococci [25, 26], while other stated that extracts from Salvia officinalis [27], S. divinorum [28], and others had no effect on the growth of Staphylococcus aureus and S. epidermidis. However the compounds of essential oil from S. sclarea—abietane diterpenoids are bactericid for the cultures of S. aureus and S. epidermidis strains [29].
3.2. Loss of 260 nm Absorbing Material
We extended our investigation of antibacterial effect of plant extracts to evaluation of its possible mechanism. The mechanism of action of terpenes from essential oils, which are main parts of tested plants, is not fully understood, but it is assumed that membrane perturbation by these lipophilic components is involved in the antibacterial action. Marked leakage of cytoplasmic material is considered indicative of gross and irreversible damage to the cytoplasmic membrane [30, 31] and is commonly quantified by the loss of intracellular material that absorb at wavelengths of 260 nm (nucleic acids). After treatment with plant extracts at increased concentrations from 0.3% to 5% (v/v) the OD260 of filtrates of all tested strains increased and the most remarkable increases occurred after 60 min treatment with 5% Salvia sclarea and 5% Salvia officinalis (Figure 1). At the same time the OD260 of control without extract and of control with ciprofloxacin were not changed. These results suggest that crude extracts from tested plants damage the cytoplasmic membrane and cause loss of intracellular components. In order to confirm the assumptions that this effect have been based on the nature of essential oils, in Figure 2 we compared the leakage of intracellular compounds in the presence of crude plant extract and of essential oil from the same plant Salvia sclarea. The effect of essential oil is significantly higher yet in lower concentrations.
Figure 1.
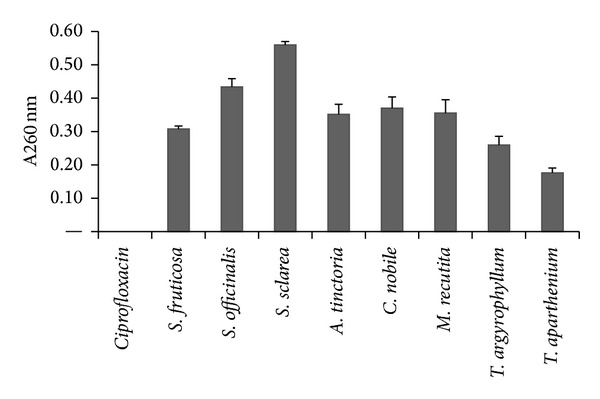
The appearance of 260 nm-absorbing material in the filtrates of S. epidermidis Sep6 after 60 min treatment with plant extracts in concentration of 5%. Ciprofloxacin was used as control. The means ± SD for at least three replicates are illustrated.
Figure 2.
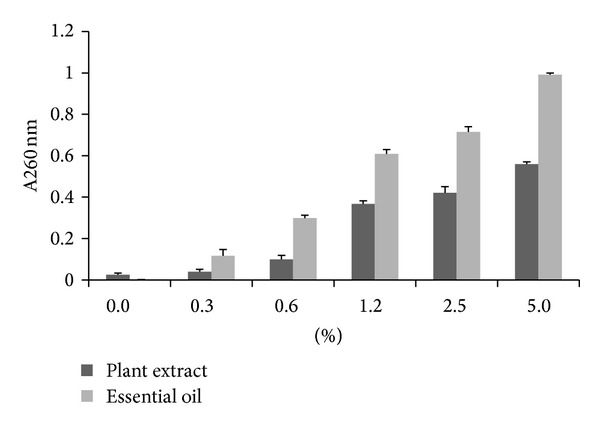
The effect of crude extract and essential oil from Salvia sclarea on the leakage of 260 nm absorbing materials from Staphylococcus epidermidis Sep6.
It has been reported that some antimicrobial agents cause gross membrane damage and provoke whole cell lysis [32]. Among these compounds can be found also essential oils from oregano, rosewood, and thyme [33], α-pinene [34], lemongrass oil [35], and tea tree oil from Melaleuca alternifolia [36].
3.3. Interactions between Plant Extracts and Oxacillin
The resistance of the six clinical strains S. epidermidis to oxacillin is obvious from the Table 2. The breakpoint for oxacillin resistance for CoNS according to Eucast 2013 [37] is MIC > 0.25 mg/L. MIC of oxacillin of our resistant strains (Sep1–Sep6) ranged from 16 mg/L to 256 mg/L, while MIC of methicillin-susceptible strain Sep7 was 0.125 mg/L. Methicillin resistance of Sep1–Sep6 strains was confirmed by determination of gene mecA (Figure 3). PCR for the mecA gene coding for methicillin resistance via penicillin binding protein 2a (PBP2a) is well established and is considered as “gold standard” for detection of methicillin resistance in comparison with phenotypic methods [38–40].
Figure 3.
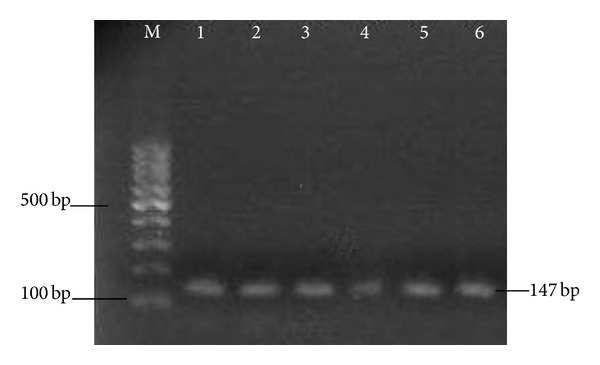
Detection of mecA gene. Lanes: M: 100 bp marker, 1–6: 147 bp PCR product of mecA gene from strains Staphylococcus epidermidis Sep1–Sep6.
The evaluation of in vitro interactions between plant extracts and oxacillin are described in terms of fractional inhibitory concentration (FIC) indices. The FICs for all used plant extracts and oxacillin against bacterial strain of methicillin-resistant S. epidermidis Sep6 are shown in Table 3. Extracts from all three Salvia species combined with oxacillin had synergistic effect. From all Asteraceae only extract from Matricaria recutita had synergistic effect with oxacillin, and all rest extracts had additive effect. It was found that the crude extract of Salvia officinalis reduced the minimum inhibitory concentration of aminoglycosides in vancomycin-resistant enterococci and then the effective compound was isolated. Carnosol, the active compound showed weak antimicrobial activity and greatly reduced the MICs of various aminoglycosides [41]. The exact mechanism for the reduction of β-lactam (methicillin) resistance by the natural antimicrobials is unknown but is likely due to some structural change in the resistant bacteria. Epigallocatechin gallate from green tea inhibited the activity of penicillinase produced by S. aureus [42] and synergistically enhanced the activity of carbapenems against methicillin-resistant S. aureus (MRSA) [43]. Tellimagrandin I from red rose (Rosa canina L.) petal extract greatly reduced the MIC of β-lactam antibiotic against MRSA [44]. Similarly, corilagin, an active compound extracted from Arctostaphylos uva-ursi, was found to reduce the MICs of oxacillin and cefmetazole against MRSA [45].
Table 3.
Interactions of plant extracts and oxacillin in effect on methicillin-resistant S. epidermidis Sep6.
| Plant extract | FIC A | FIC B | FIC | Interpretation |
|---|---|---|---|---|
| Salvia fruticosa | 0.03 | 0.17 | 0.20 | Synergistic |
| Salvia officinalis | 0.05 | 0.09 | 0.14 | Synergistic |
| Salvia sclarea | 0.06 | 0.09 | 0.15 | Synergistic |
| Anthemis tinctoria | 0.12 | 0.47 | 0.59 | Additive |
| Chamaemelum nobile | 0.13 | 0.41 | 0.54 | Additive |
| Matricaria recutita | 0.06 | 0.12 | 0.18 | Synergistic |
| Tanacetum argyrophyllum | 0.26 | 0.53 | 0.79 | Additive |
| Tanacetum parthenicum | 0.24 | 0.51 | 0.75 | Additive |
FIC A = MIC of substance A tested in combination/MIC of substance A tested alone, FIC B = MIC of substance B tested in combination/MIC of substance B tested alone. FIC = FIC A + FIC B. A—oxacillin, B—plant extract.
The synergistic effects arising from the combination of oxacillin and plant extract from Salvia species in checkerboard assays were explored in greater detail by using time-kill assays. As is shown in Figure 4, the synergistic effects (difference ≥2 Log10) could be observed starting from 10 hours of incubation and continued up to 24 hours. The results of the checkerboard and time-kill assays agreed in all cases.
Figure 4.
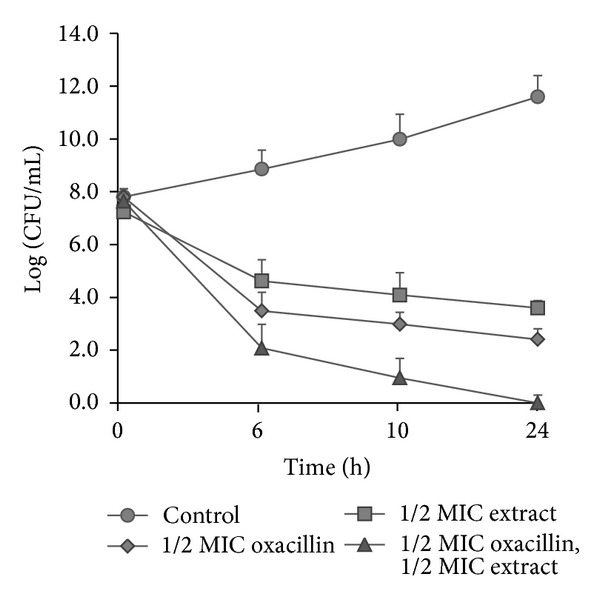
Time-kill curves of S. epidermidis Sep6 after treatment with 1/2 MIC of oxacillin, 1/2 MIC of extract from Salvia officinalis alone or in combination.
3.4. Interaction between Essential Oil from Salvia officinalis and Oxacillin
Regarding the synergistic effect of extracts from Salvia species, in this part of work we were concerned with evaluation of interactions between essential oils from Salvia officinalis and oxacillin. The results of the checkerboard method indicated synergism in all six strains of methicillin-resistant S. epidermidis for which the combination of essential oil and oxacillin were tested (Table 4). FICI 1.811 for methicillin-susceptible strain Sep7 indicated indifferent effect. This is in accordance with the fact that the combination of two agents exhibit significant synergism only if the test organism is resistant to at least one of the agents [46].
Table 4.
Interactions of essential oil from Salvia officinalis with oxacillin in effect on clinical strains of methicillin-resistant S. epidermidis.
| S. epidermidis | FIC A | FIC B | FIC | Interpretation |
|---|---|---|---|---|
| Sep1 | 0.031 | 0.125 | 0.156 | Synergistic |
| Sep2 | 0.062 | 0.250 | 0.312 | Synergistic |
| Sep3 | 0.062 | 0.125 | 0.187 | Synergistic |
| Sep4 | 0.062 | 0.250 | 0.312 | Synergistic |
| Sep5 | 0.031 | 0.250 | 0.28 | Synergistic |
| Sep6 | 0.031 | 0.125 | 0.156 | Synergistic |
| Sep7 | 0.562 | 1.250 | 1.812 | Indifferent |
FIC A = MIC of substance A tested in combination/MIC of substance A tested alone, FIC B = MIC of substance B tested in combination/MIC of substance B tested alone. FICI = FIC A + FIC B. A—oxacillin, B—essential oil.
The observed synergistic effects of plant extracts (essential oils) and oxacillin could be theoretically the results of the perturbation of the cell membrane coupled with the action of oxacillin. β-lactam antibiotic oxacillin inhibits the final stage involved in the synthesis of peptidoglycan of cell wall (transpeptidation reaction), which occurs outside the cell membrane and is mediated by alternative protein binding protein PBP2a encoded by mecA gene [47].
Compounds having synergic effect with oxacillin may inhibit the PBP2a activity or inhibit its production [45]. First mechanism can be connected with the perturbation of cell membrane, which we have confirmed in this work; the second mechanism: the inhibition of the production PBP2 by inhibition of mecA gene expression is the content of our current research and its result will be published in later stage.
4. Conclusion
The antibacterial activity of plant extracts from Asteraceae and Lamiaceae family was confirmed and contributed to the ability of contained essential oils to disturb biological membranes. Synergistic activity of extracts as well as essential oil from S. officinalis and oxacillin could suggest an alternative manner to overcome a problem of bacterial infections caused by methicillin resistant Staphylococcus epidermidis. Further research is necessary to identify active compounds and research mechanism of interaction with antibiotics.
Conflict of Interests
The authors do not have any conflict of interests regarding the content of the paper.
References
- 1.Otto M. Staphylococcus epidermidis—the “accidental” pathogen. Nature Reviews Microbiology. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. The Lancet Infectious Diseases. 2002;2(11):677–685. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 3.Viale P, Stefani S. Vascular catheter-associated infections: a microbiological and therapeutic update. Journal of Chemotherapy. 2006;18(3):235–249. doi: 10.1179/joc.2006.18.3.235. [DOI] [PubMed] [Google Scholar]
- 4.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes and Infection. 2002;4(4):481–489. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 5.Lentino JR, Narita M, Yu VL. New antimicrobial agents as therapy for resistant gram-positive cocci. European Journal of Clinical Microbiology and Infectious Diseases. 2008;27(1):3–15. doi: 10.1007/s10096-007-0389-y. [DOI] [PubMed] [Google Scholar]
- 6.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clinical Infectious Diseases. 2001;32(supplement 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 7.Miragaia M, Couto I, Pereira SFF, et al. Molecular characterization of methicillin-resistant Staphylococcus epidermidis clones: evidence of geographic dissemination. Journal of Clinical Microbiology. 2002;40(2):430–438. doi: 10.1128/JCM.40.2.430-438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarrera PM. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium) Fitoterapia. 2005;76(1):1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad I, Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiological Research. 2007;162(3):264–275. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Barbour EK, Al Sharif M, Sagherian VK, et al. Screening of selected indigenous plants of Lebanon for antimicrobial activity. Journal of Ethnopharmacology. 2004;93(1):1–7. doi: 10.1016/j.jep.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Iwu MW, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on New Crops and New Uses. Alexandria, VA, USA: AHSH Press; 1999. pp. 457–462. [Google Scholar]
- 12.Nitta T, Arai T, Takamatsu H, et al. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus . Journal of Health Science. 2002;48(3):273–276. [Google Scholar]
- 13.Gibbons S. Anti-staphylococcal plant natural products. Natural Product Reports. 2004;21(2):263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- 14.Sarac N, Ugur A. Antimicrobial activities and usage in folkloric medicine of some Lamiaceae species growing in Mugla, Turkey. EurAsian Journal of BioSciences. 2007;1(4):28–34. [Google Scholar]
- 15.Tepe EJ, Vincent MA, Watson LE. Piper: A Model Genus for Studies of Phytochemistry, Ecology, and Evolution. Kluwer Academic Publishers; 2004. Phylogenetic patterns, evolutionary trends and the origin of ant-plant associations Piper section Macrostachys: Burger’s hypotheses revisited; pp. 156–178. [Google Scholar]
- 16.Su X, Howell AB, D’Souza H. Antibacterial effects of plant-derived extracts on methicillin-resistant Staphylococcus aureus . Foodborne Pathogens and Disease. 2012;9(6):573–578. doi: 10.1089/fpd.2011.1046. [DOI] [PubMed] [Google Scholar]
- 17.Parekh J, Chanda SV. Antibacterial activity of aqueous and alcoholic extracts of 34 Indian medicinal plants against some Staphylococcus species. Turkish Journal of Biology. 2008;32(1):63–71. [Google Scholar]
- 18.Palombo EA, Semple SJ. Antibacterial activity of traditional Australian medicinal plants. Journal of Ethnopharmacology. 2001;77(2-3):151–157. doi: 10.1016/s0378-8741(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 19. European Pharmacopoeia, 3rd edition, Council of Europe, Strasbourg, France, pp. 1323–1324, Supplement 2001.
- 20. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Twenty-First Informational Supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, Pa, USA, 2011.
- 21.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus . Journal of Clinical Microbiology. 2005;43(10):5026–5033. doi: 10.1128/JCM.43.10.5026-5033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillai SK, Eliopoulos GM, Moellering RC. Antimicrobial combination. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2005. pp. 365–409. [Google Scholar]
- 23.Lee JY, Oh WS, Ko KS, et al. Synergy of arbekacin-based combinations against vancomycin hetero-intermediate Staphylococcus aureus . Journal of Korean Medical Science. 2006;21(2):188–192. doi: 10.3346/jkms.2006.21.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. Journal of Ethnopharmacology. 2010;130(1):107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Paknejadi M, Foroohi F, Yousefzadi M. Antimicrobial activities of the essential oils of five Salvia species from Tehran province, Iran. Journal of Paramedical Sciences. 2012;3(2):12–18. [Google Scholar]
- 26.Longaray Delamare AP, Moschen-Pistorello IT, Artico L, Atti-Serafini L, Echeverrigaray S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chemistry. 2007;100(2):603–608. [Google Scholar]
- 27.Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Brazilian Journal of Microbiology. 2000;31(4):247–256. [Google Scholar]
- 28.Rovinsky SA, Cizadlo GR. Salvia divinorum Eplig et Jativa-M. (Labiatae): an ethnopharmacological investigation. The McNair Scholarly Review. 1998;3:142–156. [Google Scholar]
- 29.Kuźma Ł, Rózalski M, Walencka E, Rózalska B, Wysokińska H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci . Phytomedicine. 2007;14(1):31–35. doi: 10.1016/j.phymed.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Maisnier-Patin S, Forni E, Richard J. Purification, partial characterisation and mode of action of enterococcin EFS2, an antilisterial bacteriocin, produced by a strain of Enterococcus faecalis isolated from a cheese. International Journal of Food Microbiology. 1996;30(3):255–270. doi: 10.1016/0168-1605(96)00950-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhou K, Zhou W, Li P, Liu G, Zhang J, Dai Y. Mode of action of pentocin 31-1: an antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control. 2008;19(8):817–822. [Google Scholar]
- 32.Denyer SP, Hugo WB. Biocide-induced damage to the bacterial cytoplasmic membrane. In: Denyer SP, Hugo WB, editors. Mechanisms of Action of Chemical Biocides: Their Study and Exploitation. Oxford, UK: Blackwell Scientific Publications; 1991. pp. 171–187. [Google Scholar]
- 33.Horne D, Holm M, Oberg C, Chao S, Young DG. Antimicrobial effects of essential oils on Streptococcus pneumoniae . Journal of Essential Oil Research. 2001;13(5):387–392. [Google Scholar]
- 34.Andrews RE, Parks LW, Spence KD. Some effects of Douglas fir terpenes on certain microorganisms. Applied and Environmental Microbiology. 1980;40(2):301–304. doi: 10.1128/aem.40.2.301-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onawunmi GO, Ogunlana EO. Effects of lemon grass oil on the cells and spheroplasts of Escherichia coli NCTC 9001. Microbios Letters. 1985;28(110):63–68. [Google Scholar]
- 36.Carson CF, Mee BJ, Riley TV. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrobial Agents and Chemotherapy. 2002;46(6):1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.1, 2013.
- 38.Martineau F, Picard FJ, Lansac N, et al. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis . Antimicrobial Agents and Chemotherapy. 2000;44(2):231–238. doi: 10.1128/aac.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strommenger B, Kettlitz C, Werner G, Witte W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus . Journal of Clinical Microbiology. 2003;41(9):4089–4094. doi: 10.1128/JCM.41.9.4089-4094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zmantar T, Chaieb K, Ben Abdallah F, et al. Multiplex PCR detection of the antibiotic resistance genes in Staphylococcus aureus strains isolated from auricular infections. Folia Microbiologica. 2008;53(4):357–362. doi: 10.1007/s12223-008-0055-5. [DOI] [PubMed] [Google Scholar]
- 41.Horiuchi K, Shiota S, Kuroda T, Hatano T, Yoshida T, Tsuchiya T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis . Biological and Pharmaceutical Bulletin. 2007;30(2):287–290. doi: 10.1248/bpb.30.287. [DOI] [PubMed] [Google Scholar]
- 42.Zhao WH, Hu ZQ, Hara Y, Shimamura T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2002;46(7):2266–2268. doi: 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu ZQ, Zhao WH, Asano N, et al. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy. 2002;46(2):558–560. doi: 10.1128/AAC.46.2.558-560.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiota S, Shimizu M, Mizusima T, et al. Restoration of effectiveness of β-lactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from rose red. FEMS Microbiology Letters. 2000;185(2):135–138. doi: 10.1111/j.1574-6968.2000.tb09051.x. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Shiota S, Mizushima T, et al. Marked potentiation of activity of β-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrobial Agents and Chemotherapy. 2001;45(11):3198–3201. doi: 10.1128/AAC.45.11.3198-3201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esimone CO, Iroha IR, Ibezim EC, Okeh CO, Okpana EM. In vitro evaluation of the interaction between tea extracts and penicillin G against Staphylococcus aureus . African Journal of Biotechnology. 2006;5(11):1082–1086. [Google Scholar]
- 47.Pinho MG, Lencastre H, Tomasz A. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(19):10886–10891. doi: 10.1073/pnas.191260798. [DOI] [PMC free article] [PubMed] [Google Scholar]


