Abstract
Purpose
To assess the long-term effects of prolonged-release (PR) fampridine tablets (dalfampridine extended release) in clinical practice in patients with multiple sclerosis (MS) with walking impairment.
Patients and methods
MS patients with walking impairment deemed candidates for treatment with PR-fampridine tablets were included in this case series. Clinical assessments included the Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk (T25FW), 12-item Multiple Sclerosis Walking Scale (MSWS-12), EuroQoL-5D, and the Fatigue Severity Scale (FSS). The T25FW was videotaped at each visit. Assessments were performed at baseline and after 4 weeks of treatment with PR-fampridine tablets 10 mg twice daily. Clinical benefit of treatment was defined as any improvement in T25FW or MSWS-12 score at 4 weeks. Patients who demonstrated clinical benefit continued treatment and were assessed at 3 and 6 months.
Results
Among all patients (N = 67; mean MS duration, 16.5 years; mean EDSS score, 4.8; mean T25FW, 13.9 seconds), 65, 52, and 48 completed the 4-week, 3-month, and 6-month visits, respectively. After 4 weeks, 50.7% and 32.8% of patients walked ≥10% and ≥20% faster, respectively; and in 65.7% of patients, MSWS-12 scores improved. Three patients experienced adverse events (nausea, n = 2, insomnia, n = 1) that resulted in discontinuation of treatment. After 6 months, 38.8% and 16.4% of patients walked ≥10% and ≥20% faster versus baseline, respectively; and in 59.7% of patients, MSWS-12 scores improved. Among patients who demonstrated clinical benefit of treatment at 6 months, FSS scores improved on average by 1 point and MSWS-12 scores by 10 points. Three case studies showing different outcomes of PR-fampridine treatment are detailed with a visual depiction of the changes observed.
Conclusion
In this case series, a proportion of patients demonstrated a clinical benefit of PR-fampridine treatment on walking. Determining which patients derive benefit from PR-fampridine is an important aspect of treatment. A range of clinical and patient-reported factors should be considered when assessing the clinical benefit of PR-fampridine treatment in MS patients.
Keywords: walking, treatment, multiple sclerosis, video
Introduction
Multiple sclerosis (MS) is a degenerative disease of the central nervous system that results in a range of disabilities in different functional domains, including mobility, vision, mood, and cognition.1 One of the hallmarks of MS is walking impairment,2 which affects up to 90% of patients as the disease progresses.3–5 Fatigue, leg weakness, spasticity, lack of balance and coordination, slowness of movement, and impaired sensory function contribute to walking impairment in MS.2,5 According to a study by Heesen et al,6 walking is the most highly valued functional domain in patients with MS. Although mobility is a general concept in that it refers to a change in body position and not just walking, the two are closely related.7,8 Nearly all patients with MS (93%) report problems with their mobility within 10 years of diagnosis, and 76% of patients consider mobility a significant problem.5 Indeed, studies have shown that mobility and walking impairments, including slower walking speed, impact health-related quality of life and the ability to work and perform activities of daily living.9–13
Prolonged-release (PR) fampridine tablets (Fampyra®; Biogen Idec, Maidenhead, Berkshire, UK; known as fampridine sustained or modified release in some countries and as dalfampridine extended release tablets [Ampyra®, Acorda Therapeutics, Ardsley, NY, USA] in the United States) are chemically known as 4-aminopyridine (4-AP), which is a voltage-dependent potassium channel blocker.14 The beneficial effects of PR-fampridine are believed to arise from blockade of voltage-gated potassium channels, leading to improved conduction in demyelinated nerves.14 Pooled analyses of two Phase III clinical trials of PR-fampridine showed that 38% of PR-fampridine-treated patients had consistently faster walking speed on the Timed 25-Foot Walk15 (T25FW; Timed-Walk Responders) compared with 9% of placebo-treated patients (P < 0.001).16 Furthermore, improvement in walking speed in PR-fampridine Timed-Walk Responders was associated with improvement in patient-perceived walking ability on the 12-item Multiple Sclerosis Walking Scale (MSWS-12)17 and in leg muscle strength assessed using the Lower Extremity Manual Muscle Test (LEMMT).18,19 In July 2011, PR-fampridine received conditional approval from the European Medicines Agency (EMA) for the improvement of walking in adult patients with MS with walking disability (Expanded Disability Status Scale [EDSS] 4.0–7.0).20 The European label requires that patients be reassessed for clinical benefit after 2 weeks of treatment with PR-fampridine and recommends the use of a timed test of walking, such as the T25FW.20 The label indicates that treatment should be discontinued in patients who do not demonstrate improvement or in patients who do not report a benefit from treatment.20
The ability to evaluate changes in walking is important to identify which patients are benefiting from treatment with PR-fampridine, and a range of clinical and patient-reported factors may be relevant when assessing clinical benefit of treatment. The objective of this case series was to assess the long-term clinical benefits of PR-fampridine in daily clinical practice using a variety of assessments, as well as physician observation and patient-perceived benefit. This report provides an overview of 67 patients from a single clinic in Austria who were consecutively treated with PR-fampridine and evaluated using a variety of assessment scales over the course of 6 months in a clinical practice setting. In addition, three case studies demonstrating differing results of PR-fampridine treatment on walking impairment are highlighted, providing a detailed description and video representations of the clinical changes observed in these patients. This information may assist clinicians as they assess clinical benefit in their patients who are being treated with PR-fampridine.
Methods
Patients
MS patients with walking impairment who were being treated at the MS Clinic of the Clinical Department of Neurology at Innsbruck Medical University were included in this case series. Patients who were determined to be candidates for treatment with PR-fampridine based on physician assessment at a regularly scheduled visit were consecutively included in this case series. MS patients who did not have walking impairment or those in whom PR-fampridine was contraindicated (eg, history of epilepsy or seizures, renal insufficiency) were not eligible for treatment. All patients provided informed consent to participate and to have their T25FW tests video recorded. Three patients representative of different clinical manifestations of treatment with PR-fampridine on walking (ie, definitive improvement, some improvements, and no improvement) were randomly chosen as case reports and asked for their informed consent to publish their videos.
Case series design
Treatment began in June 2011, prior to the finalization of the EMA label for PR-fampridine, thus before the requirement for the 2-week reassessment was established. All patients were evaluated before starting treatment (baseline) with PR-fampridine 10 mg twice daily taken 12 hours apart and assessed for clinical benefit of treatment after 4 weeks. Patients demonstrating clinical benefit at the 4-week visit continued treatment and were further assessed at 3 and 6 months. Assessments at each visit included the EDSS,21 T25FW,15 MSWS-12,17 EuroQoL-5D (EQ-5D),22 and, if fatigue was present, the Fatigue Severity Scale (FSS).23 The T25FW was not assessed in patients whose walking disability prevented them from performing the test according to the instructions. The T25FW was videotaped for all patients undergoing this assessment at each visit. In addition, patients were asked about their current MS disease status (eg, relapses, worsening, stable), whether they had experienced any changes in walking ability, and for detailed descriptions of these changes. Patients were also asked whether they had experienced any adverse events, including specific questions regarding adverse events in the PR-fampridine label (eg, seizure, nausea, dizziness, insomnia), and about any new concomitant treatments (eg, drugs, physiotherapy). Clinical benefit of PR-fampridine treatment was determined by assessment of walking speed on the T25FW, self-reported walking ability as assessed by the MSWS-12, and patient interview after 4 weeks of treatment. A clinical benefit from treatment at this time point was defined as any improvement on the T25FW or on the MSWS-12.
Outcome measures
The EDSS is a rating scale that assesses the degree of neurologic disability in eight functional systems (pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual, cerebral, and other) in patients with MS.21 The scale ranges from 0 (normal) to 10 (death due to MS) and the middle range of the scale (4.0–7.0) relies heavily on assessment of walking ability. An EDSS of 4.0 is indicative of significant walking disability (cannot walk more than 500 m without rest or a walking aid) and an EDSS of 7.0 is indicative of severe walking disability (cannot walk farther than 5 m with a walking aid).21 The T25FW assesses how much time in seconds it takes a patient to walk, with or without a walking aid, as quickly as they can along a well-marked 25-foot (7.62 m) linear course.15 Walking speed in m/second was calculated by dividing 7.62 m by the number of seconds it took the patient to complete the test. The MSWS-12 is a 12-item questionnaire that assesses patient-perceived walking ability and evaluates the impact of MS on walking speed, distance, and various other parameters related to walking quality.17 Item scores are summed and transformed onto a 0 to 100 scale, with higher scores indicating greater limitations in walking ability. The EQ-5D descriptive system is a generic self-administered questionnaire that assesses patient’s health state (no problems, some problems, or extreme problems) in five dimensions: mobility, self-care, pain, usual activities, and anxiety.22 Higher scores indicate worse health status. The three potential responses on each dimension were scored as follows: 1 = no problems, 2 = some problems, and 3 = extreme problems. The mean score was calculated by summing the response scores across the five dimensions and dividing by 5. Higher scores indicate greater impact on individual health status. The FSS measures the impact of fatigue on daily life and consists of nine items that are rated on a 7-point Likert-like scale.23 Scores across all nine items were averaged, with higher scores indicating greater impact of fatigue.
Statistical analyses (means, ranges, and standard deviations) and the significance of group differences (P-values, 95% confidence intervals) from baseline to each visit were evaluated using a Student’s t-test using IBM SPSS software (version 18.0; IBM Corporation, Armonk, NY, USA) and Microsoft Excel 2008 (version 12.3.5; Microsoft Corporation, Redmond, WA, USA). Statistical significance was defined as P < 0.05. The proportion of patients demonstrating clinical benefit was calculated based on the total number of patients who were treated (N = 67) using three different criteria: (1) ≥10% improvement in walking speed on the T25FW; (2) ≥20% improvement in walking speed on the T25FW; or (3) improvement in patient-reported walking ability as assessed by the MSWS-12. Any improvement in MSWS-12 score was considered improvement in patient-reported walking ability. For each criterion, the total number of patients who were treated (N = 67) was used rather than the number of patients assessed at each visit to provide an overall description of the rate of clinical response based on the initial cohort of patients treated.
To explore the relationship between patient characteristics at baseline and response to PR-fampridine, baseline clinical and demographic characteristics were evaluated in patients grouped by outcomes on walking assessments after 4 weeks or 6 months of PR-fampridine treatment. The following outcome groups were evaluated: no clinical benefit of treatment, defined as no improvement in T25FW and no improvement in MSWS-12 score at 4 weeks; moderate clinical benefit of treatment, defined as <10% improvement in T25FW and <6-point improvement in MSWS-12 score at 4 weeks or 6 months; and substantial clinical benefit of treatment, defined as >20% improvement in T25FW and ≥6-point improvement in MSWS-12 score at 4 weeks or 6 months.
Results
Summary results for the overall population
A total of 67 patients in the clinic who were consecutively treated with PR-fampridine were included in this case series. Demographic and clinical characteristics of patients prior to treatment with PR-fampridine are shown in Table 1. The majority (74.6%) of patients had a progressive form of MS (secondary-progressive or primary-progressive MS) and the mean disease duration was 16.5 years. None of the patients had a relapse in the 3 months prior to treatment. The mean EDSS score was 4.8 (range, 3.0–8.0) and patients completed the T25FW in a mean ± standard deviation of 13.9 ± 8.9 seconds (range, 5.2–47.1 seconds) at baseline. All patients had some level of walking impairment that affected walking speed, since the median time to complete the T25FW has been reported as 3.7 seconds in healthy volunteers (range, 2.8–5.2 seconds).24 About half of the patients were receiving disease-modifying drugs and/or treatment for spasticity. One patient had previously used compounded 4-AP for 2 months without any clinical benefit. Symptoms characteristic of MS were common in this cohort of patients, particularly those that potentially affect walking ability such as imbalance, lower limb weakness, and muscle tightness.
Table 1.
Demographic and MS disease characteristics at baseline
| Characteristic | Patients (N = 67)* |
|---|---|
| Age, mean (SD) y | 47.8 (8.4) |
| Women, n (%) | 46 (68.7) |
| MS disease course, n (%) | n = 67 |
| Secondary-progressive | 34 (50.8) |
| Relapsing-remitting | 17 (25.4) |
| Primary-progressive | 16 (23.9) |
| Progressive-relapsing | 0 |
| Disease duration, mean (SD) y | 16.5 (7.3) |
| Number of relapses in past 1 y | |
| Mean (SD) | 0.09 (0.29) |
| Range | 0–1 |
| Treatment of MS, n (%) | |
| MS disease-modifying therapy | 34 (50.7) |
| Therapy/drugs for spasticity | 35 (52.2) |
| History of 4-aminopyridine use | 1 (1.5) |
| Current rehabilitation therapy | 42 (62.7) |
| EDSS score | n = 67 |
| Mean (SD) | 4.8 (1.6) |
| Range | 3.0–8.0 |
| T25FW time, sec | n = 54 |
| Mean (SD) | 13.9 (8.9) |
| Range | 5.2–47.1 |
| MSWS-12 score | n = 67 |
| Mean (SD) | 77 (19) |
| Range | 2–100 |
| EQ-5D descriptive system score | n = 66 |
| Mean (SD) | 1.7 (0.3) |
| Range | 1.0–2.6 |
| FSS score | n = 38 |
| Mean (SD) | 5.2 (1.4) |
| Range | 1.2–7.0 |
| MS symptoms, n (%) | |
| Fatigue | 52 (77.6) |
| Vertigo | 18 (26.9) |
| Imbalance | 63 (94.0) |
| Lower limb pain | 26 (38.8) |
| Loss of sensation in lower limb | 41 (61.2) |
| Lower limb weakness | 48 (71.6) |
| Lower limb muscle tightness | 51 (76.1) |
| Lower limb tremor | 4 (6.0) |
| Lower limb spasticity | 34 (50.7) |
| Heat intolerance | 54 (80.6) |
| Cerebellar symptoms | 49 (73.1) |
Note:
Except where noted.
Abbreviations: EDSS, Expanded Disability Status Scale; EQ-5D, EuroQol-5D; FSS, Fatigue Severity Scale; MS, multiple sclerosis; MSWS-12, 12-item Multiple Sclerosis Walking Scale; SD, standard deviation; sec, seconds; T25FW, Timed 25-Foot Walk; y, year.
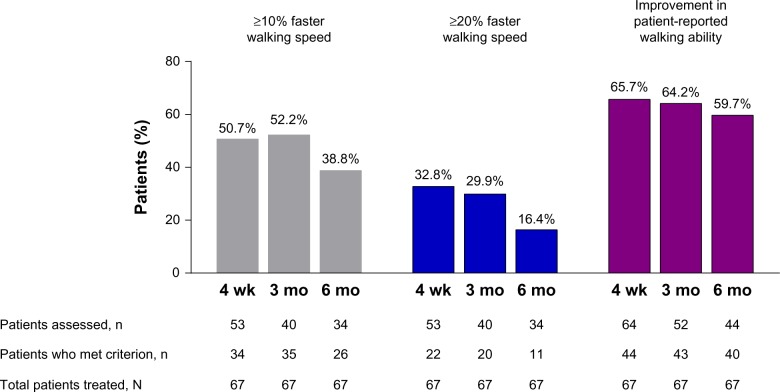
Among the 67 patients who were treated, 65 completed the 4-week visit, 52 completed the 3-month visit, and 48 completed the 6-month visit. PR-fampridine treatment was stopped in three (4.5%) patients due to adverse events (nausea [n = 2], insomnia [n = 1]) and treatment was discontinued in 16 (23.9%) patients due to lack of clinical benefit (Figure 1). The three adverse events were deemed moderate in severity and resolved within 3 days of discontinuing PR-fampridine. At the 4-week visit, 50.7% of patients demonstrated clinical benefit based on a ≥10% improvement in walking speed and 32.8% had a ≥20% improvement in walking speed (Figure 2). A higher proportion (65.7%) of patients demonstrated clinical benefit of PR-fampridine treatment after 4 weeks when improvement in patient-reported walking ability was considered (Figure 2). Patients experiencing clinical benefit typically reported improvement 2 weeks after starting treatment with PR-fampridine. The proportion of patients demonstrating clinical benefit was higher across all time points when clinical benefit was based on improvement in patient-reported walking ability versus improvement in walking speed (Figure 2).
Figure 1.
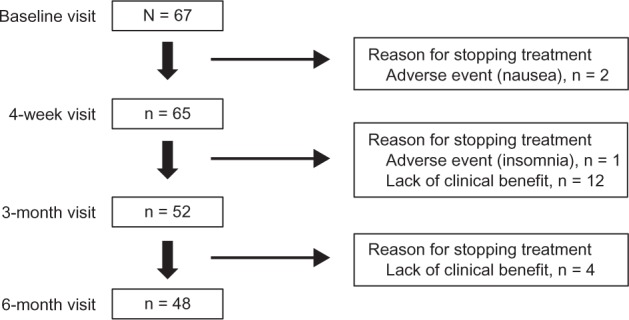
Patient disposition over 6 months.
Figure 2.
The effect of PR-fampridine on walking over 6 months.
Notes: Patients were treated with PR-fampridine 10 mg twice daily over 6 months. Walking speed was assessed using the T25FW; patient-reported walking ability was assessed using the MSWS-12. Any improvement in MSWS-12 score was considered improvement in patient-reported walking ability. For the T25FW, thresholds of ≥10% and ≥20% improvement in walking speed were used. The total number of patients who were treated with PR-fampridine (N = 67) was used to calculate the proportions of patients who demonstrated improvement in walking speed or patient-reported walking at each visit to provide an overall description of the rate of clinical response based on the initial cohort of patients treated.
Abbreviations: mo, month; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR, prolonged-release; T25FW, Timed 25-Foot Walk; wk, week.
Over the 6-month assessment period, the mean EDSS score remained stable in patients who continued treatment with PR-fampridine; two patients experienced a relapse. Among all patients (N = 67), 38.8% (26/67) of patients walked ≥10% faster, 16.4% (11/67) of patients walked ≥20% faster, and patient-reported walking ability improved in 59.7% (40/67) of patients at the 6-month visit compared with baseline (Figure 2). Among all patients assessed using the MSWS-12 after 6 months of continued treatment (n = 44), patient-reported walking ability improved by approximately 10 points (Table 2), which exceeds the four to six-point threshold used in previous studies to define a clinically meaningful change.25 Patient-reported fatigue also improved on average by one point (Table 2). Although this observational clinical practice case series was not powered to show statistical significance, the change from baseline to each follow-up visit was significant for all outcomes, with the exception of the FSS at the 4-week visit (Table 2).
Table 2.
Results of clinical assessments in the overall population over time
| Assessment | Baseline (N = 67)
|
4 wk (n = 65)
|
3 mo (n = 52)
|
6 mo (n = 48)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | P-value (95% CI)* | n | Mean (SD) | P-value (95% CI)* | n | Mean (SD) | P-value (95% CI)* | |
| T25FW | |||||||||||
| Time, sec | 54 | 13.9 (8.9) | 53 | 12.2 (10.0) | P < 0.03 (9.4, 15.0) | 40 | 9.7 (6.0) | P < 0.0001 (7.8, 11.6) | 35 | 10.4 (7.9) | P < 0.001 (7.7, 13.1) |
| Speed, m/sec | 54 | 0.55 (0.86) | 53 | 0.62 (0.76) | P < 0.0001 (0.58, 0.83) | 40 | 0.79 (1.27) | P < 0.0001 (0.72, 1.03) | 35 | 0.73 (0.95) | P < 0.0001 (0.69, 0.97) |
| MSWS-12 score | 67 | 77 (19) | 64 | 66 (20) | P < 0.0001 (61, 71) | 52 | 64 (20) | P < 0.01 (58.3, 69.7) | 44 | 66 (23) | P < 0.0001 (59, 73) |
| EQ-5D score | 66 | 1.7 (0.3) | 64 | 1.6 (0.3) | P < 0.001 (1.6, 1.7) | 51 | 1.7 (0.3) | P < 0.01 (1.6, 1.8) | 46 | 1.6 (0.3) | P < 0.001 (1.5, 1.7) |
| FSS score | 38 | 5.2 (1.4) | 36 | 4.7 (1.5) | P < 0.06 (4.2, 5.2) | 30 | 4.2 (1.5) | P < 0.01 (3.7, 4.8) | 26 | 4.2 (1.4) | P < 0.01 (3.6, 4.7) |
Notes:
Statistical analyses (P-values and 95% CIs) refer to group difference between baseline and the respective follow-up visit. Statistical significance was defined as P < 0.05.
Abbreviations: CI, confidence interval; EQ-5D, EuroQoL-5D descriptive system; FSS, Fatigue Severity Scale; mo, month; MSWS-12, 12-item Multiple Sclerosis Walking Scale; SD, standard deviation; sec, second; T25FW, Timed 25-Foot Walk; wk, week.
The baseline characteristics of patients grouped based on the extent of clinical benefit of PR-fampridine at 4 weeks or 6 months are shown in Table 3. Minimal differences were observed in baseline characteristics between the outcome groups, suggesting that it is difficult to predict whether a patient will experience a clinical benefit of PR-fampridine before initiating treatment. There may be a tendency for treatment response in patients with slightly shorter disease duration and a slightly faster T25FW at baseline; however, the differences were small and not clinically relevant to confidently predict treatment outcome.
Table 3.
Baseline characteristics of patients grouped by the level of clinical benefit of PR-fampridine treatment at week 4 or month 6
| All patients* | No clinical benefit of treatment (no improvement on T25FW and MSWS-12)
|
Moderate clinical benefit of treatment (<10% improvement on T25FW and <6 points on MSWS-12)
|
Substantial clinical benefit of treatment (>20% improvement on T25FW and ≥6 points on MSWS-12)
|
|||
|---|---|---|---|---|---|---|
| Wk 4 | Wk 4 | Mo 6 | Wk 4 | Mo 6 | ||
| Patients, n | N = 67 | n = 16 | n = 12 | n = 7 | n = 22 | n = 11 |
| Age, y | 47.8 | 46.7 | 45.3 | 50.0 | 48.9 | 47.6 |
| Women, % | 68.7 | 50.0 | 66.7 | 75.7 | 50.0 | 54.5 |
| MS disease course, n (%) | ||||||
| Secondary-progressive | 34 (50.8) | 11 (68.7) | 6 (50.0) | 3 (42.8) | 9 (40.9) | 5 (45.4) |
| Relapsing-remitting | 17 (25.4) | 3 (18.8) | 5 (41.7) | 2 (28.6) | 6 (27.3) | 3 (27.3) |
| Primary-progressive | 16 (23.9) | 2 (12.5) | 1 (8.3) | 2 (28.6) | 7 (31.8) | 3 (27.3) |
| MS duration, y | 16.5 | 16.3 | 15.6 | 15.8 | 14.7 | 14.0 |
| EDSS score | 4.8 | 5.1 | 4.3 | 4.7 | 4.8 | 4.6 |
| T25FW, sec | 13.9 | 16.4 | 12.2 | 15.5 | 15.1 | 13.5 |
| MSWS-12 score | 77.0 | 80.3 | 74.3 | 71.8 | 76.5 | 74.8 |
Notes:
Values are the mean unless otherwise noted. Patients with an intermediate clinical benefit of treatment (≥10% but ≤20% improvement on T25FW) are not shown.
Abbreviations: EDSS, Expanded Disability Status Scale; mo, month; MS, multiple sclerosis; MSWS-12, 12-item Multiple Sclerosis Walking Scale; PR, prolonged-release; sec, second; T25FW, Timed 25-Foot Walk; wk, week; y, year.
Case report 1: definitive improvement in walking
Patient #1 was a 54-year-old white man who had MS for 15 years and secondary-progressive MS for the past 3 years. He had previously received antispasticity drugs, steroids, and rehabilitation therapy, but not compounded 4-AP or disease-modifying therapies for MS. The patient reported progression of his walking impairment over the last year, limitations in walking distance without an aid (150 m), and that he used two walking sticks for longer distances. He reported several MS-related symptoms (fatigue, imbalance, lower limb weakness and muscle tightness, heat intolerance, cerebellar symptoms, and urinary hesitancy and urgency) and that work exhausted him. He was currently receiving oral baclofen 25 mg twice daily for spasticity and physical rehabilitation therapy once weekly for an hour. Physician assessment at baseline noted that his walking ability, particularly walking distance and balance, were impaired due to spastic paresis in the left leg and ataxia (see Supplementary Video Patient #1). Clinical examination showed an EDSS score of 5.5, slight spasticity but markedly diminished muscle strength (LEMMT grade 3 out of 5) in his left leg, and a T25FW time of 17.1 seconds (walking speed, 0.45 m/second).
At the 4-week visit, patient #1 reported noticeable improvement in his walking ability within the first few days after starting PR-fampridine treatment (eg, walked faster, walked farther without exhaustion, and climbed stairs more easily). The patient no longer used his walking sticks for routine walking, and only required them for longer distances to help him maintain his balance. He reported that others also noticed improvement in his walking ability. This patient reported no adverse events following initiation of PR-fampridine. Clinical assessment performed at 4 weeks showed a dramatic improvement in T25FW walking speed (64.4%) and improvement in MSWS-12, EQ-5D, and FSS scores (Table 4). Visually, the overall quality of the patient’s walking had improved (eg, walking was smoother, less spastic, more effortless, and more balanced) and he appeared more confident (see Supplementary Video Patient #1). Based on physician assessment and patient report, Patient #1 was considered to have clear clinical benefit of PR-fampridine treatment.
Table 4.
Results of clinical assessments in the three case studies over time
| Assessment | Baseline | 4 wk | 3 mo | 6 mo |
|---|---|---|---|---|
| Patient #1 (54-year-old white man with SPMS, EDSS score 5.5) | ||||
| T25FW | ||||
| Time, sec | 17.1 | 10.3 | 7.5 | 7.6 |
| Speed, m/sec | 0.45 | 0.74 | 1.02 | 1.00 |
| Change in walking speed from baseline,* % | – | +64.4 | +126.7 | +122.2 |
| MSWS-12 score | 83 | 56 | 54 | 58 |
| EQ-5D score | 1.8 | 1.8 | 1.4 | 1.2 |
| FSS score | 5.67 | 5.11 | 4.67 | 4.33 |
| Patient #2 (49-year-old white woman with SPMS, EDSS score 4.0) | ||||
| T25FW | ||||
| Time, sec | 7.1 | 6.6 | 6.2 | 5.9 |
| Speed, m/sec | 1.07 | 1.15 | 1.23 | 1.29 |
| Change in walking speed from baseline,* % | – | +7.5 | +15.0 | +20.6 |
| MSWS-12 score | 77 | 50 | 50 | 48 |
| EQ-5D score | 1.4 | 1.4 | 1.4 | 1.4 |
| FSS score | 6.78 | 6.33 | 4.44 | 5.11 |
| Patient #3 (39-year-old white woman with SPMS, EDSS score 6.5) | ||||
| T25FW | ||||
| Time, sec | 29.4 | 52.7 | NA | NA |
| Speed, m/sec | 0.26 | 0.14 | NA | NA |
| Change in walking speed from baseline,* % | – | −46.2 | NA | NA |
| MSWS-12 score | 65 | 65 | NA | NA |
| EQ-5D score | 1.4 | 2.0 | NA | NA |
| FSS score | 3.56 | 5.78 | NA | NA |
Notes:
Positive (+) percentage change from baseline in walking speed indicates faster walking speed compared with baseline; negative (−) percentage change from baseline indicates slower walking speed compared with baseline. Patients who did not demonstrate clinical benefit of prolonged-release fampridine after 4 weeks of treatment discontinued treatment and were not followed-up on.
Abbreviations: EDSS, Expanded Disability Status Scale; EQ-5D, EuroQoL-5D descriptive system; FSS, Fatigue Severity Scale; mo, month; MSWS-12, 12-item Multiple Sclerosis Walking Scale; NA, not available; sec, second; SPMS, secondary-progressive multiple sclerosis; T25FW, Timed 25-Foot Walk; wk, week.
By the 3-month visit (see Supplementary Video Patient #1), the patient had further improvement in walking speed, patient-reported walking ability, and overall health status (Table 4). The patient no longer had spasticity in his left leg, so baclofen was discontinued, and his left leg strength had improved (LEMMT grade 4 out of 5). The patient reported that he had been on vacation, was more physically active than in the past 3 years, and his walking endurance was better. He reported feeling “safer” when walking and was satisfied with the improvements in his walking.
At the 6-month visit (see Supplementary Video Patient #1), improvements in walking ability were maintained from the 3-month visit (Table 4), and the patient still had no spasticity in his left leg, and improvements in lower left leg strength were sustained. Patient #1 was pleased with his walking ability versus before treatment with PR-fampridine, and he felt that his walking impairment was no longer progressing but instead had improved.
Case report 2: some improvements in walking
Patient #2 was a 49-year-old white woman with a 27-year history of MS and secondary-progressive MS for the past 2 years. She had previously received intramuscular interferon β-1a, steroids, and rehabilitation therapy, but not compounded 4-AP. She was only receiving rehabilitation therapy once weekly at the time of the baseline visit. Patient #2 reported several MS-related symptoms (fatigue; imbalance; heat intolerance; and loss of sensation, weakness, muscle tightness, and spasticity in her lower limbs) and that her walking speed and distance had deteriorated over the past 2 years. She sometimes tripped and felt her walking was powerless and exhausting, which aggravated her existing fatigue. Her walking impairment made activities of daily living more difficult and exhausting to perform. Clinical examination at baseline revealed an EDSS score of 4.0; walking impairments that impacted distance, endurance, and balance; spastic paresis in her left leg, and slightly diminished left leg strength (LEMMT grade 4 out of 5); and a T25FW time of 7.1 seconds (walking speed, 1.07 m/second: see Supplementary Video Patient #2).
After 4 weeks of treatment with PR-fampridine, the patient reported that some days she noticed improvement in her walking speed and balance and experienced less exhaustion, but there were other days where she did not notice these improvements. She reported no change in fatigue. She wanted to continue treatment despite the lack of a consistent benefit. Clinical assessment at the 4-week follow-up visit found slight (7.5%) improvement in T25FW walking speed, a clinically meaningful 27-point improvement in MSWS-12 score (Table 4 and see Supplementary Video Patient #2), and no changes in leg spasticity and strength. Based on physician assessment and patient report, this patient was considered to demonstrate modest clinical benefit with PR-fampridine treatment. It was agreed that the patient should continue treatment with PR-fampridine and be re-evaluated at the next visit.
At the 3-month visit, the patient showed a 15% improvement in T25FW walking speed versus baseline and sustained improvements in MSWS-12 score (Table 4). Furthermore, the patient reported that her walking endurance and balance had more clearly improved, her walking was smoother, and overall she felt more confident and powerful in her walking. She could now walk for 30 minutes without interruption, resting, or stopping. She reported that her fatigue had improved and she could participate more actively in daily tasks and leisure activities, which made her happy. Physician assessment showed no change in lower limb spasticity and strength from baseline, further improvement in T25FW walking speed versus baseline, and sustained improvement in MSWS-12 score (Table 4). Additionally, improvements in FSS score at 3 months compared with baseline reflected the patient’s verbal report of improvements in fatigue. The patient reported that mild dizziness, which she had initially reported at the 4-week visit, had resolved; however, she still experienced exacerbation of constipation that was an issue before starting PR-fampridine.
At the 6-month visit the patient reported that previous improvements in walking were sustained over the last 3 months of treatment and overall her walking was better with improved balance and self-confidence. Clinical assessments confirmed the patient report, which showed additional improvement in walking speed after 6 months of treatment (20.6% versus baseline), maintenance of benefits on patient-perceived walking ability, and fatigue (Table 4), and smoother walking as assessed by the treating physician versus baseline (see Supplementary Video Patient #2). The patient also reported that her constipation was better and she was no longer experiencing dizziness.
Case report 3: worsening of walking and decreased walking speed
Patient #3 was a 39-year-old white woman with a 26-year history of MS and secondary-progressive MS for the past 9 years. She had previously received intramuscular interferon β-1a, intravenous immunoglobulin, baclofen for spasticity, steroids, and rehabilitation therapy, but had not been treated with compounded 4-AP. Patient #3 reported fatigue; heat intolerance; cerebellar symptoms; vertigo; imbalance; loss of vision associated with nystagmus; and pain, parathesias, loss of sensation, weakness, spasticity, and muscle tightness in her legs. The patient had sensory impairment, imbalance, limitations in leg muscle strength, and bilateral visual impairment that together contributed to a severe walking impairment and made it difficult for her to walk a great distance. She reported that she could walk a maximum of 20 m using two walking crutches. The patient was currently receiving rehabilitation therapy but not disease-modifying therapy for MS or drugs for spasticity. Clinical assessment revealed that she had severe walking impairment due to spastic paraparesis and ataxia (see Supplementary Video Patient #3), an EDSS score of 6.5, severe spasticity in both legs, proximal and distal paraparesis (LEMMT grades 4 out of 5 and 2 out of 5, respectively), and a T25FW time of 29.4 seconds (walking speed, 0.26 m/second).
After 4 weeks of treatment with PR-fampridine, patient #3 reported experiencing dizziness, confusion, and difficulties with short-term memory tasks after initiating PR-fampridine. She had a slower walking speed on the T25FW (decrease of 46.2%), no change on the MSWS-12, and worse fatigue and overall health status (Table 4 and see Supplementary Video Patient #3). She continued to have severe spasticity in both legs, and her paraparesis remained unchanged proximally but worsened distally (LEMMT grade 1 out of 5). Interestingly, this patient did report an improvement in her vision and physician assessment at the 4-week visit found no detectable nystagmus. Based on physician assessment and patient report, this patient was considered to have no clinical benefit with PR-fampridine treatment and she reported potentially treatment-related side effects. Treatment with PR-fampridine was stopped and the patient was no longer followed-up on.
Discussion
The range of improvements with PR-fampridine varies among patients, as evidenced by this case series. Among the 67 patients who were treated, 60% demonstrated clinical benefit of PR-fampridine treatment based on improvement in patient-reported walking ability alone, and 39% demonstrated clinical benefit of treatment based on $10% improvement in walking speed after 6 months of treatment. In this case series, the extent of the clinical benefit of PR-fampridine was not predictable based on the demographic or clinical characteristics of patients or the level of walking disability at baseline. Furthermore, post hoc analyses of the Phase III clinical trial data demonstrated that response to PR-fampridine was consistent across a range of patient baseline characteristics and the presence or absence of a range of MS-related symptoms (eg, heat intolerance) at baseline.16,26,27 The case studies presented in detail describe three different results of PR-fampridine treatment in the clinical setting. Patient #1 demonstrated clear clinical benefit of PR-fampridine treatment on walking based on increases in objectively measured walking speed and patient-reported benefits that appeared within a few weeks of initiating treatment. For patient #2, the benefits of PR-fampridine on walking took longer to become apparent. Although clinical benefits of PR-fampridine treatment were modest at the 4-week visit, patient #2 showed clear improvements in walking speed, self-reported walking ability, and fatigue at the 3-month visit that were sustained at the 6-month visit. Patient #3 did not experience any clinical benefits of PR-fampridine treatment after 4 weeks and experienced potentially treatment-related adverse events, providing a clear example of a patient who should discontinue treatment.
There is no single method to assess the clinical benefit of PR-fampridine treatment. Defining improvements in walking ability should not be limited to a single clinical assessment, but rather should also include patient-reported assessment of benefit. A patient-reported assessment such as the MSWS-12 score or the patient narrative should be the fundamental basis for judging improvement in walking ability. This is similar to the assessment of neurologic symptoms such as pain, headache, and unspecified vertigo, in which the neurologist must rely on patient-reported assessment of the severity or a change in the symptom. In most cases and particularly over the long-term, physician-based assessment using the T25FW is consistent with the patient-reported clinical benefit of treatment with PR-fampridine. Thus, it is important for neurologists to document patient-reported outcomes and ask patients specific questions to elicit information about different dimensions of walking, including strength, endurance, balance, speed, falls, fatigability, feeling insecure, and overall confidence in and perception of their walking ability. Physicians should also assess the impact of any of these symptoms on patients’ activities of daily living and quality of daily life and consider using the MSWS-12 and the T25FW or another short-format test of walking speed (eg, 10-Meter Walk Test)28 to assess the clinical benefit of PR-fampridine on walking. Both assessments take just a few minutes and are easy to perform in daily routine clinical practice. Furthermore, because of the importance of patient-reported outcomes and the ultimate goal of improving health-related quality of life, it should be assessed after 6 months of treatment to document any change.
Clinical benefits of treatment with PR-fampridine should generally become apparent after 2 weeks.20 In the current case series, patient #1 reported clinical benefits of PR-fampridine treatment that occurred within a few days of initiating treatment. In contrast, the clinical benefits experienced by patient #2 were modest at the 4-week visit but became more apparent at the 3- and 6-month visits. The reason behind the variability in response to PR-fampridine in people with MS is not clear.29 There has been some speculation that the variability could be attributed to lesion location, with responders having demyelinated lesions in areas of the central nervous system relevant to walking, or that it may be a result of some unknown genetic differences in potassium channel subunits that produce different drug susceptibilities.29
When assessing patients over time, one must keep in mind the progressive nature of MS and the underlying worsening of walking ability.30 In placebo-treated patients with secondary-progressive MS (EDSS score range, 3.5–6.5) in the IMPACT study, walking speed declined on average by 10% after 12 months and 19% after 24 months.30 Because the magnitude of walking improvements may decrease over time compared with their original baseline status potentially due to MS disease progression, patients may still be benefiting from treatment in that their walking ability is improved in comparison to what it would be without treatment, although it may have worsened in comparison to pretreatment levels. If a decline in walking ability is observed, physicians should consider an interruption to treatment in order to reassess the benefits of PR-fampridine.20 The re-evaluation should include withdrawal of treatment and subsequent assessment of walking ability, including a walking test.20 PR-fampridine should be discontinued if patients no longer receive a walking benefit.20
The T25FW, or another similar short-format timed walking test, is useful for objectively measuring the benefit of PR-fampridine treatment in the clinical setting.31 Improvement in walking speed may be associated with improvement in a patient’s ability to be productive and undertake activities of daily living.5,11,12 Some patients experience improvement in self-perceived walking ability with or without remarkable changes in walking speed that may be apparent on visual inspection or are reported by the patient, similar to what was observed with patient #2. Patients who show no benefit to PR-fampridine across clinical factors and who do not report any improvement in walking should stop treatment. Determining adequate clinical benefit of any MS therapy requires physicians to use their judgment based on a range of clinical factors, including patient self-report and the current clinical presentation of the patient, in addition to the information provided by MS-specific assessment scales.
This was an open-label clinical practice case series of patients from a single MS clinic, and, thus, the results may be subject to several sources of bias. To minimize the selection bias, patients were enrolled in a consecutive manner after being deemed candidates for treatment with PR-fampridine. This was a small population that was not randomly selected and was, therefore, subject to sample bias. The results observed in this case series may not be generalizable to other clinics or the overall population of patients with MS with walking impairment. Although patient interviews were standardized, information gained during these interviews may have been prone to recall error on behalf of the patient or interviewer bias. Despite the potential for bias, this case series provides useful information about the long-term treatment of MS patients with walking impairment with PR-fampridine in a real-world setting.
In the current case series, five of the 67 patients with walking impairment who were treated with PR-fampridine had a baseline EDSS score just outside the indicated range in the final EMA label. There are currently no known clinical characteristics to predict if a patient will experience a clinical benefit of PR-fampridine, thus, it is unlikely that these few patients influenced the observed rate of clinical benefit to PR-fampridine. In fact, a post hoc analysis of data from the Phase III clinical studies of PR-fampridine showed consistency of response to PR-fampridine in terms of the proportion of patients who were Timed-Walk Responders (the definition of response from Phase III studies) among patients with EDSS scores of 5.5 or less, 6.0, or 6.5 or more.16 Additionally, not all improvements in these patients can be necessarily attributable to PR-fampridine treatment because some improvement may be a result of the normal variability of MS symptoms over time. Furthermore, there is the possibility that some improvements were due to a placebo effect because all patients knowingly received active treatment; however, the prolonged clinical benefits on walking observed in this case series over a period of 6 months appear unlikely to be wholly a result of a placebo effect.
Conclusion
Walking impairment is common in patients with MS and treatment of walking impairment is cited as an unmet need. PR-fampridine may provide improvements in walking ability for patients with MS based on objective clinical measures, patient-reported outcome measures, or informal patient report of benefit. Determining which patients are benefiting from treatment with PR-fampridine is an important aspect for the appropriate management of MS patients with walking impairment. The case reports presented here illustrate the range of clinical and patient-reported factors that can be used to assess clinical benefit of PR-fampridine in MS patients with walking impairment.
Acknowledgments
Biogen Idec provided funding for editorial support in the development of this paper; Alison Gagnon from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Joanne King from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Videos were recorded by the authors; video editing services were provided by Nigel Cowley at Just Communicate Ltd and were funded by Biogen Idec. Biogen Idec reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper, and provided their final approval of all content.
Footnotes
Disclosure
PR-fampridine was provided by Biogen Idec. Michael Prugger reports no conflicts of interest in this work. Thomas Berger has participated as a consultant in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Allergan, Allmiral, AOP, Baxter, Bayer, Biogen Idec, Biotest, CSL Behring, Merck Serono, Novartis, ratiopharm, Sanofi-Aventis, and Teva. Thomas Berger and his institution have received financial support by unrestricted research grants from Allergan, AOP, Biogen Idec, Berlex, Bayer, Biotest, CSL Behring, Merck Serono, Novartis, ratiopharm, and Sanofi-Aventis and for participation in clinical trials in multiple sclerosis sponsored by Bayer, Biogen Idec, Merck Serono, Novartis, Octapharma, Roche, Sanofi-Aventis, and Teva. The authors report no other conflicts of interest in this work.
References
- 1.NINDS Multiple Sclerosis Information Page [webpage on the Internet] Bethesda, MD: National Institute of Neurological Disorders and Stroke; 2013Available from: http://www.ninds.nih.gov/disorders/multiple_sclerosis/multiple_sclerosis.htmAccessed May 16, 2012 [Google Scholar]
- 2.Panitch H, Applebee A. Treatment of walking impairment in multiple sclerosis: an unmet need for a disease-specific disability. Expert Opin Pharmacother. 2011;12(10):1511–1521. doi: 10.1517/14656566.2011.586338. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 4.Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914–1929. doi: 10.1093/brain/awq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Asch P. Impact of mobility impairment in multiple sclerosis 2 – patient perspectives. Eur Neurol Rev. 2011;6(2):115–120. [Google Scholar]
- 6.Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14(7):988–991. doi: 10.1177/1352458508088916. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther. 2011;35(1):26–33. doi: 10.1097/NPT.0b013e3182097190. [DOI] [PubMed] [Google Scholar]
- 8.Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin. 2010;26(1):109–119. doi: 10.1185/03007990903433528. [DOI] [PubMed] [Google Scholar]
- 9.Jones CA, Pohar SL, Warren S, Turpin KV, Warren KG. The burden of multiple sclerosis: a community health survey. Health Qual Life Outcomes. 2008;6:1. doi: 10.1186/1477-7525-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajagopalan K, Jones EC, Pike J, Jackson J. Emergence of walking and mobility problems among patients with multiple sclerosis and its impact on quality of life: a cross-sectional assessment in 5 EU countries. Mult Scler. 2010;16(Suppl 10):S335. Available from: http://msj.sagepub.com/content/16/10_suppl/S7.full.pdf+html (page S335) [Google Scholar]
- 11.Pike J, Jones E, Rajagopalan K, Piercy J, Anderson P. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol. 2012;12:94. doi: 10.1186/1471-2377-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yildiz M. The impact of slower walking speed on activities of daily living in patients with multiple sclerosis. Int J Clin Pract. 2012;66(11):1088–1094. doi: 10.1111/ijcp.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther. 2009;26(12):1043–1057. doi: 10.1007/s12325-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 14.Blight AR. Treatment of walking impairment in multiple sclerosis with dalfampridine. Ther Adv Neurol Disord. 2011;4(2):99–109. doi: 10.1177/1756285611403960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5(4):244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 16.Stourac P, Putzki N. Prolonged-release fampridine shows consistent efficacy across sub-groups of multiple sclerosis patients. J Neurol. 2012;259:S152. [Google Scholar]
- 17.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60(1):31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Goodman AD, Brown TR, Edwards KR, et al. MSF204 Investigators A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68(4):494–502. doi: 10.1002/ana.22240. [DOI] [PubMed] [Google Scholar]
- 19.Goodman AD, Brown TR, Krupp LB, et al. Fampridine MS-F203 Investigators Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373(9665):732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- 20.Biogen Idec Limited Fampyra 10 mg prolonged-release tablets. [summary of product characteristics] Full prescribing information Maidenhead, UK: Biogen Idec Limited; 2011Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002097/WC500109956.pdfAccessed December 21, 2012 [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.EuroQoL Group [homepage on the Internet] EQ-5D: a standardized instrument for use as a measure of health outcome EuroQoL Group; Rotterdam, the Netherlands: 2012Available from: http://www.euroqol.org/Accessed December 21, 2012 [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Phan-Ba R, Pace A, Calay P, et al. Comparison of the timed 25-foot and the 100-meter walk as performance measures in multiple sclerosis. Neurorehabil Neural Repair. 2011;25(7):672–679. doi: 10.1177/1545968310397204. [DOI] [PubMed] [Google Scholar]
- 25.Hobart J. Prolonged-release fampridine for multiple sclerosis: was the effect on walking ability clinically significant? Mult Scer. 2010;16(Suppl 10):S172. Available from: http://msj.sagepub.com/content/16/10_suppl/S7.full.pdf+html (page S172) [Google Scholar]
- 26.Ziemssen T, Berger T, Potts J, Putzki N, Goodman A. Relationship between heat intolerance and response to prolonged-release fampridine in patients with multiple sclerosis. Neurology. 2012;78(Suppl 1):P07.078. [Google Scholar]
- 27.Short C, Putzki N, Goodman A. Response to sustained-release fampridine in multiple sclerosis patients with various walking-related MS symptoms. Neurology. 2012;78(Suppl 1):P07.079. [Google Scholar]
- 28.Vaney C, Blaurock H, Gattlen B, Meisels C. Assessing mobility in multiple sclerosis using the Rivermead Mobility Index and gait speed. Clin Rehabil. 1996;10(3):216–226. [Google Scholar]
- 29.Goodman AD, Stone RT. Enhancing neural transmission in multiple sclerosis (4-aminopyridine therapy) Neurotherapeutics. 2013;10(1):106–110. doi: 10.1007/s13311-012-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan A, Goodman AD, Potts J, Cohen J. Health-related quality of life is reduced in multiple sclerosis patients whose walking speed declines over time. Neurology. 2012;78(Suppl 1):P07.096. [Google Scholar]
- 31.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. 2012;18(7):914–924. doi: 10.1177/1352458512444498. [DOI] [PubMed] [Google Scholar]