Abstract
The use of plants as natural medicines in the treatment of type II diabetes mellitus (T2DM) has long been of special interest. In this work, we developed a docking score-weighted prediction model based on drug-target network to evaluate the efficacy of medicinal plants for T2DM. High throughput virtual screening from chemical library of natural products was adopted to calculate the binding affinity between natural products contained in medicinal plants and 33 T2DM-related proteins. The drug-target network was constructed according to the strength of the binding affinity if the molecular docking score satisfied the threshold. By linking the medicinal plant with T2DM through drug-target network, the model can predict the efficacy of natural products and medicinal plant for T2DM. Eighteen thousand nine hundred ninety-nine natural products and 1669 medicinal plants were predicted to be potentially bioactive.
1. Introduction
Type II Diabetes mellitus (T2DM) has been a major global health problem and affects a large population worldwide [1, 2]. T2DM is a multifactorial and genetically heterogeneous disease caused by various risk factors such as insulin resistance, β-cell dysfunction, and obesity [2–5]. Moreover, T2DM may cause acute cardiovascular disease, retinopathy, nephropathy, neuropathy, and kidney-related complications [5–7]. Therefore, it demands effective drugs with minimal toxicity. The herbal medicines have been used for T2DM for thousands of years and accumulated a great deal of clinical experience. A herbal formula comprises several medicinal plants or animals and thus can affect the biological system through interactions between compounds and cellular targets [3, 8–17]. The main mechanisms of herbal medicines in treating T2DM are that it increases insulin secretion and the sensitivity of insulin, inhibits glucose absorption, and reduces radicals caused by lipid peroxidation [8]. However, the major problem of herbal medicines is lack of scientific and clinical data to evaluate their efficacy and safety.
Network pharmacology proposed by Hopkins is a holistic approach to understand the function and behavior of a biological system at systems level in the context of biological networks and would be the next paradigm for drug discovery [18–20]. Several efforts have been made to explore the mechanism of herbal medicines such as prediction of the active ingredients and potential targets [21–26] and screening synergistic drug combinations [11, 27, 28]. The drug-target network (DTN) which connects drugs and their target proteins is an important biological network and provides an overview of polypharmacology of drugs [29–32]. Since medicinal plants have multiple compounds and a compound would have several target proteins, the DTN may bridge the gap between medicinal plants and diseases. In this work, we developed a computational approach based on DTN to evaluate the efficacy of medicinal plants.
2. Materials and Methods
2.1. Data Collection and Molecular Docking
The pathogenesis of T2DM is concerned with various proteins. We retrieved the information of these proteins from KEGG Pathway database [33] and DrugBank [34] (Figure 1). The pathway of T2DM was downloaded from the KEGG website (http://www.genome.jp/dbget-bin/www_bget?hsa04930), and the information of T2DM-related proteins was collected. In DrugBank, we first retrieved the FDA-approved drugs for T2DM and then found the target proteins for each drug. Then we searched the ligand-protein complex structure (x-ray or NMR) for each protein from RCSB protein data bank (http://www.rcsb.org/pdb/home/home.do). Finally, thirty-three proteins and their information were listed in Table 1.
Figure 1.
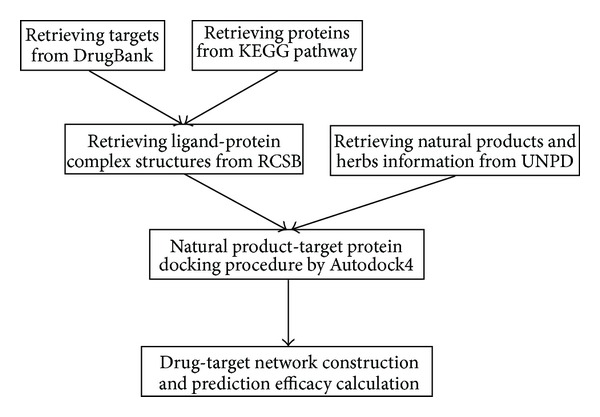
The work flow of this approach.
Table 1.
List of 33 proteins related with T2DM for molecular docking.
| Index | UniProt entry | PDB entry | Protein name |
|---|---|---|---|
| 1 | O43451 | 3CTT | Maltase-glucoamylase, intestinal |
| 2 | P01308 | 1TYM | Insulin |
| 3 | P01375 | 2AZ5 | Tumor necrosis factor alpha |
| 4 | P04150 | 3H52 | Glucocorticoid receptor |
| 5 | P04746 | 1XDO | Pancreatic alpha-amylase |
| 6 | P05121 | 3UT3 | Plasminogen activator inhibitor 1 |
| 7 | P06213 | 3EKN | Insulin receptor |
| 8 | P07339 | 1LYW | Cathepsin D |
| 9 | P08069 | 3I81 | Insulin-like growth factor 1 receptor |
| 10 | P11474 | 3K6P | Steroid hormone receptor ERR1 |
| 11 | P12821 | 3L3N | Angiotensin-converting enzyme |
| 12 | P13569 | 3GD7 | Cystic fibrosis transmembrane conductance regulator |
| 13 | P14410 | 3LPP | Sucrase-isomaltase, intestinal |
| 14 | P14618 | 3BJF | Pyruvate kinase isozymes M1/M2 |
| 15 | P14735 | 3E4A | Insulin-degrading enzyme |
| 16 | P19367 | 1DGK | Hexokinase-1 |
| 17 | P27361 | 2ZOQ | Mitogen-activated protein kinase 3 |
| 18 | P27487 | 3G0D | Dipeptidyl peptidase 4 |
| 19 | P27986 | 4A55 | Phosphatidylinositol 3-kinase regulatory subunit alpha |
| 20 | P28482 | 3I5Z | Mitogen-activated protein kinase 1 |
| 21 | P30613 | 2VGF | Pyruvate kinase isozymes R/L |
| 22 | P35557 | 3IMX | Glucokinase |
| 23 | P35568 | 2Z8C | Insulin receptor substrate 1 |
| 24 | P37231 | 3H0A | Peroxisome proliferator-activated receptor gamma |
| 25 | P42336 | 3HHM | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform |
| 26 | P42345 | 1FAP | Serine/threonine-protein kinase mTOR |
| 27 | P43220 | 3C59 | Glucagon-like peptide 1 receptor |
| 28 | P45983 | 3PZE | Mitogen-activated protein kinase 8 |
| 29 | P45984 | 3NPC | Mitogen-activated protein kinase 9 |
| 30 | P48736 | 3SD5 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform |
| 31 | P53779 | 3TTI | Mitogen-activated protein kinase 10 |
| 32 | P62508 | 2P7A | Estrogen-related receptor gamma |
| 33 | Q9BYF1 | 1R4L | Angiotensin-converting enzyme 2 |
The 3D structures of natural products contained in medicinal plants were retrieved from the Universal Natural Product Database (UNPD) which comprised more than 208 thousands of natural products [54, 55]. The AutoDock 4.0 [56, 57] was adopted to perform the virtual screening, and binding free energy-based docking score (pK i) was used to evaluate the affinity between each compound and each protein. For each protein, the hetero atoms of the ligand-protein complex structure were deleted and the polar hydrogen atoms were added. The binding site of each protein was defined as a 40 × 40 × 40 Å cube around the original ligand with a spacing of 0.375 Å between the grid points. The center of binding site was located in the center of the original ligand. The molecular docking was conducted according to the protocol described previously [58].
2.2. Drug-Target Network Construction and Analysis
The drug-target network was constructed by linking the compound with target protein if the docking score satisfied the thresholds that were used to determine whether the interaction between compound and protein was strong. According to our previous study, the thresholds were set as follow: the docking score should be greater than 7.00 and the score of original ligand of corresponding protein and the top percentage of rank of docking score should be less than 10% [54]. The edge value was the docking score of corresponding compound and protein. Finally, the DTN consisted of 32 target proteins, 18999 compounds (the UNPD ID, chemical name, formula, molecular weight, and CAS registry number of each compound were listed in Table S1, see Table S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2013/203614), and 35076 edges (Supplementary Table S2). The glucocorticoid receptor (P04150) did not have any compounds. The compounds were derived from 1669 medicinal plants distinguished by Latin names. The DTN of potentially active compounds and proteins related with T2DM was used as a bridge to build the relationship between compound or medicinal plant and T2DM.
2.3. Chemical Space Analysis
The analysis of the distribution of compounds in the chemical space was conducted by principal component analysis (PCA) module in Discovery Studio. The PCA model was built with 8 descriptors: A log P, molecular weight, number of hydrogen-bond donors, number of hydrogen-bond acceptors, number of rotatable bonds, number of rings, number of aromatic rings, and molecular fractional polar surface area. The variances of PC1, PC2, and PC3 for compounds in Figure 2 were 0.488, 0.186, and 0.145, respectively. The PCA of 25 FDA-approved small-molecule drugs retrieved from DrugBank was performed in the same process as above.
Figure 2.
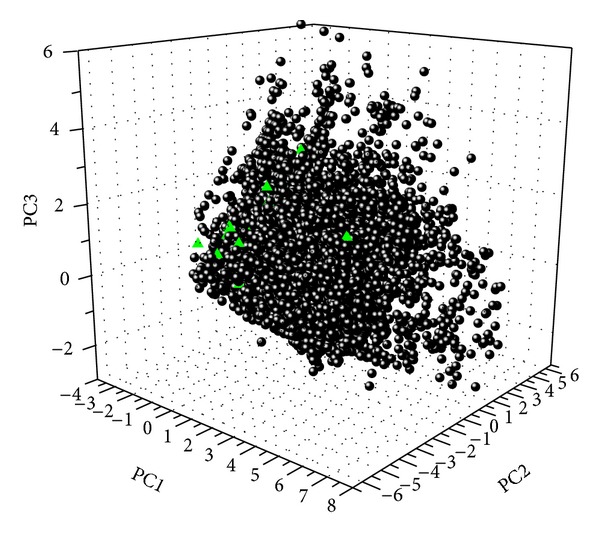
The distribution in chemical space according to PCA of natural products contained in medicinal plants and 25 FDA-approved drugs for T2DM. The black dots and green triangles represent natural products and FDA-approved drugs, respectively.
2.4. Prediction Model
Natural products are multitarget agents. The average number of target proteins was 1.84 in the DTN. Therefore, we proposed that the prediction efficacy (PE) of a compound for T2DM was the sum of its all edge values (docking scores) in the DTN:
| (1) |
where P was the set of proteins related to T2DM and scorej was the docking score between this compound and jth protein. The PEcompound for each compound was listed in Table S3.
Similarly, the prediction efficacy of a medicinal plant was defined as the sum of PE of compounds contained in this plant:
| (2) |
where N denoted the number of compounds contained in the medicinal plant. The PEplant for each medicinal plant was listed in Table S4.
3. Results and Discussion
3.1. Drug-Likeness of Medicinal Natural Products for T2DM
The natural products contained in medicinal plants for T2DM had good drug-like properties. Lipinski CA and colleagues proposed the “rule of five” (molecular weight (MW) less than 500 Da, the number of hydrogen bond acceptors (HBA) less than 10, the number of hydrogen bond donors (HBD) less than 5, and octanol-water partition coefficient (A log P) less than five) [59, 60] to estimate solubility and permeability of compounds in drug discovery. That is, a compound was unlikely to be a drug if it disobeyed the rules. The mean and median of MW, HBA, HBD, and A log P of these compounds were 540.43, 494.62; 6.3, 5; 2.5, 2; and 4.94, 5.07; respectively. It indicated that most compounds would be drug-like. The wide distribution of natural products in chemical space (Figure 2) showed that there would be vast property (structural and functional) diversity. Moreover, the large overlap between natural products and 25 FDA-approved small-molecule drugs for T2DM demonstrated that natural products contained in these medicinal plants had a hopeful prospect for drug discovery for T2DM.
3.2. Prediction Efficacy of Natural Product and Medicinal Plant
Herb medicines could simultaneously target multiple physiological processes through interactions between multiple compounds and cellular target proteins. For example, there were 105 distinct compounds contained in Hypericum perforatum, and 21 compounds existed in DTN. The herbal medicines could influence the biological system through interactions between multi-component and multi-target and thus reverse the biological networks from disease state to health state. Since a group of compounds contained in the herbal medicine could play a therapeutic role, the dosage could be reduced to reduce toxicity and side effects. For example, UNPD43323 (ormojine), UNPD194973 (ormosinin), and UNPD194973 (strychnohexamine) were the top three potential compounds (Supplementary Table S3). ormojine, ormosinin, and strychnohexamine had 27, 24, and 23 targets, respectively. The polypharmacology of natural products was very common.
The predicted efficacy of the top twenty medicinal plants for T2DM was listed in Table 2. There were five plants (Hypericum perforatum, Ganoderma lucidum, Holarrhena antidysenterica, Celastrus orbiculatus, and Murraya euchrestifolia) where prediction efficacy was higher than 1000. We searched the literatures which reported the anti-T2DM bioactivities of the top twenty medicinal plants (Table 2) and found that 15 medicinal plants had information of definite effectiveness against T2DM. For example, Arokiyaraj and colleagues evaluated the antihyperglycemic activity of Hypericum perforatum in diabetic rats, and it produced significant reduction in plasma glucose level [35].
Table 2.
Top twenty potential medicinal plants.
| Rank | Latin name | PEplant | Reported bioactivity |
|---|---|---|---|
| 1 | Hypericum perforatum | 1777.81 | [35, 36] |
| 2 | Ganoderma lucidum | 1560.05 | [37] |
| 3 | Holarrhena antidysenterica | 1147.22 | [38, 39] |
| 4 | Celastrus orbiculatus | 1089.44 | N/A |
| 5 | Murraya euchrestifolia | 1066.97 | N/A |
| 6 | Melia azedarach | 980.47 | [40] |
| 7 | Datura metel | 894.36 | [41, 42] |
| 8 | Ficus microcarpa | 837.65 | [43] |
| 9 | Tripterygium wilfordii | 785.30 | [44] |
| 10 | Pachysandra terminalis | 740.38 | N/A |
| 11 | Calendula officinalis | 729.77 | [45] |
| 12 | Vitis vinifera | 719.77 | [46] |
| 13 | Melia toosendan | 711.49 | N/A |
| 14 | Mangifera indica | 677.08 | [47] |
| 15 | Piper nigrum | 667.41 | [48] |
| 16 | Solanum dulcamara | 667.12 | [49] |
| 17 | Garcinia hanburyi | 641.41 | N/A |
| 18 | Momordica charantia | 632.37 | [50, 51] |
| 19 | Lantana camara | 625.64 | [52] |
| 20 | Ceriops tagal | 623.13 | [53] |
3.3. Clinical Herbal Formula
Tangminling which was a widely used herbal formula in China to treat T2DM comprised eleven medicinal herbs (Trichosanthes kirilowii, Citrus sinensis, Bupleurum chinense, Rheum officinale, Astragalus membranaceus, Pinellia ternata, Scutellaria discolor, Crataegus pinnatifida var. major, Paeonia albiflora, Prunus mume, and Picrorhiza kurroa) [3]. The prediction efficacy of each medicinal plant was 493.04, 199.26, 36.06, 29.08, 15.12, 14.80, 7.83, 7.09, 7.07, 7.06, and 7.04, respectively. It indicated that all plants could play a role in the treatment of T2DM. However, the prediction efficacy of eleven herbs differed considerably from each other. It meant that Trichosanthes kirilowii and Citrus sinensis played major roles (sovereign herbs). Meanwhile, The others worked as assistants which may strengthen the efficacy of sovereign herbs or reduce the toxicity.
4. Conclusions
Medicinal plants are potentially important for novel therapeutic drugs. It is currently estimated that approximately 420,000 plant species exist in nature [61]. However, only 10,000 of all plants have documented medicinal use [62]. Therefore, there are potentially many more important pharmaceutical applications of plants to be exploited. Traditional method (from selecting plants to separating compounds following bioassay) is time-consuming. In this work, we developed a molecular docking score-weighted prediction model based on drug-target network to evaluate the efficacy of natural products and medicinal plants for T2DM. Natural products contained in the medicinal plants would target several cellular target proteins. The prediction efficacy of this model took into account all potential interactions between multicomponents and targets. Therefore, the prediction efficacy was an overall evaluation at systems level. Fifteen out of the top twenty medicinal plants had reported bioactivity against T2DM in literatures. This approach may promote the research on the use of medicinal plants to treat T2DM and drug discovery from natural products.
Supplementary Material
The supplementary materials comprise four tables of large datasets. Table S1 listed the identification information of 18999 natural products. Table S2 listed the natural products-target proteins interaction network (DTN). Table S3 and Table S4 listed the prediction efficacy of natural products and medicinal plants for T2DM, respectively.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This work was financially supported by the National Key Special Project of Science and Technology for Innovation Drugs (Grant nos. 2012ZX09501001-004 and 2013ZX09402202). The calculations were performed on TianHe-1(A) at the National Supercomputer Center in Tianjin.
References
- 1.Lin Y, Sun Z. Current views on type 2 diabetes. Journal of Endocrinology. 2010;204(1):1–11. doi: 10.1677/JOE-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. The Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 3.Gu J, Zhang H, Chen L, Xu S, Yuan G, Xu X. Drug-target network and polypharmacology studies of a Traditional Chinese Medicine for type II diabetes mellitus. Computational Biology and Chemistry. 2011;35(5):293–297. doi: 10.1016/j.compbiolchem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta U, Ukil S, Dimitrova N, Agrawal S. Expression-based network biology identifies alteration in key regulatory pathways of type 2 diabetes and associated risk/complications. PLoS ONE. 2009;4(12) doi: 10.1371/journal.pone.0008100.e8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SC., Jr. Multiple risk factors for cardiovascular disease and diabetes mellitus. The American Journal of Medicine. 2007;120(3):S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. The Lancet. 2008;371(9626):1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian S, Li Y, Li D, et al. Modeling compound-target interaction network of traditional chinese medicines for type II diabetes mellitus: insight for polypharmacology and drug design. Journal of Chemical Information and Modeling. 2013;53(7):1787–1803. doi: 10.1021/ci400146u. [DOI] [PubMed] [Google Scholar]
- 8.Zhang TT, Jiang JG. Active ingredients of traditional chinese medicine in the treatment of diabetes and diabetic complications. Expert Opinion on Investigational Drugs. 2012;21(11):1625–1642. doi: 10.1517/13543784.2012.713937. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H-L, Tong PCY, Chan JCN. Traditional Chinese medicine in the treatment of diabetes. Nutritional Management of Diabetes Mellitus and Dysmetabolic Syndrome. 2006;11:15–29. doi: 10.1159/000094399. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Lu C, Jiang M, et al. Traditional chinese medicine-based network pharmacology could lead to new multicompound drug discovery. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11 pages. doi: 10.1155/2012/149762.149762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zhang B, Zhang N. Network target for screening synergistic drug combinations with application to traditional Chinese medicine. BMC Systems Biology. 2011;( 1)(1, article S10) doi: 10.1186/1752-0509-5-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma T, Tan C, Zhang H, Wang M, Ding W, Li S. Bridging the gap between traditional Chinese medicine and systems biology: the connection of Cold Syndrome and NEI network. Molecular BioSystems. 2010;6(4):613–619. doi: 10.1039/b914024g. [DOI] [PubMed] [Google Scholar]
- 13.Li S. Network systems underlying traditional Chinese medicine syndrome and herb formula. Current Bioinformatics. 2009;4(3):188–196. [Google Scholar]
- 14.Wu X, Jiang R, Zhang MQ, Li S. Network-based global inference of human disease genes. Molecular Systems Biology. 2008;4, article 189 doi: 10.1038/msb.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian S, Li YY, Wang JM, et al. Drug-likeness analysis of traditional chinese medicines: 2. Characterization of scaffold architectures for drug-like compounds, non-drug-like compounds, and natural compounds from traditional chinese medicines. Journal of Cheminformatics. 2013;5(1) doi: 10.1186/1758-2946-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen MY, Tian S, Li YY, et al. Drug-likeness analysis of traditional chinese medicines: 1. Property distributions of drug-like compounds, non-drug-like compounds and natural compounds from traditional chinese medicines. Journal of Cheminformatics. 2012;4(1, article 31) doi: 10.1186/1758-2946-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S, Wang JM, Li YY, et al. Drug-likeness analysis of traditional chinese medicines: 3. Prediction of drug-likeness using machine learning approaches. Molecular Pharmaceutics. 2012;9(10):2875–2886. doi: 10.1021/mp300198d. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins AL. Network pharmacology. Nature Biotechnology. 2007;25(10):1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biology. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Zhang B. Traditional chinese medicine network pharmacology: theory, methodology and application. Chinese Journal of Natural Medicines. 2013;11(2):110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 21.Tao WY, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of chinese herbal radix curcumae formula for application to cardiovascular disease. Journal of Ethnopharmacology. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Li S. Network-based relating pharmacological and genomic spaces for drug target identification. PLoS ONE. 2010;5(7) doi: 10.1371/journal.pone.0011764.e11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu J, Chen Y, Li S, Li Y. Identification of responsive gene modules by network-based gene clustering and extending: application to inflammation and angiogenesis. BMC Systems Biology. 2010;4, article 47 doi: 10.1186/1752-0509-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei LX, Bao YW, Liu S, et al. Material basis of chinese herbal formulas explored by combining pharmacokinetics with network pharmacology. Plos ONE. 2013;8(2) doi: 10.1371/journal.pone.0057414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang A, Sun H, Yang B, Wang X. Predicting new molecular targets for rhein using network pharmacology. BMC Systems Biology. 2012;6, article 20 doi: 10.1186/1752-0509-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhao J, Jiang P, Zhang W. Molecular networks for the study of TCM pharmacology. Briefings in Bioinformatics. 2009;11(4):417–430. doi: 10.1093/bib/bbp063. [DOI] [PubMed] [Google Scholar]
- 27.Yan H, Zhang B, Li S, Zhao Q. A formal model for analyzing drug combination effects and its application in TNF-α-induced NFκB pathway. BMC Systems Biology. 2010;4, article 50 doi: 10.1186/1752-0509-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Zhang B, Jiang D, Wei Y, Zhang N. Herb network construction and co-module analysis for uncovering the combination rule of traditional Chinese herbal formulae. BMC Bioinformatics. 2010;11(11, article S6) doi: 10.1186/1471-2105-11-S11-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildirim MA, Goh K-I, Cusick ME, Barabási A-L, Vidal M. Drug-target network. Nature Biotechnology. 2007;25(10):1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 30.Janga SC, Tzakos A. Structure and organization of drug-target networks: insights from genomic approaches for drug discovery. Molecular BioSystems. 2009;5(12):1536–1548. doi: 10.1039/B908147j. [DOI] [PubMed] [Google Scholar]
- 31.Mestres J, Gregori-Puigjané E, Valverde S, Solé RV. The topology of drug-target interaction networks: implicit dependence on drug properties and target families. Molecular BioSystems. 2009;5(9):1051–1057. doi: 10.1039/b905821b. [DOI] [PubMed] [Google Scholar]
- 32.Vogt I, Mestres J. Drug-target networks. Molecular Informatics. 2010;29(1-2):10–14. doi: 10.1002/minf.200900069. [DOI] [PubMed] [Google Scholar]
- 33.Kanehisa M, Goto S, Sato Y, et al. Kegg for integration and interpretation of large-scale molecular data sets. Nucleic Acids Research. 2012;40(1):D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for “Omics” research on drugs. Nucleic Acids Research. 2011;39(1):D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arokiyaraj S, Balamurugan R, Augustian P. Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine. 2011;1(5):386–390. doi: 10.1016/S2221-1691(11)60085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasanein P, Shahidi S. Effects of Hypericum perforatum extract on diabetes-induced learning and memory impairment in rats. Phytotherapy Research. 2011;25(4):544–549. doi: 10.1002/ptr.3298. [DOI] [PubMed] [Google Scholar]
- 37.You Y-H, Lin Z-B. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmacologica Sinica. 2002;23(9):787–791. [PubMed] [Google Scholar]
- 38.Ali KM, Chatterjee K, De D, et al. Efficacy of aqueous extract of seed of holarrhena antidysenterica for the management of diabetes in experimental model rat: a correlative study with antihyperlipidemic activity. International Journal of Applied Research in Natural Products. 2009;2(3):13–21. [Google Scholar]
- 39.Mana S, Singhal S, Sharma N, Singh D. Hypoglycemic effect of Holarrhena antidysenterica seeds on streptozotocin induced diabetic rats. International Journal of PharmTech Research. 2010;2(2):1325–1329. [Google Scholar]
- 40.Samudram P, Vasuki R, Rajeshwari H, Geetha A, Sathiya Moorthi P. Antioxidant and antihepatotoxic activities of ethanolic crude extract of Melia azedarach and Piper longum. Journal of Medicinal Plant Research. 2009;3(12):1078–1083. [Google Scholar]
- 41.Krishna Murthy B, Nammi S, Kota MK, Krishna Rao RV, Koteswara Rao N, Annapurna A. Evaluation of hypoglycemic and antihyperglycemic effects of Datura metel (Linn.) seeds in normal and alloxan-induced diabetic rats. Journal of Ethnopharmacology. 2004;91(1):95–98. doi: 10.1016/j.jep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Murch SJ, Alan AR, Cao J, Saxena PK. Melatonin and serotonin in flowers and fruits of Datura metel L. Journal of Pineal Research. 2009;47(3):277–283. doi: 10.1111/j.1600-079X.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 43.Asok Kumar K, Uma Maheswari M, Sivashanmugam AT, Subhadra Devi V, Prasanth NV, Ravi TK. Hypoglycemic effect of Ficus microcarpa leaves (Chinese Banyan) on alloxan-induced diabetic rats. Journal of Biological Sciences. 2007;7(2):321–326. [Google Scholar]
- 44.Ge Y, Xie H, Li S, et al. Treatment of diabetic nephropathy with tripterygium wilfordii hook f extract: a prospective, randomized, controlled clinical trial. Journal of Translational Medicine. 2013;11, article 134 doi: 10.1186/1479-5876-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakraborthy GS, Arora R, Majee C. Antidiabetic and antihyperlipidaemic effect of hydro-alcoholic extract of calendula officinalis. International Research Journal of Pharmacy. 2011;2(1):61–65. [Google Scholar]
- 46.Orhan N, Aslan M, Orhan DD, Ergun F, Yeşilada E. In-vivo assessment of antidiabetic and antioxidant activities of grapevine leaves (Vitis vinifera) in diabetic rats. Journal of Ethnopharmacology. 2006;108(2):280–286. doi: 10.1016/j.jep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Aderibigbe AO, Emudianughe TS, Lawal BAS. Evaluation of the antidiabetic action of Mangifera indica in mice. Phytotherapy Research. 2001;15(5):456–458. doi: 10.1002/ptr.859. [DOI] [PubMed] [Google Scholar]
- 48.Kaleem M, Sheema S, Sarmad H, Bano B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian Journal of Physiology and Pharmacology. 2005;49(1):65–71. [PubMed] [Google Scholar]
- 49.Asano N, Nash RJ, Molyneux RJ, Fleet GWJ. Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry. 2000;11(8):1645–1680. [Google Scholar]
- 50.Ooi CP, Yassin Z, Hamid T-A. Momordica charantia for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews. 2010;2 doi: 10.1002/14651858.CD007845.pub2. [DOI] [PubMed] [Google Scholar]
- 51.Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. British Journal of Nutrition. 2009;102(12):1703–1708. doi: 10.1017/S0007114509992054. [DOI] [PubMed] [Google Scholar]
- 52.Kazmi I, Rahman M, Afzal M, et al. Anti-diabetic potential of ursolic acid stearoyl glucoside: a new triterpenic gycosidic ester from Lantana camara. Fitoterapia. 2012;83(1):142–146. doi: 10.1016/j.fitote.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Tiwari P, Tamrakar AK, Ahmad R, et al. Antihyperglycaemic activity of Ceriops tagal in normoglycaemic and streptozotocin-induced diabetic rats. Medicinal Chemistry Research. 2008;17(2-7):74–84. [Google Scholar]
- 54.Gu JY, Gui YS, Chen LR, et al. Use of natural products as chemical library for drug discovery and network pharmacology. Plos ONE. 2013;8(4) doi: 10.1371/journal.pone.0062839.e62839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao X, Hou T, Zhang W, Guo S, Xu X. A 3D structure database of components from Chinese traditional medicinal herbs. Journal of Chemical Information and Computer Sciences. 2002;42(3):481–489. doi: 10.1021/ci010113h. [DOI] [PubMed] [Google Scholar]
- 56.Jiang X, Kumar K, Hu X, Wallqvist A, Reifman J. DOVIS 2.0: an efficient and easy to use parallel virtual screening tool based on AutoDock 4.0. Chemistry Central Journal. 2008;2(1, article 18) doi: 10.1186/1752-153X-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris GM, Ruth H, Lindstrom W, et al. Software news and updates AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. Journal of Computational Chemistry. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu JY, Li Q, Chen LR, et al. Platelet aggregation pathway network-based approach for evaluating compounds efficacy. Evidence-Based Complementary and Alternative Medicine. 2013;2013:8 pages. doi: 10.1155/2013/425707.425707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 60.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 61.Vuorela P, Leinonen M, Saikku P, et al. Natural products in the process of finding new drug candidates. Current Medicinal Chemistry. 2004;11(11):1375–1389. doi: 10.2174/0929867043365116. [DOI] [PubMed] [Google Scholar]
- 62.McChesney JD, Venkataraman SK, Henri JT. Plant natural products: back to the future or into extinction? Phytochemistry. 2007;68(14):2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary materials comprise four tables of large datasets. Table S1 listed the identification information of 18999 natural products. Table S2 listed the natural products-target proteins interaction network (DTN). Table S3 and Table S4 listed the prediction efficacy of natural products and medicinal plants for T2DM, respectively.


