Abstract
Bone tissue is continuously renewed throughout adult life by a process called 'remodeling', which involves a dynamic interplay among bone cells including osteoclasts, osteoblasts and osteocytes. For example, a tight coupling between bone resorption and formation is essential for the homeostasis of the skeletal system. Studies on the coupling mechanism in physiological and pathological settings have revealed that osteoclasts or osteoclastic bone resorption promote bone formation through the production of diverse coupling factors. The classical coupling factors are the molecules that promote bone formation after resorption, but there may be distinct mechanisms at work in various phases of bone remodeling. A recent study revealed that the Semaphorin 4D expressed by osteoclasts inhibits bone formation, which represents a mechanism by which coupling is dissociated. Furthermore, it has been demonstrated that osteoblastic expression of Semaphorin 3A exerts an osteoprotective effect by both suppressing bone resorption and increasing bone formation. Thus, recent advances have made it increasingly clear that bone remodeling is regulated by not only classical coupling factors, but also molecules that mediate cell–cell communication among bone cells. We propose that such factors be called bone cell communication factors, which control the delicate balance of the interaction of bone cells so as to maintain bone homeostasis.
Introduction
The bony skeleton enables various crucial processes, such as locomotive activity, the storage of calcium and the harboring of hematopoietic stem cells. Bone is a dynamic organ that is continuously being broken down by osteoclasts and subsequently rebuilt with new bone by osteoblasts throughout the course of one's adult life. These activities occur in response to various hormones, cytokines, chemokines and biomechanical external stimuli.1,2 This process, called bone remodeling, is a prerequisite for the normal bone homeostasis that maintains both bone quality and strength.2 An imbalance of bone resorption and formation is often central to metabolic bone diseases in humans and animals. For example, excessive resorption by osteoclasts results in pathological bone destruction, as seen in osteoporosis, autoimmune arthritis, Paget's disease, periodontitis and bone tumors.1,3,4 Therefore, bone remodeling must be regulated both temporally and spatially so that the aged or damaged bone is replaced by an essentially equivalent amount of new bone.1,5,6 The bone remodeling is carried out within the temporary anatomic structures, so-called basic multicellular unit (BMU), which consists of a group of osteoclasts in front forming the cutting cone and a group of osteoblasts behind forming the closing cone, by associating with blood supply and the peripheral innervation.7,8 The bone remodeling is a cycle consisting of three phases: 'initiation' of bone resorption by osteoclasts, the 'transition' from resorption to new bone formation (also well known as a 'reversal' period) and the 'bone formation' (Figure 1).6,9 The entire process is achieved by the coordinated actions of osteoclasts, osteoblasts and other osteoblast lineage cells, including bone-lining cells and osteocytes. The most obvious example is the osteoclastogenesis regulatory system by receptor activator of nuclear-κB ligand (RANKL) and its decoy receptor osteoprotegerin (OPG), which are produced by osteoblast lineage cells. However, key questions remain unanswered as to how the timing and location of the specific bone formation functions are decided, how osteoprogenitor cells are recruited to the resorption site and how the amount of the bone produced is so precisely controlled. The concept of coupling mechanisms, in which bone resorption promotes the bone formation which follows, can only partially answer these questions. Furthermore, the recent studies on the role of Semaphorin 4D (Sema4D) and Semaphorin 3A (Sema3A) in bone remodeling provided the genetic evidence of the novel mechanisms to understand how osteoclasts and osteoblasts exert their functions, each by avoiding the interruption from the other. This review will summarize the current knowledge of the coupling mechanisms established by genetic studies and highlight a newly recognized mode of the regulation of bone remodeling through bone cell communication factors, which mediate cell–cell communication among bone cells.
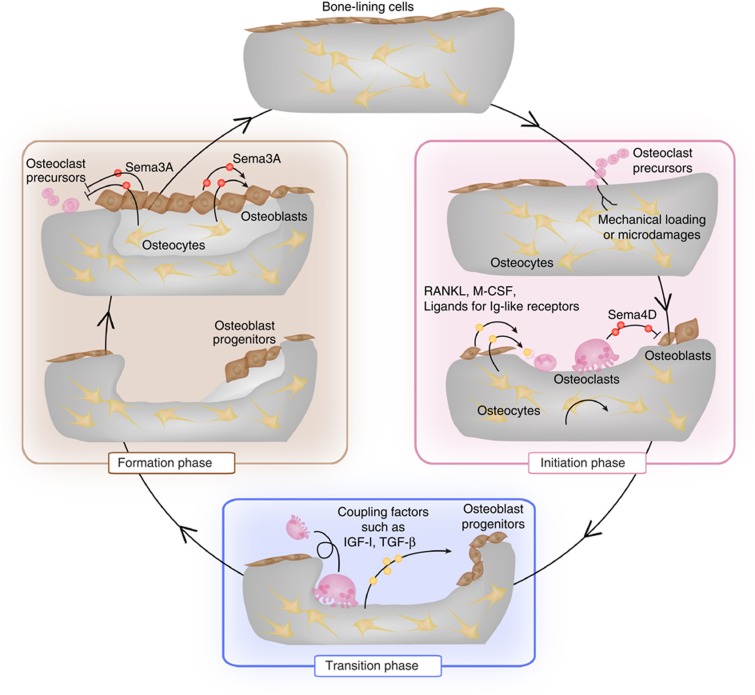
Figure 1. The bone remodeling cycle.
The bone remodeling process is divided into the initiation, transition and formation phases. In the initiation phase, mechanical loading and microdamage are sensed by osteocytes, which stimulate the recruitment of osteoclast precursor cells. Osteoclastogenesis is stimulated by the RANKL, macrophage colony-stimulating factor (M-CSF) and ligands for immunoglobulin-like receptors, which are produced by osteoblast lineage cells including osteocytes, and bone resorption starts. Osteoclasts inhibit bone formation during bone resorption through the expression of Sema4D. In the transition phase, classical coupling factors, including IGF-I and TGF-β1, stimulate the migration of osteoprogenitors to the resorbed sites and promote differentiation into osteoblasts. In the bone formation phase, osteoblasts replenish the resorbed area with new bone. Sema3A, which is produced by osteoblast lineage cells, inhibits osteoclastogenesis and simultaneously promotes bone formation in this phase.
The bone remodeling cycle
In the initiation phase, osteoclast precursor cells, which originate from cells of hematopoietic lineage, are recruited to the specific bone surface areas and differentiate into mature osteoclasts. It has long been suggested that when osteocytes sense mechanical loading or microdamage, they stimulate the recruitment of osteoclast precursor cells to the bone surface. Recent studies demonstrated that osteocytes act as major source of RANKL to promote osteoclastogenesis in adult bone remodeling,10,11 whereas RANKL expressed on osteoblasts or chondrocytes is essential for the skeletal development (Figure 1). Although stromal cells also have the ability to support osteoclastogenesis through RANKL production, the contribution of these cells as RANKL source in bone remodeling is still unclear. After having finished bone resorption, osteoclasts undergo apoptosis and the bone resorption phase is terminated.
In the subsequent transition phase, the osteoprogenitor cells of the mesenchymal lineage migrate to the resorbed sites and differentiate into bone-forming osteoblasts. The migration, differentiation and bone formation activity of osteoblasts are regulated by a large number of paracrine, autocrine and endocrine factors. These include bone morphogenetic proteins, Wnts, parathyroid hormone, prostaglandins, growth factors, such as insulin-like growth factors (IGFs), transforming growth factor-β (TGF-β), and acidic and basic fibroblast growth factors, and angiogenic factors such as vascular endothelial growth factor and endothelin-1.12 Among these, recent studies using genetically modified mice showed that the IGFs and TGF-β are stored in bone matrix function and upon release act as coupling factors, as discussed below (Figure 1).
In the formation phase, osteoblasts deposit new bone matrix called osteoid and subsequently mineralize osteoid. When the resorbed lacunae are replenished with new bone, some of the osteoblasts disappear because of apoptosis. A part of osteoblasts become quiescent as bone-lining cells on the surface of the newly formed bone, or further differentiate into osteocytes embedded in the bone matrix (Figure 1). As osteoblast lineage cells produce OPG, which potently suppresses osteoclastogenesis by masking RANKL, they may inhibit the initiation of additional bone remodeling cycle activity in the BMU. Osteocytes may also contribute to the termination of the remodeling cycle through the production of Sclerostin, which inhibits the bone formation induced by Wnt signaling in osteoblasts,13 but future studies will be needed to provide evidence for these possibilities.
Evidence for the existence of coupling mechanisms
How is bone formation stimulated after bone resorption? In 1981, Baylink and Colleagues14 first proposed the hypothesis that there are soluble coupling factors stored in the bone matrix. The coupling mechanism is now generally accepted, based on the accumulating evidence from human and mouse genetics, in addition to pharmacological studies.3,15 In the patients with osteoclast-poor osteopetrosis, the reduced bone resorption results in decreased bone formation.15 These findings support the idea that bone resorption leads to a stimulation of bone formation. On the other hand, in patients with osteoclast-rich osteopetrosis, the osteoclast number is often increased and the number of bone-forming osteoblasts is also increased.15 These patients do have osteoclasts, but they are dysfunctional because of a mutation in certain bone resorption-related genes. These findings suggest that not only bone matrix-derived but also osteoclast-derived factors stimulate osteoblast differentiation and bone formation.
Bone matrix-derived coupling factors
A number of osteotropic factors, such as IGFs, TGF-β, bone morphogenetic proteins, fibroblast growth factor and platelet-derived growth factor have been thought to function as coupling factors, because they are stored in large amounts in bone matrix and liberated by bone resorption. 16 In vitro experiments have shown that these growth factors exert diverse effects on the chemotaxis, proliferation, differentiation and bone-forming activity of osteoblast lineage cells (Table 1). However, the specific physiological significance of these factors has not been well elucidated.
Table 1. Bone cell communicating factors in bone remodeling.
|
Based on the in vivo studies | ||||
|---|---|---|---|---|
| Sources | Effects on osteoclasts | Effects on osteoblasts | References | |
| Factors involved in the bone cell communication in the initiation phase | ||||
| Sema4D | Osteoclasts | No effect | Inhibition of differentiation and bone formation Increase in cell motility | 28 |
| RANKL | Osteoblast lineage cells | Activation of differentiation | Unknown (direct effect) (decrease in bone formation in knockout mice) | 43 |
| Sema3B | Osteoblast lineage cells | Activation of differentiation and bone resorption | Activation of differentiation and bone formationa (no effect in osteoblast-specific Tg mice) | 31 |
| Factors involved in the bone cell communication in the transition phase (classical coupling factors) | ||||
| TGF-β | Bone matrix | Activation of differentiationa (increase in osteoclast number in CED mice) | Increase in cell migration Activation of differentiation (early stage) | 12,16,17 |
| IGF-I | Bone matrix | Activation of differentiation and bone resorptiona | Activation of bone formation | 12,16,19 |
| CT-1 | Osteoclasts | Unknown (increase in number and size of dysfunctional osteoclasts) | Activation of differentiation (during bone modeling) | 24 |
| Factors involved in the bone cell communication in the bone formation phase | ||||
| Sema3A | Osteoblasts | Inhibition of differentiation Repulsion of osteoclast precursors | Activation of differentiation (inhibition of adipogenesis) | 39 |
| OPG | Osteoblastic lineage cells | Inhibition of differentiation Inhibition of bone resorption | Unknown (direct effect) (increase in bone formation in knockout mice) | 44 |
| Sclerostin | Osteocytes | Unknown | Inhibition of bone formation | 13 |
| |
|
|
|
|
|
Based on the in vivo studies | ||||
| |
Sources |
Effects on osteoclasts |
Effects on osteoblasts |
References |
| Ephrin A2 | Osteoclasts | Activation of differentiation | Inhibition of differentiation | 45 |
| BMP (2,6,7) | Bone matrix | Inhibition of differentiation | Activation of bone formation Inhibition of bone formation (BMP receptor 1 KO mice) | 12,16,25,46,47 |
| Wnt10b | Osteoclasts | Unknown (decrease in osteoclast number in knockout mice) | Activation of differentiation | 25,26,27 |
| PDGF-BB | Osteoclasts | Inhibition of differentiation through OPG expression | Inhibition of differentiation Increase in chemotaxis | 48,49 |
| FGF | Bone matrix | Activation of differentiation through RANKL expression | Increase in proliferation Inhibition of differentiation | 12 |
| PDGF | Bone matrix | Activation of osteoclastogenesis | Increase in proliferation Increase in chemotaxis | 12 |
| VEGF | Bone matrix | Activation of differentiation and bone resorption Increase in cell migration | Increase in chemotaxis | 50,51 |
| Ephrin B2 | Osteoclasts | Inhibition of differentiation No effect (ephrin B2 KO mice) | Activation of differentiation No effect (ephrin B2 KO mice) | 20 |
| Mim-1 | Osteoclasts | Unknown | Increase in chemotaxis and differentiation | 52 |
| Sphingosin 1-phosphate | Osteoclasts | Increase in chemotaxis | Increase in cell migration and bone formation Increase in survival | 25,53 |
| HGF | Osteoclasts | Increase in cell migration and proliferation | Increase in proliferation | 54 |
| TRAP | Osteoclasts | Unknown | Activation of differentiation | 55 |
| Sema3E | Osteoblasts | Inhibition of differentiation | Inhibition of migration | 36 |
| Sema7A |
Osteoclasts |
Activation of bone resorption |
Increase in cell migration |
32,33 |
Abbreviations: BMP, bone morphogenetic protein; CED, Camurati-Engelmann disease; CT-1, cardiotrophin-1; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF-I, insulin-like growth factor-I; KO, knockout; OPG, osteoprotegerin; PDGF, platelet-derived growth factor; RANKL, receptor activator of nuclear factor-κB ligand; Sema, Semaphorin; Tg, transgenic; TGF-β, transforming growth factor-β; TRAP, tartrate-resistant acid phosphatase; VEGF, vascular endothelial growth factor.
aIn vitro analysis.
TGF-β1 is deposited in bone matrix as an inactive, latent complex along with latency-associated protein. During bone resorption, osteoclasts acidify the resorption pits and secrete proteolytic enzymes, such as cathepsin K and matrix metalloproteinases, so as to degrade and dissolve the bone matrix. As a result, TGF-β1 is released and activated by the dissociation of latency-associated protein. Mice lacking TGF-β1 exhibited a significant decrease in the osteoblast number on the bone surface, but an abnormal accumulation of osteoblasts in the marrow space.17 A mutation in the Tgfb1 gene has been identified in patients with a skeleton remodeling disorder, Camurati–Engelmann disease. Mice carrying a Camurati–Engelmann disease-derived mutation in the Tgfb1 gene had a high level of prematurely active TGF-β1 in the bone marrow microenvironment and displayed the progressive diaphyseal dysplasia seen in the human disease.17 These findings indicate that TGF-β1 promotes the migration of osteoprogenitors to the resorbed bone surface where this osteoblast differentiation is induced.
IGF-I and IGF-II are the most abundant growth factors stored in bone, and it is well established that IGFs has a critical role in bone development and remodeling. The action of IGFs in bone is inhibited by IGF binding proteins (IGFBP1, 2, 4, 6), which sequester IGFs from their receptor. The cleavage of such inhibitory IGFBPs is needed to the IGF action.18 However, as IGFs act as an endocrine hormone released from the liver, it has been difficult to determine the contribution of local osteoblast-produced IGFs to bone remodeling. Mice lacking IGF-I specifically in the liver, in which circulating IGF-I was reduced by 90%, displayed reduced skeletal growth and bone size, but the skeletal phenotype was much less severe than that observed in total Igf1−/− mice. This result suggested that the IGF-I produced in the local bone microenvironment is required for bone formation. A study using mice lacking IGF-I specifically in osteoblast lineage cells further supported this interpretation,19 but it has not been defined how much IGF-1 in the bone matrix decreased. As most of the osteotropic factors, such as IGF-I, function also as systemic factors, future studies using conditional deletion specific in the bone are needed to establish the local activity of coupling.
Osteoclast-derived coupling factors
The membrane-bound ligand ephrinB2, which is an axon guidance molecule, was found to be expressed on mature osteoclasts, whereas the tyrosine kinase receptor EphB4 was found to be expressed on osteoblast lineage cells.20 The ephrin/Eph system transduces bidirectional signals through the receptor (forward signaling) and through the ligand (reverse signaling), depending on certain specific cell–cell contacts. The binding of ephrinB2 to EphB4 stimulates osteoblast differentiation through the activation of the small GTPase RhoA. The reverse signaling through ephrinB2 into osteoclasts suppressed osteoclastogenesis by inhibiting the induction of c-Fos and NFATc1.20 These results suggested that ephrinB2/EphB4 signaling may stimulate the termination of bone resorption and simultaneously activate osteoblast differentiation in the transition phase. However, as there was no abnormality in bone of mice lacking ephrinB2 in myeloid cells,20 it is still unclear how ephrinB2/EphB4 signaling contributes to the bone remodeling under physiological conditions and whether bone-forming osteoblasts communicate with bone-resorbing osteoclasts via the cell–cell contact. On the other hand, mice expressing EphB4 specifically in osteoblasts showed increased bone mass through the enhancement of bone formation. It was reported that ephrinB2 is also expressed on osteoblast lineage cells and parathyroid hormone, and that parathyroid hormone-related protein and Wnt3a further enhance ephrinB2 expression.21,22 Furthermore, the ephrinB2/EphB4 axis has been shown to be implicated in certain pathological settings such as osteoarthritis and multiple myeloma.22,23 It is possible that the osteoblastogenic effect induced by ephrinB2/EphB4 signaling is also exerted through the osteoblast–osteoblast interaction in the bone formation phase.
Previous in vitro studies have demonstrated that several factors regulating mineralization or migration of osteoblastic cells are produced by osteoclasts (Table 1). Cardiotrophin-1 (CT-1), a member of the interleukin-6 cytokine family, is found to be produced by osteoclasts and promotes osteoblast differentiation through its receptor complex consisting of gp130 and the leukemia inhibitory factor receptor. Neonatal mice lacking CT-1 display an osteopenic phenotype due to decreased bone formation, despite the presence of impaired bone resorption.24 These mice have a large number of dysfunctional osteoclasts, which do not appear to stimulate bone formation. These observations suggest that CT-1, at least in neonates, has a predominant role in promoting osteoblast differentiation over other stimulating factors derived from osteoclasts. However, as the CT-1-knockout mice aged, no defects were observed in the osteoblastic function, but impaired bone resorption caused an osteopetrotic phenotype. Bone-increasing effect of CT-1 as a coupling factor is not obvious in adult bone remodeling.24 Wnt10b was also identified in the culture medium of human osteoclasts as a stimulator of mineralization activity of osteoblastic cells.25 Wnt10b is known to be involved in the canonical Wnt/β-catenin pathway that is essential for osteoblast differentiation and bone formation. As a previous study demonstrated that mice lacking Wnt10b exhibit low bone mass due to decreased bone formation without any change in the osteoclast number or bone resorption,26,27 osteoclastic expression of Wnt10b may couple bone resorption with formation. However, it remains to be determined whether osteoclasts are the major source of these molecules.
The genetic deletion of these factors specifically in osteoclast lineage cells may be a powerful strategy for elucidating the local factors involved in the communication among bone cells. However, caution should be exercised when using conditional knockout mice because bone formation may be influenced not only directly by the absence of a putative coupling factor but also indirectly by a cell-autonomous osteoclast defect, which results in an alteration of production of other coupling factors. Therefore, it may prove challenging to obtain direct evidence for coupling functions in vivo.
Inhibition of coupling in the initiation phase
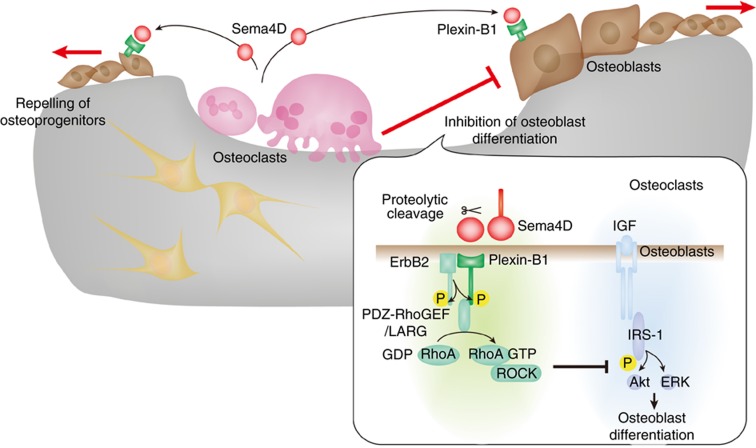
Once bone resorption is initiated, various coupling factors are produced and stimulate bone formation. However, in order to completely remove the damaged or aged bone in the initiation phase, osteoblast differentiation and formation within the BMU needs to be suppressed until bone resorption is accomplished. A recent study provided evidence that osteoclasts suppress bone formation through the expression of Sema4D (Figure 2).28
Figure 2. Inhibition of bone formation by the osteoclastic expression of Sema4D in the initiation phase.
During bone resorption, osteoclast-derived Sema4D inhibits osteoblast differentiation in the proximity of osteoclasts and repels osteoblasts by increasing their motility. The binding of Sema4D to its receptor complex, consisting ErbB2 and Plexin-B1, activates RhoA through RhoGEFs, including PDZ-RhoGEF and LARG. The Rho-associated protein kinase Rho-associated kinase (ROCK) inhibits IRS-1 phosphorylation, which is the crucial step in IGF-1 signaling for osteoblast differentiation. The motility of osteoblasts is also controlled by the activation of RhoA-ROCK.
Semaphorins are originally described as axonal guidance repellents that induce growth cone collapse during neuronal development and subsequently recognized as attractant and repellent cues for multiple cell types.29,30 However, they were detected in a wide variety of tissues and shown to be implicated in diverse biological processes including organogenesis, cardiovasucular development, the immune response and tumor progression. It has been suggested that the Semaphorins are also involved in the cell–cell communication between osteoclasts and osteoblasts during bone remodeling.31,32,33,34,35,36 Mice overexpressing Sema3B in osteoblasts exhibited an osteoporotic phenotype because of enhanced osteoclastogenesis.31 In addition, it was reported that Sema7a polymorphisms were associated with low bone mineral density and high risk of vertebral fracture in postmenopausal women.32 However the functions of these Semaphorins have not been clearly demonstrated in vivo in loss-of-function genetic models.
The Semaphorins compose a large family of secreted and membrane-bound glycoproteins characterized by a conserved amino-terminal 'Sema' domain. On the basis of structural features and amino-acid sequences similarity, Semaphorins are divided into eight subclasses, of which classes III–VII are vertebral Semaphorins. Sema4D belongs to the class 4 Semaphorin based on its structure of membrane-bound form, but also acts as a soluble factor after proteolytic cleavage. Sema4D, also called CD100, was first identified in the activated T cells and has been extensively explored in the immune system.37 Sema4D regulates the activation and survival of B cells and dendritic cells, and inhibits monocyte migration minly through its low-affinity receptor CD72. Recent studies demonstrated Sema4D is also involved in the angiogenesis and tumor progression through its high-affinity receptor Plexin-B1.
In bone, Sema4D was highly and selectively produced by the osteoclasts and its receptor, Plexin-B1 (encoded by the Plxnb1 gene), was expressed on osteoblasts. The soluble form of Sema4D potently inhibited osteoblast differentiation, whereas it did not affect osteoclastogenesis. The differentiation of osteoblasts was markedly enhanced by the addition of the culture supernatant of Sema4d−/− osteoclasts or coculturing with Sema4d−/− osteoclasts, whereas either the addition of the culture supernatant of wild-type osteoclasts or coculturing with wild-type osteoclasts did not influence bone formation, suggesting that osteoclasts suppress bone formation through the expression of Sema4D. Considering the finding of the absence of an effect of wild-type osteoclasts on bone formation, it is concluded that osteoclasts generate factors that promote bone formation, which is obvious only in the absence of Sema4D, but Sema4D exerts a predominating effect that antagonizes this bone-forming activity. As osteoclasts produce Sema4D as long as they are present in the BMU, bone formation does not start even in the presence of the osteogenetic factors until Sema4D disappears, which is accompanied by osteoclast apoptosis.
Sema4d−/− mice had high bone mass due to augmented bone formation without any abnormality in bone resorption, suggesting that osteoclastic expression of Sema4D inhibits osteoblastic bone formation and is a mediator of osteoclast–osteoblast communication. Furthermore, wild-type mice engrafted Sema4D-dificient bone marrow cells including osteoclast precursor cells also exhibited osteosclerotic phenotype, showing that the bone phenotype in Sema4d−/− mice resulted from a defect in hematopoietic lineage cells including osteoclasts.28,38 It will be required to further investigate the role of Sema4D in other cell types including vascular and neural cells in the BMU.
The binding of Sema4D to Plexin-B1 was shown to result in the activation of the tyrosine kinase ErbB2, which activates Plexin-B1 by phosphorylation. RhoA was subsequently activated by RhoGEF, including PDZ-RhoGEF and LARG, which associate with Plexin-B1. Plxnb1−/− mice and mice expressing a dominant-negative RhoA, specifically in osteoblast lineage cells, exhibited an osteosclerotic phenotype because of enhanced bone formation, which recapitulates the bone phenotype of the Sema4d−/− mice, providing evidence for the inhibitory regulation of bone formation by the Sema4D–Plexin-B1–RhoA pathway.28
What are the mechanisms by which Sema4D inhibits bone formation? Sema4D decreased the phosphorylation of insulin receptor substrate-1 (IRS-1) and its downstream molecules, including Atk and ERK, which is the crucial step in IGF signaling that favors osteoblast differentiation. Activation of RhoA or its effector Rho-associated kinase (ROCK) inhibited the IGF-I-stimulated IRS-1 pathway, whereas suppression of RhoA or ROCK activity activated IRS-1 signaling. Collectively, the Sema4D–Plexin-B1–RhoA pathway suppressed osteoblast differentiation by attenuating IGF-I signaling (Figure 2). In addition, Sema4D-induced RhoA activation promoted osteoblast motility. In bone tissue, clusters of osteoblasts lie at certain distance from osteoclasts at the bone surface, and there is usually an intervening quiescent surface in between. However, in Sema4d−/− mice, as well as Plxnb1−/− and dominant-negative RhoA conditional transgenic mice, bone-forming osteoblasts resided in close vicinity to osteoclasts. These findings suggest that osteoclast-derived Sema4D is required for the proper localization of osteoblasts and the maintenance of the requisite quiescent surface. Thus, osteoclast-derived Sema4D inhibits osteoblast differentiation in the proximity of osteoclasts and repels osteoblasts by increasing their motility during bone resorption.42 Sema4D acts as a guidance molecule for osteoblast positioning and protects the resorption area against bone formation in the initiation phase.
Previous in vitro studies have also proposed an inhibitory regulation of coupling between bone resorption and formation (Table 1). Although the genetic evidence for the function of dissociation of coupling must await future study, the findings on the inhibitory mechanisms underlying the dissociation of coupling give rise to a new mode of understanding bone remodeling.
Inhibition of bone resorption in the bone formation phase
During bone formation, the generation of additional sites of initiation of the bone remodeling cycle needs to be prevented within the BMU. It is well known that osteoblast lineage cells inhibit osteoclastogenesis by secreting OPG. However, a recent study reported that a substantial anti-osteoclastogenic effect was observed in the conditioned medium of OPG-deficient calvarial cells and they identified another Semaphorin family member Sema3A as the osteoblast-secreted inhibitors of osteoclast differentiation by mass spectrometry.39
Sema3A, which is secreted protein, is the first identified vertebral Semaphorin and has been the most extensively studied in the nervous system, but now is also recognized to be involved in multiple physiological and pathological processes. Recent reports demonstrated that the Sema3A suppresses the onset of autoimmune diseases such as SLE, rheumatoid arthritis and multiple myeloma, as well as tumor progression.40,41 Sema3a−/− mice exhibited abnormal bone and cartilage development, with vertebral fusions and partial rib duplication, suggesting that Sema3A contribute to the skeletal patterning in the embryonic stage.42
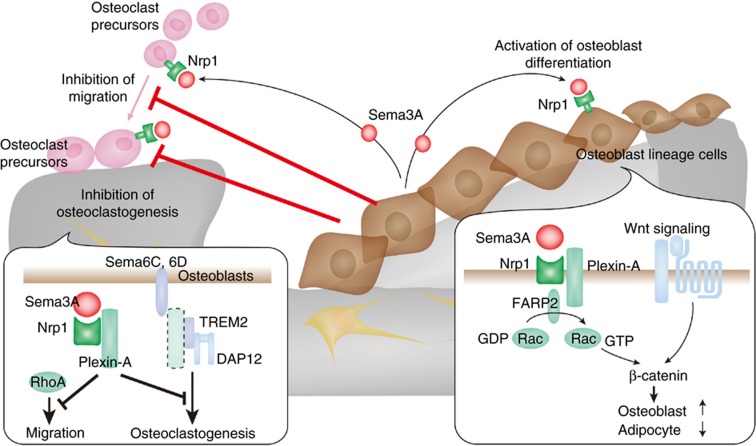
Sema3A binds to a receptor complex of the ligand-binding subunit Neuropilin-1 and one of the class A Plexins. In a recent study, Sema3A was shown to be predominantly produced by osteoblast lineage cells, whereas the receptor complex was expressed both on osteoblast lineage cells and osteoclast precursor cells.39 It has been reported that Plexin-A1 promotes osteoclast differentiation by activating ITAM signaling through the formation of the Plexin-A1–TREM-2–DAP12 complex.34 The binding of Sema3A to Neuropilin-1 resulted in abrogation of the differentiation capacity of osteoclast precursor cells by sequestering Plexin-A1 from TREM2 so as to suppress ITAM signaling. Sema3A also inhibited the migration of osteoclast precursor cells by suppressing RhoA activation. On the other hand, osteoblast differentiation was stimulated by Sema3A through the activation of the canonical Wnt/β-catenin pathway while suppressing adipogenesis (Figure 3). The binding of Sema3A to Nrp1 resulted in the activation of Rac1 through a Rac-specific GEF FARP2, which enhances the nuclear accumulation of β-catenin. Thus, osteoblasts may have a crucial role in the bone formation phase, in which osteoblasts extensively produce bone, and at the same time restrain osteoclasts from migrating to the formation sites and starting to resorb the newly formed bone.
Figure 3. Inhibition of bone resorption by the osteoblastic expression of Sema3A in the bone formation phase.
During bone formation, osteoblast-derived Sema3A inhibits osteoclast differentiation and migration, and at the same time stimulates bone formation. The binding of Sema3A to Nrp1 on osteoclasts inhibits ITAM signaling by sequestering Plexin-A1 from TREM-2. Sema3A–Nrp1–Plexin-A1 also inhibits the migration of osteoclast precursor cells by suppressing RhoA activation. The binding of Sema3A to Nrp1 on osteoblasts activates Rac1 through the RacGEF FARP2. Rac1 activation enhances the Wnt-mediated nuclear localization of β-catenin, which is essential for osteoblast differentiation.
Viable Sema3a−/− mice exhibited a severe osteopenic phenotype due to an increase in bone resorption accompanied by decreased bone formation.39 Furthermore, knock-in mice in which the Nrp1 gene was replaced by mutant Nrp1 lacking the Sema-binding site recapitulated the bone phenotype of Sema3a−/− mice. As Sema3A may have a role in the regulation of innervations and blood vessel invasion,35 future studies using osteoblast lineage-specific Sema3a−/− conditional knockout mice will be informative.
New therapeutic approaches to bone diseases
Anti-resorptive drugs such as bisphosphonates have been the primary therapy for osteoporosis and other remodeling diseases, but a general problem with the existing anti-resorptive treatments is an associated decrease in bone formation resulting from the coupling of resorption with formation. Therefore, it would be desirable to develop a strategy that effectively targets either bone resorption or formation by dissociating resorption from formation. It was clearly demonstrated that the treatment with the Sema4D-specific neutralizing antibody and recombinant Sema3A not only effectively protected against bone loss in mouse model of osteoporosis, but also restored the lost bone by increasing bone formation.28,39 Considering the involvement of Sema4D and Sema3A in the pathological setting, including cancer progression and autoimmune diseases, the blocking of Sema4D or treatment with Sema3A promise as a strategy for the design of agents with pleiotropic effects on bone loss associated with autoimmune diseases and bone tumors.
Conclusions
The whole cycles of bone remodeling are finely controlled by multiple communication pathways between osteoblast and osteoclast lineage cells at many stages of differentiation and function in these cells. The coupling mechanism has traditionally been considered to regulate the cell–cell communication at the transition stage. However, now the molecules that mediate intercellular signaling at various stages of bone remodeling may be collectively called bone cell communication factors, which include classical coupling factors. In addition to such factors derived from bone cells, the bone is additionally under the control of factors that are related to the immune, vascular and nervous systems.2,6,8 Therefore, the understanding of the complicated network mediated by cell–cell communication would be extremely helpful for the design of novel treatments for bone diseases.
Acknowledgments
This work was supported in part by Grants-in-Aid for the Global Center of Excellence Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and ERATO, Takayanagi Osteonetwork Project from the Japan Science and Technology Agency.
Footnotes
The authors declare no conflict of interest.
References
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 2007;7:292–304. [DOI] [PubMed] [Google Scholar]
- Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 1965;206:489–490. [DOI] [PubMed] [Google Scholar]
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol 2011;6:121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Delmas PD. Bone quality-the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–2261. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11:76–81. [DOI] [PubMed] [Google Scholar]
- Sims NA, Gooi JH. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol 2008;19:444–451. [DOI] [PubMed] [Google Scholar]
- Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res 1969;3:211–237. [DOI] [PubMed] [Google Scholar]
- Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 2008;473:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys 2008;473:201–209. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011;17:1231–1234. [DOI] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 20011;17:1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Growth factor control of bone mass. J Cell Biochem 2009;108:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 2004;199:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard GA, Bottemiller BL, Turner RT, Rader JI, Baylink DJ. Parathyroid hormone stimulates bone formation and resorption in organ culture: evidence for a coupling mechanism. Proc Natl Acad Sci USA 1981;78:3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia-Silvestre T, Neutzsky-Wulff AV, Sorensen MG, Christiansen C, Bollerslev J, Karsdal MA et al. Advances in osteoclast biology resulting from the study of osteopetrotic mutations. Hum Genet 2009;124:561–577. [DOI] [PubMed] [Google Scholar]
- Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone 1996;19:1S–12S. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z et al. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009;15:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol 2005;20:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1α2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology 2007;148:5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006;4:111–121. [DOI] [PubMed] [Google Scholar]
- Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 2008;23:1170–1181. [DOI] [PubMed] [Google Scholar]
- Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD Jr, Barlogie B et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood 2009;114:1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan Tat S, Pelletier JP, Amiable N, Boileau C, Lajeunesse D, Duval N et al. Activation of the receptor EphB4 by its specific ligand ephrin B2 in human osteoarthritic subchondral bone osteoblasts. Arthritis Rheum 2008;58:3820–3830. [DOI] [PubMed] [Google Scholar]
- Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JM et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res 2008;23:2025–2032. [DOI] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 2008;105:20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 2005;102:3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res 2007;22:1924–1932. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 2011;17:1473–1480. [DOI] [PubMed] [Google Scholar]
- Derijck AA, Van Erp S, Pasterkamp RJ. Semaphorin signaling: moclular switches at the midline. Trends Cell Biol 2010;20:568–576. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol 2007;23:263–292. [DOI] [PubMed] [Google Scholar]
- Sutton A, Zhang X, Dowd DR, Kharode YP, Komm BS, Macdonald PN. Semaphorin 3B is a 1,25-dihydroxyvitamin D3-induced gene in osteoblasts that promotes osteoclastogenesis and induces osteopenia in mice. Mol Endocrinol 2008;22:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JM, Oh B, Lee JY, Lee JK, Kimm K, Kim GS et al. Association study of semaphorin 7a (sema7a) polymorphisms with bone mineral density and fracture risk in postmenopausal Korean women. J Hum Genet 2006;51:112–117. [DOI] [PubMed] [Google Scholar]
- Delorme G, Saltel F, Bonnelye E, Jurdic P, Machuca-Gayet I. Expression and function of semaphorin 7A in bone cells. Biol Cell 2005;97:589–597. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 2006;8:615–622. [DOI] [PubMed] [Google Scholar]
- Gomez C, Burt-Pichat B, Mallein-Gerin F, Merle B, Delmas PD, Skerry TM et al. Expression of Semaphorin-3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev Dyn 2005;234:393–403. [DOI] [PubMed] [Google Scholar]
- Hughes A, Kleine-Albers J, Helfrich MH, Ralston SH, Rogers MJ. A class III semaphoring (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcif Tissue Int 2012;90:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol 2008;9:17–23. [DOI] [PubMed] [Google Scholar]
- Dacquin R, Domenget C, Kumanogoh A, Kikutani H, Jurdic P, Macguca-Gayet I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS One 2011;6:e26627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature 2012;3:69–74. [DOI] [PubMed] [Google Scholar]
- Catalano A. The neuroimmune semaphoring-3A reduces inflammation and progression of experimental autoimmune arthritis. J Immunol 2010;185:6373–6383. [DOI] [PubMed] [Google Scholar]
- Vadasz Z, Haj T, Halasz K, Rosner I, Slobodin G, Attias D et al. Semaphorin 3A is a marker for disease activity and a potential immunoregulator in systemic lupus erythematosus. Arthritis Re Ther 2012;14:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bone and heart. Nature 1996;383:525–528. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999;397:315–323. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998;12:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 2009;284:14637–14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM et al. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 2008;135:3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Murai J, Imai Y, Ikegami D, Kamiya N, Kato S et al. Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice. J Bone Miner Res 2011;26:2511–2522. [DOI] [PubMed] [Google Scholar]
- Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Miner Res 2002;17:257–265. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One 2008;3:e3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Curr Top Dev Biol 2005;65:169–187. [DOI] [PubMed] [Google Scholar]
- Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 2002;30:472–477. [DOI] [PubMed] [Google Scholar]
- Falany ML, Thames AM 3rd, McDonald JM, Blair HC, McKenna MA, Moore RE et al. Osteoclasts secrete the chemotactic cytokine mim-1. Biochem Biophys Res Commun 2001;281:180–185. [DOI] [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009;458:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci USA 1996;93:7644–7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Fornari R, Van Wesenbeeck L, de Freitas F, Timmermans JP, Peruzzi B et al. A new heterozygous mutation (R714C) of the osteopetrosis gene, pleckstrin homolog domain containing family M (with run domain) member 1 (PLEKHM1), impairs vesicular acidification and increases TRACP secretion in osteoclasts. J Bone Miner Res 2008;23:380–391. [DOI] [PubMed] [Google Scholar]