Abstract
Vacuum-assisted closure therapy is an alternative method for a massive subcutaneous emphysema treatment. It is easily applicable and shows rapid effectiveness in massive subcutaneous emphysema, intractable with chest tube drainage.
Keywords: Subcutaneous emphysema, Device, Negative pressure
Subcutaneous emphysema is a potential complication after thoracic surgical procedures. Most cases of emphysema are self-limiting, requiring no specific management, but some cases can be massive, rapidly inflating, severely disturbing the airway, and becoming life-threatening. The main risk of subcutaneous emphysema is rapid and massive accumulation of air in the deep fascial planes at the level of the thoracic inlet [1]. Therefore, a massive accumulated air can compress the trachea and the great vessels, which can severely compromise the airway, venous return, and blood flow to the head and neck. A few treatments for subcutaneous emphysema, such as needle aspiration, small-bore-catheter insertion, chest tube insertion, and multiple skin incision-like 'blow-holes,' have been reported [2]. Recently, negative pressure wound therapy, as known as vacuum-assisted closure (VAC), has been emerging as an effective treatment modality for wounds ranging from simple wounds to complex septic wounds. This therapeutic method, firstly introduced by Argenta and Morykwasin in 1997, is based on the effects of a negative pressure of 75 to 150 mmHg on wound healing. Many different terms, such as vacuum therapy, topical negative pressure wound therapy, and VAC, have been used to describe it since then. The vacuum device has demonstrated great utility in decreasing edema via a negative pressure gradient that pulls fluid out of wounds. Therefore, we applied this technique for subcutaneous emphysema with the placement of a VAC dressing to decrease the subcutaneous air.
CLINICAL SUMMARY AND TECHNIQUE
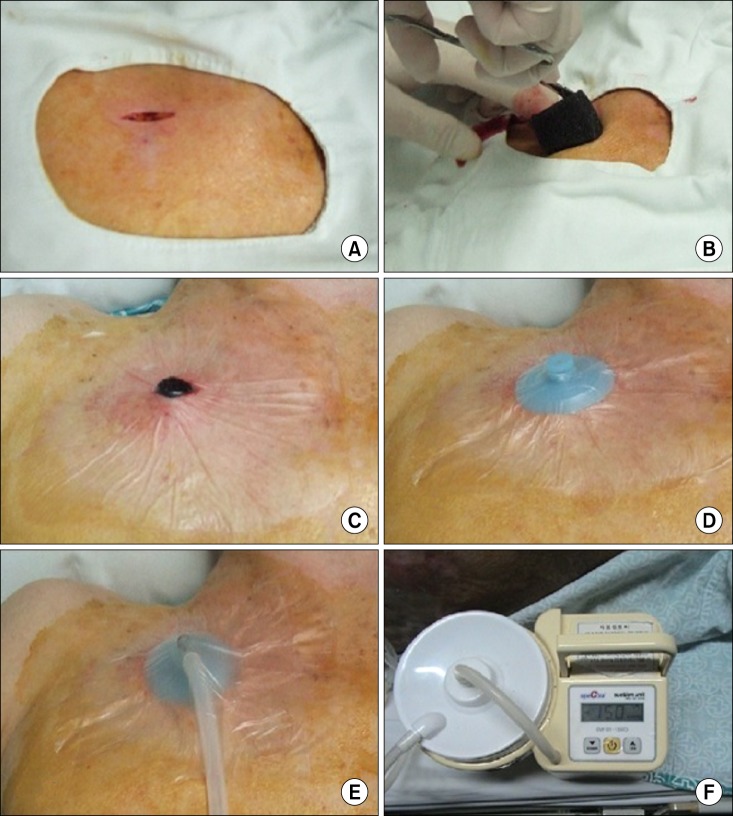
A total of 4 patients who all had been suffering from secondary pneumothorax with massive subcutaneous emphysema that was intractable despite chest tube drainage underwent VAC therapy at the department of thoracic and cardiovascular surgery of Eulji University Hospital. All were male. The median age of the patients was 73.3 years (range, 63 to 87 years). Table 1 shows that all of the patients had chronic obstructive pulmonary disease with emphysematous lungs. The patients were first treated by closed-tube thoracostomy, but subsequently abrupt massive subcutaneous emphysema occurred. A 2-cm 'blow-hole' incision was made below the right or left infraclavicular region through the skin and the prepectoral fascia was exposed sufficiently at the bedside under local anesthesia. Sterile polyurethane foam was trimmed to shape and placed into the blow-hole incision (Fig. 1A, B). An adhesive drape was used to cover the foam and an additional 3 to 5 cm of surrounding intact skin. An opening hole was created in the adhesive drape and a non-collapsible tube connector was placed directly over the hole in the drape (Fig. 1C, D). The tube was connected at the opposite end to an electronic vacuum pump (CuraVAC™, Daewoong Pharm. Co., Ltd., South Korea) and negative pressure was applied. As a result, the VAC device absorbed subcutaneous air via the blow-hole incision. The topical VAC dressing was set at a continuous suction of 150 mmHg (Fig. 1E, F). The VAC dressing changes were performed at the bedside using a sterile technique every other day. The mean time to VAC therapy was 1.5 post-closed thoracostomy days (range, 0 to 3 days) and the mean duration of VAC therapy was 3.0 days (range, 2 to 4 days). After application of the VAC therapy, we performed a chest X-ray scan and physical examination daily to measure the subcutaneous emphysema. All of the patients experienced a dramatic reduction in trapped air after 24 hours of VAC therapy (Fig. 2). Two patients were able to have the VAC device removed within 48 hours, and all of the VAC machines were withdrawn within 4 days. The VAC device was removed when near-complete resolution of subcutaneous emphysema was confirmed by physical examination and repeated chest X-ray scans, and when there was no more progression of air accumulation when the VAC therapy was stopped. An air leak still remained but was adequately managed by the chest tube. There were no recurrence of subcutaneous emphysema and no complications associated with VAC therapy including mortality or morbidity.
Table 1.
Patient characteristics and results
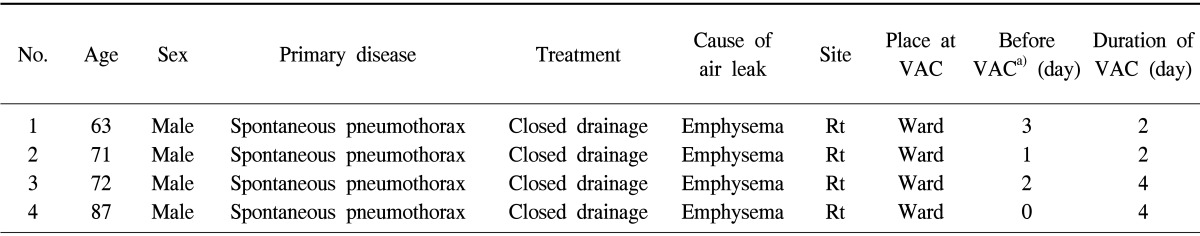
VAC, vacuum-assisted closure; Rt, right.
a)Duration of subcutaneous emphysema before VAC therapy.
Fig. 1.
Technique of vacuum-assisted closure (VAC) preparation. (A) A 2-cm 'blow-hole' incision is made below the infraclavicular region. (B) Sterile polyurethane foam is inserted into blow-hole incision. (C) Adhesive drape cover the foam and additional surrounding skin. (D) After opening hole is created in the prior drape, the non-collapsible tube connector is placed over the hole, and then another adhesive drape cover the connector. (E) The tube is connected from VAC machine. (F) See the electronic vacuum pump (CuraVAC) is on.
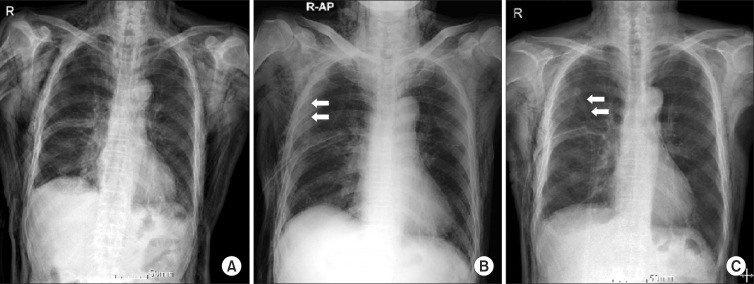
Fig. 2.
Changes of chest X ray finding in subcutaneous emphysema after vacuum-assisted closure (VAC) therapy (arrow shows the VAC connection tube). (A) Initial massive subcutaneous emphysema before VAC therapy. (B) Decreased subcutaneous emphysema after 24 hours of VAC therapy. (C) After 72 hours after VAC therapy, subcutaneous emphysema was much more improved. AP, anterior-posterior.
COMMENT
The clinical symptoms of subcutaneous emphysema vary depending on the severity and extent of trapped air. Patients may have mild disfigurement of their chest wall contour with mild to moderate pain. Large air leaks are usually responsible for the development of subcutaneous emphysema. The air leaks move cephalad along the tissue planes to the root of the neck. Therefore, the optimal site for draining the collecting air is at the level of the thoracic inlet [1]. The main objective of subcutaneous emphysema treatment is to decrease the source of air insufflating into the subcutaneous space, which allows the proper expansion of the lung and apposition of the pleura surfaces, which, in turn, allows the injured parenchyma to recover [3]. Treatment modalities include observation, tissue squeezing, fenestrated angiocatheter insertion into the subcutaneous space, larger semi-rigid catheter insertion, incising the skin and subcutaneous fascia to create a 'blow-hole' [2] to allow air to escape, or trocar chest tubes and VATS or open thoracotomy with repair of parenchymal injury [4].
There are few scientific studies comparing different subcutaneous emphysema treatment modalities. With observation alone, in which air is gradually reabsorbed by soft tissues, it can take several weeks for significant subcutaneous emphysema to resolve. Subcutaneous insertion of a small- or large-bore catheter with fenestrations is widely used as a form of drainage of subcutaneous emphysema. These methods used fenestrated angiocatheters or drains placed subcutaneously and set to continuous suction and can significantly reduce subcutaneous emphysema in several days. However, the catheter can easily become blocked by blood, and may need to be re-inserted [3]. A 'blow-hole' incision is widely performed to release the trapped subcutaneous air and to minimize further progression of air dissection into the face and neck. In this method, a small (approximately 2 cm) incision in the infraclavicular area skin and blunt dissection is used to create short tracks down to the level of the prepectoral fascia at the bedside under local anesthesia. This method is frequently used and relies on the passive diffusion of trapped air out of the incision. The tracks work for a short time, but factors such as tissue recoil and plasma clot can cause them to collapse and close the tracks [3]. Therefore, the wound is typically packed with gauze and the dressing is changed twice a day. Blow-hole placement can be effective in decreasing massive subcutaneous emphysema over the course of several days; however, complete resolution in cases of significant subcutaneous emphysema can take several days or more. The most invasive method of subcutaneous emphysema treatment requires surgical repair and control of the source of leaking air. A prospective trial by Cerfolio et al. [4] has shown VATS to be an excellent means of controlling an air leak site.
VAC therapy was originally developed as an alternative treatment for debilitated patients with chronic wounds in a wide variety of clinical settings beyond uncomplicated wound care and complicated wounds such as necrotizing fasciitis or diabetic foot [5]. These devices have shown an extracellular effect, such as increased blood flow and edema reduction; a cellular effect, such as granulation tissue formation and cellular synthesis; and complex effects, such as infection control and flap survival [5]. Among the effects, the edema reduction mechanism is pulling fluid out of wounds via a negative pressure gradient. Following the same theory, a blow-hole incision was in continuity with the source of air escaping from sites, so the occlusive suction dressing in the skin incision would allow for aspiration of the trapped air. Therefore, VAC therapy enabled the rapid resolution of both subcutaneous emphysema and the air leak.
VAC placement for subcutaneous emphysema allows for adequate control of the trapped air via the subfacial track, and it is an important process enabling expansion of the lung, apposition of the pleura surfaces, resolution of lung parenchymal injury, and cessation of air leakage from the thorax. According to our results, subcutaneous emphysema decreased in 4 patients after VAC therapy was started. Furthermore, in all of the cases, the subcutaneous emphysema was resolved 2 to 4 days after VAC therapy and the subcutaneous emphysema was not re-aggravated when the VAC therapy was turned off. The key to successful VAC therapy is sufficient dissection of the prepectoral fascial plane for aspiration of trapped subcutaneous air.
VAC therapy did not replace tube thoracostomy in these 4 cases; it is an adjunct to therapy for subcutaneous emphysema once a chest tube has been placed. Wound site pain is a common complication after VAC therapy. It is controlled with pressure adjustment between 75 mmHg and 150 mmHg. Other considerations, such as increased wound care needs, the potential for development of wound infections, and equipment costs must be carefully evaluated prior to initiation of VAC therapy for subcutaneous emphysema.
CONCLUSION
In various methods of massive subcutaneous emphysema treatment, VAC therapy is easily usable and rapidly effective for evacuating subcutaneous trapped air in large air leak patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sherif HM, Ott DA. The use of subcutaneous drains to manage subcutaneous emphysema. Tex Heart Inst J. 1999;26:129–131. [PMC free article] [PubMed] [Google Scholar]
- 2.Herlan DB, Landreneau RJ, Ferson PF. Massive spontaneous subcutaneous emphysema: acute management with infraclavicular "blow holes". Chest. 1992;102:503–505. doi: 10.1378/chest.102.2.503. [DOI] [PubMed] [Google Scholar]
- 3.Sciortino CM, Mundinger GS, Kuwayama DP, Yang SC, Sussman MS. Case report: treatment of severe subcutaneous emphysema with a negative pressure wound therapy dressing. Eplasty. 2009;9:e1. [PMC free article] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, Bryant AS, Maniscalco LM. Management of subcutaneous emphysema after pulmonary resection. Ann Thorac Surg. 2008;85:1759–1763. doi: 10.1016/j.athoracsur.2007.12.079. [DOI] [PubMed] [Google Scholar]
- 5.Schintler MV. Negative pressure therapy: theory and practice. Diabetes Metab Res Rev. 2012;28(Suppl 1):72–77. doi: 10.1002/dmrr.2243. [DOI] [PubMed] [Google Scholar]