Abstract
Background:
Nowadays Vitamin D deficiency is a notable medical condition world-wide and also in Iran. Since, vitamin D can have renoprotective effect by inhibiting the renin-angiotensin system; it appears that low vitamin D level can worsen the renal injury in diabetic patients. This study demonstrates the effect of vitamin D3 therapy on reducing proteinuria in diabetic patients with concomitant diabetic nephropathy and vitamin D deficiency after controlling hypertension and use of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II type receptor blockers (ARBs).
Materials and Methods:
In this randomized double blinded parallel groups clinical trial, 51 diabetic patients with proven nephropathy and vitamin D deficiency/insufficiency and stable hypertension, dyslipidemia, and hyperglycemic treatment were enrolled. The patients were divided randomly into two groups (treatment and placebo group). Patients received oral vitamin D3 (pearl 50000 IU) or placebo one pearl every week for 12 weeks. Patients were assessed at baseline and 12 weeks after intervention from the point of 25(OH) D level, and urine albumin/creatinine ration (UACR).
Results:
Mean serum 25(OH) D concentrations were 14.06 ng/ml and 16.05 ng/ml before treatment. Furthermore, after intervention, its levels were risen to 71.23 and 17.63 in drug and placebo groups, respectively. Whereas, UACR as the main variable did not change significantly after intervention in both groups (P = 0.919).
Conclusion:
According to our finding, there was not a decrease in proteinuria in diabetic patients who received vitamin D for a period of 3 months.
Keywords: Diabetic nephropathy, vitamin D deficiency, proteinuria, diabetes
INTRODUCTION
Nowadays Vitamin D deficiency is a notable medical condition world-wide. It seems that the limitation of sun exposure is the main cause of vitamin D deficiency.[1,2] The process of vitamin D photosynthesis begins in the sun exposed skin and then vitamin D will be activated by hydroxylase enzymes in the liver and kidney respectively. In Iran, vitamin D deficiency is also prevalent. A study by Hovsepian et al. in Isfahan (center of Iran) showed high prevalence of vitamin D deficiency (50.8%) and insufficiency (19.6%) among the adult population.[3] Another study by Kaykhaei et al. showed that the prevalence of vitamin D deficiency and insufficiency is about 94.7% in Zahedan.[4]
Vitamin D has the prominent role more than calcium and phosphate metabolism in the different organ. It can affect musculoskeletal and cardiovascular system; innate immunity and body metabolism. It also has a renoprotective effect.[5,6,7,8,9,10,11,12] Vitamin D can also inhibit the renin-angiotensin system (RAS) as shown in Li et al. study.[13,14] A study by Zhang et al. confirmed the effective role of vitamin D on RAS to protect from kidney injury.[15] In the other hand, Diabetic nephropathy is a problematic challenge as a leading cause of end stage renal disease (ESRD) world-wide. In the pathogenesis of diabetic nephropathy, multiple pathways are engaged, and the intrarenal RAS is also activated. It is proved that drugs that inhibit the RAS, can prevent from renal damage and proteinuria.[16] As the traditional management, treatment with angiotensin converting enzyme inhibitors (ACEIs) or Angiotensin II type receptor blockers (ARBs) can reduces the progression of proteinuria in diabetic patients.
Due to mentioned role for vitamin D, it appears that low vitamin D level can worsen the renal injury in the diabetic patients. Many of the studies showed the effect of activated forms of vitamin D on the diabetic nephropathy, but little information is available using vitamin D3.[17,18,19,20,21] This study demonstrates the effect of vitamin D3 therapy on reducing proteinuria in diabetic patients with concomitant diabetic nephropathy and vitamin D deficiency after controlling hypertension and use of ACEIs or ARBs.
MATERIALS AND METHODS
Study design and patients
In this randomized double blinded parallel groups clinical trial, 60 diabetic patients with proven nephropathy and vitamin D deficiency/insufficiency were enrolled. In our study, the sample size was calculated by considering a 90% statistical power for detecting an effect size of 0.8 in urine albumin/creatinine ration (UACR) (based on percentage changes from the baseline between treatment and placebo groups) and 5% as a type one error rate.[19] All of them were selected randomly among patients who regularly followed-up in the Isfahan endocrine and metabolism research center during 2011. The Ethics Committee of the Isfahan Medical University approved the study (project number: 390287) and all participants signed a written informed consent. Vitamin D deficiency and vitamin D insufficiency is defined as 25(OH) D levels below 20 ng/mL and 20 to 29 ng/mL respectively.[22] Plasma levels of 25(OH) D were determined by direct competitive chemiluminescence as a routine laboratory test carried out in diabetic patients followed in Isfahan endocrine and metabolism disease research center.
In this study, nephropathy was defined as proteinuria determined in fasting urine sample by a UACR more than 30 mg/g Consistent with American Diabetes Association guidelines.
Other inclusion criteria included age older than 20 years; stable hypertension, dyslipidemia, and hyperglycemic treatment that not changed at least 3 months before the examination and the patients were receiving permanent doses of ACEIs or ARBs for 3 months or more. Glomerular filtration rate (GFR) was assessed by Cockcroft equation and patients with stable GFR were eligible for enrollment. Patients were excluded if there they had glomerulonephritis, serum phosphorus level greater than 5.2 mg/dl, serum calcium level (adjusted for albumin) greater than 10.5 mg/dl, malignancy, uncontrolled hypertension or chronic heart failure.
Procedure and measurements
A total of 60 patients were divided randomly into two groups (30 patients in treatment and plcebo). The randomization was carried out using permutated random blocks. Patients in the treatment group received oral vitamin D3 (pearl 50,000 IU) one pearl every week for 12 weeks. Furthermore, participants in the control group received placebo, one pearl weekly for 12 weeks; finally 51 patients completed the treatment period. 9 of the enrolled patients did not follow the prescribed study's protocol. The vitamin D and placebo pearls were formulated in Zahravi pharmaceutical company. The placebo pearls were completely identical in appearance to vitamin D pearl.
Detailed history was taken and physical examination was carried out at the beginning of the study. Patients were assessed at baseline and 12 weeks after intervention from the point of 25(OH) D level, lipid profile, hemoglobin A1C (Hb A1C) and UACR. The patients’ blood pressure and body mass index were also evaluated. At the end of follow-up period, 28 patients in the drug group and 23 patients in the placebo group completed the study.
Statistical analysis
Quantitative variables are represented as Mean ± SD (standard deviation) and qualitative ones as number (percentage). Normality of quantitative studied variables was assessed using Kolmogorov-Smirnov test and p-p plot. Logarithmic transformation was conducted for normalizing the distribution of variables as required. In our study, the transformation was conducted for Bun, serum Cr and 25(OH) D. Accordingly between groups comparisons, based on the absolute percentage changes, were conducted using two-independent samples t-test. The absolute percentage change for each variable was calculated by the formula |(E-B/B)×100|, where E was the end of treatment value and B was the baseline value and within group comparisons using the paired samples t-test. Between groups comparisons based on qualitative variables were done using Chi-square statistical test. P < 0.05 was considered as a statistically significant level. All statistical analyses were done using SPSS software version 16 (SPSS, Chicago IL).
RESULTS
From 51 diabetic patients included in study, 34 (66.7%) patients used oral hypoglycemic drugs and 17 (33.3%) were on insulin injection. Hypertension was determined from the patients’ medical history documents. All patients received ACEI or ARBs. 30 (58.8%) patients of 51 participants had the history of hypertension and all of them used ACEI or ARBs as anti-hypertensive drugs. Patients without hypertension also received ACEI or ARBs drugs as reno-protective agent. 20 (39.9%) patients had the history of diabetic retinopathy in the recent ophthalmologic examination and were treated properly. Among 51 patients, 18 (35.3%) had the history of thyroid dysfunction but all of them had normal thyroid function test before the study. The demographic and basic clinical characteristics of all study participants were evaluated at the start and end of the study period. No statistically significant differences were seen in terms of basic clinical characteristics in each studied groups as well as between case and control groups [Table 1].
Table 1.
basic and clinical characteristics of participants in drug and placebo groups before intervention
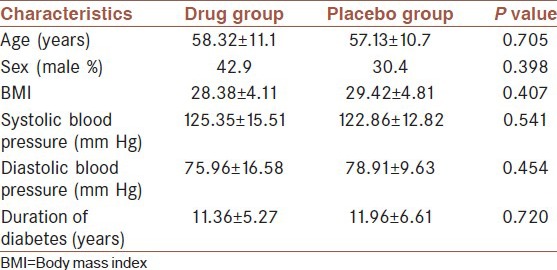
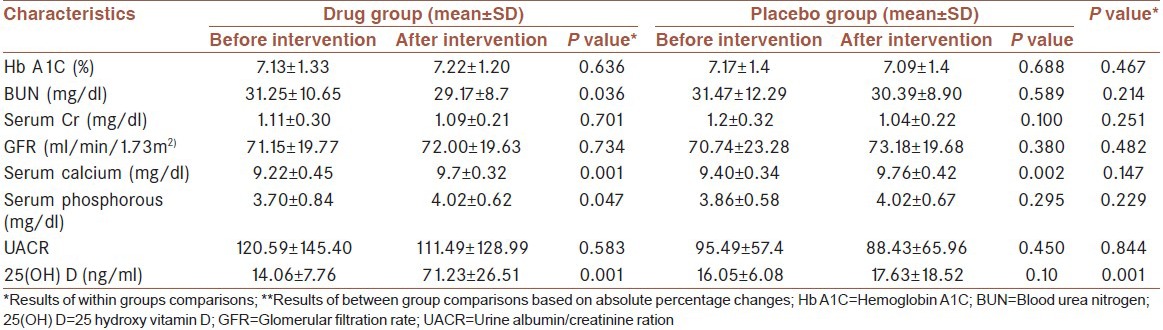
Table 2 presents the results of within and between groups’ comparisons in terms of studied variables. As can be seen from Table 2, within groups’ comparisons only statistically significant is for vitamin D. The mean of calcium before and after intervention was 9.22 ± 0.4 mg/dl and 9.75 ± 0.32 mg/dl (P < 0.0001) and the mean of phosphorous was 3.70 ± 0.84 mg/dl and 4.02 ± 0.62 mg/dl (P < 0.05) respectively. However, between groups comparisons in terms of absolute percentage changes for these variables were not statistically significant. Mean systolic blood pressures changed significantly throughout the course of study in the treatment group. Mean systolic blood pressure in the vitamin D group was 125.35 ± 15.51 mm Hg at baseline and at last evaluation, it was 119.67 ± 16.74 mm Hg (P = 0.033). In the placebo group, the mean systolic blood pressure was 122.86 ± 12.8 and 118.17 ± 17.25 before and after intervention (P = 0.022). However, it showed no significant differences between two groups before and after treatment changes. In the control group, neither baseline nor final differences were statistically significant. Mean serum 25(OH) D concentrations were 14.06 ng/ml and 16.05 ng/ml before treatment, which was in the range of vitamin D deficiency and insufficiency in both groups. However, after intervention, its levels were risen to 71.23 and 17.63 in drug and placebo groups, respectively. Between groups comparison in terms of absolute percentage changes was statistically significant at P < 0.0001. Whereas, UACR as the main variable did not change significantly after intervention in both groups (P = 0.919). The studied groups did not show significant differences in terms of lipid profile, Hb A1C and GFR. None of the patients had the albumin-corrected calcium above 10.5 or phosphorous above 5.2 after treatment. No adverse events were reported in the placebo and drug groups.
Table 2.
Results of within and between group comparisons in terms of studied variables
DISCUSSION
25(OH) D is not only a vitamin, but also known as a hormone in different pathological pathways. The anti-inflammatory role of 25(OH) D in addition to its effect on decreasing the cytokines is becoming evident on daily bases. In National Health And Nutrition Examination Study (NHANES) III study, there is a relationship between the increase in albuminuria and decrease in plasma 25(OH) D.[23] Zehnder et al. studied the relationship between inflammatory markers in chronic kidney disease (CKD) with the level of 1, 25(OH) D.[24] They found out that there is a relationship between the CKD and the level of 1, 25(OH) D. The higher levels of 25(OH) D consequently results in lower inflammation. On the other hand, in CKD patients, a decrease in vitamin D metabolites is related to increase in inflammation. The active metabolites of vitamin D directly influence the endothelial function and inversely related to the degree of arterial calcification in CKD patients. Therefore, decrease in 25(OH) D in CKD patients leads to poor prognosis and eventually progression to ESRD.[25]
There are evidences demonstrating that vitamin D decreases the renal fibrosis progression.[26] The mechanism might be due to decrease in podocytes and increase in glomerulosclerosis, which both increase the risk of albuminuria.[17] There are many studies showing that active forms of vitamin D such as calcitriol and paricalcitrol decreases the albuminuria rate in patients with type 2 diabetes mellitus. The mechanism might be due to decrease in renal expression of renin as well as curbing the Tumor necrosis alpha effect. The vitamin D analogues are known to have reno-protective effect.
As far as our investigation there is no clinical trial that has studied the effect of 25(OH) D on decreasing the proteinuria in patients with type 2 diabetes.
In this study, patients with stable hypertension, dyslipidemia and hyperglycemic treatment, and vitamin D deficiency have been treated with Pearl 50,000 IU vitamin D3 on weekly bases. The patients were compared with placebo groups. Although, the level of 25(OH) D in patients receiving the treatment significantly increased, there was no significant decrease in proteinuria or a change in GFR after 3 months of treatment was observed. The short period of follow-up and small population under study might be the result of insignificant albuminuria in these patients. However, the patients will be evaluated in periods of 6 months and 1 year after receiving the treatment. There are evidences showing that taking vitamin D is beneficial in decreasing the systolic and diastolic blood pressures. However, the difference between the two groups was not significant.
CONCLUSION
According to our finding, there was not a decrease in proteinuria in diabetic patients who have been receiving vitamin D for a period of 3 months.
ACKNOWLEDGMENT
We should appreciate zahravi Pharmaceutical factory that provide vitamin D pearls and the similar placebo. We also thank Dr. Farmani that help us performing this study.
Footnotes
Source of Support: Isfahan University of Medical Sciences, Isfahan Endocrine and Metabolism Research Center
Conflict of Interest: None declared.
REFERENCES
- 1.Mori H, Okada Y, Tanaka Y. Mutual interaction between vitamin D and lifestyle-related diseases in women. J UOEH. 2012;34:323–9. doi: 10.7888/juoeh.34.323. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 3.Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29:149–55. doi: 10.3329/jhpn.v29i2.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaykhaei MA, Hashemi M, Narouie B, Shikhzadeh A, Rashidi H, Moulaei N, et al. High prevalence of vitamin D deficiency in Zahedan, southeast Iran. Ann Nutr Metab. 2011;58:37–41. doi: 10.1159/000323749. [DOI] [PubMed] [Google Scholar]
- 5.John S, Adams W, Hewison M. Update in Vitamin D. Clin Endocrinol Metab. 2010;95:471–8. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz VA, Mainous AG, 3rd, Carek PJ, Wessell AM, Everett CJ. The association of vitamin D deficiency and insufficiency with diabetic nephropathy: Implications for health disparities. J Am Board Fam Med. 2009;22:521–7. doi: 10.3122/jabfm.2009.05.080231. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4:1523–8. doi: 10.2215/CJN.02010309. [DOI] [PubMed] [Google Scholar]
- 8.Tian J, Liu Y, Williams LA, de Zeeuw D. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant. 2007;22:321–8. doi: 10.1093/ndt/gfl595. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MC, Cooper ME. Into the light? Diabetic nephropathy and vitamin D. Lancet. 2010;376:1521–2. doi: 10.1016/S0140-6736(10)61304-9. [DOI] [PubMed] [Google Scholar]
- 10.Li YC. Vitamin D and diabetic nephropathy. Curr Diab Rep. 2008;8:464–9. doi: 10.1007/s11892-008-0080-4. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara S, Kantharidis P, Cooper ME. What are new avenues for renal protection, in addition to RAAS inhibition? Curr Hypertens Rep. 2012;14:100–10. doi: 10.1007/s11906-012-0251-1. [DOI] [PubMed] [Google Scholar]
- 12.Jódar-Gimeno E, Muñoz-Torres M. Vitamin D hormone system and diabetes mellitus: Lessons from selective activators of vitamin D receptor and diabetes mellitus. Endocrinol Nutr. 2013;60:87–95. doi: 10.1016/j.endonu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxy vitamin D (3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163–71. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 16.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 17.Bonakdaran S, Hami M, Hatefi A. The effects of calcitriol on albuminuria in patients with type-2 diabetes mellitus. Saudi J Kidney Dis Transpl. 2012;23:1215–20. doi: 10.4103/1319-2442.103562. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896–901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet. 2010;376:1543–51. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 20.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis. 2009;54:647–52. doi: 10.1053/j.ajkd.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Aperis G, Paliouras C, Zervos A, Arvanitis A, Alivanis P. The role of paricalcitol on proteinuria. J Ren Care. 2011;37:80–4. doi: 10.1111/j.1755-6686.2011.00229.x. [DOI] [PubMed] [Google Scholar]
- 22.Michael F, Holick MF. Vitamin D and health: Evolution, biologic functions, and recommended dietary intakes for vitamin D. Clin Rev Bone Miner Metab. 2009;7:2–19. [Google Scholar]
- 23.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343–53. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.London GM, Guérin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, et al. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxy vitamin D deficiency. J Am Soc Nephrol. 2007;18:613–20. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 26.Llach F, Yudd M. Pathogenic, clinical, and therapeutic aspects of secondary hyperparathyroidism in chronic renal failure. Am J Kidney Dis. 1998;32:S3–12. doi: 10.1053/ajkd.1998.v32.pm9808139. [DOI] [PubMed] [Google Scholar]


