Abstract
Background:
High prevalence of obesity and the importance of this issue as a risk factor for chronic diseases such as severe cardiovascular diseases, diabetes, and cancer necessitate the need for treatment. The aim of this study was the evaluation of acarbose effect on the weight loss in non diabetic overweight or obese patients in Kerman.
Materials and Methods:
A double blind randomized controlled clinical trial was performed on 66 patients with the body mass index (BMI) between 25 and 35 kg/m2. Patients were divided in treatment and control groups using the randomization. The treatment group took 100 mg acarbose 3 times a day for 20 weeks in combination with the low calorie diet and exercise. Control group was given placebo, low calorie diet, and exercise. BMI was measured after 20 weeks. The analyses were carried out using t-test and repeated measured ANOVA.
Results:
Patients in acarbose and placebo group had a non significant difference in BMI at baseline. Reducing in weight was considered in every month in both groups, but this reduction was higher in the treatment group. At the 5th month, the difference of BMI in the treatment group was significantly lower than the placebo group (2.31 ± 0.6 vs. 0.76 ± 0.6 kg/m2, P < 0.001).
Conclusion:
Acarbose, especially in combination with the low calorie diet and exercise, seems to lose weight effectively in obese and overweight patients in communities that have a high carbohydrate intake (like Persian diet).
Keywords: Acarbose, clinical trial, non-diabetics obese, weight loss
INTRODUCTION
Nowadays, more than 1.4 billion adults, 20 and older, were overweight. Of these over 200 million men and nearly 300 million women were obese.[1] Obesity is a common problem of industrial societies and developing countries. Based on the World Health Organization reports, obesity is increasing more rapidly in developing countries than developed countries. This disease is a risk factor for developing hyperlipidemia. These risk factors consequently lead to cardiovascular diseases as the most common cause of death in the world.[2] Obesity and over weight is a major health problem world-wide. Obesity also is an important risk factor for cardiovascular diseases, diabetes type 2 (DM II), osteoarthritis, hyperlipidemia, apnea, and etc., Although studies in the second half of the 20th century about the etiology, pathophysiology and treatment of obesity increased our knowledge in this field of science, but effective methods for treatment and prevention of obesity remains unknown.[3] Studies show that the prevalence of obesity has being elevated in Iran in the recent years due to increasing urbanization. In controlling weight gain, diet is an important factor. Carbohydrate and fat are two most effective component of diet in obese individuals in different societies; carbohydrate in Iran and fat in most Asian countries and in the Americans and Europeans.[4]
Acarbose is an anti-diabetic drug in DM II. This agent inhibits α-glucosidase activity in the small intestine and pancreas and causes reduced glucose release from the disaccharides and oligosaccharides. These mechanisms result in control of the blood glucose after eating.[5] Acarbose has been known as an overweight medication in some studies with mechanisms including appetite reduction and fat or calorie malnutrition especially in long time use.[6] However, some studies reported non-significant weight loss using this drug in non-Asians. This drug is better tolerated in the Asians.[7,8,9] It is shown to reduce weight in DM II patients with high carbohydrate diet. On the other hand, acarbose consumption alone will not lead to low blood sugar and hypoglycemia.[10,11]
There are some drugs with confirmed effect on weight loss world widely with the high cost for consumption in Iran. Acarbose is a non-expensive and reachable drug for Iranian patients. The cost of acarbose is less than the other anti-obesity drugs and procedures.
High controversy about the effect of acarbose on the weight loss in different countries indicated a well-designed study in the Iranian patients with the high carbohydrate diet.[6,4,7,8,9,12] This investigation is designed to evaluate the effect of acarbose on weight loss in non-diabetics overweight or obese in Iran.
MATERIALS AND METHODS
Patients
This study was a double-blind randomized controlled clinical trial. From all obese patients referred to the endocrinology clinic of Afzalipour hospital and office for treatment from November 2009 to April 2010, 66 non-diabetic subjects were recruited to this study. Inclusion criteria were age equal or more than 18 years, willingness to participate in the study, body mass index (BMI) (weight (kg)/height (m)2) between 25 and 35, no evidence of cardiovascular disease, diabetes, gastrointestinal disease (inflammatory bowel disease [IBD], intestinal ulcers, bowel obstruction), liver disease and renal disease, no history of medication for lowering weight, women should not be pregnant or milking. The exclusion criteria were no desire to continue the treatment, development of renal, gastrointestinal and liver disease during treatment and beginning to utilize antihypertensive drugs such as β blocker and thiazides. All patients gave written consensus when they were included to the study. At the beginning of study, the baseline characteristics of all selected subjects including height, weight, total cholesterol, and triglyceride (TG) and fasting plasma glucose (FPG) were recorded. The study protocol was approved by the ethics committee of Kerman University of Medical Sciences (k/88/106) and this trial was registered on the Iranian Registry of Clinical Trials (IRCT) website (the registration number of the trial is IRCT ID: IRCT201011151774N3). We tried this randomized controlled trials conform to the Consort statement and flow diagram.
Randomization
A double-blind randomized, parallel group, placebo-controlled design was used. Patients were randomly assigned to receive acarbose or placebo. Block randomization was performed centrally by a computer program with minimization for height, weight and BMI according to the study center. After baseline assessment, the eligible patients were allocated by computer generated random numbers to 1 of 2 treatment groups and followed-up at 1 week. The letter A and B was selected for two groups; physician referred the patient to nurse. The nurse had several envelope the contains the letter A or B in a random manner (for example AABB/ABAB/…etc.,). The letter A or B belongs to patient in this way and the drug gives to patient by another nurse.
Treatment group was given acarbose pills (100 mg) and other group took lactose contained placebo (100 mg). Acarbose and placebo were packed in sachets of identical appearance; patients and trial nurses and physician and statistician were all unaware of the study-group assignment until after analysis.
Treatment
Acarbose pills (100 mg) and lactose contained placebo (100 mg) were prepared from Exir co. and were similar in shape and packing. Patients were asked to take medicines in the first 2 weeks with 1½ of a tablet daily and from 3rd week with 3 tablets daily (to reduce gastrointestinal side-effects). They were asked to take the pills at the beginning of a meal. Patients in the placebo and treatment groups had nutrition consultation for reducing 500-800 Kcal at first and the every 45 days. A training exercise CD was given to each person and they planned to exercise at least ½ h daily.
Outcome measures
Subjects were evaluated every month for adverse effects, weight loss, and BMI for 5 months. Once every 3 months and a baseline blood sample for FPG, TG, and cholesterol was taken from patients.
Analyses
The analyses were performed by paired t-test and repeated measured ANOVA and also they were adjusted for baseline BMI. The probability value of less than 0.05 was considered significant. All analysis was carried out using the SPSS v. 13 software.
RESULTS
From 66 enrolled patients, 33 were assigned to treatment and 33 to placebo group. Of these patients, 21 (63%) in the placebo group and 19 (58%) in the acarbose group received the assigned intervention completely. The most common reason for not continuing study intervention was the occurrence of side-effects. The side-effects were abdominal pain, diarrhea, and flatulence. No severe side-effects such as hepatitis or thrombocytopenia were seen. Other reasons are reported in Figure 1.
Figure 1.
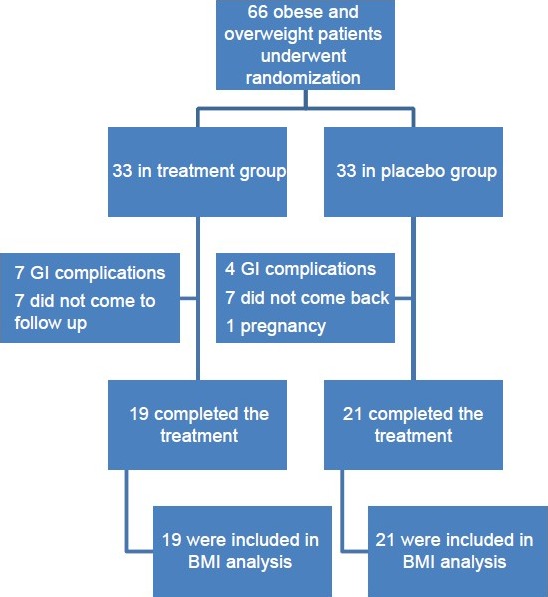
The study fellow chart; enrollment and outcomes
The subjects consisted of 10 men (25%) and 30 women (75%) with a mean age of 31 ± 8.8 years. The mean weight was 77.8 ± 10.1 kg and BMI was 30.1 ± 2.6 kg/m2.
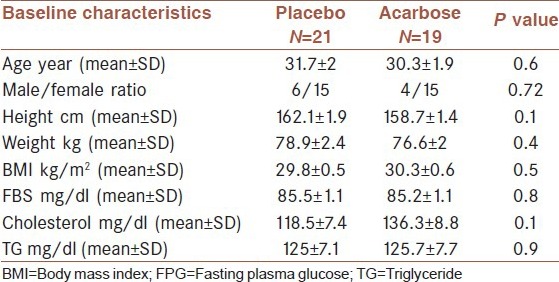
As shown in Table 1, there was no significant difference in the baseline characteristics of two groups.
Table 1.
Baseline characteristics of the study population
As could be seen in Figure 2 the BMI reduced in both groups during the study, but this reduction was higher in the acarbose group (P < 0.001). The differences in each interval are shown in Table 2. And the effect of acarbose was constant and progressive [Figure 3].
Figure 2.

Body mass index in each treatment in the treatment and placebo groups
Table 2.
Difference of BMI changes in each 1 month interval in two groups (mean±SD, kg/m2)

Figure 3.
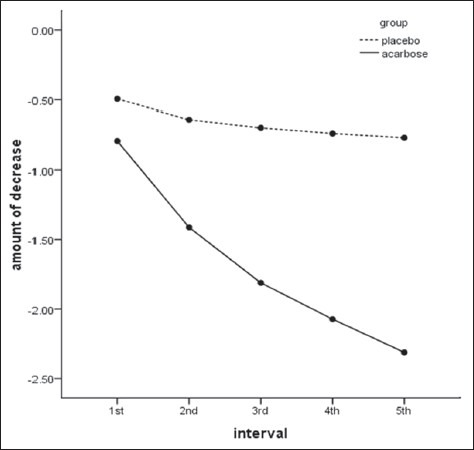
Body mass index changes during intervals in the treatment and placebo group
DISCUSSION
This study showed that acarbose can reduce weight in over weight and obese non-diabetic people; this effect is continuous and progressive. Considering the high prevalence of overweight and obesity in society and the importance of this as one of the major risk factors for chronic diseases such as severe cardiovascular diseases, diabetes and cancer, the requirement for therapy is obvious. Variety of treatments for obesity and overweight has been suggested. One of these drugs is α glycosidase inhibitors including acarbose. Acarbose is an anti-hyperglycemic agent in DM II and lower the weight of many diabetic patients reportedly.[13] There is controversy about the effect of acarbose on weight loss in different societies with different diet. In this study, the effect of this drug was investigated on the Iranians patients as a society with the high carbohydrate utilization in the meals. As shown in the results, the patients treated with acarbose have a higher rate of weight loss after 2 months. Several clinical trials have shown similar results to the present study. The results of a study by Hauner et al. to investigate the effect of acarbose on stability after low calorie diet induced weight loss confirmed the finding of our study. In that double-blind controlled clinical trial, patients with a BMI between 32 and 38 kg/m2 were treated with the low calorie diet for 10-16 weeks and then 26 weeks with acarbose.[8] However, an investigation on 2002 in Taiwan and several Asian countries showed the acarbose has higher effect on reducing Hemoglobin A1c and post-prandial glucose in DM II patients than sulfonylurease. Furthermore, acarbose was shown to be well-tolerated and safe but did not significantly lower the BMI than other treatment.[5] In that study, the DM II patients did not get low calorie diet in contrast to the present study in non-diabetic patients with low calorie diet. In another trial by Rachmani et al., acarbose was not shown to significantly decrease weight than placebo. Although, their diet was very different and low calorie diet was not administered for the subjects.[14] Our study had some limitation. First, the sample size was too small to compare the sex difference in acarbose induced weight loss. Second, no data was available about the intake of patients.
CONCLUSION
Consequently combination of acarbose administration with the low calorie diet seems to be an effective treatment for lowering weight in the overweight and obese patients with the high carbohydrate utilization.
ACKNOWLEDGMENTS AND CONFLICTS OF INTEREST
This study was a part of a common project and sponsored by the vice chancellor and Physiology Research Center Kerman University of Medical Sciences and Exir Pharma Company. This study approved as a medical student thesis (proposal no. 87/147). The authors would like to thank all participant of this study. There was no conflict of interest for authors. The Exir co. just prepared the drug and placebo.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fact sheet: Obesity and overweight. [Internet] World Health Organization (WHO) 2013. Mar, Avalilable from: http://www.who.int/mediacentre/factsheets/fs311/en .
- 2.Hadaegh F, Zabetian A, Tohidi M, Ghasemi A, Sheikholeslami F, Azizi F. Prevalence of metabolic syndrome by the Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions and their association with coronary heart disease in an elderly Iranian population. Ann Acad Med Singapore. 2009;38:142–9. [PubMed] [Google Scholar]
- 3.Krummel, Dabra A. Krause's Food, Nutrition and Diet Therapy. 11th ed. Philadelphia: WB Saunders; 2004. “Medical nutrition therapy in cardiovascular disease”; pp. 860–99. [Google Scholar]
- 4.Kelishadi R, Ardalan G, Gheiratmand R, Gouya MM, Razaghi EM, Delavari A, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN Study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin BJ, Wu HP, Huang HS, Juang JH, Sison A, bin Abdul Kadir DK, et al. Efficacy and tolerability of acarbose in Asian patients with type 2 diabetes inadequately controlled with diet and sulfonylureas. J Diabetes Complications. 2003;17:179–85. doi: 10.1016/s1056-8727(02)00258-1. [DOI] [PubMed] [Google Scholar]
- 6.Siraj ES. Is there a role for metformin or acarbose as a weight-loss agent in the absence of diabetes? Cleve Clin J Med. 2003;70:702–4. doi: 10.3949/ccjm.70.8.702. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktar F, Hamulu F, Ozgen AG, Yilmaz C, Tüzün M, Kabalak T, et al. Acarbose treatment in obesity: A controlled study. Eat Weight Disord. 1998;3:46–9. doi: 10.1007/BF03339987. [DOI] [PubMed] [Google Scholar]
- 8.Hauner H, Petzinna D, Sommerauer B, Toplak H. Effect of acarbose on weight maintenance after dietary weight loss in obese subjects. Diabetes Obes Metab. 2001;3:423–7. doi: 10.1046/j.1463-1326.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H, Degerblad M, Thorell A, Nygren J, Ståhle A, Kuhl J, et al. Combined treatment with exercise training and acarbose improves metabolic control and cardiovascular risk factor profile in subjects with mild type 2 diabetes. Diabetes Care. 2006;29:1471–7. doi: 10.2337/dc05-2513. [DOI] [PubMed] [Google Scholar]
- 10.Pan CY, Gao Y, Chen JW, Luo BY, Fu ZZ, Lu JM, et al. Efficacy of acarbose in Chinese subjects with impaired glucose tolerance. Diabetes Res Clin Pract. 2003;61:183–90. doi: 10.1016/s0168-8227(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 11.Kasper DL, Braunward E, Fauci AS, Hauser S, Longo DL, Jameson J, editors. 16th ed. United States: Mc Graw Hill; 2005. Harrison's Principle of Internal Medicine; pp. 2380–5. [Google Scholar]
- 12.Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, et al. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes Relat Metab Disord. 1997;21:756–63. doi: 10.1038/sj.ijo.0800468. [DOI] [PubMed] [Google Scholar]
- 13.Halpern A, Mancini MC, Suplicy H, Zanella MT, Repetto G, Gross J, et al. Latin-American trial of orlistat for weight loss and improvement in glycaemic profile in obese diabetic patients. Diabetes Obes Metab. 2003;5:180–8. doi: 10.1046/j.1463-1326.2003.00262.x. [DOI] [PubMed] [Google Scholar]
- 14.Rachmani R, Bar-Dayan Y, Ronen Z, Levi Z, Slavachevsky I, Ravid M. The effect of acarbose on insulin resistance in obese hypertensive subjects with normal glucose tolerance: A randomized controlled study. Diabetes Obes Metab. 2004;6:63–8. doi: 10.1111/j.1463-1326.2004.00317.x. [DOI] [PubMed] [Google Scholar]


