Abstract
Purpose
Many women with hormone receptor-positive breast cancer discontinue effective aromatase inhibitor (AI) treatment due to joint symptoms.
Methods
We conducted a single-arm, open-label, phase II study evaluating glucosamine-sulfate (1,500 mg/day)+ chondroitin-sulfate (1,200 mg/day) for 24 weeks to treat joint pain/stiffness in postmenopausal women with early stage breast cancer who developed moderate-to-severe joint pain after initiating AIs. The primary endpoint was improvement in pain/stiffness at week 24 assessed by the Outcome Measure in Rheumatology Clinical Trials and Osteoarthritis Research Society International (OMERACT-OARSI) criteria. Secondary endpoints assessed changes in pain, stiffness, and function using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index for hips/knees and the Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (M-SACRAH) for hands/wrists. The Brief Pain Inventory (BPI) assessed pain interference, severity, and worst pain.
Results
Of 53 patients enrolled, 39 were evaluable at week 24. From baseline to week 24, 46 % of patients improved according to OMERACT-OARSI criteria. At week 24, there were improvements (all P<0.05) in pain and function as assessed by WOMAC and M-SACRAH, and in pain interference, severity, and worst pain as assessed by BPI. Estradiol levels did not change from baseline. The most commonly reported side effects were headache (28 %), dyspepsia (15 %), and nausea (17 %).
Conclusions
In this single-arm study, 24 weeks of glucosamine/chondroitin resulted in moderate improvements in AI-induced arthralgias, with minimal side effects, and no changes in estradiol levels. These results suggest a need to evaluate efficacy in a placebo-controlled trial.
Keywords: Breast cancer, Aromatase inhibitors, Arthralgias, Dietary supplement, Glucosamine, Chondroitin
Introduction
In postmenopausal women, aromatase inhibitors are more effective than tamoxifen for reducing the recurrence of hormone-sensitive breast cancers [1–6]. However, work by our group and others has shown that up to 50 % of women on aromatase inhibitors report joint pain and or stiffness that starts or worsens after initiating treatment and causes significant morbidity [7–11]. These side effects can lead to early discontinuation of aromatase inhibitors and thus prevent women from obtaining the full survival benefit of this effective treatment for hormone-sensitive breast cancers [12, 13]. There is a need for safe, effective treatments to reduce this common drug-related toxicity.
Recent estimates show that up to two thirds of individuals in the general population who suffer pain associated with arthritis and other musculoskeletal disorders have used complementary and alternative treatments in an attempt to control their symptoms, but data on the efficacy of such use are limited [14–16]. Glucosamine and chondroitin are popular dietary supplements frequently used with the goal of treating arthritic pain; in 2007, 19.9 % of US adults reported using glucosamine in the previous 30 days. Glucosamine and chondroitin are both natural compounds found in healthy cartilage. Glucosamine is an aminomonosaccharide substrate used in the synthesis of glycosaminoglycan and proteoglycans present in the cartilage matrix and synovial fluid. Chondroitin is a major component of aggrecan, a large glycoprotein present in the extracellular matrix of connective tissue. Both are hypothesized to have local anti-inflammatory effects within joints, and glucosamine may increase the synthesis of proteoglycans in articular cartilage [16–24]. A National Institutes of Health-funded, large-scale, randomized, placebo-controlled trial of glucosamine and chondroitin sulfate for painful knee osteoarthritis (n = 1,583), the Glucosamine/chondroitin Arthritis Intervention Trial (GAIT), reported no effect at week 24 when data from all participants were examined. However, when the analyses were restricted to patients with moderate to severe pain at baseline, the rate of response was significantly higher with combined therapy than with placebo (79.2 % versus 54.3 %, p = 0.002) [25].
To date, all studies that have examined glucosamine and chondroitin have examined its effect on osteoarthritic pain. We conducted a single-arm phase II study to test the effect of 24 weeks of glucosamine plus chondroitin on aromatase inhibitor-induced joint pain in postmenopausal women with a history of hormone receptor-positive breast cancer.
Methods and procedures
Participants
Women were recruited from the Columbia University Medical Center (CUMC) breast oncology clinic. Potentially eligible women were referred by their breast oncologists to be screened by study staff for the following eligibility criteria—age, 21 years or older; postmenopausal (defined as cessation of menses for >1 year, follicle-stimulating hormone>20 mIU/mL, or bilateral oophorectomy); previous diagnosis of stage I–III breast cancer with no evidence of metastatic disease; current use of a third-generation aromatase inhibitor (anastrozole, letrozole, exemestane) for ≥3 months; self-reported knee and/or hand joint pain and/or stiffness for ≥3 months prior to study entry; ongoing musculoskeletal pain/stiffness in hand and/or knee joints (≥4 on a ten-point scale assessing worst joint pain/stiffness in the past 7 days) that started or increased since initiating aromatase inhibitor therapy and has been present for ≥3 months; if taking bisphosphonates, on a stable dose for ≥3 months and tolerating the dose; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; hemoglobin A1c<8 within past 12 months; ability to read and/or understand English and/or Spanish language. Ineligibility criteria were as follows: use of glucosamine or chondroitin within the past 3 months; current use of medications other than acetaminophen and ibuprofen for joint pain; concurrent medical/arthritic disease that could confound or interfere with evaluation of joint pain or efficacy (women with preexisting osteoarthritis whose joint pain worsened after initiating aromatase inhibitor therapy were eligible for participation); history of significant joint, ligament, or bone disease and/or injury in 6 months prior to study entry; and allergy to shellfish.
The CUMC institutional review board approved the study, and all participants provided written informed consent in English or Spanish. Study staff was bilingual.
Clinical data collection
Eligible participants were scheduled for a baseline visit where they completed the following procedures: questionnaire assessing demographic information, reproductive history, joint symptoms, quality of life, aromatase inhibitor use, use of pain medications, and attitudes towards and use of complementary and alternative therapies; history and physical examination, including hand grip and pinch strength; and a blood draw. Follow-up clinic visits took place at 6, 12, and 24 weeks; a phone follow-up took place at 18 weeks. The week 6 clinic visit assessed aromatase inhibitor use, use of pain medications, and hand grip and pinch strength. At the 12 and 24 week follow-up clinic visits, participants completed the following procedures: questionnaire assessing joint symptoms, quality of life, aromatase inhibitor use, and use of pain medications; hand grip and pinch strength; and a blood draw. The 18 week phone call assessed aromatase inhibitor use and use of pain medications.
Measures
Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index
The WOMAC index was used to measure changes in hip and knee joint pain and stiffness [26]. The WOMAC index is a validated questionnaire that consists of 24 questions related to three subscales: pain, stiffness, and physical function. Five questions assess joint pain, two questions assess stiffness, and 17 questions assess limitation of physical function in the 7 days prior to assessment. In addition, there is a single global assessment question which asks “How well are you doing?” Each question is answered by participants using a standardized 10-cm-long visual analog scale (VAS) with terminal descriptors. Subscale scores are normalized into a 0–100-point scale.
Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands (M-SACRAH)
The M-SACRAH was used to measure changes in hand joint pain and stiffness [27]. This scale consists of three domains assessing pain, stiffness, and functional status in patients suffering from hand osteoarthritis and rheumatoid arthritis. Subscale scores are normalized into a 0–100-point scale.
Brief Pain Inventory Short Form (BPI-SF)
The BPI-SF is a 14-item questionnaire that assesses various dimensions of general pain over the past week, including pain interference, pain severity, and worst pain [28]. We modified the questionnaire to ask specifically about joint pain and/or stiffness.
Functional Assessment of Cancer Therapy-Breast and Endocrine System (FACT-B/ES)
The FACT-B was used to measure self-reported functional well-being and physical well-being [29]. We used the Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES) to monitor endocrine side effects caused by aromatase inhibitors, including hot flashes, night sweats, etc. [30]. The FACT scales have five response levels (“not at all” to “very much”), where higher scores reflect higher states of wellbeing and fewer symptoms [29].
Hand grip and pinch strength
At baseline, 6, 12 and 24 weeks, hand grip and pinch strength was measured using a Martin dynamometer, which involved squeezing a rubber ball and recording the pressure measured in kilopascals. Three different types of strength were measured: pinch grip, tripod grip, and power grip. For each measure, the best of three attempts was recorded.
Serum estradiol
At baseline, 12, and 24 weeks, blood samples were collected to measure serum total estradiol. Samples were batch-analyzed using liquid chromatography tandem mass spectroscopy in the Columbia University Clinical Translational Service Award Biomarkers Core Laboratory.
Adverse events
Adverse event severity and frequency were assessed at every clinic visit using National Cancer Institute (NCI) Common Terminology Criteria Version 3.0 [31].
Glucosamine and chondroitin capsules
Study capsules were manufactured by Thorne Research (Sand-point, ID) using pharmaceutical-grade raw materials under dietary supplement Good Manufacturing Process standards and US Food and Drug Administration Investigational New Drug number 79,502/S0005. Each capsule contained 250 mg glucosamine sulfate potassium chloride (shellfish source) and 200 mg chondroitin sulfate sodium (bovine source) (both obtained from TSI Health Sciences, Missoula, MT). The chondroitin sulfate sodium was certified as being obtained from a country free of bovine spongiform encephalopathy.
At each clinic visit, participants provided with a supply of glucosamine sulfate and chondroitin sulfate to last until the following clinic visit. To achieve a daily dose of 1,500 mg glucosamine and 1,200 mg chondroitin and to increase adherence rates, participants could choose to take either two capsules three times daily or three capsules two times daily.
Daily diaries and rescue pain medication
Participants were provided with a daily diary to log the number of pills taken and use of rescue pain medications. Participants were permitted to use up to 3,000 mg acetaminophen per day (using 325 mg tablets) or 2,400 mg ibuprofen per day (using 200 mg tablets). The study did not provide acetaminophen or ibuprofen to participants.
Statistical analysis
The Outcome Measures in Rheumatology Clinical Trials and Osteoarthritis Research Society International (OMERACT-OARSI) Criteria were used to assess response to treatment at 12 and 24 weeks, with the primary endpoint being response at 24 weeks [32]. This measure was chosen as the primary endpoint so that results could be compared with the GAIT study results. A response is classified as (1) an improvement in pain or function of at least 50 % and a decrease of at least 20 mm on the VAS for pain or function, or (2) the occurrence of at least two of the following: (a) a decrease in pain of at least 20 % and at least 10 mm on the VAS; (b) an improvement in function of at least 20 % and a decrease of at least 10 mm on the VAS; and (c) an increase in the patient’s global assessment score by at least 20 % and at least 10 mm on the VAS. In this study, we used both the WOMAC and the M-SACRAH VAS subscales to evaluate pain and function. To evaluate global assessment of disease status, we used the 100 mm VAS used in the GAIT study.
Efficacy was assessed by the proportion of patients who showed a clinical response based upon the OMERACT-OARSI criteria at week 24. Our a priori hypothesis was that this treatment would be of interest for further evaluation if more than 17 responses were observed in the 46 evaluable patients (36.9 % response rate). We assumed a 15 % dropout rate and targeted 53 patients for enrollment.
Paired t tests were used to compare all continuous data from baseline to the end of follow-up. To assess the OMERACT-OARSI measure, we used the last-observation-carried-forward and last-observation-carried backward method in the analysis of all outcomes among patients who made at least one follow-up visit but who did not complete the 24-week follow-up.
Analyses were conducted using SAS 9.2 (Cary, NC).
Results
Recruitment, retention, and baseline characteristics
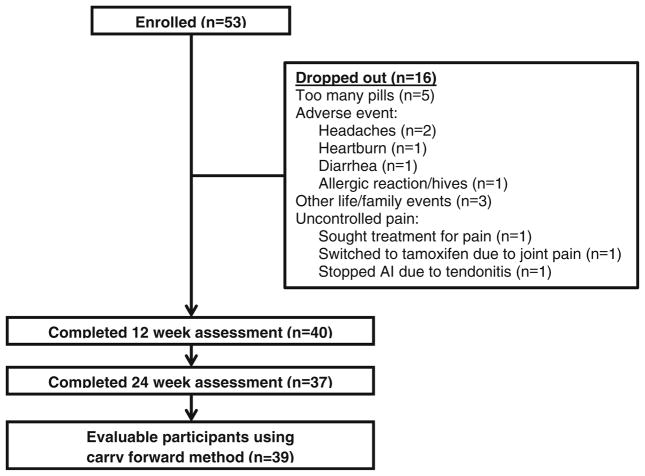
Between October 2008 and November 2010, 53 women were enrolled into the study. Of these 53 women, 37 (69.8 %) were retained in the study for the full 24 weeks (see Fig. 1 for CONSORT diagram). The main reasons for drop-out were burden of taking six capsules per day (n = 5), uncontrolled pain (n = 3), and headaches (n = 2). Table 1 shows demographic and clinical characteristics of the 40 women who were evaluated at 12 and/or 24 weeks. The average age of participants was 61.4 years (range, 40.7–83.2 years). Forty percent of participants were Hispanic, and 41.5 % were non-Hispanic white. The majority of participants (67.5 %) reported some college education. Participants were on average 3.6 years beyond breast cancer diagnosis (range, 0.5–15.2 years). At baseline, 87.5 % of women were taking anastrozole (Arimidex); 7.5 % of women were taking exemestane (Aromasin), and 5 % of women were taking letrozole (Femara). At baseline, 57.5 % of women reported any prior use of complementary and alternative medicine, and 32.5 % reported using any form of an ingestible dietary supplement specifically to treat their joint pain. There were no statistically significant (P<0.05) differences in demographic and clinical characteristics between the 40 women in Table 1 and the 13 women who dropped out of the study (data not shown).
Fig. 1.
CONSORT diagram
Table 1.
Demographic and clinical characteristics of participants who completed 12 and/or 24 weeks of the study (n = 40a)
| n | % | |
|---|---|---|
| Demographic characteristics | ||
| Age, years | ||
| 40–49 | 7 | 17.5 |
| 50–59 | 15 | 37.5 |
| 60–69 | 12 | 27.5 |
| ≥70 | 7 | 17.5 |
| Race | ||
| Non-Hispanic white | 17 | 42.5 |
| Hispanic | 16 | 40 |
| Black | 6 | 15 |
| Asian | 1 | 2.5 |
| Education | ||
| <HS grad | 5 | 12.5 |
| GED/HS grad | 8 | 20 |
| Associate/some college | 9 | 22.5 |
| College degree | 7 | 17.5 |
| Master/doctorate | 11 | 27.5 |
| Marital status | ||
| Married | 24 | 60 |
| Single/divorced | 13 | 32.5 |
| Widowed | 3 | 7.5 |
| Clinical characteristics | ||
| BMI, kg/m2 | ||
| <18.5 | 1 | 2.5 |
| 18.5–24.9 | 10 | 25 |
| 25–29.9 | 15 | 37.5 |
| 30–34.9 | 9 | 22.5 |
| ≥35 | 5 | 12.5 |
| Years since diagnosis | ||
| <2 years | 12 | 30 |
| 2–5 | 21 | 52.5 |
| ≥5 years | 7 | 17.5 |
| Stage at diagnosis | ||
| I | 21 | 52.5 |
| II | 17 | 42.5 |
| III | 2 | 5.0 |
| Her2/neu-positive | 4 | 15 |
| Received chemotherapy | 23 | 57.5 |
| Type of aromatase inhibitor | ||
| Anastrozole/Arimadex | 35 | 87.5 |
| Exemestane/Aromasin | 3 | 7.5 |
| Letrozole/Femara | 2 | 5.0 |
| Prior tamoxifen therapy | 10 | 25.0 |
| Prior HRT | 6 | 15.0 |
| Baseline joint conditions | 23 | 57.5 |
| Typeb | ||
| Osteoarthritis | 14 | 35.0 |
| Chronic low back pain | 2 | 5.0 |
| Rheumatoid arthritis | 1 | 2.5 |
| Joint surgery | 5 | 12.5 |
| Other | 3 | 7.5 |
Data included for 40 participants who provided follow-up data at 12 and/or 24 weeks, out of 53 participants who enrolled in the study
Categories not mutually exclusive
Joint symptoms
At week 24, 18 of the 39 (46.2 %) evaluable participants met the OMERACT-OARSI criteria for self-reported improvements in their joint pain symptoms related to pain and/or function as assessed by WOMAC and/or M-SACRAH; at week 12, 15/39 (38.5 %) of participants met the criteria.
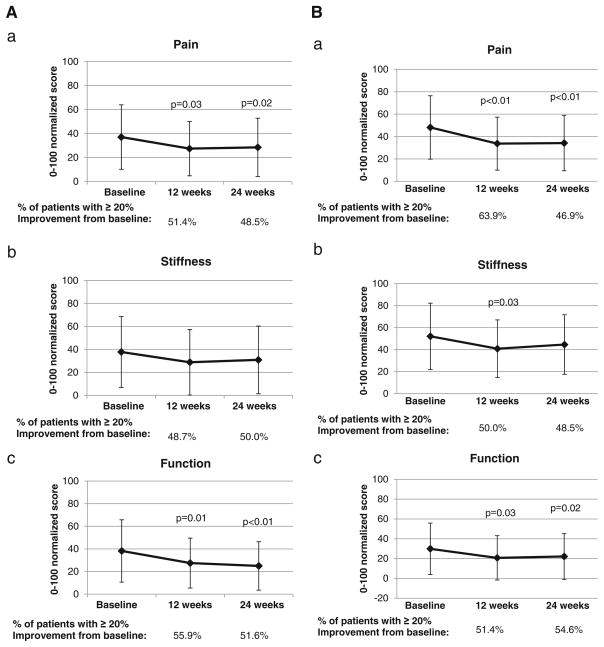
Comparing week 12 to baseline, 51.4 % of participants reported a ≥20 % decrease in their hip and knee pain as assessed by WOMAC (Fig. 2a), and 63.9 % of participants reported a decrease in their hand and wrist pain as assessed by M-SACRAH (Fig. 2b); these changes were maintained at week 24. Similar changes were seen in measures of stiffness and function in these WOMAC and M-SACRAH. Comparing week 12 to baseline, 36.8 % of participants reported a ≥20 % decrease in pain severity, and 43.6 % reported a ≥20 % decrease in worst pain (Fig. 2c); these effects also held at week 24.
Fig. 2.
Changes in study endpoints. a Change in WOMAC scores for symptoms in hips and knees. a pain, b stiffness, and c function. P values calculated using paired t tests. b Change in M-SACRAH scores for symptoms in hands and wrists. a pain, b stiffness, and c function. P values calculated using paired t tests. c Change in BPI. a Pain severity, b pain interference, and c worst pain. P values calculated using paired t tests; all P values>0.05. d Change in grip strength via Martin dynamometer. a Pinch grip strength, b tripod grip strength, and c power grip strength. The black line corresponds to the left hand. The gray line corresponds to the right hand. P values calculated using paired t tests; all P-values<0.05. Abbreviations: WOMAC Western Ontario & McMaster Universities Osteoarthritis Index; M-SACRAH Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands; BPI Brief Pain Inventory; KPAs kilopascals
Compared with baseline, improvements were seen in mean scores at week 12 in the WOMAC index for function (P = 0.01) and pain (P = 0.03); the M-SACRAH index for function (P = 0.03), pain (P<0.01), and stiffness (P = 0.03); and the BPI for pain severity (P = 0.05) and worst pain (P = 0.02) (Table 2). Similar patterns were observed when comparing means scores at week 24 to baseline with improvements in the WOMAC index for function (P<0.01) and pain (P = 0.02); the M-SACRAH index for function (P = 0.02) and pain (P<0.01); and the BPI for pain interference (P<0.01).
Table 2.
Mean values of WOMAC, BPI, M-SACRAH, grip strength, and quality of life at baseline, weeks 12 and 24
| Baseline
|
Week 12 (n = 38)
|
Week 24 (n = 36)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean difference from baseline | P valuea | Mean | SD | Mean difference from baseline | P valuea | |
| WOMAC—hips/knees | ||||||||||
| Function (0–100) | 38.2 | 27.5 | 27.5 | 22.1 | −10.7 | 0.01 | 24.9 | 21.4 | −13.3 | 0.004 |
| Pain (0–100) | 37.0 | 26.9 | 27.4 | 22.7 | −9.6 | 0.03 | 28.4 | 24.4 | −10.7 | 0.02 |
| Stiffness (0–100) | 37.8 | 30.9 | 28.8 | 28.5 | −9.0 | 0.10 | 30.9 | 29.5 | −7.5 | 0.15 |
| M-SACRAH—hand/wrists | ||||||||||
| Function (0–100) | 29.9 | 26.0 | 20.7 | 22.4 | −9.2 | 0.03 | 22.1 | 23.1 | −8.5 | 0.02 |
| Pain (0–100) | 48.1 | 28.3 | 33.7 | 23.7 | −14.4 | 0.0011 | 34.6 | 24.5 | −13.8 | 0.0008 |
| Stiffness (0–100) | 52.1 | 30.1 | 40.8 | 26.1 | −11.3 | 0.03 | 44.6 | 27.0 | −7.9 | 0.13 |
| BPI | ||||||||||
| Pain severity (0–10) | 4.9 | 2.2 | 4.2 | 2.1 | −0.7 | 0.05 | 4.3 | 2.0 | −0.6 | 0.07 |
| Pain interference (0– 10) | 4.1 | 2.3 | 3.5 | 2.2 | −0.6 | 0.06 | 3.2 | 2.2 | −1.0 | 0.0003 |
| Worst pain (0–10) | 6.2 | 2.3 | 5.3 | 2.5 | −0.9 | 0.02 | 5.3 | 2.6 | −1.2 | 0.02 |
| Grip strength (kPAs) | ||||||||||
| Pinch grip strength | ||||||||||
| Right hand | 31.3 | 10.6 | 33.8 | 8.7 | 2.5 | 0.07 | 32.2 | 9.6 | 2.0 | 0.22 |
| Left hand | 28.6 | 9.1 | 31.2 | 8.1 | 2.6 | 0.02 | 28.9 | 8.5 | 1.5 | 0.22 |
| Tripod grip strength | ||||||||||
| Right hand | 34.1 | 11.0 | 37.9 | 9.9 | 3.8 | 0.02 | 35.6 | 9.6 | 2.7 | 0.06 |
| Left hand | 33.2 | 10.3 | 37.2 | 10.3 | 4.1 | 0.02 | 35.3 | 9.9 | 2.8 | 0.11 |
| Power grip strength | ||||||||||
| Right hand | 37.7 | 12.9 | 42.6 | 11.9 | 4.9 | 0.01 | 41.8 | 11.6 | 3.9 | 0.0005 |
| Left hand | 35.9 | 11.7 | 40.6 | 11.5 | 4.6 | 0.02 | 39.2 | 10.7 | 4.3 | 0.02 |
| FACT | ||||||||||
| Functional well-being | 18.8 | 5.7 | 22.6 | 6.7 | 3.8 | 0.0001 | 18.1 | 6.0 | −0.2 | 0.79 |
| Physical well-being | 17.8 | 5.4 | 19.2 | 5.4 | 1.4 | 0.14 | 18.4 | 5.3 | 1.0 | 0.16 |
| Endocrine well-being | 52.0 | 11.5 | 54.1 | 9.8 | 2.1 | 0.09 | 50.9 | 10.7 | −1.2 | 0.36 |
WOMAC Western Ontario and McMaster Universities Osteoarthritis Index, M-SACRAH Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands, BPI Brief Pain Inventory, FACT Functional Assessment of Cancer Therapy, kPAs kilopascals
P values calculated using paired t tests to compare differences in group means at baseline and follow-up
Grip and pinch strength
Using the Martin dynamometer, at week 12, approximately one third of women demonstrated ≥20 % improvement in pinch, tripod, and power grip strength (Fig. 2d), which was maintained at week 24. Overall, participants also demonstrated improvements in mean scores in pinch (right hand, P = 0.07), tripod (right hand, P = 0.02), and power (right hand, P = 0.01) grip strength (Table 2).
Quality of life
At week 12, participants reported an improvement in mean levels of functional well-being (P = 0.0001), which was not maintained at week 24 (Table 2). There were no changes in physical well-being or the endocrine symptom sub-scale from baseline to 12 or week 24.
Use of pain medications
At baseline, 47.5 % of participants reported using analgesics for pain relief, including aspirin, acetaminophen, ibuprofen, naproxen, and other nonsteroidal anti-inflammatory agents. There were no reported differences in use of analgesics from baseline to 12 or week 24 (all P>0.05, data not shown).
Safety
The normal range of estradiol for postmenopausal women is <10 pg/mL. At baseline, the mean estradiol concentration was 6.4 (SD 18.8)pg/mL, and there were no clinically meaningful changes at week 12 (mean, 7.0 [SD 19.8]pg/mL) or week 24 (5.7 [SD 16.3)]pg/mL).
Adverse events and toxicities were assessed at every clinical encounter. Participants reported improvements in arthralgias and myalgias at weeks 12 and 24 (all P<0.05, data not shown), but no changes in hot flashes or vaginal dryness. The most commonly reported adverse events that were at least possibly related to study drug were grade 1 headaches (n = 13), grade 1 dyspepsia (n = 9), and grade 1 nausea (n = 8) (Table 3). Reported related or possibly related grade II toxicities included nausea (n = 1), heartburn (n = 1), gastrointestinal disorder (n = 1), headache (n = 1), fatigue (n = 1), and an allergic reaction (hives) (n = 1).
Table 3.
Worst measurable related and possibly related toxicities during 24-week trial period (n = 53)
| Toxicity | Grade 1 | Grade 2 | Total
|
|
|---|---|---|---|---|
| n | % | |||
| Gastrointestinal | ||||
| Nausea | 8 | 1 | 9 | 17% |
| Dyspepsia | 9 | 0 | 9 | 17% |
| Diarrhea | 4 | 0 | 4 | 8% |
| Flatulence | 3 | 0 | 3 | 6% |
| Heartburn | 1 | 1 | 2 | 4% |
| Gastrointestinal disorder | 0 | 1 | 1 | 2% |
| Abdominal distention | 1 | 0 | 1 | 2% |
| Gastritis | 1 | 0 | 1 | 2% |
| Hiccough | 1 | 0 | 1 | 2% |
| Stomach pain | 1 | 0 | 1 | 2% |
| Metabolic | ||||
| Weight gain | 1 | 0 | 1 | 2% |
| Neurologic | ||||
| Headache | 13 | 1 | 14 | 26% |
| Constitutional | ||||
| Fatigue | 2 | 1 | 3 | 6% |
| Dermatologic | ||||
| Allergic reaction | 0 | 1 | 1 | 2% |
| Vascular | ||||
| Bleeding at biopsy site | 1 | 0 | 1 | 2% |
If a participant experienced the same toxicity more than once over the course of the study, it is only accounted for once in this table
As measured using the NCI Common Terminology Criteria Version 3.0
Discussion
In this phase II clinical trial of glucosamine and chondroitin to treat moderate-to-severe aromatase inhibitor-induced joint pain, we observed that 46.2 % of participants reported a clinically meaningful response after 6 months, as assessed by the OMERACT-OARSI criteria. After 3 months, approximately 50 % of participants self-reported a ≥20 % improvement in pain, stiffness, and function in their hips/knees and hands/wrists, and approximately one third of participants demonstrated ≥20 % improvement in clinically measured grip strength; these improvements were maintained at 6 months. The intervention was well-tolerated with minimal toxicities. Reassuringly, we did not observe an increase in serum estradiol with glucosamine and chondroitin supplementation. Changes in estradiol were assessed to ensure that glucosamine and chondroitin did not interfere with the anti-tumor effects of aromatase inhibitors.
These results contribute to the growing body of literature examining agents and approaches to prevent and/or treat aromatase inhibitor-induced joint pain and stiffness. Reports have been published on the effects of orally administered agents, including vitamin D [33–35] and a serotonin-norepinephrine reuptake inhibitor, duloxetine [36], as well as mind–body approaches, including acupuncture [37–39] and yoga [40]. Others have examined the effects of switching aromatase inhibitors [41]. However, many of these studies have been small and uncontrolled. The magnitude of the intervention effects in these small trials is variable, and primary outcomes differ between studies. For example, in the uncontrolled trial of duloxetine, 72 % of patients reported ≥30 % reduction in pain after 8 weeks [36]. In our sham-controlled trial of acupuncture, we observed an average 50 % improvement in pain scores among women receiving true acupuncture [37]. An informal cross-trials comparison suggest that glucosamine plus chondroitin may be less effective than duloxetine and acupuncture, but definitive controlled trials are needed to adequately assess effects and side effects of these interventions. As a result, phase III clinical trials studying the effects of acupuncture, essential fatty acids, androgen therapy, and duloxetine on aromatase inhibitor-induced joint pain are opening within the NCI cooperative group setting (www.ClinicalTrials.gov). In addition, there are ongoing studies examining the effects of vitamin E, physical activity, and behavioral counseling on aromatase inhibitor-induced joint pain.
Glucosamine and chondroitin are of particular interest as agents to treat and prevent aromatase inhibitor-induced arthralgias because of their potential efficacy in conjunction with their low toxicity profile and low cost. The magnitude of change in symptoms observed in this study was on par with the magnitude of change observed in the sub-group analyses of participants with moderate-to-severe osteoarthritis in the GAIT study [25]. The grades 1 and 2 toxicities reported in this study were also reflective of the side effect profile of glucosamine and chondroitin as reported in other trials [16]. Although the GAIT trial did not show benefit as assessed by its primary outcome, recent trials of glucosamine and chondroitin alone have shown benefit for hand [42] and knee [18, 19] osteoarthritis, but not low back pain [43].
The primary limitation of this study is that it is uncontrolled, and the observed benefit may have been due to the placebo effect and/or chance. The phase II study design was chosen to conduct a quick and efficient trial to determine whether glucosamine and chondroitin demonstrated enough of an effect on treating aromatase inhibitor-induced joint pain to warrant moving forward to a larger phase III trial. Our a priori criteria for moving forward to a larger trial stated that we needed to observe ≥36.9 % of participants reporting a substantial improvement in pain and/or function at week 24, based upon the OMERACT-OARSI criteria [32]. Since initiating the trial, additional criteria have been published by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group. [44] For pain intensity, the IMMPACT group considers reductions in pain of ≥30 % as moderately clinically important and reductions in pain of ≥50 % as a substantially clinically important. In a post hoc analysis using these criteria, 31–47 % had a moderate clinical improvement, and 25–38 % had a substantial clinical improvement depending on the outcome assessed. In addition, trials need to conservatively estimate drop-out rates. We assumed a 15 % drop-out rate, but observed a 30 % drop-out rate, partly due to the large number (n = 6) of capsules participants needed to take per day. Future study formulations may be able to decrease the number of capsules needed per day to decrease participant burden.
In summary, this phase II trial demonstrated that glucosamine and chondroitin supplementation may be beneficial for the treatment of moderate-to-severe aromatase inhibitor-induced joint pain. The study agents were well tolerated with minimal toxicities. Glucosamine and chondroitin supplementation was safe, as it did not change estradiol levels in postmenopausal breast cancer survivors on aromatase inhibitors. Since effective agents are needed to prevent and treat aromatase inhibitor-induced joint pain, glucosamine and chondroitin should be pursued in a randomized, phase III, placebo-controlled trial to understand its true effect.
Acknowledgments
We thank the women who participated in the study. This research was supported by AstraZeneca (D.L.H.), the Breast Cancer Research Foundation (D.L.H.), the National Cancer Institute (K23CA141052 to H.G.), and Grant Number UL1 RR024156 from the National Center for Research Resources, a component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Footnotes
Ethical standards The CUMC institutional review board approved the study, and all participants provided written informed consent in English or Spanish.
Conflict of interest The authors declare that they have no conflict of interest. We have full control of all primary data, and we agree to allow the journal to review the data if requested.
Contributor Information
Heather Greenlee, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA. Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Katherine D. Crew, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA. Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA
Theresa Shao, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Grace Kranwinkel, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Kevin Kalinsky, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Matthew Maurer, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Lois Brafman, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Beverly Insel, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA.
Wei Yann Tsai, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA. Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, NY, USA.
Dawn L Hershman, Email: dlh23@columbia.edu, Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY, USA. Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA. Columbia University, 161 Fort Washington Avenue, Room 1068, New York, NY 10032, USA.
References
- 1.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T, Group AT. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after 5 years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 3.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 4.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardly A, Price KN, Goldhirsch A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 7.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25(25):3877–3883. doi: 10.1200/JCO.2007.10.7573. 10.1200/ JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 8.Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, Buzdar AU. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9(9):866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 9.Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes DF, Storniolo AM, Clauw DJ. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111(2):365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115(16):3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortimer JE. Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol. 2010;22(1):56–60. doi: 10.1097/GCO.0b013e328334e44e. 10.1097/ GCO.0b013e328334e44e. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Lin Gomez S, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med. 1999;131(6):409–416. doi: 10.7326/0003-4819-131-6-199909210-00003. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1–23. [PubMed] [Google Scholar]
- 16.Chou R, McDonagh MS, Nakamoto E, Griffin J. AHRQ comparative effectiveness reviews. Vol. 38. Agency for Healthcare Research and Quality (US); Rockville (MD): 2011. Analgesics for osteoarthritis: an update of the 2006 comparative effectiveness review [Internet] [PubMed] [Google Scholar]
- 17.Kapoor M, Mineau F, Fahmi H, Pelletier JP, Martel-Pelletier J. Glucosamine sulfate reduces prostaglandin E2 production in osteoarthritic chondrocytes through inhibition of microsomal PGE synthase-1. J Rheumatol. 2011 doi: 10.3899/jrheum.110621. [DOI] [PubMed] [Google Scholar]
- 18.Petersen SG, Beyer N, Hansen M, Holm L, Aagaard P, Mackey AL, Kjaer M. Nonsteroidal anti-inflammatory drug or glucosamine reduced pain and improved muscle strength with resistance training in a randomized controlled trial of knee osteoarthritis patients. Arch Phys Med Rehabil. 2011;92(8):1185–1193. doi: 10.1016/j.apmr.2011.03.009. 10.1016/ j.apmr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Wildi LM, Raynauld JP, Martel-Pelletier J, Beaulieu A, Bessette L, Morin F, Abram F, Dorais M, Pelletier JP. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis. 2011;70(6):982–989. doi: 10.1136/ard.2010.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setnikar I, Cereda R, Pacini MA, Revel L. Antireactive properties of glucosamine sulfate. Arzneimittelforschung. 1991;41(2):157–161. [PubMed] [Google Scholar]
- 21.Shikhman AR, Kuhn K, Alaaeddine N, Lotz M. N-acetyl-glucosamine prevents IL-1 beta-mediated activation of human chondrocytes. J Immunol. 2001;166(8):5155–5160. doi: 10.4049/jimmunol.166.8.5155. [DOI] [PubMed] [Google Scholar]
- 22.Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage. 1998;6(6):427–434. doi: 10.1053/joca.1998.0146. [DOI] [PubMed] [Google Scholar]
- 23.Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113–2123. doi: 10.1001/archinte.162.18.2113. [DOI] [PubMed] [Google Scholar]
- 24.Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res. 2000;381:229–240. doi: 10.1097/00003086-200012000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, Bradley JD, Bingham CO, 3rd, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR, Jr, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 26.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 27.Sautner J, Andel I, Rintelen B, Leeb BF. Development of the M-SACRAH, a modified, shortened version of SACRAH (Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands) Rheumatology (Oxford) 2004;43 (11):1409–1413. doi: 10.1093/rheumatology/keh360. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 29.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 30.Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- 31.Anderson JW, Nicolosi RJ, Borzelleca JF. Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem Toxicol. 2005;43(2):187–201. doi: 10.1016/j.fct.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat. 2011;129(1):107–116. doi: 10.1007/s10549-011-1644-6. [DOI] [PubMed] [Google Scholar]
- 34.Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, Klemp JR, Fabian CJ. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119(1):111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-Garcia M, Diez-Perez A, Albanell J, Tusquets I, Nogues X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;125 (3):869–878. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- 36.Henry NL, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, Griggs JJ, Hayes DF. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117(24):5469–5475. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 37.Crew KD, Capodice JL, Greenlee H, Brafman L, Fuentes D, Awad D, Yann Tsai W, Hershman DL. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol. 2010;28(7):1154–1160. doi: 10.1200/JCO.2009.23.4708. 10.1200/ JCO.2009.23.4708. [DOI] [PubMed] [Google Scholar]
- 38.Crew KD, Capodice JL, Greenlee H, Apollo A, Jacobson JS, Raptis G, Blozie K, Sierra A, Hershman DL. Pilot study of acupuncture for the treatment of joint symptoms related to adjuvant aromatase inhibitor therapy in postmenopausal breast cancer patients. J Cancer Surviv. 2007;1(4):283–291. doi: 10.1007/s11764-007-0034-x. 10.1007/ s11764-007-0034-x. [DOI] [PubMed] [Google Scholar]
- 39.Mao JJ, Bruner DW, Stricker C, Farrar JT, Xie SX, Bowman MA, Pucci D, Han X, DeMichele A. Feasibility trial of electro-acupuncture for aromatase inhibitor-related arthralgia in breast cancer survivors. Integr Cancer Ther. 2009;8(2):123–129. doi: 10.1177/1534735409332903. 10.1177/ 1534735409332903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galantino ML, Desai K, Greene L, Demichele A, Stricker CT, Mao JJ. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther. 2011 doi: 10.1177/1534735411413270. [DOI] [PubMed] [Google Scholar]
- 41.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (Articular Tolerance of Letrozole) study. Breast Cancer Res Treat. 2010;120(1):127–134. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 42.Gabay C, Medinger-Sadowski C, Gascon D, Kolo F, Finckh A. Symptomatic effects of chondroitin 4 and chondroitin 6 sulfate on hand osteoarthritis: a randomized, double-blind, placebo-controlled clinical trial at a single center. Arthritis Rheum. 2011;63(11):3383–3391. doi: 10.1002/art.30574. [DOI] [PubMed] [Google Scholar]
- 43.Wilkens P, Scheel IB, Grundnes O, Hellum C, Storheim K. Effect of glucosamine on pain-related disability in patients with chronic low back pain and degenerative lumbar osteoarthritis: a randomized controlled trial. JAMA. 2010;304(1):45–52. doi: 10.1001/jama.2010.893. 10.1001/ jama.2010.893. [DOI] [PubMed] [Google Scholar]
- 44.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. 10.1016/ j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]