Abstract
Following a mass-casualty nuclear disaster, effective medical triage has the potential to save tens of thousands of lives. In order to best use the available scarce resources, there is an urgent need for biodosimetry tools to determine an individual’s radiation dose. Initial triage for radiation exposure will include location during the incident, symptoms, and physical examination. Stepwise triage will include point of care assessment of less than or greater than 2 Gy, followed by secondary assessment, possibly with high throughput screening, to further define an individual’s dose. Given the multisystem nature of radiation injury, it is unlikely that any single biodosimetry assay can be used as a stand-alone tool to meet the surge in capacity with the timeliness and accuracy needed. As part of the national preparedness and planning for a nuclear or radiological incident, we reviewed the primary literature to determine the capabilities and limitations of a number of biodosimetry assays currently available or under development for use in the initial and secondary triage of patients. Understanding the requirements from a response standpoint and the capability and logistics for the various assays will help inform future biodosimetry technology development and acquisition. Factors considered include: type of sample required, dose detection limit, time interval when the assay is feasible biologically, time for sample preparation and analysis, ease of use, logistical requirements, potential throughput, point-of-care capability, and the ability to support patient diagnosis and treatment within a therapeutically relevant time point.
Keywords: Dosimetry, cytogenetics, dose assessment, emergency planning
INTRODUCTION
Effective planning for the medical response to a radiological or nuclear incident such as the detonation of an improvised nuclear device (IND) in a metropolitan setting is complex and requires an in-depth understanding of how medical triage and treatment will occur. The details of a response plan are often referred to as the concept of operations (CONOPS) and include: the goals of the different phases of the response, time course of the response, responsibilities for specific tasks, means of communications and coordination, and required resources (Coleman et al. 2009). Based on modeling a 10 KT (kiloton) detonation in a range of conditions there may be hundreds of thousands of people receiving an absorbed dose of ionizing radiation. It is likely that up to one million people would require screening for radiation exposure with a subset deemed at higher risk of clinically significant exposure. Moderate exposures to ionizing radiation can be survived with early and appropriate medical intervention based on the received dose of radiation (Knebel et al. 2011). Radiation disasters, particularly a terrorist detonation of a nuclear weapon, have the potential to create a ‘scarce resources setting’ where the available resources are fewer than those needed. The availability of rapid biodosimetry diagnostics will save lives by allowing more accurate triage and targeting of personnel, equipment, medical countermeasures, and expertise to those most likely to benefit (Casagrande et al. 2011).
The threshold level of exposure that causes radiation sickness is approximately 0.75 – 1 Gy. Individuals receiving this dose of radiation may still require medical management and treatment for symptoms, but delay of treatment could be considered since the level of exposure is not expected to pose immediate danger to life, allowing for judicious use of scarce resources in a radiation mass casualty incident. Those individuals receiving >2 Gy of exposure will have greater biological damage, are at higher risk for the acute radiation syndrome (ARS) than those with 1 Gy of exposure, and will benefit greatly from prompt treatment. Early medical intervention for ARS has been shown to improve the survival of individuals after radiation exposure (Goans et al. 1997), and some medical countermeasures are most effective when administered within the first 24 hours (Vijay-Kumar et al. 2008, Chen et al. 2010, Farese et al. 2012). Because exposure to doses of radiation above 2 Gy increase the probability of an individual experiencing ARS, this is currently the planned threshold for administering limited supplies of myeloid cytokines for mitigation of the hematopoietic syndrome in the immediate aftermath of an IND. (Homeland Security Council 2010, DiCarlo et al. 2011). Biodosimetry is essential to distinguish those who need immediate medical intervention from those who are candidates for delayed treatment, only require long-term follow-up, or possibly require no medical care.
At present, there is no biodosimetry method approved by the U.S. Food and Drug Administration (FDA). In the event of a radiological catastrophe, immediate triage would be accomplished through a combination of physical dosimetry, history of an individual’s location, clinical signs and symptoms, and individual hematology assessment, with other methods such as the dicentric chromosome assay (DCA) used for long-term risk assessment. The Biomedical Advanced Research and Development Authority (BARDA) within the U.S. Department of Health and Human Services (DHHS) is working to identify emerging technologies to enhance the available biodosimetry capabilities. Understanding how these methods could be used operationally for triage or mass casualty situations will strengthen the resulting technologies. All methods have their limitations. Physical dosimetry may not be readily available for a number of hours after an incident, clinical signs such as time-to-emesis are not necessarily accurate (Demidenko et al. 2009), and no biodosimetry method can be used as a stand-alone tool to meet the required surge in capacity with the timeliness needed. A combination of assays would be likely be necessary to triage the large number of individuals. First, a point of care (POC) diagnostic device for initial sorting of patients by exposure, second, a high throughput assay to refine the initial dose estimate so that people deemed at risk for manifesting ARS over the next few weeks are referred for care, and third, an assay that is not time sensitive and could be used for estimating long-term cancer risk for those with some radiation exposure. Currently, the only available POC test is the complete blood count with white blood cell differential (CBC Differential) that produces the absolute lymphocyte count used in the lymphocyte depletion kinetic (LDK) assay. However, even with the available hematology instrumentation present within a local or regional area, it would be difficult to obtain the number of tests required for those needing assessment.
The operational issues for all assays that must be addressed include: (1) sample collection, transport, tracking, and processing; (2) assay detection limits; and (3) patient tracking. In this paper we discuss currently available and emerging methods of biodosimetry and how they may fit into the current federal operational and logistical framework for medical response to a nuclear disaster, considering the availability of limited resources. Gaps in technology are identified that would strengthen further biodosimetry development. Issues discussed include the dose range where the assay gives usable results, time lag before the sample can be taken and provide a useful result, time before the assay produces a dose estimate, and the time interval during which the assay will give accurate results.
METHODS
Information for the time from sample acquisition to dose estimation was gathered by thorough examination of the methods sections of the primary literature, and when available, internationally accepted standards and technical manuals (IAEA 2001, ISO 2004, ISO 2008, IAEA 2011). In this manner, the time zero was kept constant between the assays, and time needed for sample preparation and readout was taken into account in the overall estimation. Much of the available literature focused on determination of the threshold level of detection, and less information was available for the upper limits. Additionally, few studies focused on the length of time for which the assay would give usable results. When not specifically stated or examined, this was indicated in the text. As much as possible, all information was gathered from literature that used human samples rather than animal model data.
RESULTS
Biological assays
Biodosimetric methods based on cellular changes due to ionizing radiation have the capability to be very specific for radiation, such as that the DCA, and also reflect the amount of biological damage done to an individual rather than just indicating the dose of radiation received. However, most of the currently available methods are not very specific to ionizing radiation exposure, and their results can be confounded by a variety of factors such as age, disease status, stress, lifestyle, and gender (Fenech 1993, Blakely et al. 2010a, Blakely et al. 2010b, Vral et al. 2011). Nonetheless, after a mass casualty incident, they would be used to sort people into triage categories for further medical assessment and treatment. The utility of the method is dependent on the throughput of the assay and the time interval during which it will give accurate results. Most methods require specialized laboratories for sample preparation and time to complete the assays, making the logistics of providing large numbers of these tests challenging. Depending on the biological response pathway being assessed, there is a limited timeframe during which the biodosimeter will be usable and it may not be logistically feasible to perform the test in this time period. Figs 1-2 and Table 1 summarize the operational characteristics of common biodosimetry methods, and are discussed more in depth below.
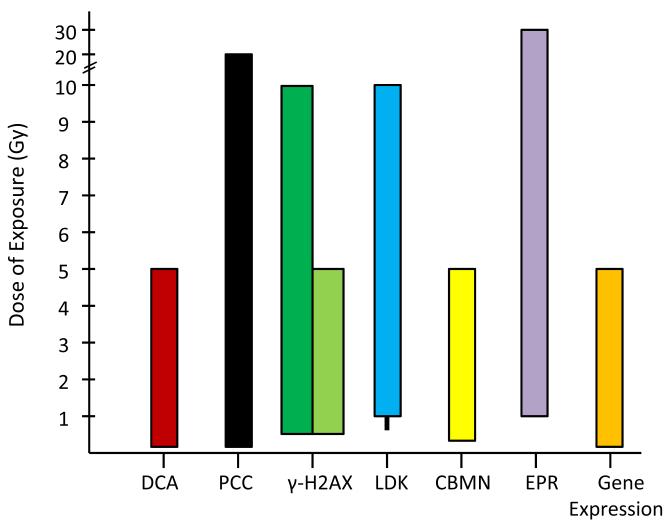
Fig.1. Dose range in which the indicated assays are able to estimate absorbed radiation dose.
The dotted line represents conflicting dose estimate reports for the LDK assay which indicate the range begins at either 0.5 or 1 Gy. For the gamma-H2AX method; the black column represents the dose range for the microscopy method, and the white column is representative of the dose range for the flow cytometry method of measurement.
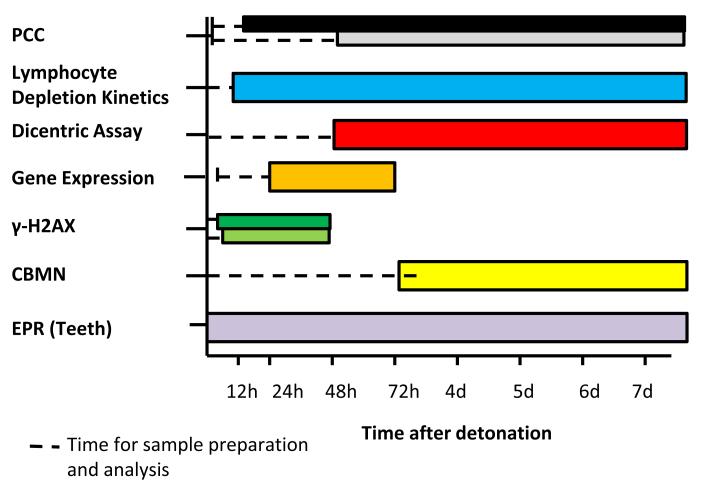
Fig. 2. Time phased comparison of currently available dosimetry methods out to one week after detonation.
Dotted lines represent time needed for sample preparation to occur before any results become available. Note: Some lymphocyte based assays recommend 12hours before drawing blood to allow lymphocyte circulation from tissues. For the gamma-H2AX method; the black bar represents the microscopy method, and the white bar is representative of the flow cytometry method of measurement. For PCC; the black bar represents the CHO method and the white bar represents the chemical stimulation method.
Table 1. Comparison of biodosimetry method properties.
| Method | Dose Range (Gy) |
Time before sample can be taken |
Time to dose estimate |
Time period when assay will give results |
|---|---|---|---|---|
| Dicentric Chromosome | 0.1–5 | Immediately | 55 + h | 3–6+ mo |
| Assay | 0.5–5 (triage) | 52+ h | ||
|
Cytokinesis Block
Micronucleus |
0.3–5 | Immediately | 3+ d | 6 mo –1 y |
| γ–H2AX | Immediately | 24–48 h | ||
| Microscope | 0.5–5 | 4–6 h | ||
| FACS | 0.5–10 | 2 h | ||
|
Lymphocyte Depletion
Kinetics Assay |
0.5/1–10 | Immediately for baseline, time needed for depletion to occur |
<1 h per test. Serial testing requires 8-48 h. |
12 h– 7 d |
| Premature Chromosome Condensation | 0.2–20 | Immediately | CHO fusion: 2–3 h Chemical stimulation: 51 h |
At least 1 wk, likely 6+ mo |
| Gene Expression | 0.1–5 | Immediately, depending on when gene is upregulated |
9–36 h | Through 48–72 h |
| EPR (tooth) | 1–30 | Immediately | 5–25 min | Lifetime and longer |
Hematological biodosimetry
Lymphocyte depletion kinetic assay
The LDK assay is currently the only biodosimetric test that can be performed outside a specialized laboratory. The CBC Differential required for this method is automated and routinely performed in point of care (POC) settings such as hospitals, oncology centers, and urgent care facilities. Blood samples drawn at off-site locations can be sent for testing at large commercial referral labs. Lymphocyte depletion in the peripheral blood can occur after exposure to a radiation dose above 0.5 Gy with an exponential decline in lymphocytes occurring 12 or more hours after exposure (Goans et al. 2001, Azizova et al. 2008, Ainsbury et al. 2011). The kinetics of lymphocyte depletion have been shown to be directly related to the absorbed radiation dose (Goans et al. 1997, Dainiak et al. 2003, Waselenko et al. 2004). Early literature recommended that the absolute lymphocyte count initially be measured as soon as possible and then every 2-3 hours to accumulate the 3-4 data points necessary to generate a LDK curve (Goans et al. 1997). More recent literature has indicated that the dose calculation can be simplified and estimated using two lymphocyte counts and the time between blood samplings (Parker and Parker 2007). For just-in-time use, the Radiation Emergency Medical Management (REMM) web site (NLM 2012) contains a dose estimation tool derived from parts of the Armed Forces Radiobiology Research Institute’s (AFRRI) Biodosimetry Assessment Tool (BAT) which can estimate the dose based on the time of incident and at least one lymphocyte count (Blakely et al. 2010a, Goans 2010, Sandgren et al. 2010). When using only one blood sample, the absolute lymphocyte count is compared to a set value from the normal range calculated for the population to determine the depletion kinetics. However, normal lymphocyte counts in healthy individuals vary within a range based on age, gender, race, etc. Therefore, when possible serial blood counts should be used since this will provide a more accurate dose estimation than a single count (Goans et al. 1997, Parker and Parker 2007).
Currently, there are multiple algorithms in the literature that can be used to calculate the rate of lymphocyte depletion (Goans et al. 1997, Goans et al. 2001, Goans and Waselenko 2005, Parker and Parker 2007, Sandgren et al. 2010). Given the same absolute lymphocyte count and timepoint after exposure, the dose or dose ranges estimated may vary to some extent based on the algorithms used. This is because the sets of data used for the normal and exposed populations were different when the algorithms were derived. In order to overcome this limitation in a mass casualty incident, it will be essential for the same algorithm to be used for consistent dose assessments, and by extension medical treatments across the population.
The LDK assay is useful for estimating preliminary doses in the range of approximately 0.5 Gy – 10 Gy (Goans et al. 1997, Goans et al. 2001, Dainiak 2002, Waselenko et al. 2004, Blakely et al. 2005, Goans and Waselenko 2005, HPA 2009, Ainsbury et al. 2011). To perform the LDK assay, blood should be drawn as soon as possible after exposure in order to establish a baseline level at a medical triage setting. This may be difficult to do within the time needed so the draw could be used as a single data point with the population average used as a baseline. Once blood is drawn, a point-of-care device could provide a CBC Differential result in less than an hour. The time to dose estimate will be greater if the blood sample is sent to an outside testing facility. Based on findings in the literature, the LDK assay will give results up to 7-9 days after the radiation exposure has occurred depending on the dose of radiation (Waselenko et al. 2004, Blakely et al. 2005, Riecke et al. 2010). This makes it a useful method for triage in the early time period after the event, but not for retrospective dose determination.
Ratio of neutrophils to lymphocytes
Recently, there has been interest in using the ratio of neutrophils to lymphocytes (N/L) to determine the level of radiation exposure (Blakely et al. 2007, Blakely et al. 2010b, Graessle and Fliedner 2010). An increase in the number of granulocytes, including neutrophils, is seen in the first few days after moderate radiation exposure followed by a decline, the time of which is dependent on the dose of exposure (Fliedner et al. 2001, Fliedner 2006, Graessle and Fliedner 2010). The neutrophil level in a patient can be determined using a CBC Differential, which would allow this method to be used in the same manner as the LDK assay. The N/L ratio could be used in the first days after an exposure along with the LDK assay for early phase patient triage, but additional experimental data and information are needed to further validate this method before it could be put into routine use. Neutrophil baseline values show inter- and intra-individual variability similar to that seen for the LDK assay, and would require knowledge of the patient’s baseline neutrophils count before use. Additionally, patients with burns or other trauma may have an elevated neutrophil count complicating the use of this method after an improvised nuclear device incident (HPA 2009).
Use of the total granulocyte count for medical triage guidance is seen in the Medical Treatment Protocols for Radiation Accident Victims as a Basis for a Computerized Guidance System (METREPOL) (Fliedner et al. 2001). This system is supported by the European Commission and uses assessment of hematological (H), neurovascular (N), cutaneous (C), and gastrointestinal (G) damage early after exposure to predict the prognosis of the patient and prescribe corresponding medical treatment. Using the 4 systems (H, N, C, G), the potential for multi-organ failure can be identified. Each system is graded on a scale of 1-4, with 4 representing a severe impact on the specific organ system. The hematological damage level in this system is evaluated using platelet, granulocyte, and lymphocyte levels from blood counts and includes use of the lymphocyte depletion assay. The most important distinction is between patients graded H1, H2, and H3, with those graded H4, although the differentiation between H3 and H4 would not be necessary for triage purposes. The H4 category represents irreversible damage to the bone marrow stem cell compartment, and damage to the hematopoietic system is fatal without the appropriate treatment. Additional information about the METREPOL system, including how the 4 systems can be used to triage patients can be found in (Fliedner et al. 2001, Fliedner et al. 2008). This system requires serial multi-organ assessment so it would be useful for individual patient management in an expert setting but not for initial mass casualty triage (Fliedner et al. 2009).
Cytogenetic biodosimetry
Dicentric chromosome assay
The (DCA) is currently considered the “gold standard” for determining the absorbed dose of radiation and has been used in the evaluation of hundreds of people with suspected and actual radiation exposures. The assay is very specific to ionizing radiation and low background levels of dicentric chromosomes allow it to be highly sensitive. This is one of the few assays that can identify a partial-body radiation exposure (Lloyd et al. 2000, Prasanna et al. 2010). The DCA method has undergone standardization by the International Organization for Standardization (ISO), although it is suggested that each laboratory should establish its own calibration curve due to inter-laboratory variability (IAEA 2001, ISO 2004, ISO 2008, IAEA 2011). A dicentric chromosome results from the fusion of two centromere-containing fragments of chromosome. This type of chromosomal damage is considered an unstable aberration and at later time periods after exposure requires the use of a correction factor to account for the removal rate. (IAEA 2001, Thierens et al. 2005, IAEA 2011, Vral et al. 2011).
For the dicentric chromosome assay, blood samples are drawn and cultured in the presence of mitogen for 48 hours in an incubator. After cell processing, slides are made to obtain metaphase spreads then stained for observation under a microscope. The metaphase spreads are scored for the presence of dicentric chromosomes. Traditionally, the chromosomes of 500 to 1,000 metaphase spreads are counted. However, this is extremely time consuming. Improvements have been made to streamline workflow in the laboratory. These include the use of barcoded sample containers, robotic liquid handlers, and automated metaphase cell harvesters, metaphase cell spreaders, slide stainers and coverslippers (Martin et al. 2007). Two main approaches have been used in order to decrease the time needed to estimate a dose. First, the employment of automated metaphase finders, and second, reductions in the number of metaphases scored.
The triage DCA method reduces the amount of time at the microscope by scoring only 50 metaphases or 30 dicentrics (Lloyd et al. 2000, Prasanna et al. 2010). The scoring of 50 metaphases results in an uncertainty of ± 0.5-1.0 Gy (Voisin et al. 2001, IAEA 2011) and increases throughput up to 20 times (IAEA 2011). After initial triage, more cells can be counted to assess the risk of radiation-induced cancer. The DCA QuickScan method further increases scoring speed (Flegal et al. 2010). With no appreciable difference in accuracy compared to the triage-DCA method, the QuickScan approach allows six times as many initial dose estimates to be completed within the first day after samples are processed compared to the classic DCA (Flegal et al. 2010). Depending on the amount of automation, it has been estimated to take 5-25 hours for one cytogeneticist to score 500-1,000 spreads, 1-2.5 hours for the 50 cell triage mode, and 20 minutes for the QuickScan technique (Flegal et al. 2010). An additional method for increasing the throughput of DCA analyses has been the creations of laboratory networks that can electronically share digital pictures of metaphase spreads allowing cytogeneticists in distant laboratories to score samples and provide a dose estimate. This method has been tested by laboratories in the U.S. and Canada, and has proven reliable (IAEA 2011, Flegal et al. 2012).
The DCA has a dose range of approximately 0.1-5 Gy (HPA 2009, AFRRI 2010, Flegal et al. 2010, Pinto et al. 2010, IAEA 2011, Romm et al. 2011, Flegal et al. 2012). At doses greater than 5-6 Gy there is no longer a clear dose-effect relationship causing uncertainty in high dose estimates. Large doses of radiation cause impaired cell proliferation leading to fewer cells reaching metaphase and therefore fewer scorable cells. A blood sample can be drawn immediately after exposure has occurred, although it is suggested to wait 12-24 hours if there could be partial body radiation exposure so that lymphocytes have time to circulate into the blood from the affected organs (IAEA 2011). It is estimated that the first available dose estimate can be determined on the third day after the sample has reached the laboratory, and may take longer depending on the amount of time required to score enough metaphases (Romm et al. 2009, Flegal et al. 2010, Pinto et al. 2010). Due to the unstable nature of dicentric chromosomes, the DCA will give results up to 3-6 months post-exposure, with the length of time highly dependent on the dose of radiation exposure. Analysis after this time period uses dose-modification techniques or fluorescence in situ hybridization (FISH) for the analysis of stable translocations (Romm et al. 2009, AFRRI 2010, IAEA 2011).
Premature chromosome condensation assay
In the premature chromosome condensation (PCC) assay, chemical agents or mitotic Chinese hamster ovary (CHO) cells are used to cause interphase chromatin to condense into distinct chromosomes. This condensation allows chromosome aberrations to be visualized directly in lymphocytes, removing the requirement for progression through mitosis. The number of PCC fragments above the normal 46 chromosomes is counted. The presence of chromosomal breaks, dicentrics, rings and translocations can also be measured. The PCC method using CHO cells is technically more difficult than the chemically induced PCC assay, but provides a quicker dose estimate because it does not require culturing or stimulation by mitogens.
Conventional microscope scoring of the PCC assay is time consuming because similar to the dicentric assay, metaphase spreads must be made and identified before any counting can begin. An automated metaphase finder can be used to speed up the scoring component of the assay. A triage scoring method has been developed, with 50 rings or 300 PCC cells analyzed per sample (Lindholm et al. 2010). Lindholm et al. (2010) have compared the DCA and the PCC assay, and have found that the dicentric assay provides a better estimate at doses less than 6 Gy, while the PCC assay is better for exposures greater than 6 Gy.
The PCC assay is linear in the dose range of 0.2-20 Gy (Kanda et al. 1999, HPA 2009, Lindholm et al. 2010). A blood sample can be drawn immediately after radiation exposure, although DNA repair processes must be taken into account if there is a delay before the sample is processed. The CHO method for the PCC assay will give a dose estimate in 2-3 h, while the chemically induced method requires 51 hours before a dose estimate is available (Kanda et al. 1999, HPA 2009). The PCC method has been used up to 7 days after an incident (Darroudi et al. 1998a), although depending on the stability of the induced chromosomal damage, it may be possible for the assay to be useful for months, similar to the DCA and cytokinesis block micronucleus (CBMN) assays. The PCC assay can also be used to asses exposure from partial-body radiation exposure (Darroudi et al. 1998b).
DNA damage assays
Radiation exposure causes a variety of cellular responses including delay in cell cycle progression, double-stranded DNA breaks, and base lesions (Rothkamm and Horn 2009). Therefore, various types of DNA damage might serve as potential biomarkers. A concern with these biomarkers for their use after an IND incident is their kinetics of appearance and disappearance subsequent to radiation injury may make it difficult to utilize their expression within the time window required.
Gamma-H2AX foci assay
As a result of double-stranded DNA breaks, H2AX protein in the vicinity of the double strand break becomes phosphorylated during the DNA repair process. The amount of phosphorylated gamma-H2AX protein peaks approximately 30 minutes after radiation exposure, declines rapidly in the first 24 hours, and returns to baseline within a few days depending on the dose of radiation (HPA 2009). It is therefore advised that processing should be performed within 1-2 days of the radiation exposure with the best time point at about 30 minutes to 1 hour post-exposure (Andrievski and Wilkins 2009, HPA 2009, Rothkamm and Horn 2009, Garty et al. 2010). Antibodies to gamma-H2AX have been developed and can be used to detect the presence of the protein by flow cytometry and microscopy. Microscopy provides the more sensitive analysis at low doses of radiation, but is time consuming requiring 4-6 hours for a dose estimate, while flow cytometry methods can give a result in about 2 hours. The dose range in which gamma-H2AX provides an accurate assessment is 0.5-5 Gy (HPA 2009, Garty et al. 2010, Riecke et al. 2010) for microscopy and 0.5-10 Gy for flow cytometry (Andrievski and Wilkins 2009), although it has been shown that the total gamma-H2AX intensity levels are dose dependent and approximately linear up to a dose of 100 Gy (Ismail et al. 2007). The short time window in which gamma-H2AX can be used as a valid biomarker reduces its viability as a biodosimetry method after an IND incident.
Cytokinesis block micronucleus assay (CBMN)
Micronuclei are formed when chromosome segments fail to separate properly during mitosis and remain in the cytoplasm instead of passing to the daughter nuclei (Fenech and Morley 1985, Fenech and Morley 1986). Micronuclei are stable chromosome aberrations and disappear with a half-life around 1 year, though they are not specific to radiation exposure (Fenech 1993, Vral et al. 2011). Cells producing micronuclei die quickly after large doses of radiation exposure, and combined with delays in cell cycle progression result in fewer cells reaching mitosis. After 72 hours of culture time, cell processing occurs followed by slide preparation. Scoring of micronuclei is easier than dicentrics and requires less skilled technical staff (AFRRI 2010). For standard biodosimetry it is recommended that 1,000 binuclear cells be scored with Voisin et al. (2001) indicating that this counting can be performed in 2 hours. Recent work by Lindholm et al. (2010) has demonstrated that scoring of 200 binuclear cells allowed the identification of doses of radiation >1 Gy, and took about 15 minutes. Despite some of the drawbacks of the CBMN assay, new improvements make the assay more specific to radiation (Fenech 2010), and enable automated scoring of the assay for high-throughput analysis (Decordier et al. 2009, Willems et al. 2010).
The CBMN assay has a dose range of approximately 0.3-5 Gy, although it has been suggested that a range of 0.1-15 Gy may be attainable with modifications to the method (Darroudi et al. 1998a, Darroudi et al. 1998b, AFRRI 2010, Fenech 2010, Pinto et al. 2010, Willems et al. 2010, IAEA 2011, Vral et al. 2011). Use of an automated scoring system results in a lower limit of dose estimation of 1 Gy, although the uncertainty in the measurement is quite large. A blood sample can be drawn immediately, with the first results available 3-4 days after culturing and processing has begun (Voisin et al. 2001, Fenech 2010, Willems et al. 2010). Experiments have shown that this method is reliable up to 6 mo after exposure (Thierens et al. 2005), but with a correction factor the time can be extended to approximately 1 year (Fenech et al. 1990, Fenech 1993, Vral et al. 2011). ISO standardization is currently pending for this method, although scoring standards have been published (Fenech et al. 2003).
Emerging methods -“omic” assays
Many of the novel “omic” science approaches focus on molecular biomarkers that quantify radiation-induced injury and dose-response. These include proteomics, genomics (messenger RNA, microRNA), metabolomics, and lipidomics. The use of molecular biomarkers for estimation of radiation dose and injury for biodosimetry applications can be challenging to develop for biodosimetry applications. Inherent biomarker variables that must be addressed include: (1) genetic and epigenetic influences; (2) temporal and inter-individual variability of biomarker expression; (3) variance of baseline expression; and (4) other confounders (e.g. medications or supplements being taken, radiation treatment for cancer, age, or gender). These potential biomarkers from assessable tissues such as blood need to be validated in relevant animal and human models.
Gene expression biomarkers
Microarray analysis has been used to identify radiation-responsive genes and signaling pathways in peripheral blood that have an altered response after radiation exposure including the p53-dependent DNA repair pathway and genes in the injury repair pathway (Amundson et al. 2001, Paul and Amundson 2008, Meadows et al. 2010). Additional techniques such as quantitative PCR (qPCR) and quantitative Nuclease Protection Assay (qNPA) have been used to validate and combine this information into a gene set that can be used to estimate the dose of radiation to which a person has been exposed (Grace et al. 2002, Grace et al. 2003, Turtoi et al. 2008, Brengues et al. 2010).
Currently, published results have indicated that biodosimetry using gene expression profiles are relevant in the 0.1-5 Gy dose range (Riecke et al. 2010). Gene expression biodosimeters are proposed for point-of-care and high throughput diagnostic device screening tools. Depending on the method used, preparation needed, and amount of automation available, the first dose estimate results should be available 9-36 hours after the blood sample arrives at the laboratory for analysis (Paul and Amundson 2008, Paul et al. 2011). Current gene expression sets are available for use 48-72 hours after exposure, (Amundson et al. 2001, Amundson and Fornace 2001, Brengues et al. 2010) although some newer techniques should extend this time period out to at least 7 days (Meadows et al. 2008).
MicroRNA expression
MicroRNAs are small, non-protein coding RNAs that are regulatory in nature. They represent a family of highly conserved, small non-protein coding RNAs that generally negatively regulate protein coding genes (Lewis et al. 2003, Lewis et al. 2005). Further studies are needed to achieve the in-depth understanding of the complete dynamics of ‘miRNAome’ patterns in regulating cellular differentiation, proliferation, and apoptosis, as well as bystander effects and tissue specificities, and their potential use in partial-body exposure scenarios. MicroRNA (miRNAs) can be found and are stable in urine, saliva, plasma, serum, and whole blood samples possibly allowing development of a biodosimeter based off of a more easily collected fluid than blood. Ionizing radiation is reported to alter expression of a small conserved group of miRNA targets over extended time (Ishii and Saito 2006, Marsit et al. 2006, Weidhaas et al. 2007, Grace 2008, Jacob et al. 2013).
Protein biomarkers
Through an increase in the understanding of the cellular pathways involved in the radiation response and advances in technology, changes in the protein profile can be determined in blood or urine samples (Marchetti et al. 2006, Sharma et al. 2010). Currently, research is being done using a variety of techniques including western blotting, enzyme-linked immunosorbent assays (ELISA), flow cytometry, and mass spectrometry to identify sets of proteins that undergo a change after radiation exposure. Plasma proteins may be used in combinations that allow mathematical algorithms to use their concentrations differentially on specific days post-irradiation. Alternatively, multiple markers protein could be used together with a hematological test such as the LDK assay to enhance their specificity and diagnostic utility (Blakely et al. 2007, Ossetrova et al. 2007, Blakely et al. 2010b). Platforms for using protein biomarkers are under development, and markers are being validated.
Metabolic biomarkers
Information from genomics, transcriptomics, and proteomics are advanced by information provided by the relatively new field of metabolomics. Metabolomic responses result from proteomic and transcriptomic processes at the cellular level and may be sensitive indicators of system health of the individual in biological fluids such as urine and saliva (Coy et al. 2011). As seen with gene and protein expression, the metabolome is context dependent, differing from cell type to cell type, organ to organ, and individual to individual (Patterson et al. 2010). Radiation triggers complex molecular and cellular processes that alter the concentration of metabolites, or small molecules left behind by metabolic processes in the cell. A number of radiation responsive metabolites have been identified in the urine of mice, rats, and non-human primates, though few identified metabolites are found to be markers in all species. Changes in metabolite concentration were only seen in a limited dose range, primarily 24-36 hours after exposure in non-human primates (Johnson et al. 2012) These data combined with high-sensitivity, differential mobility mass spectrometry (DMS-MS) ion mobility spectrometry show potential for the development of metabolite biomarkers and deployable instrumentation.
Bio-physically based dosimetry
It is well established that physical changes in dental enamel from extracted teeth are the most accurate biodosimetric approach for retrospective dose reconstruction. Non-invasive electron paramagnetic resonance (EPR)-based methods (e.g., in vivo teeth) appear to span all the relevant and life-threatening radiation doses (0.5-10 Gy) that are required for triage. Optically stimulated luminescence (OSL) of tooth enamel has been suggested as an alternative method to EPR. The technology is unique as a physical, in-situ dosimetry assay for assessing absorbed doses of ionizing radiation.
Electron paramagnetic resonance
EPR, also known as Electron Spin Resonance, is a biodosimetry method that is based on physical changes that occur in tissues independently of any biological responses to radiation (Swartz et al. 2010). Ionizing radiation creates a signal proportionate to the dose of radiation absorbed that is stabilized for a long period of time (108 -1011 years) if they are in a matrix such as teeth or bones (Swartz et al. 2007). The signal is also seen in the keratin of fingernails or toenails but are less stable, decaying within a few weeks (Symons et al. 1995). Additionally, the process of cutting nails for ex vivo analysis causes the identification of the radiation induced signal to become overly complex (Trompier et al. 2009, Black and Swarts 2010, Romanyukha et al. 2010, He et al. 2011).
Originally designed to measure radiation dose on isolated exfoliated teeth many years after an exposure, the recent development of lower frequency EPR has enabled the measurement of teeth in vivo. Additionally, a field-deployable unit and methods for using either in vivo fingernails or fingernail cuttings are in the process of being developed and evaluated (Swartz et al. 2007, Trompier et al. 2009, Black and Swarts 2010, Romanyukha et al. 2010, He et al. 2011, Williams et al. 2011a). One of the advantages of EPR is that it is a non-invasive procedure and does not require any blood draw to be performed. However, it is necessary for the person to have teeth present that can be appropriately measured. Additionally, the EPR signal cannot differentiate radiation delivered over a short time period, such as that found during an IND incident, from an amount accumulated over time from dental x rays or CT scans.
The dose response for EPR is linear from 1 Gy up to at least 30 Gy with an uncertainty of ±0.5–1.0 Gy, although higher dose levels have been reported (Derosiers and Schauer 2001, Swartz et al. 2006, Fattibene and Callens 2010, Williams 2010, Williams et al. 2011a, Williams et al. 2011b). Scanning of teeth can be done immediately after the exposure as there are no biological processes that need to occur before sampling. The scanning process takes about 5-25 minutes per casualty, depending on the number of spectra necessary to obtain a good signal to noise ratio (Swartz et al. 2007, Williams 2010). Operationally, only one sample can be scanned at a time, with an estimated 275 people able to be scanned per machine system per day (Williams et al. 2011a). Therefore in order to measure 500,000 people in 48 hours, 1,000 platforms would need to be operated concurrently, although location and medical symptomatology could sort out those at highest risk. The logistics of operating a large number of platforms such as this becomes complex very quickly, and the cost to provide the tests will increase with the additional instruments. Casualties would have to be funneled through distinct testing sites, which would hamper evacuation efforts. Additionally, if the teeth were not in the field of radiation exposure, this method may not be able to adequately assess the dose of exposure. At this time, ISO standardization is in progress for this method (Ainsbury et al. 2011).
Optically stimulated luminescence (OSL)
In OSL, material exposed to radiation (e.g. teeth) gives a signal with an intensity that is proportional to the adsorbed dose (Pass 1997, DeWitt et al. 2010). Measurements are non-invasive and can be used to obtain immediate results for rapid triage of exposed and non-exposed populations without the confounding effects of inflammation, stress, or radiation combined injuries, such as wounds or burns. While this approach eventually might be applicable to in vivo analyses, it is still relatively early in its development. Recent literature has indicated that OSL signal from tooth enamel decreases substantially within the first 12 hours when kept in a wet environment, such as that found in the mouth (DeWitt et al. 2010, Geber-Bergstrand et al. 2012), and has questioned its usability for acute exposure dosimetry, suggesting that the sample would need to be processed in the first 24 hours (Sholom et al. 2011). Variability has also been found between distinct teeth and in different parts of the same tooth. The primary advantages when compared to EPR are that there is no spectral deconvolution required and the equipment needed is said to be simpler and more easily made field-ready. In addition to teeth, OSL has been performed on fingernails and non-biologic samples such as buttons, business cards, and cellular phones (Beerten et al. 2011, Sholom et al. 2011). These materials may be more easily assessed since they do not require in vivo testing, and can be tested on equipment in the laboratory setting.
DISCUSSION
Operational and logistical considerations for biodosimetry use after a radiological or nuclear incident
When planning for the response to an IND detonation, the following assumptions are used. The IND detonation will produce a fireball that will create a bright flash of light followed by a blast wave and thermal pulse. This will be followed by ignition of flammable materials, destruction of surrounding infrastructure, car crashes, and glass breakage. These conditions will not only result in flash blindness, burns, and physical trauma, but also in blocked transportation access making it extremely difficult to get needed supplies and personnel into the damaged areas, as well as those needing medical care out. Another aspect of a detonation, the electromagnetic pulse (EMP), poses no hazard to human health, but may produce a high-voltage surge that would impact electronics. Radiation exposure will occur through two methods; prompt radiation near the site of the detonation, which gives off radiation at a high dose for a short period of time, and residual radiation, also termed fallout, which has a lower dose rate, and the total absorbed dose is dependent on location and duration of exposure. Sheltering-in-place during the first hours to one day can markedly reduce exposure (Buddemeier 2010, Homeland Security Council 2010). There will be the “Dangerous Fallout Zone” (Homeland Security Council 2010) in which people will be at risk for ARS, and this footprint will reach is maximum size in 1-2 hours and then rapidly shrink. Fallout will be carried beyond the initial blast site by atmospheric winds and therefore expose those more distant from the blast site, however, most of them will not be at risk for ARS. After a detonation, the initial medical response will be performed at the local and state level followed by federal intervention when requested. The first federal aid could occur as early as 24 hours post-detonation, with more comprehensive aid requiring at least 72 hours. To protect the general population from fallout a broad shelter-in-place directive should be given for the first 24 hours.
After the incident, including any time spent sheltering, individuals who have the ability will self-evacuate to a nearby hospital or medical center to seek care. This will constitute the first wave of patients who will seek care and begin to potentially consume resources. Many will have no physical or radiation injury but will be appropriately fearful. With very few available beds in hospitals even in routine circumstances, the first wave of those who need immediate medical care will likely overwhelm the hospital capacity (DiCarlo et al. 2011).
Early triage will consist of a combination of medical history, including location during the incident, time to emesis, physical examination, and blood counts as available. In general, the goal of the first dose estimate is to allow for quick assessment in order to provide information to physicians so they can make treatment decisions, and to allay the fears of those who have not undergone any significant or measurable exposure. This first method may have a large uncertainty in the result, but initial dose estimation will not be the only factor in their treatment course. Biodosimetry is meant to assist medical professions in patient management, not determine a specified treatment regimen. Each affected person will be managed medically by how the course of their symptoms and signs changes over time with modification of treatment as necessary. Secondary or tertiary biodosimetry methods that require additional time and expertise such as the DCA or CBMN will give a more precise dose estimate although they may be most useful when the need for results is not urgent. Further dose refinement may be used to refer ARS patients to the Radiation Injury Treatment Network (RITN 2012) or other major centers for monitoring.
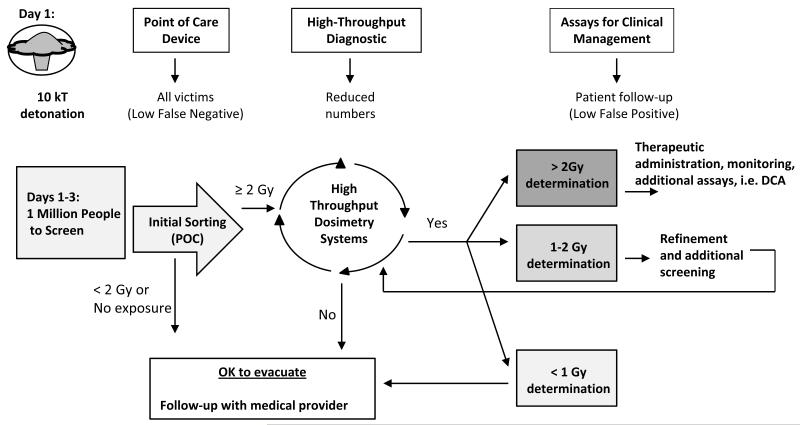
A model for the planned use of biodosimetry is illustrated in Fig. 3 and includes the different roles for Point of Care (POC) and High Throughput Screening (HT) techniques. Table 2 details the target product profiles in the BARDA Broad Agency Announcement with the notable differences between POC and HT (DHHS BARDA 2012). Some technologies may be useable for both screening modes in that a variation on the assay itself may be useful for initial triage and a more refined analysis will be useful for injury assessment and medical treatment. Sample preparation that is more labor intensive and methods with a longer time to result can be tolerated in a high throughput system.
Fig 3. Biodosimetry model for using Point of Care and High Throughput Screening Systems for medical triage after an IND detonation.
After the event it is anticipated that one million people could require biodosimetry screening to determine their dose of exposure to radiation. A point of care device with the use profile shown in Table 2 is preferred for the initial triage. After the first round of biodosimetry, it is anticipated that the number of people requiring additional testing will be reduced to around 400,000. More complex methods can then be used on this smaller population to refine initial dose estimates, with a high-throughput system being preferred.
Table 2. Target product profiles for Point of Care of High Throughput biodosimetry devices.
| Point of Care Device (POC) | High Throughput Device (HT) | |
|---|---|---|
| Type of result: | Qualitative | Quantitative (accuracy ± 0.5 Gy) |
| CONOPS: | Initial Triage / Sorting | Injury Assessment / Treatment |
| Exposure level: | 2 Gy (threshold) | Range: 0.5–10 Gy |
| Ease of operation: | Easy to operate, minimal complexity, requires minimal training. CLIA waived |
Laboratory instrument – more labor intensive, requires training |
|
Device
characteristics |
Integrated components – no separate sample preparation |
May include separate components as needed. High automation desired. |
| Intended use: | Tents, shelters, open settings | Labs, hospitals, fixed facilities |
| # Patients / Event | Up to 1,000,000 in 6 days | Up to 400,000 (may need multiple assessments) |
| Time to result: | Rapid but individual sample result (15 to 30 min) |
Up to 24 hours |
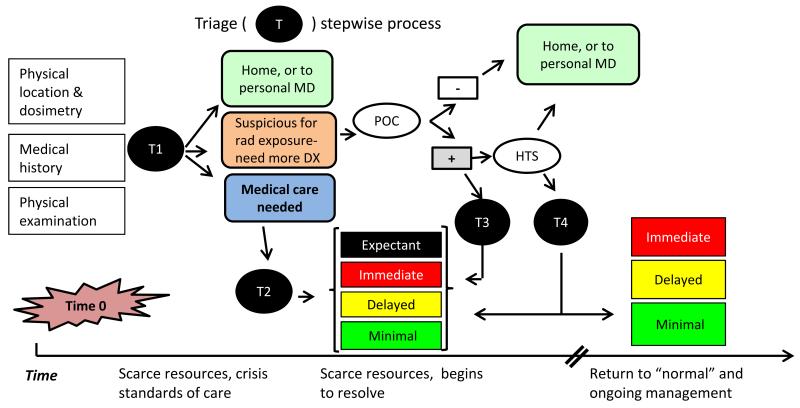
Medical triage following a nuclear detonation will be a stepwise process and an illustration as to how this will occur is found in Fig. 4. The general concept is captured by the acronym “SALT”- Sort, Assess, Lifesaving Interventions, Treatment/Transport, in which there is an initial Sort (T1 in this Fig.) followed by further assessment, interventions, and treatment. For a nuclear detonation and in a scarce resource setting, there would be repeated triage as more information and data are obtained and the resource setting improves. For those who definitely need medical care the second triage (T2) is intended to separate into prognostic groups, based on their medical condition and available resources. This is discussed in detail in Coleman et al. (2011). For some, the dose will be sufficiently high so that they are then triaged (T3) into a specific treatment group, while others may need the more accurate discrimination before being triaged (T4). The time line for this type of scarce-resources based triage is approximately four days as it is anticipated that the need for crisis standards of care will be over by then, even in the more heavily damaged zones.
Fig. 4. Stepwise triage process for use after an IND detonation.
T- triage, DX – differential diagnosis, MD- medical doctor; triage categories are per Coleman et al. (2011) The time from time 0 until “normal” standards of care are in force will vary on distance from the incident.
The inherent characteristics of a biodosimetry method must be understood to evaluate where they will deployed and how they will be used in the planned concept of operations for medical triage shown in Fig. 4. Ideal characteristics for POC and HT are listed in Table 3. The most important parameters for a POC method are the time that it takes to provide a dose estimate and the time period in which the assay is useful. A method that has a short time to result and is usable for at least a week would be advantageous in this setting. Additionally, if the time between the testing and result is minimal, data management and movement of casualties through the triage system will be easier. These and other parameters such as ease of sample collection, type of personnel required to collect or process the sample, and the complexity of the assay have a direct effect on the method’s capacity or throughput. For example, a venous blood draw is more difficult and takes longer than a simple fingerstick. Methods requiring long culture times or microscopic analysis can become labor intensive, and there are currently few people qualified to perform the assays.
Table 3. Important Characteristics for biodosimetry methods to be used after an IND incident.
| Characteristic | Reason |
|---|---|
| Simple sample collection method | Requires fewer specially trained personnel |
| Field-readiness | Samples can be run on site, little sample preparation required |
| Short time to result | Results can be used to guide treatment more quickly |
| High-capacity | A large number of samples can be run on one instrument |
| Time to result | Medical care can begin earlier with a fast time to result |
| Dual-utility | Method in routine use, return on investment, personnel will be trained and used to performing test |
| Method standardization | Ease of comparing results across laboratories, systematic training |
| Specificity for radiation | Low background signal, not affected by confounders such as inflammation or infection |
| Low inter-individual variation | Baseline would not need to be determined for each segment of the population (e.g. age, gender) |
| Low intra-individual variation | Decreased need for repeated tests, test not confounded by infection or other medical treatments |
| Low uncertainty in the result | Decreased need for repeated tests |
| Stable output signal | Increases the amount of time the assay can be used |
| Inexpensive sample processing | Number of tests required will be large and cost will add up quickly |
Currently, no single biodosimetry method would be able to meet the testing capacity required after an IND incident in the timeframe needed. This may be the case after further research and development as well. Throughput needs can be partially offset by having more instruments, although this also requires space, reagents, and technicians at an increased cost. At the moment, the LDK assay has the greatest ability to meet this need, with some point of care hematology devices already developed and in use. Cooperative agreements with large commercial laboratories could be used to meet demand requirements (Adalja et al. 2011). Regional, national, and international partnerships with clinical cytogenetic facilities can also be used enhance capacity. Many nations have begun to establish cytogenetic reference laboratories as well as national and regional networks for support (IAEA 2011). Additionally, United Nations agencies that provide international cooperation such as the International Atomic Energy Agency (IAEA) and World Health Organization (WHO) have established cytogenetic networks (IAEA 2011). However, the use of outside laboratories requires sample transportation and a method for connecting results back to patients, both of which would be difficult in an environment with impaired transportation and communications systems.
Additional challenges such as approval of a new biodosimeter through the regulatory process (Flood et al. 2007) and the potential challenge of measuring radiation dose in a special population such as children, pregnant women, or patients who also have burns or trauma injuries in addition to radiation exposure are also worth noting in technology development and deployment. These complexities will all need to be addressed before appropriate biodosimetry architecture can be created.
CONCLUSIONS
Given the size and complexity of a nuclear detonation incident and the triage process for matching people with resources, biodosimetry assessment is an essential component of medical triage and overall management of the incident. It is unlikely that a single biodosimetry method would have all the necessary characteristics for POC and HT use. Therefore, an integrated system will be necessary consisting of complementary tools that when used together would give the desired information in a short time span. In addition to using multiple methods, clinical and laboratory networks will help increase the numbers of technicians and scientists available to perform the testing and read and interpret the results. While biodosimetry is extraordinarily useful, it is but one component of medical management. Effective preparation for a nuclear detonation requires a thorough understanding of the complexities that will result from this type of incident, including damage to infrastructure, the needs of the population for medical treatment, and logistics of transportation of patients and responders. Here, we have focused on understanding the details of current and upcoming biodosimetry methods and fitting them with the CONOPS, essentially overlaying the timelines of Figs. 1-3.
The authors anticipate coordinated evolution in CONOPS, biodosimetry technology and medical care that will model likely flow of people out of the involved area with the potential impact on a very large region, even the entire country. The ability to accurately and rapidly triage casualties and concerned individuals will enhance the efficacy of the response, reduce the scarce-resources imbalance, provide care and reassurance as appropriate to the individual patients and increase the number of lives saved.
Acknowledgments
We would like to thank Alicia Livinski, biomedical librarian, National Institutes of Health Library, for assistance with the preparation of this article.
Footnotes
Conflicts of Interest and Source of Funding: For all authors, none declared.
Disclaimer: The content of this article represents the personal views of the individual authors and does not necessarily express the opinion or policy of the U.S. Department of Health and Human Services or its components. No statement in this article should be construed as an official position of the U.S. Department of Health and Human Services or its components.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adalja AA, Watson M, Wollner S, Toner E. A possible approach to large-scale laboratory testing for acute radiation sickness after a nuclear detonation. Biosecur Bioterror. 2011;9:345–350. doi: 10.1089/bsp.2011.0042. [DOI] [PubMed] [Google Scholar]
- Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V, Darroudi F, Fattibene P, Gruel G, Guclu I, Horn S, Jaworska A, Kulka U, Lindholm C, Lloyd D, Longo A, Marrale M, Monteiro Gil O, Oestreicher U, Pajic J, Rakic B, Romm H, Trompier F, Veronese I, Voisin P, Vral A, Whitehouse CA, Wieser A, Woda C, Wojcik A, Rothkamm K. Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat Prot Dosim. 2011;147:573–592. doi: 10.1093/rpd/ncq499. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Bittner M, Meltzer P, Trent J, Fornace AJ., Jr. Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res. 2001;156:657–661. doi: 10.1667/0033-7587(2001)156[0657:iogeaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Fornace AJ., Jr. Gene expression profiles for monitoring radiation exposure. Radiat Prot Dosim. 2001;97:11–16. doi: 10.1093/oxfordjournals.rpd.a006632. [DOI] [PubMed] [Google Scholar]
- Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol. 2009;85:369–376. doi: 10.1080/09553000902781147. [DOI] [PubMed] [Google Scholar]
- Armed Forces Radiobiology Research Institute (AFRRI) Medical management of radiological casualites handbook. AFRRI; Bethesda, MD, USA: 2010. Online 3rd Edition. [Google Scholar]
- Azizova TV, Osovets SV, Day RD, Druzhinina MB, Sumina MV, Pesternikova VS, Teplyakov, Zhang A, Kuniak M, Vasilenko EK, Wald N, Slaughter DM, Okladnikova ND, Schall LC. Predictability of acute radiation injury severity. Health Phys. 2008;94:255–263. doi: 10.1097/01.HP.0000290833.66789.df. [DOI] [PubMed] [Google Scholar]
- Bauchinger M, Schmid E, Braselmann H. Time-course of translocation and dicentric frequencies in a radiation accident case. Int J Radiat Biol. 2001;77:553–557. doi: 10.1080/09553000010022382. [DOI] [PubMed] [Google Scholar]
- Beerten K, Reekmans F, Schroeyers W, Lievens L, Vanhavere F. Dose reconstruction using mobile phones. Radiat Prot Dosim. 2011;144:580–583. doi: 10.1093/rpd/ncq343. [DOI] [PubMed] [Google Scholar]
- Black PJ, Swarts SG. Ex vivo analysis of irradiated fingernails: Chemical yields and properties of radiation-induced and mechanically-induced radicals. Health Phys. 2010;98:301–308. doi: 10.1097/HP.0b013e3181b0c045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely WF, Madrid JP, Sandgren DJ. Biodosimetry medical recording-use of the biodosimetry assessment tool. Health Phys. 2010a;99:S184–S191. doi: 10.1097/HP.0b013e3181f26895. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Ossetrova NI, Manglapus GL, Salter CA, Levine IH, Jackson WE, Grace MB, Prasanna PGS, Sandgren DJ, Ledney GD. Amylase and blood cell-count hematological radiation-injury biomarkers in a rhesus monkey radiation model—use of multiparameter and integrated biological dosimetry. Radiat Meas. 2007;42:1164–1170. [Google Scholar]
- Blakely WF, Ossetrova NI, Whitnall MH, Sandgren DJ, Krivokrysenko VI, Shakhov A, Feinstein E. Multiple parameter radiation injury assessment using a nonhuman primate radiation model— biodosimetry applications. Health Phys. 2010b;98:153–159. doi: 10.1097/HP.0b013e3181b0306d. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Salter CA, Prasanna PG. Early-response biological dosimetry—recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Phys. 2005;89:494–504. doi: 10.1097/01.hp.0000175913.36594.a4. [DOI] [PubMed] [Google Scholar]
- Brengues M, Paap B, Bittner M, Amundson S, Seligmann B, Korn R, Lenigk R, Zenhausern F. Biodosimetry on small blood volume using gene expression assay. Health Phys. 2010;98:179–185. doi: 10.1097/01.HP.0000346706.44253.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddemeier B. Reducing the consequences of a nuclear detonation - recent research. The Bridge. 2010;40:28–38. [Google Scholar]
- Casagrande R, Wills N, Kramer E, Sumner L, Mussante M, Kurinsky R, McGhee P, Katz L, Weinstock DM, Coleman CN. Using the model of resource and time-based triage (mortt) to guide scarce resource allocation in the aftermath of a nuclear detonation. Disaster Med Public Health Prep. 2011;5:S98–110. doi: 10.1001/dmp.2011.16. [DOI] [PubMed] [Google Scholar]
- Chen BJ, Deoliveira D, Spasojevic I, Sempowski GD, Jiang C, Owzar K, Wang X, Gesty-Palmer D, Cline JM, Bourland JD, Dugan G, Meadows SK, Daher P, Muramoto G, Chute JP, Chao NJ. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PLoS One. 2010;5:e11056. doi: 10.1371/journal.pone.0011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CN, Hrdina C, Bader JL, Norwood A, Hayhurst R, Forsha J, Yeskey K, Knebel A. Medical response to a radiologic/nuclear event: Integrated plan from the Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services. Ann Emerg Med. 2009;53:213–222. doi: 10.1016/j.annemergmed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Coleman CN, Weinstock DM, Casagrande R, Hick JL, Bader JL, Chang F, Nemhauser JB, Knebel AR. Triage and treatment tools for use in a scarce resources-crisis standards of care setting after a nuclear detonation. Disaster Med Public Health Prep. 2011;5:S111–S121. doi: 10.1001/dmp.2011.22. [DOI] [PubMed] [Google Scholar]
- Coy SL, Cheema AK, Tyburski JB, Laiakis EC, Collins SP, Fornace AJ. Radiation metabolomics and its potential in biodosimetry. Int J Radiat Biol. 2011;87:802–823. doi: 10.3109/09553002.2011.556177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology / ASH Education Program Book. 2003;2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- Darroudi F, Fomina J, Meijers M, Natarajan AT. Kinetics of the formation of chromosome aberrations in x-irradiated human lymphocytes, using PCC and FISH. Mutat Res. 1998a;404:55–65. doi: 10.1016/s0027-5107(98)00095-5. [DOI] [PubMed] [Google Scholar]
- Darroudi F, Natarajan AT, Bentvelzen PA, Heidt PJ, Van Rotterdam A, Zoetelief J, Broerse JJ. Detection of total- and partial-body irradiation in a monkey model: A comparative study of chromosomal aberration, micronucleus and premature chromosome condensation assays. Int J Radiat Biol. 1998b;74:207–215. doi: 10.1080/095530098141582. [DOI] [PubMed] [Google Scholar]
- Decordier I, Papine A, Plas G, Roesems S, Vande Loock K, Moreno-Palomo J, Cemeli E, Anderson D, Fucic A, Marcos R, Soussaline F, Kirsch-Volders M. Automated image analysis of cytokinesis-blocked micronuclei: An adapted protocol and a validated scoring procedure for biomonitoring. Mutagenesis. 2009;24:85–93. doi: 10.1093/mutage/gen057. [DOI] [PubMed] [Google Scholar]
- Demidenko E, Williams BB, Swartz HM. Radiation dose prediction using data on time to emesis in the case of nuclear terrorism. Radiat Res. 2009;171:310–319. doi: 10.1667/RR1552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosiers M, Schauer DA. Electron paramagnetic resonance (EPR) biodosimetry. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2001;184:219–228. [Google Scholar]
- DeWitt R, Klein DM, Yukihara EG, Simon SL, McKeever SW. Optically stimulated luminescence (OSL) of tooth enamel and its potential use in post-radiation exposure triage. Health Phys. 2010;98:432–439. doi: 10.1097/01.HP.0000347997.57654.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN, Weinstock DM. Radiation injury after a nuclear detonation: Medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5:S32–S44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2012;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattibene P, Callens F. EPR dosimetry with tooth enamel: A review. Appl Radiat Isot. 2010;68:2033–2116. doi: 10.1016/j.apradiso.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Fenech M. The cytokinesis-block micronucleus technique: A detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- Fenech M. The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry. Health Phys. 2010;98:234–243. doi: 10.1097/HP.0b013e3181b85044. [DOI] [PubMed] [Google Scholar]
- Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Fenech M, Denham J, Francis W, Morley A. Micronuclei in cytokinesis-blocked lymphocytes of cancer patients following fractionated partial-body radiotherapy. Int J Radiat Biol. 1990;57:373–383. doi: 10.1080/09553009014552471. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147:29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- Fenech M, Morley AA. Cytokinesis-block micronucleus method in human lymphocytes: Effect of in vivo ageing and low dose x-irradiation. Mutat Res. 1986;161:193–198. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Flegal FN, Devantier Y, Marro L, Wilkins RC. Validation of quickscan dicentric chromosome analysis for high throughput radiation biological dosimetry. Health Phys. 2012;102:143–153. doi: 10.1097/HP.0b013e3182307758. [DOI] [PubMed] [Google Scholar]
- Flegal FN, Devantier Y, McNamee JP, Wilkins RC. Quickscan dicentric chromosome analysis for radiation biodosimetry. Health Phys. 2010;98:276–281. doi: 10.1097/HP.0b013e3181aba9c7. [DOI] [PubMed] [Google Scholar]
- Fliedner TM. Nuclear terrorism: The role of hematology in coping with its health consequences. Curr Opin Hematol. 2006;13:436–444. doi: 10.1097/01.moh.0000245696.77758.e6. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Chao NJ, Bader JL, Boettger A, Case C, Jr., Chute J, Confer DL, Ganser A, Gorin NC, Gourmelon P, Graessle DH, Krawisz R, Meineke V, Niederwieser D, Port M, Powles R, Sirohi B, Weinstock DM, Wiley A, Coleman CN. Stem cells, multiorgan failure in radiation emergency medical preparedness: A U.S. / European consultation workshop. Stem Cells. 2009;27:1205–1211. doi: 10.1002/stem.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedner TM, Friesecke I, Beyrer K. Medical management of radiation accidents — manual on the acute radiation syndrome. British Institute of Radiology; London: 2001. [DOI] [PubMed] [Google Scholar]
- Fliedner TM, Powles R, Sirohi B, Niederwieser D, European Group for Blood and Marrow Transplantation (EBMT) Nuclear Accident Committee (NAC) Radiologic and nuclear events: The metrepol severity of effect grading system. Blood. 2008;111:5757–5758. doi: 10.1182/blood-2008-04-150243. author reply 5758-5759; [DOI] [PubMed] [Google Scholar]
- Flood AB, Bhattacharyya S, Nicolalde RJ, Swartz HM. Implementing EPR dosimetry for life-threatening incidents: Factors beyond technical performance. Radiat Meas. 2007;42:1099–1109. doi: 10.1016/j.radmeas.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Brenner DJ. The RABiT: A rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber-Bergstrand T, Bernhardsson C, Mattsson S, Raaf CL. Retrospective dosimetry using OSL of tooth enamel and dental repair materials irradiated under wet and dry conditions. Radiat Environ Biophys. 2012;13:13. doi: 10.1007/s00411-012-0434-9. [DOI] [PubMed] [Google Scholar]
- Goans RE. Clinical application of the AFRRI BAT computer program. Health Phys. 2010;99:S192–S196. doi: 10.1097/HP.0b013e3181ebcef7. [DOI] [PubMed] [Google Scholar]
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment following severe radiation accidents. Health Phys. 1997;72:513–518. doi: 10.1097/00004032-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment in criticality accidents. Health Phys. 2001;81:446–449. doi: 10.1097/00004032-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Goans RE, Waselenko JK. Medical management of radiological casualties. Health Phys. 2005;89:505–512. doi: 10.1097/01.hp.0000172144.94491.84. [DOI] [PubMed] [Google Scholar]
- Grace MB. Genetic molecular markers for radiation exposure: Applications of the gene expression bioassay; Partial-Body Radiation Diagnositc Biomarkers and Medical Management of Radiation Injury Workshop; 2008. [Google Scholar]
- Grace MB, McLeland CB, Blakely WF. Real-time quantitative RT-PCR assay of gadd45 gene expression changes as a biomarker for radiation biodosimetry. Int J Radiat Biol. 2002;78:1011–1021. doi: 10.1080/09553000210158056. [DOI] [PubMed] [Google Scholar]
- Grace MB, McLeland CB, Gagliardi SJ, Smith JM, Jackson WE, IIIrd, Blakely WF. Development and assessment of a quantitative reverse transcription-PCR assay for simultaneous measurement of four amplicons. Clin Chem. 2003;49:1467–1475. doi: 10.1373/49.9.1467. [DOI] [PubMed] [Google Scholar]
- Graessle DH, Fliedner TM. Computer-assisted severity of effect assessment of hematopoietic cell renewal after radiation exposure based on mathematical models. Health Phys. 2010;98:282–289. doi: 10.1097/HP.0b013e3181b08ed3. [DOI] [PubMed] [Google Scholar]
- He X, Gui J, Matthews TP, Williams BB, Swarts SG, Grinberg O, Sidabras J, Wilcox DE, Swartz HM. Advances towards using finger/toenail dosimetry to triage a large population after potential exposure to ionizing radiation. Radiat Meas. 2011;46:882–887. doi: 10.1016/j.radmeas.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Protection Agency (HPA) High dose radiation effects and tissue injury, report of the independent advisory group on ionizing radiation. Health Protection Agency; Oxfordshire, United Kingdom: 2009. [Google Scholar]
- Homeland Security Council, Interagency Policy Coordination Subcommittee for Preparedness and Response to Radiological and Nuclear Threats [Accessed March 7, 2013];Planning guidance for response to a nuclear detonation [online] Available at: http://hps.org/hsc/documents/Planning_Guidance_for_Response_to_a_Nuclear_Detonation-2nd_Edition_FINAL.pdf.
- International Atomic Energy Agency (IAEA) Cytogenetic analysis for radiation dose assessment: A manual. IAEA; Vienna: 2001. Technical Reports Series No. 405. [Google Scholar]
- International Atomic Energy Agency (IAEA) Cytogenetic dosimetry: Applications in preparedness for and response to radiation emergencies. IAEA; Vienna: 2011. [Google Scholar]
- International Organization For Standardization (ISO) Radiation protection—performance criteria for service laboratories performing biological dosimetry by cytogenetics. ISO; Geneva: 2004. ISO 19238. [Google Scholar]
- International Organization For Standardization (ISO) Radiation protection—performance criteria for laboratories performing cytogenetic triage for assessment of mass casualties in radiological or nuclear emergencies - general principles and application to dicentric assay. ISO; Geneva: ISO 21243; 2008. [Google Scholar]
- Ishii H, Saito T. Radiation-induced response of microRNA expression in murine embryonic stem cells. Med Chem. 2006;2:555–563. doi: 10.2174/1573406410602060555. [DOI] [PubMed] [Google Scholar]
- Ismail IH, Wadhra TI, Hammarsten O. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 2007;35:e36. doi: 10.1093/nar/gkl1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob NK, Cooley JV, Yee TN, Jacob J, Alder H, Wickramasinghe P, Maclean KH, Chakravarti A. Identification of sensitive serum microRNA biomarkers for radiation biodosimetry. PLoS One. 2013;8:e57603. doi: 10.1371/journal.pone.0057603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, Luecke H, Gonzalez FJ, Blakely WF, Idle JR. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012;178:328–340. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda R, Hayata I, Lloyd DC. Easy biodosimetry for high-dose radiation exposures using drug-induced, prematurely condensed chromosomes. Int J Radiat Biol. 1999;75:441–446. doi: 10.1080/095530099140366. [DOI] [PubMed] [Google Scholar]
- Knebel AR, Coleman CN, Cliffer KD, Murrain-Hill P, McNally R, Oancea V, Jacobs J, Buddemeier B, Hick JL, Weinstock DM, Hrdina CM, Taylor T, Matzo M, Bader JL, Livinski AA, Parker G, Yeskey K. Allocation of scarce resources after a nuclear detonation: Setting the context. Disaster Med Public Health Prep. 2011;5:S20–S31. doi: 10.1001/dmp.2011.25. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lindholm C, Stricklin D, Jaworska A, Koivistoinen A, Paile W, Arvidsson E, Deperas-Standylo J, Wojcik A. Premature chromosome condensation (PCC) assay for dose assessment in mass casualty accidents. Radiat Res. 2010;173:71–78. doi: 10.1667/RR1843.1. [DOI] [PubMed] [Google Scholar]
- Lloyd DC, Edwards AA, Moquet JE, Guerrero-Carbajal YC. The role of cytogenetics in early triage of radiation casualties. Appl Radiat Isot. 2000;52:1107–1112. doi: 10.1016/s0969-8043(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Eddy K, Kelsey KT. Microrna responses to cellular stress. Cancer Res. 2006;66:10843–10848. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- Martin PR, Berdychevski RE, Subramanian U, Blakely WF, Prasanna PG. Sample tracking in an automated cytogenetic biodosimetry laboratory for radiation mass casualties. Radiat Meas. 2007;42:1119–1124. doi: 10.1016/j.radmeas.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SK, Dressman HK, Daher P, Himburg H, Russell JL, Doan P, Chao NJ, Lucas J, Nevins JR, Chute JP. Diagnosis of partial body radiation exposure in mice using peripheral blood gene expression profiles. PLoS One. 2010;5:e11535. doi: 10.1371/journal.pone.0011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SK, Dressman HK, Muramoto GG, Himburg H, Salter A, Wei Z, Ginsburg G, Chao NJ, Nevins JR, Chute JP. Gene expression signatures of radiation response are specific, durable and accurate in mice and humans. PLoS One. 2008;3:e1912. doi: 10.1371/journal.pone.0001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossetrova NI, Farese AM, MacVittie TM, Manglapus GL, Blakely WF. The use of discriminant analysis for evaluation of early-response multiple biomarkers of radiation exposure using non-human primate 6-Gy. Radiat Meas. 2007;42:1158–1163. [Google Scholar]
- Parker DD, Parker JC. Estimating radiation dose from time to emesis and lymphocyte depletion. Health Phys. 2007;93:701–704. doi: 10.1097/01.HP.0000275289.45882.29. [DOI] [PubMed] [Google Scholar]
- Pass B. Collective radiation biodosimetry for dose reconstruction of acute accidental exposures: A review. Environ Health Perspect. 1997;105:1397–1402. doi: 10.1289/ehp.97105s61397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AD, Lanz C, Gonzalez FJ, Idle JR. The role of mass spectrometry-based metabolomics in medical countermeasures against radiation. Mass Spectrom Rev. 2010;29:503–521. doi: 10.1002/mas.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. International Journal of Radiation Oncology, Biology, & Physics. 2008;71:1236–1244. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Barker CA, Turner HC, McLane A, Wolden SL, Amundson SA. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175:257–265. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto MM, Santos NF, Amaral A. Current status of biodosimetry based on standard cytogenetic methods. Radiat Environ Biophys. 2010;49:567–581. doi: 10.1007/s00411-010-0311-3. [DOI] [PubMed] [Google Scholar]
- Prasanna PG, Moroni M, Pellmar TC. Triage dose assessment for partial-body exposure: Dicentric analysis. Health Phys. 2010;98:244–251. doi: 10.1097/01.HP.0000348020.14969.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radiation Injury Treatment Network (RITN) [Accessed Septmeber 3, 2012];Radiation injury treatment network webpage [online] Available at: http://www.ritn.net/
- Riecke A, Ruf CG, Meineke V. Assessment of radiation damage-the need for a multiparametric and integrative approach with the help of both clinical and biological dosimetry. Health Phys. 2010;98:160–167. doi: 10.1097/HP.0b013e3181b97306. [DOI] [PubMed] [Google Scholar]
- Romanyukha A, Reyes RA, Trompier F, Benevides LA. Fingernail dosimetry: Current status and perspectives. Health Phys. 2010;98:296–300. doi: 10.1097/01.HP.0000347999.01948.74. [DOI] [PubMed] [Google Scholar]
- Romm H, Oestreicher U, Kulka U. Cytogenetic damage analysed by the dicentric assay. Ann Ist Super Sanita. 2009;45:251–259. [PubMed] [Google Scholar]
- Romm H, Wilkins RC, Coleman CN, Lillis-Hearne PK, Pellmar TC, Livingston GK, Awa AA, Jenkins MS, Yoshida MA, Oestreicher U, Prasanna PG. Biological dosimetry by the triage dicentric chromosome assay: Potential implications for treatment of acute radiation syndrome in radiological mass casualties. Radiat Res. 2011;175:397–404. doi: 10.1667/RR2321.1. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Horn S. Gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 2009;45:265–271. [PubMed] [Google Scholar]
- Sandgren DJ, Salter CA, Levine IH, Ross JA, Lillis-Hearne PK, Blakely WF. Biodosimetry assessment tool (BAT) software-dose prediction algorithms. Health Phys. 2010;99:S171–S183. doi: 10.1097/HP.0b013e3181f0fe6c. [DOI] [PubMed] [Google Scholar]
- Sharma M, Halligan BD, Wakim BT, Savin VJ, Cohen EP, Moulder JE. The urine proteome for radiation biodosimetry: Effect of total body vs local kidney irradiation. Health Phys. 2010;98:186–195. doi: 10.1097/HP.0b013e3181b17cbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholom S, Dewitt R, Simon SL, Bouville A, McKeever SW. Emergency dose estimation using optically stimulated luminescence from human tooth enamel. Radiat Meas. 2011;46:778–782. doi: 10.1016/j.radmeas.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Burke G, Coey M, Demidenko E, Dong R, Grinberg O, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo KM, Nicolalde RJ, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell C, Romanyukha A, Schauer DA. In vivo EPR for dosimetry. Radiat Meas. 2007;42:1075–1084. doi: 10.1016/j.radmeas.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB. A critical assessment of biodosimetry methods for large-scale incidents. Health Phys. 2010;98:95–108. doi: 10.1097/HP.0b013e3181b8cffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikhov I, Khan N, Lesniewski P, Thomas J, Romanyukha A, Schauer D, Starewicz P. In vivo EPR dosimetry to quantify exposures to clinically significant doses of ionising radiation. Radiat Prot Dosim. 2006;120:163–170. doi: 10.1093/rpd/nci554. [DOI] [PubMed] [Google Scholar]
- Symons MCR, Chandra H, Wyatt JL. Electron paramagnetic resonance spectra of irradiatied finger-nails: A possible mearsure of accidental exposure. Radiat Prot Dosim. 1995;58:11–15. [Google Scholar]
- Thierens H, De Ruyck K, Vral A, de Gelder V, Whitehouse CA, Tawn EJ, Boesman I. Cytogenetic biodosimetry of an accidental exposure of a radiological worker using multiple assays. Radiat Prot Dosim. 2005;113:408–414. doi: 10.1093/rpd/nch483. [DOI] [PubMed] [Google Scholar]
- Trompier F, Romanyukha A, Kornak L, Calas C, LeBlanc B, Mitchell C, Swartz H, Clairand I. Electron paramagenetic resonance radiation dosimetry in fingernails. Radiat Meas. 2009;44:6–10. doi: 10.1016/j.radmeas.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtoi A, Brown I, Oskamp D, Schneeweiss FH. Early gene expression in human lymphocytes after gamma-irradiation-a genetic pattern with potential for biodosimetry. Int J Radiat Biol. 2008;84:375–387. doi: 10.1080/09553000802029886. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Library of Medicine (NLM) [Accessed 3 September 2012];Radiation emergency medical management: REMM [online] Available at: http://remm.nlm.gov.
- U.S. Department of Health and Human Services (DHHS), Office of theSecretary, Office of the Assistant Secretary for Preparedness and Response (ASPR), Acquisitions Management, Contracts, & Grants (AMCG) BARDA broad agency announcement for the advanced research and development of chemical, biological, radiological, and nuclear medical countermeasures [Accessed September 10, 2012]; Solicitation number: BARDA-CBRN-BAA-12-100-sol-00011 [online]. Available at: https://www.fbo.gov/index?s=opportunity&mode=form&tab=core&id=c54fe27341e8b06882d5 c89c04c0a709&_cview=0.
- Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. The Journal of Immunology. 2008;180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- Voisin P, Benderitter M, Claraz M, Chambrette V, Sorokine-Durm I, Delbos M, Durand V, Leroy A, Paillole N. The cytogenetic dosimetry of recent accidental overexposure. Cellular and Molecular Biology (Noisy-Le-Grand, France) 2001;47:557–564. [PubMed] [Google Scholar]
- Vral A, Fenech M, Thierens H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis. 2011;26:11–17. doi: 10.1093/mutage/geq078. [DOI] [PubMed] [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N. Medical management of the acute radiation syndrome: Recommendations of the strategic national stockpile radiation working group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ. Micrornas as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–11116. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems P, August L, Slabbert J, Romm H, Oestreicher U, Thierens H, Vral A. Automated micronucleus (MN) scoring for population triage in case of large scale radiation events. Int J Radiat Biol. 2010;86:2–11. doi: 10.3109/09553000903264481. [DOI] [PubMed] [Google Scholar]
- Williams BB. Development of in vivo tooth EPR for individual radiation dose estimation and screening. Health Phys. 2010;98:327–338. doi: 10.1097/HP.0b013e3181a6de5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Dong R, Flood AB, Grinberg O, Kmiec M, Lesniewski PN, Matthews TP, Nicolalde RJ, Raynolds T, Salikhov IK, Swartz HM. A deployable in vivo EPR tooth dosimeter for triage after a radiation event involving large populations. Radiat Meas. 2011a;46:772–777. doi: 10.1016/j.radmeas.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Dong R, Nicolalde RJ, Matthews TP, Gladstone DJ, Demidenko E, Zaki BI, Salikhov IK, Lesniewski PN, Swartz HM. Physically-based biodosimetry using in vivo EPR of teeth in patients undergoing total body irradiation. Int J Radiat Biol. 2011b;87:766–775. doi: 10.3109/09553002.2011.583316. [DOI] [PMC free article] [PubMed] [Google Scholar]