Abstract
Aims
The need for ongoing and lifelong follow-up (FU) of patients with cardiac implantable electric devices (CIED) requires significant resources. Remote CIED management has been established as a safe alternative to conventional periodical in-office FU (CFU). An economic model compares the long-term cost and consequences of using daily Home Monitoring® (HM) instead of CFU.
Methods and results
A cost–consequence evaluation comparing HM vs. CFU was performed using a Markov cohort model and data relating to events and costs identified via a systematic review of the literature. The model is conservative, without assuming a reduction of cardiovascular events by HM such as decompensated heart failure or mortality, or considering cost savings such as for transportation. Also cost savings due to an improved timing of elective device replacement, and fewer FU visits needed in patients near device replacement are not considered. Over 10 years, HM is predicted to be cost neutral at about GBP 11 500 per patient in either treatment arm, with all costs for the initial investment into HM and fees for ongoing remote monitoring included. Fewer inappropriate shocks (−51%) reduce the need for replacing devices for battery exhaustion (−7%); the number of FU visits is predicted to be halved by HM.
Conclusion
From a UK National Health Service perspective, HM is cost neutral over 10 years. This is mainly accomplished by reducing the number of battery charges and inappropriate shocks, resulting in fewer device replacements, and by reducing the number of in-clinic FU visits.
Keywords: Implantable defibrillator, Cardiac resynchronization therapy, Remote monitoring, Markov chain, Cost-effectiveness analysis, Health-care costs
Introduction
Cardiac implantable electronic devices (CIEDs) are frequently used for the treatment of bradycardia and heart failure as well as for preventing sudden cardiac death. Following device implantation, conventional care requires patients to regularly attend calendar-based in-clinic follow-up (FU) visits to monitor and optimize device function, to evaluate device diagnostics, and to assess patient health status.1 However, the vast majority (>90%) of all calendar-based FU visits does not identify any issue and therefore does not result in further action.2 Thus, they are an unnecessary burden for patients, their caregivers, and the health-care system as a whole.
The number of patients with CIEDs has been steadily increasing, and this trend is likely to continue.3 The burden on the health-care system generated by conventional follow-up (CFU) is thus expected to rise in the future, further straining the budgetary and technical capacity of health-care systems to provide ongoing care for patients with CIEDs. According to recently modelled estimates, the number of CIED patients in the UK was about 225 000 in 2010. At the current annual growth rate of about 8%, the patient pool is expected to reach ∼334 000 in 2015.4 All these patients will require FU services for as long as they have their device implanted, which is usually lifelong.
Professional societies have endorsed remote FU as a safe replacement for in-office services, and hospitals have developed workflow schemes that improve efficiency and suit their local needs.1,5,6 Remote management reduces the travel burden for patients, thus potentially improving long-term adherence when compared with in-office FU.2 However, adoption of remote CIED management in Europe has been hindered by the lack of reimbursement.7,8 Uncertainty regarding the long-term economic consequences of remote CIED management has been cited as a hurdle against public funding.7,9 The model presented here aims to address this gap.
The objective of this evaluation is to assess the long-term costs and consequences of using remote CIED management in patients implanted with implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds) for either primary or secondary prevention. This evaluation takes a UK National Health Service (NHS) perspective.
Methods
Remote cardiac implantable electronic device management
Although systems from different CIED manufacturers share some common features, there are inherent differences in technology and function.9 For the sake of simplicity and consistency, we chose to limit our analysis to the BIOTRONIK Home Monitoring® system (HM), as it currently has the most published data and since we had access to additionally required but unpublished information. Home Monitoring® provides remote monitoring with clinical and technical data transmitted automatically on a daily basis via the mobile phone network, plus instant automated alert transmission in case of a pre-specified parameter deviation (e.g. abnormal lead impedance, atrial fibrillation burden etc). This allows for an earlie detection, review and intervention in case of relevant clinical and technical events, compared with a scheduled in-office FU regimen.2 Home Monitoring® is a fully automated system that neither requires patient interaction for data transmission nor a manual re-set after alert interrogation.
Modelling approach
The economic evaluation was performed as a cost–consequence analysis comparing long-term FU using either HM or CFU in ICD and CRT-D patients.
A cost–consequence analysis is a variation of the cost effectiveness approach that provides costs and outcomes (consequences) in disaggregated form, and thus is more transparent than, e.g. an analysis reporting cost per quality-adjusted life-year. It also leaves the decision regarding the relative importance of different outcomes to the reader.
Twelve consequences were examined in the model. These events were either clinical events or device-related technical events, namely scheduled and unscheduled FU visits, battery replacements, lead malfunctions, atrial fibrillation/flutter (AF), lead-related inappropriate shocks, non lead-related inappropriate shocks, stroke, hospital admission for acute decompensated heart failure (ADHF), sustained ventricular arrhythmia (SVA), appropriate shocks triggered by SVA, and death. Cost of the HM system and costs of managing the included consequences were captured in the model.
A deterministic Markov cohort model was developed, using TreeAge Pro 2009 (TreeAge Software Inc.). The Markov cycle length was 1 year, with a base case modelling period of 10 years, ensuring that device replacement would be captured by the model. Half-cycle correction was applied to all costs except to the initial one-off investment for the HM transmitter (CardioMessenger II, BIOTRONIK) in the first cycle (HM arm only). Costs and outcomes were discounted at 3.5% per annum, in line with the UK guideline.10
Model structure
The patient population represented in the model consists of patients who have undergone an ICD or CRT-D implantation and are managed in an outpatient setting. One treatment arm represents patients managed by HM, while the other arm consists of patients managed with CFU. Four main health states were included: Well, Post-stroke, Post-ADHF, and Dead as an absorbing state (see Figure 1). Stroke and ADHF were deemed to have long-term clinical and/or economic implications, warranting separate ‘post’ health states. If patients in the ‘Post-ADHF’ state experienced a stroke, they were re-assigned to ‘Post-stroke’. Patients who entered the ‘Post-stroke’ health state stayed in this health state until death (or model termination).
Figure 1.
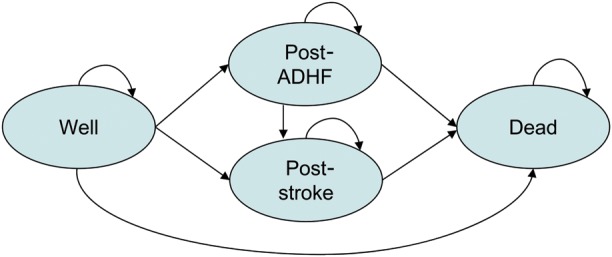
Transition diagram for the presented model.
In every Markov cycle and health state (except ‘Dead’), the probabilities of experiencing the clinical and technical events (see above) were applied. The model consists of individual modules for exposing every patient to all possible events (e.g. battery life module, lead issue module, non-lead issue-related shock module, and cardiovascular (CV) events module; refer to online Appendix for the exact structure). The order of these modules does not indicate a certain order of events in a patient. In fact, the modules may be re-arranged with no effect on the results, as the actual sequence and timing of multiple events within one cycle is irrelevant for estimating health outcomes and costs.
The model structure was identical in the two compared FU options (see online Appendix for model structure).
Model data inputs
Clinical and cost data were identified via structured searches using MEDLINE and systematic review of the identified sources. While most of the event data were taken from randomized controlled trials (RCTs) for HM; all costs were specific for the UK. Data from multiple sources were pooled or meta-analysed. Where required, data specific for ICD and CRT-D patients or for gender were weighted, based on the number of procedures performed in the UK or the gender split in the UK population. Further data on e.g. patient age at implantation were gathered from relevant sources. While the complete data appraisal cannot be reported here in detail, key model input data are provided in Table 1, with the complete model input data reported in online Appendix.
Table 1.
Essential model data
| Variable | Model input (HM) | Model input (CFU) | Reference/comments |
|---|---|---|---|
| General population characteristics | |||
| Age at implantation | 65 years | Central Cardiac Audit Database (A.D. Cunningham, personal communications) | |
| ICD: CRT-D | 62.8%: 37.2% | Central Cardiac Audit Database (A.D. Cunningham, personal communications) | |
| Follow-up service patterns and costs | |||
| Scheduled in-office FU visits | 1 p.a. | 3 p.a. | Wilkoff et al. (2008)1 |
| Unscheduled in-office FU visits | 0.78 p.a. | 0.50 p.a. | Varma et al. (2010)2 |
| Remote monitoring service | 0.78 p.a. | – | Assuming remote FU of HM alert, outside of FU visit |
| Participation in office FU visits | 20% cardiologist/100% technician | Assumption based on local practice | |
| 82% cardiologist/68% technician for sensitivity analysis | Average for ICD/CRT based on Boriani et al.7 | ||
| Fee for in-office FU visit | GBP 99 (cardiologist), GBP 96 (technician) | Assumption | |
| Fee for remote monitoring service | GBP 75 | – | Assumption |
| Transmitter device | GBP 1334 | – | List price including transmission costs and data storage |
| Lead issue treatment | |||
| Probability of lead issue | 0.015 p.a. | Varma et al.19 and TRUST Clinical Study Report27 | |
| Lead issue surgery costs (no replacement) | GBP 1085 | Fox et al.28/Costs inflated to 2010 | |
| Lead replacement cost | GBP 1335 | Fox et al.28/Weighted by relative use of ICD/CRT-D Costs inflated to 2010. | |
| Battery replacement | |||
| Device replacement (including procedure, device, no leads) | GBP 14 993 | Fox et al.28/Inflated to 2010. Weighted average for ICD/CRT-D. | |
| Cardiovascular events and treatment | |||
| AF probability | 0.17 p.a. | Pooled results from Varma et al.2 and data on file | |
| Warfarin treatment costs (in case of AF) | GBP 615 p.a. (ongoing) | NICE29/Costing report for warfarin treatment. Inflated to 2010 | |
| Major bleedings due to warfarin treatment | GBP 1710 for major bleedings (2.4% p.a.) | NICE29 | |
| Minor bleedings due to warfarin treatment | GBP 95 for minor bleedings (15.8% p.a.) | NICE28 | |
| Inpatient treatment of SVA | GBP 936 | DoH30/Weighted average of HRG tariffs HRG EB07H and EB07I | |
| SCD cost | GBP 1424 | DoH30/HRG tariff EB05Z | |
| Stroke treatment costs (initial and recurrent) | GBP 16,005 (year 1) | Ward et al.31 and | |
| GBP 4303 (year 2 onwards) | Luengo-Fernandez et al.32 | ||
| GBP 14 006 (fatal stroke) | |||
| Incidence of ADHF | 0.198 p.a. (first year following implantation) | Pooled results from Goldenberg et al.33 (MADIT II), Higgins et al.34 (CONTAK CD), Young et al.35 (MIRACLE ICD), Moss et al.36 (MADIT CRT), Tang et al.37 (RAFT), FDA38 (COMPANION). Weighted by relative ICD and CRT-D use. | |
| 0.252 p.a. (subsequent years) | |||
| ADHF treatment costs (initial and recurrent) | GBP 1820 | DoH30/Weighted average of HRG tariffs EB03H and EB03I | |
For a full list of model inputs including event rates and all references see Supplementary material online, Appendix.
ADHF, acute decompensated heart failure; AF, atrial fibrillation or flutter; CFU, conventional follow-up; CRT-D, cardiac resynchronisation therapy defibrillator; FU, follow-up; GBP, British Pound; HM, Home Monitoring®; ICD, implantable cardioverter defibrillator; p.a., per annum; SCD, sudden cardiac death; SVA, sustained ventricular arrhythmia; DoH, Department of Health; NICE, National Institute for Health and Clinical Excellence.
Event rates were converted into annual probabilities.11 Some of these probabilities remain constant, while others change over time depending on age or prior clinical events.
While there are some data suggesting that HM can reduce the risk of CV events such as stroke2,12 these data are still at early stages of development. To this end, no differential risks of CV events were applied to the two compared strategies. The model can, however, easily host new clinical data as they become available over time.
As the RCTs comparing HM with conventional FU had a relatively short FU, they were not suited to provide data on mortality. Saxon et al.13 report overall survival in ICD and CRT-D patients based on the ALTITUDE registry. A matched pair analysis of 10 272 patients showed a 50% reduction in mortality in patients on remote monitoring over the first 4 years. However, due to the inherent limitations of a registry we did not attribute a survival benefit with HM, but considered the mortality as observed for conventionally followed patients for both FU groups.
By providing earlier detection of clinical and technical complications that drain the battery reserve, HM prolongs the life of a CIED and thus reduces the necessity for device replacement when compared with CFU. In an analysis of the ECOST trial, HM reduced appropriate and inappropriate shocks by 71% and the number of capacitor charges by 76%.14 Battery life of CIEDs is primarily determined by two factors; frequency of capacitor charging and percentage of pacing. No extra battery consumption attributable to the operation of HM was considered, given that it is negligible (equivalent to about a single 30 J shock over the entire device lifetime).15 Capacitor charges occur for routine capacitor formatting, for aborted shocks, and for delivered shocks (appropriate, inappropriate, and ineffective). The relationship between capacitor charging, percentage of pacing, and battery drainage was incorporated in the model, and as described for the BIOTRONIK device models most widely used in the UK: the LUMAX 540 DR-T at 0% pacing was considered as ICD, the LUMAX 540 HF-T at 100% pacing as CRT-D.16
Predictive validity of the model was tested by assessing how well event numbers from the pivotal RCTs were predicted by the model. Debugging of the model was undertaken by entering zero or extreme values for mortality, number of FU visits, and shock probabilities. Multiple sensitivity analyses were performed to address data uncertainty.
Results
The results of the 10-year base case analyses are presented in Table 2 (event numbers) and Table 3 (costs).
Table 2.
Expected number of events
| Number of events per 1000 patients over 10 years |
||||||
|---|---|---|---|---|---|---|
| Undiscounted |
Discounted |
|||||
| HM | CFU | Difference (%) | HM | CFU | Difference (%) | |
| Death and cardiovascular events | ||||||
| Death (all cause) | 596 | 596 | 0 (0) | 542 | 542 | 0 (0) |
| Stroke | 26 | 26 | 0 (0) | 22 | 22 | 0 (0) |
| ADHF | 796 | 796 | 0 (0) | 724 | 724 | 0 (0) |
| Sustained ventricular arrhythmia | 2313 | 2313 | 0 (0) | 2035 | 2035 | 0 (0) |
| AF | 983 | 983 | 0 (0) | 865 | 865 | 0 (0) |
| Shock events | ||||||
| Inappropriate shock (total) | 116 | 237 | −121 (−51) | 102 | 209 | −107 (−51) |
| Due to lead issuesa | 12 | 52 | −40 (−77) | 10 | 46 | −36 (−78) |
| In AFa | 104 | 185 | −81 (−44) | 92 | 163 | −71 (−44) |
| Appropriate shock for SVAa | 364 | 364 | 0 (0) | 320 | 320 | 0 (0) |
| Device-related events | ||||||
| Battery replacement | 467 | 502 | −35 (−7) | 367 | 409 | −42 (−10) |
| Lead issues | 87 | 87 | 0 (0) | 76 | 76 | 0 (0) |
| Follow-up services | ||||||
| Number of visits (total) | 11 355 | 22 328 | −10 973 (−49) | 10 018 | 19 699 | −9681 (−49) |
| Unscheduled | 4976 | 3190 | 1786 (56) | 4390 | 2814 | 1576 (56) |
| Scheduled | 6379 | 19 138 | −12 759 (−67) | 5628 | 16 885 | −11 257 (−67) |
All estimates have been discounted at 3.5% per annum. The sample size of 1000 patients was chosen to facilitate the reporting of small numbers and to allow for easy breakdown to smaller samples as desired by the reader.
ADHF, acute decompensated heart failure; AF, atrial fibrillation or flutter; CFU, conventional follow-up; HM, Home Monitoring®; SVA, sustained ventricular arrhythmia
aShown are the number of occasions at which shocks occurred. The actual number of shocks is different as patients might experience one or more shocks during a single event.
Table 3.
Costs results
| Cost per patient over 10 years (GBP) |
||||||
|---|---|---|---|---|---|---|
| Undiscounted |
Discounted |
|||||
| HM | CFU | Difference (%) | HM | CFU | Difference (%) | |
| Costs | ||||||
| Total | 13 608 | 13 660 | −52 (−0.4) | 11 452 | 11 486 | −34 (−0.3) |
| Device and patient management | 10 091 | 10 143 | −52 (−0.5) | 8356 | 8389 | −33 (−0.4) |
| CV events | 3517 | 3517 | – | 3096 | 3097 | – |
Discounting at 3.5% per annum. Ten-year modelling period. Rounding differences may occur.
CFU, conventional follow-up; CV, cardiovascular; GBP, British Pound; HM, Home Monitoring®.
Our model reflects what is reported from the clinical trials: HM reduces the number of patients affected by inappropriate shocks due to lead issues and AF (as well as the actual number of shocks in case of shock events but that is not reported as an outcome here). Home Monitoring® also reduces the need for battery replacements (as a result of less battery charges), and lowers the number of in-office FU visits significantly. The increase in the number of unscheduled visits is caused by the need to FU automatic alerts issued by the HM system. Overall, HM is cost neutral compared with CFU, with only a minor cost advantage predicted for HM over CFU (GBP 33 over 10 years). This includes all costs for the initial investment in the HM technology and all ongoing remote CIED management services.
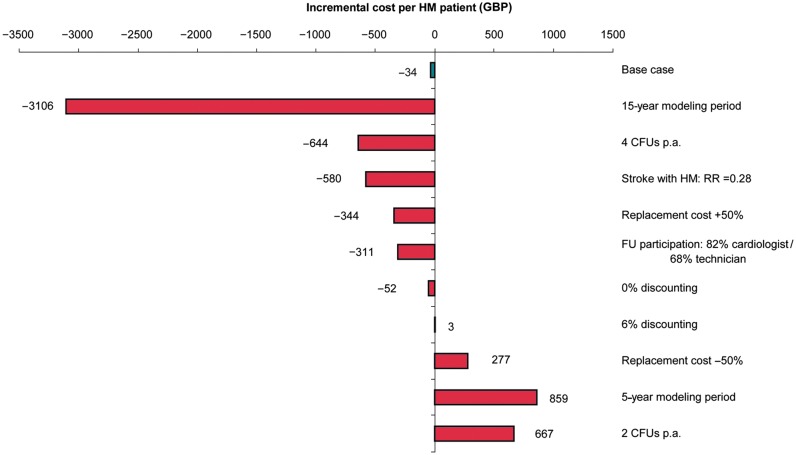
The findings of the sensitivity analyses are shown in Figures 2 and 3. The data relate to the incremental changes in the discounted cost with HM vs. CFU. A number of scenarios were identified where a change to the model input data would result in notable changes to the incremental cost with HM: Considerable cost savings could be expected over a 15-year modelling period, if patients would routinely attend four or more CFU visits per annum, if HM reduces stroke risk (RR = 0.28) as reported in the TRUST trial,2 if device replacement costs were higher than considered for the base case, and if cardiologists were to participate more frequently in in-office FU visits (82% instead of 20%). Home Monitoring® would cause additional costs if replacement costs were lower than considered for the base case, if a 5-year modelling period would be considered, and if patients would routinely attend two CFU per annum. The base case results were insensitive to changes in the rate of SVA, costs for treating lead issues, and patient age at implantation (not shown).
Figure 2.
Univariate sensitivity analysis: discounted incremental costs per patient on HM compared with conventional in-clinic FU, negative data indicate cost savings with HM.
Figure 3.
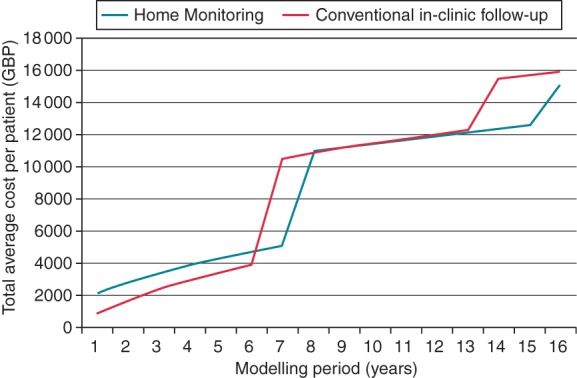
Univariate sensitivity analysis for modelling period: Discounted total costs per patient on HM vs. conventional in-clinic FU.
A separate analysis of different modelling periods was performed (Figure 3). If shorter modelling periods are considered, the initial investment in HM results in a higher cost per patient. The effect of extended battery longevity with HM is clearly visible at year 7, when the majority of CFU patients require box changes for battery depletion, which occurs at year 8 for HM patients (resulting in cost savings of GBP 5433). For use of HM for 8–9 years, additional costs are negligible (GBP 56 and GBP 9, respectively). If used for 10 years or longer, HM becomes increasingly cost saving.
Discussion
Main findings
This is the first comprehensive long-term economic model for any CIED remote management system. A Markov cohort model assuming no mortality benefit predicts cost neutrality when applying HM in the FU of ICD and CRT-D patients. Apart from being cost neutral in the UK NHS environment, HM reduces the demand for in-office services, thereby allowing to redirect health-care services. This study fills the gap of a thorough long-term economic assessment of costs and consequences which often has been cited as a hurdle towards reimbursement.
Previous studies
The impact of HM on health-care resource utilization and clinical outcomes has been studied and reported before as part of observational studies and RCTs such as Home ICD,17 OEDIPE,18 TRUST,2,19 COMPAS,12 and ECOST.14,20 Abbreviated cost assessments have been undertaken in the past, for example, by Fauchier et al.21 who estimated the costs of using HM in France, including costs for in-office FU visits, associated medical services, and transportation. However, the report did not consider costs for clinical and technical issues requiring unscheduled visits, or reimbursement for remote FU.
An evidence review undertaken by the NHS Purchasing and Supply Agency in 2005 provided a modelled economic analysis from a UK NHS perspective, and for patients with prior AF and fitted with a CIED.22 Based on the evidence available at the time, and not considering technical issues requiring medical intervention, changes in battery longevity, or a survival gain from remote monitoring (RM), it was concluded that the initial investment into RM would not be offset by cost savings within 10 years—a finding that is contrary to the comprehensive economic assessment presented here. However, the Centre for Evidence-based Purchasing assessment also concluded that a relative reduction in stroke incidence by RM of as little as 5% would be sufficient for it to be cost-effective by National Institute for Health and Clinical Excellence standards. Ongoing studies (IN-TIME and IMPACT) assessing the impact of HM on major CV events including stroke will provide clarification. Previous RCTs have already pointed towards a favourable effect on stroke risk, although they were not powered for this endpoint,2,12 A Monte Carlo simulation by Ricci et al.23 also showed a reduction in risk of stroke by HM. This early evidence warranted considering a stroke risk reduction in our model as part of the sensitivity analysis, with cost savings by HM of 580 GBPs per patient (see Figure 2).
The main progress of the economic model presented here is that it captures the clinical, technical, and economic consequences of using HM to monitor CIEDs, and details the balance of costs and consequences. For the first time, the impact of HM on the need for device replacement has been considered. By taking a long-term perspective, our model extends and translates the available clinical evidence and applies it to the UK NHS-insured population.
Study limitations
In the model, every CV event was considered to be treated individually, whereas in reality multiple (successive) events might have occurred and been treated during one hospitalization. Such possibility could not be explored further due to lack of information on the relative timing of the individual events. Another limitation of the input data might be the constant probability of lead issues, whereas in reality this probability might increase with time.24
Our model focused on economic aspects, and did not take into account patient-perceived preferences for different FU strategies and outcomes.
Considering that almost all clinical evidence in this model comes from randomised controlled trials for the BIOTRONIK HM system, this evaluation could be considered applicable to HM only. For example, a recent report with different remote monitoring systems showed that 49.2% of all scheduled remote transmissions were not received due to patient non-compliance, requiring considerable resources to contact these patients by phone.25 By contrast, 93% of the daily HM transmissions in the TRUST trial were successfully received.19 Also, data reported from RCTs may not reflect outcome in a routine clinical setting.
Possible additional fields of cost savings by Home Monitoring®
Reimbursement decision makers might disregard inappropriate shocks as clinically relevant outcome (and focus predominantly on ‘hard’ outcomes such as CV events including mortality). However, apart from affecting device longevity, the psychological consequences resulting from inappropriate shocks may result in increased use of medical resources.
Our economic analysis may be conservative due to the fact that we did not consider any reduction in duration of hospital stay with HM (an 18% reduction was observed in the remote monitoring arm of the CONNECT trial).26 The NHS savings presented here may also be underestimated as we did not include transportation costs (which are often subsidized by the NHS), and also did not consider improvements in efficiency of the device clinic resulting from HM. Likewise, we did not take into account the possible increase in device longevity resulting from delaying box change in patients on HM (in whom automatic alerts are available to warn of low battery charge), as well as an increase in FU frequency in patients with CFU in whom the battery is reaching the elective replacement indicator.
In conclusion, the presented model establishes HM as an economically viable technology when applied within the UK NHS system.
Supplementary material
Funding
This work was supported by Biotronik. H.B was supported in part by a grant from la Tour Foundation for Cardiovascular Research.
Conflict of interest: H.B. has received consulting fees and speaker honoraria from Biotronik, Boston Scientific, Medtronic, Sorin and St Jude Medical. C.S. has received consulting fees and speaker honoraria from Biotronik, Boston Scientific, Medtronic, Sorin, and St Jude Medical. J.W. has given educational lectures on behalf of Biotronik, Boston Scientific, St Jude Medical, and Medtronic, and received travel and accommodation from all four companies at international conferences. A.S. is an employee of BIOTRONIK SE & Co KG, Berlin, Germany. K.M. and D.T. have received consultancy honoraria from BIOTRONIK SE & Co KG, Berlin, Germany.
Supplementary Material
References
- 1.Wilkoff B. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–25. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 3.Stoepel C, Boland J, Busca R, Saal G, Oliveira M. Usefulness of remote monitoring in cardiac implantable device follow-up. Telemed J E Health. 2009;15:1026–30. doi: 10.1089/tmj.2009.0068. [DOI] [PubMed] [Google Scholar]
- 4.Smala A, Gessler M, Stoepel C. Demand for routine in office follow-up visits for cardiac implantable electric devices (CIED) in Germany and the United Kingdom. Value Health. 2011;14:A256. [Google Scholar]
- 5.Fuhrer J, Babotai I, Bauersfeld U, Burri H, Gloor H, Schläpfer J, et al. Richtlinien für die Fernüberwachung (Remote Monitoring) implantierter Geräte zur Diagnostik und Therapie von Rhythmusstörungen und Herzinsuffizienz. Cardiovasc Med. 2011;14:13–5. [Google Scholar]
- 6.Vogtmann T, Stiller S, Marek A, Kespohl S, Gomer M, Kühlkamp V, et al. Workload and usefulness of daily centralised Home Monitoring for patients treated with implantable cardiac pacing devices: Results of the MoniC (Model Project Monitor Centre) prospective multicentre study. Europace. 2013;15:219–26. doi: 10.1093/europace/eus252. [DOI] [PubMed] [Google Scholar]
- 7.Boriani G, Burri H, Mantovani LG, Maniadakis N, Leyva F, Kautzner J, et al. Device therapy and hospital reimbursement practices across European countries: a heterogeneous scenario. Europace. 2011;13:ii59–65. doi: 10.1093/europace/eur080. [DOI] [PubMed] [Google Scholar]
- 8.Burri H, Heidbüchel H, Jung W, Brugada P. Remote monitoring: a cost or an investment? Europace. 2011;13:ii44–48. doi: 10.1093/europace/eur082. [DOI] [PubMed] [Google Scholar]
- 9.Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009;11:701–9. doi: 10.1093/europace/eup110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence (NICE) 2008. Guide to the methods of technology appraisal Ref No N1618 http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. April 2011, date last accessed.
- 11.Sonnenberg FA, Beck JR. Markov models on medical decision making: a practical guide. Med Decis Making. 1993;13:322–39. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 12.Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, et al. A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial) Eur Heart J. 2011;33:1105–11. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–67. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 14.Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida JS, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators. Safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34:605–14. doi: 10.1093/eurheartj/ehs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BIOTRONIK GmbH & Co KG. 2010;19 LUMAX 500/540 Technical Specifications, Doc ID TKD-118–047 Rev E, [Google Scholar]
- 16.BIOTRONIK GmbH & Co KG. 2008. pp. 232–233. LUMAX 540 Function Manual, Doc ID 363 986—B,
- 17.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;9:III/3–III/9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 18.Halimi F, Clémenty J, Attuel P, Dessenne X, Amara W. Optimised post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace. 2008;10:1392–9. doi: 10.1093/europace/eun250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol. 2010;3:428–36. doi: 10.1161/CIRCEP.110.951962. [DOI] [PubMed] [Google Scholar]
- 20.Guédon-Moreau L, Chevalier P, Marquié C, Kouakam C, Klug D, Lacroix D, et al. ECOST trial Investigators. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31:2246–52. doi: 10.1093/eurheartj/ehq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauchier L, Sadoul N, Kouakam C, Briand F, Chauvin M, Babuty D, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. PACE. 28:S255–9. doi: 10.1111/j.1540-8159.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 22.Centre for Evidence-based Purchasing (CEP) Evidence review: Implantable cardiac devices with remote monitoring facilities. CEP08047 report. April 2007 http://www.parliament.uk/deposits/depositedpapers/2009/DEP2009-3160.pdf. January 2011, date last accessed.
- 23.Ricci R, Morichelli L, Gargaro A, Laudadio MT, Santini M. Home Monitoring in patients with implantable cardiac devices: Is there a potential reduction of stroke risk? Results from a computer model tested through Monte Carlo simulations. J Cardiovasc Electrophysiol. 2009;20:1244–51. doi: 10.1111/j.1540-8167.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 24.Eckstein J, Koller MT, Zabel M, Kalusche D, Schaer BA, Osswald S, et al. Necessity for surgical revision of defibrillator leads implanted long-term – Causes and Management. Circulation. 2008;117:2727–33. doi: 10.1161/CIRCULATIONAHA.107.740670. [DOI] [PubMed] [Google Scholar]
- 25.Cronin EM, Ching EA, Varma N, Martin DO, Wilkoff BL, Lindsay BD. Remote monitoring of cardiovascular devices: a time and activity analysis. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2012.08.002. Article in press. [DOI] [PubMed] [Google Scholar]
- 26.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH The CONNECT (Clinical Evaluation of remote notification to reduce time to clinical decision) trial. The value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–9. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 27.BIOTRONIK Inc. The TRUST Study. 2009;53 Final clinical report. 30 November 30. [Google Scholar]
- 28.Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess. 2007;11:1–264. doi: 10.3310/hta11470. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Clinical Excellence (NICE) Atrial fibrillation: the management of atrial fibrillation, Costing Report. 2006. Implementing NICE guidance in England. (NICE clinical guideline No 36) http://www.nice.org.uk/nicemedia/pdf/CG036costingreport.pdf. January 2011, date last accessed.
- 30.Department of Health (UK) Appendix NSRC04: NHS trust and PCT combined reference cost schedule. 2011. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_123458.xls. February 2011, date last accessed.
- 31.Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11:1–166. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 32.Luengo-Fernandez R, Gray AM, Rothwell PM. Population-based study of determinants of initial secondary care costs of acute stroke in the United Kingdom. Stroke. 2006;37:2579–87. doi: 10.1161/01.STR.0000240508.28625.2c. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg I, Moss AJ, Hall WJ, McNitt S, Zareba W, Andrews ML, et al. Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Investigators. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 34.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–9. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 35.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. J Am Coll Cardiol. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 36.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 37.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–95. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration (FDA) Summary of Safety and Effectiveness Data (SSED): Cardiac Resynchronization Therapy -Defibrillators (CRT-Ds) Application by Guidant Corp. 2004. Report No.: P010012/S026 http://www.accessdata.fda.gov/cdrh_docs/pdf/P010012S026b.pdf. May 2011, date last accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.