Abstract
The degradation of various formulations of the racemic mixture and the enantiomers (including mefenoxam) of metalaxyl in typical soils from Germany and Cameroon in controlled incubation experiments was studied. The kinetics of the degradation or transformation was determined by means of reversed phase HPLC, while the enantiomeric ratios were measured by HPLC with a chiral Whelk O1 column. The dynamics of the quantitative changes in microbiological properties induced by the addition of these fungicides at their recommended field rates were determined in the soils during a 120-day incubation experiment. The degradation followed first-order kinetics (R2≥0.96). Higher metalaxyl acid metabolite concentrations were found in German than in Cameroonian soils. The enantiomers of the fungicide had different degradation rates in both soils, with half-lives ranging from 17 to 38 days. All forms of metalaxyl had lower degradation rates in the Cameroonian soil than in the German soil. The degradation of the R-enantiomer was much faster than the S-enantiomer in the German soil and slower than the S-enantiomer in the Cameroonian soil, suggesting that different microbial populations, which may be using different enzymes, have different degradation preferences. The type of soil significantly influenced the effect of these fungicides on the soil parameters studied. Incorporation of these fungicides resulted in a change in the ecophysiological status of the soil microbial community as expressed by microbial activities. The activity of phosphatases and β-glucosidase, the mineralization and availability of N and most plant nutrients in soils were stimulated, whereas the activity of dehydrogenase and the availability of NO3−, were generally adversely affected. The soil NH4+, NO3−, and enzymes activities values in general did not correlate with the degradation of metalaxyl in both soils. However, the degradation of formulated and unformulated metalaxyl was positively correlated to the activity of acid phosphatase in the German soil (R2, 0.84 and 0.94 respectively) and in the Cameroonian soil (R2, 0.97 and 0.96 respectively).
Keywords: Mefenoxam, Metalaxyl, Soil enzymes, Soil biochemical parameters, Plant nutrients, Degradation kinetics, Soils
Introduction
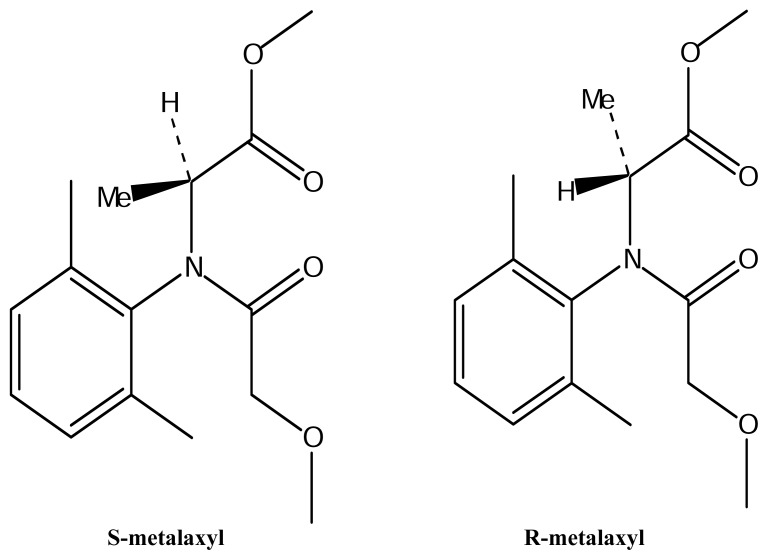
The phenylamide fungicide, metalaxyl, is a chiral compound (structural formula in Fig. 1), which is marketed in its racemic as well as in its enantiopure R form (trade names, e.g., mefenoxam, ridomil etc.). It is manufactured by Syngeta and is used as a seed treatment for banded or broadcast soil application and as a foliar spray in combination with protectant type fungicides such as copper or folpet. It has activity against fungal pathogens of the order Peronosporales which cause late blight, downy mildew, damping off, and stem and fruit rots of many plants. The compound is taken up by roots, leaves, green stems and shoots and transported acropetally within the plant, and inhibits the fungal protein synthesis [1, 2]. It is stable under a broad range of pH, temperature and light [3].
Figure 1.
Structures of the two metalaxyl enantiomers.
Because of its broad-spectrum activity, metalaxyl is registered for use on a wide range of crops and in several countries in temperate, subtropical and tropical regions. The addition of metalaxyl, while enhancing the plant growth and crop yield [1], can affect the homeostasis of the soil system [4]. Any perturbation is likely to lead to a shift in the equilibrium of the system while directly affecting the structure and function of the soil microbial community. This is evidenced by previous observations [5], showing that applications of metalaxyl on vineyard soils over 3 years, markedly decreased microbial numbers and decreased the activity, and increased the number of micro-organisms involved in the mineralization of organic matter. It was reported that the systemic application of metalaxyl induced a brief stimulation and a subsequent suppression of soil fungi and actinomycetes [6].
Metalaxyl has two enantiomers which are expected to be formed in a 1:1 ratio if synthesized from racemic materials [7]; however, the enantiomeric distribution should be checked, as some assumed racemates do not give 1:1 peak ratios. Many reports have documented the microbially-mediated degradation of metalaxyl in soils [8–10], and the faster degradation of the R-metalaxyl in temperate soil [7, 11–13]. These reports have not addressed degradation behaviour of the enantiomers applied in various formulations of metalaxyl in soils of different climatic environments. Information about pesticide dissipation with time is essential in assessing environmental risks [14]. The replacement of racemic metalaxyl by, e.g., mefenoxam [15], means that knowledge of and data on the persistence of this enantiomer are essential for use, management and its registration, especially in the tropical regions of Africa, e.g., Cameroon, where metalaxyl is heavily used in cocoa farming [16]. Additionally, knowledge on the degradation mechanism is a prerequisite for the registration in new fields of application.
Mefenoxam (also called R-metalaxyl) is the R-enantiomer of metalaxyl and has been on the market since 1996 under various formulations and trade names including Ridomil gold, Fonganil gold, Apron XL, Subdue, MAXX. It provides the same level of efficacy as metalaxyl but at half the application rate. The introduction of mefenoxam may contribute to risk reduction for metalaxyl [17]. Thus, mefenoxam is to replace technical metalaxyl in parts of the world where the registration of metalaxyl has not been renewed. Many indicators of soil quality and health have been suggested including, potentially mineralizable N and soil enzymes [18–19]. The importance of soil enzymes resides in their relationship to soil biology, their ease of measurement, and their rapid response to changes in soil management. Concern over the effects of these fungicides on soil processes is based on the fact that many of the reactions in nutrient cycling are mediated by microbes [20]; there is also the possibility that these chemicals can enter into the food chain and, thus, affect higher organisms including humans.
Application rates of metalaxyl range from 0.151 to 8.970 ai (active ingredient) kg ha−1 for agricultural crops, from 0.154 to 0.700 g ai kg−1 seed for agricultural seed treatment, from 1.00 to 8.07 kg a.i ha−1 for ornamental trees and plants. Multiple applications (depending on plant) are approved. This intensive use of metalaxyl has not been without problems. Reports have shown that strains of fungi resistant to metalaxyl may develop [21]. Metalaxyl has been shown to affect soil biology adversely [6, 22]. Moreover, metalaxyl has been found in the water supply (sea and ground water) [23–24] and in food [25].
Mefenoxam is a new product and quantitative studies on its fate and effects are required. Numerous studies have documented changes in the soil ecosystem as a result of pesticide application [26]. Information is scarce concerning the effect of mefenoxam and metalaxyl on soil quality. Since phenylamide fungicides are among the fungicides most frequently employed worldwide, it is important to consider their possible impact on soil response to changes in soil management. Enzyme analyses integrate chemical, physical and biological characteristics and can be used to monitor the effects of soil management, including pesticide use on long-term productivity. Several enzyme activities were measured simultaneously, in order to obtain a more valid estimate of the metabolic response of soil to fungicide stress following a reported suggestion [27].
Pesticide degradation studies are essential to evaluate its impact in the environment and on non-target organisms. In this study, a combination of chemical and biological soil properties have been used for the evaluation of soil properties changes resulting from a single application of the maximum commercial recommended field rate of mefenoxam and metalaxyl to tropical and temperate agricultural soils originating from Cameroon and Germany respectively. The specific objectives of this study were (i) to study the enantioselective degradation and persistence of the racemic and enantiopure forms of metalaxyl in tropical and temperate soils using reverse phase and chiral high-performance chromatography (HPLC); (ii) and to determine the effects of mefenoxam and metalaxyl amendments on selected soil property parameters including nitrogen transformation processes, and soil enzyme activities. We hypothesized that in cases where these fungicides are not toxic to microbiological processes, they would serve as carbon and nitrogen inputs to the soil. This could result in significant increases in soil microbial biomass and some of the more labile soil organic matter fraction [28]. These changes could eventually be followed by an increase in soil organic matter and nutrient availability [29]. Our secondary objectives were (i) to gain information on the effect of the formulation on degradation and (ii) to determine which of the soil property parameters tested could be used specifically as early warning indicators of any side-effects for mefenoxam and metalaxyl on soil biological activity.
Materials and Methods
Soil
Soils used in this study originated from Cameroon and Germany. One soil was collected from Monheim, Germany (Temperate soil-German soil). It is an agricultural sandy loam soil, which is used regularly by Bayer AG for adsorption and degradation studies. This part of Germany receives an annual average rainfall of 750 mm, with an annual mean temperature of about 10°C [30]. The soil, originating from Cameroon (Tropical soil-Cameroonian soil), was collected from the experimental research farm of the Institute of Agronomic Research for Development (IRAD) in Nkolbisson, near Yaounde. It is a sand-clay loam soil. The red ferralitic soil in this site covers 60% on the national surface area of the country. This site receives an annual rainfall from 1400 to 1600 mm and the annual temperature ranges from 19 to 28°C [31].
These two soils, which had not received any pesticide applications for at least 5 years, were taken from the surface layer (0–10cm), air-dried, disaggregated manually, passed through a 2-mm screen sieve, and mixed to achieve homogeneity. The moisture content of the air-dried soil was determined by difference in pre-and post-oven dried weights. The organic matter content of the soils was determined using the “Loss –On Ignition” method [32]. The soil main properties are listed in Table 1.
Table 1.
Selected physico-chemical properties of soils
| Texture Analysis (USDA) | Sand Clay Loam | Sandy Loam |
|---|---|---|
| Clay (< 2 μm) (%) | 31 | 5 |
| Silt (< 50 - 2μm) (%) | 24 | 23 |
| Sand (2000 - 50μm) (%) | 45 | 72 |
| pH (water, ratio 1:2.5) | 4.80 | 7.20 |
| pH (0.01M CaCl2, ratio 1:2.5) | 4.16 | 6.75 |
| Corg. (%) | 3.01 | 1.69 |
| Ntot. (%) | 0.17 | 0.09 |
| P (mg P2O5/100g dry weight) | 0.11 | 57 |
| Cation Exchange Capacity (Meq/100g dry weight) | 9.76 | 8.00 |
| Maximum Water Holding Capacity (MWHC) (%) | 54.4 | 34.4 |
| Density (g/ml) | 1.35 | 2.50 |
| Origin | Yaounde | Monheim |
| Minkoameyos | Laacher Hof Axxa | |
| Cameroon | Germany | |
| Designation | Cameroonian soil (Tropical soil) | German soil (Temperate Soil) |
Test Substances
Pure metalaxyl, analytical standard grade (purity, >99%), (P-metalaxyl), was obtained from Riedel-de-Haen, Germany. Formulated metalaxyl (F-metalaxyl) and mefenoxam were emulsifiable concentrate (EC) formulations containing 24% and 48% of metalaxyl and R-metalaxyl respectively. They were obtained from Novartis Agro GmbH, Frankfurt, Germany. The metalaxyl acid metabolite (CGA 62826) analytical grade (99%)) was obtained from Novartis Crop Protection AG, Basel, Switzerland. All other chemicals used in the study were analytical grade from Merck Co. or Aldrich Chemical Co.
F-metalaxyl contains metalaxyl as active ingredient, which is a racemic mixture of R- and S-enantiomers, whereas mefenoxam contains only the active R-enantiomer. Both compounds, having the same molecular weight (279.34), empirical formula (C15H21NO4) and structural formulae (Figure 1), have been compared on a μg/g basis.
Application of Test Substances in Soil
Degradation Studies
Racemic metalaxyl, in its pure and formulated form, as well as formulated R-metalaxyl were spiked in different experiments to the two soils. The amounts administered correspond to field application rates of roughly 1 kg/ha racemic metalaxyl assuming a penetration depth of 5 cm and a soil density of 1,5 g/cm3 (details in Table 2). To avoid potential effects of solvents upon the microbiological activity of the soil, the volumes of the application solution were limited to 200–900μl and were dispensed onto portions of ~30 g air-dry soil in porcelain dishes, the treated sub samples of soils were thoroughly mixed with a spatula until the solvent was completely evaporated (~10min) and the respective compounds were evenly distributed. The respective sub samples were subsequently added to the total soil mass of the corresponding soils (700–1500g). Subsequently, the soil gross mass for each test chemical was mixed in a tumbling mixer for 1hr. This was followed by the adjustment of the moisture content of the soil to 60% of the maximum water holding capacity, to allow optimal conditions for activity of aerobic soil micro-organisms to occur. Aliquots were then taken. Batches of 100 g of soil each (based on dry weight) were incubated in the dark in an Erlenmeyer flask, under controlled temperature (20 ± 2°C) for 120 days. The moisture content in each flask was checked gravimetrically every 2 weeks and at each sampling period. During incubation, flasks corresponding to the appropriate period were removed for analyses of fungicide residues. Each incubation was carried out in duplicate.
Table 2.
Rates of application and measured final concentration of the investigated fungicides in soilsa.
| Soil Type | P-Metalaxyl | F-Metalaxyl | Mefenoxam | |||
|---|---|---|---|---|---|---|
|
| ||||||
| German | Cameroonian | German | Cameroonian | German | Cameroonian | |
| Application rate | 1220.2 | 1297.5 | 1221.0 | 1297.5 | 609 | 642.0 |
| Final concentration (μg a.i./100 g of dry soil) | 162.7 | 173.0 | 162.8 | 173.0 | 81.2 | 85.6 |
Active ingredient (a.i.) refers to concentration of pure metalaxyl
Effect Studies
P-metalaxyl and F-metalaxyl (144 μg of active ingredient/100 g of soil on a dry weight basis each) and mefenoxam (72 μg of active ingredient/g 100 g of soil on a dry weight basis) respectively were mixed thoroughly and separately into the soils at the recommended commercial application rate for cocoa crop in Cameroon of 1,080 g active ingredient (a.i)/ha for P-metalaxyl, F-metalaxyl and 540 g a.i/ha for mefenoxam (Novartis, 2000, pers. communication). These application rates were based on the maximum single use rate, assuming a depth in the soil of 5 cm and a soil density of 1.5 g/cm3. To avoid the potential effects of solvents upon the microbiological activity of the soil, the calculated volumes of the application solution, 553μl and 550.5μl of the solution of P-metalaxyl in methanol for the German soil and for the Cameroonian soil respectively; 949μl and 814μl of solution of F-metalaxyl in water for the German soil and for the Cameroonian soil, respectively; 237.5μl and 203.5μl of solution of mefenoxam in water for the German soil and for the Cameroonian soil respectively, were dispensed onto portions of ~30g air-dry soil in porcelain dishes. The treated subsamples of soils were thoroughly mixed with a spatula until the solvent had completely evaporated (~20min). The subsamples were subsequently added to the total soil mass of the corresponding field moist soils [1114 and 1146g for German soil and for Cameroonian soil respectively for P-metalaxyl; 1912 and 1694 g for German soil and for Cameroonian soil respectively for F-metalaxyl and mefenoxam. Subsequently, the gross soil mass for each test chemical rate was mixed in a tumbling mixer for 1hr. This was followed by adjustment of the moisture content of the soil to 60% of the maximum water holding capacity, prior to sampling, to allow optimal conditions for activity of aerobic soil micro-organisms. Batches of 100 g of each soil (equivalent dry weight) were incubated in the dark in 750-ml glass jars, under controlled temperature (20 ± 2°C) for 120 days. The jars were kept covered with perforated parafilm. The moisture content in each flask was checked gravimetrically each week and at each sampling period. During the incubation, jars were removed periodically and sampled once for analyses of soil chemical and biochemical parameters. Each experiment was carried out in duplicate.
Degradation Kinetics Studies
Extraction and Clean-up of Incubated Soil Samples
Methanol Suprasolv (Merck, Darmstadt, Germany) (200 mL) was added to the respective Erlenmeyer flask containing incubated soil. The methanolic soil suspension was acidified with formic acid (400μl) (p.a. Merck, Darmstadt, Germany). The flask was subsequently shaken on an overhead shaker (Gelhardt, Rotierapparat RS20, Germany) at speed 6 for 1h. After the extraction the resulting mixture was spiked with 50μl of internal standard solution (metazachlor, 100μg/mL). The suspension was hand-mixed for about 15 seconds and allowed to settle for 15 minutes. The clear supernatant was decanted through a glass fibre filter (Gelman Sciences Type A/E Glass 142 mm) into a 500mL round-bottom flask. The residual soil slurry was re-extracted following the same procedure with 100mL methanol and 100μl formic acid. The filter was rinsed with 6mL methanol. The pooled methanolic extract was evaporated to ~10mL on a rotary evaporator at 300mbar and 60°C. The resulting extract was cleaned up using a preconditioned (6mL methanol followed by 6 mL water) C18 SPE cartridge (500mg, 6mL Baker, Deventer, Netherlands). The analytes were then eluted with 6mL methanol. The eluate was concentrated to ~5mL by means of a rotary evaporator at 150 mbar and 60°C, to yield the final soil extract. Twenty μl aliquots of this solution were analysed by reverse phase high-performance liquid chromatography (HPLC).
Reverse Phase HPLC Analysis
The degradation of metalaxyl compounds and the formation of their metabolites were monitored by HPLC-DAD using a Gynkotek/Dionex HPLC system consisting of a degassing unit (ERC-3822), a Gina 50 autosampler, a Dionex P 580 pump, a column oven at 20°C and a diode array detector (UVD 340S) operated at 205 nm. The system was operated under control of the Chromeleon 6.0 software package. An Ultrasep PAK (L=250mm i.d 3mm and pore size 6μm) C18RP column was used for non-chiral separation. The mobile phase (0.5 mL/min) consisted of water and acetonitrile, both HPLC grade (Baker, Deventer, Netherlands) with 0.1% formic acid. The gradient was programmed: 0–13 min: 42% acetonitrile; 13–14 min: 42–>50%; 15–18 min: 50–>100%; 18–19 min:100%, 19–20 min: 100->42%; and 20–25 min: 42%. The calibration was performed as multilevel internal standard calibration (IS= metazachlor) by using metalaxyl and its acid metabolite prepared in HPLC-grade methanol. The procedure gave recoveries of 100.0% with 4.1 % RSD for metalaxyl and 96.0% with 7.2 % for its acid metabolite (recovery rates obtained from spiked German soil). The limit of determination in the analysis was 0.05 μg/g soil for both metalaxyl and its acid metabolite. Blanks were determined to be below this limit of determination. The data reported are uncorrected for recoveries.
The identity of the compounds found in the samples was confirmed by comparing retention time and UV-spectra to those obtained from standard solutions in the same sequence. Additional confirmation of the identity of the compounds was obtained by HPLC-MS/MS in selected samples. The operating parameters were as follows: HPLC: Phenomex Luna 3 C18 100 A (150mm × 2mm) column, mobile phase; same as described above; flow 0.250mL/min. MS: TSQ 7000 (Finnigan MAT, Bremen, Germany) equipped with an ESI II and APCI source. For ESI, the ionisation voltage was set at 5 kV and transfer capillary temperature at 220°C. For APCI, a vaporizer temperature of 450°C and a transfer capillary temperature of 230°C were used. The ionisation current and detector voltage were set to 5μA and 1.3kV, respectively. For the selected reaction monitoring (SRM) scans, a dwell time of 20 ms and a scanning time of 0.5 s were applied. SRM transitions of 280->220 amu (metalaxyl) and 266->220 (acid metabolite) amu were utilized [9].
Chiral HPLC Analysis
Aliquots of the extracts that were prepared for RP-HPLC (0.5 mL) were conditioned for chiral HPLC by solvent exchange to n-hexane:2-propanol (80:20) by means of careful evaporation to dryness at room temperature under vacuum using an Eppendorf Concentrator 5301 (Eppendorf, Hamburg, Germany). The residues were redissolved in 0.1 mL n-hexane:2-propanol (80:20). A 5μl aliquot of this solution was then analysed by chiral HPLC using the same HPLC-DAD instrument as before for reverse phase analyses. In particular, a chiral column 250 × 4 mm of (R,R) Whelk-01 (5μm), obtained from Merck, Darmstadt, Germany, equipped with a diol pre-column 4×4 mm (Merck) was used. The mobile phase consisted of n-hexane:2-propanol (73:27) (isocratic) mobile phase. The flow rate was set to 0.9 mL/min. The calibration was performed by using high-purity metalaxyl and enantiopure R-metalaxyl standards prepared in HPLC-grade n-hexane:2-propanol (80:20). The (R, R) Whelk-01 column resolved the enantiomers of rac-metalaxyl (see Figures 2, 3). The elution order was S (10.25 min) before R (11.88 min). The enantiomer ratios in samples were determined using their peak area ratios. As in some cases racemates do not give peak ratios of 1:1 at all concentrations, the chiral separation was tested for concentration dependencies concerning the peak area ratios. Ratios of 1:1 were determined for the racemate for the whole range of concentrations that were analysed.
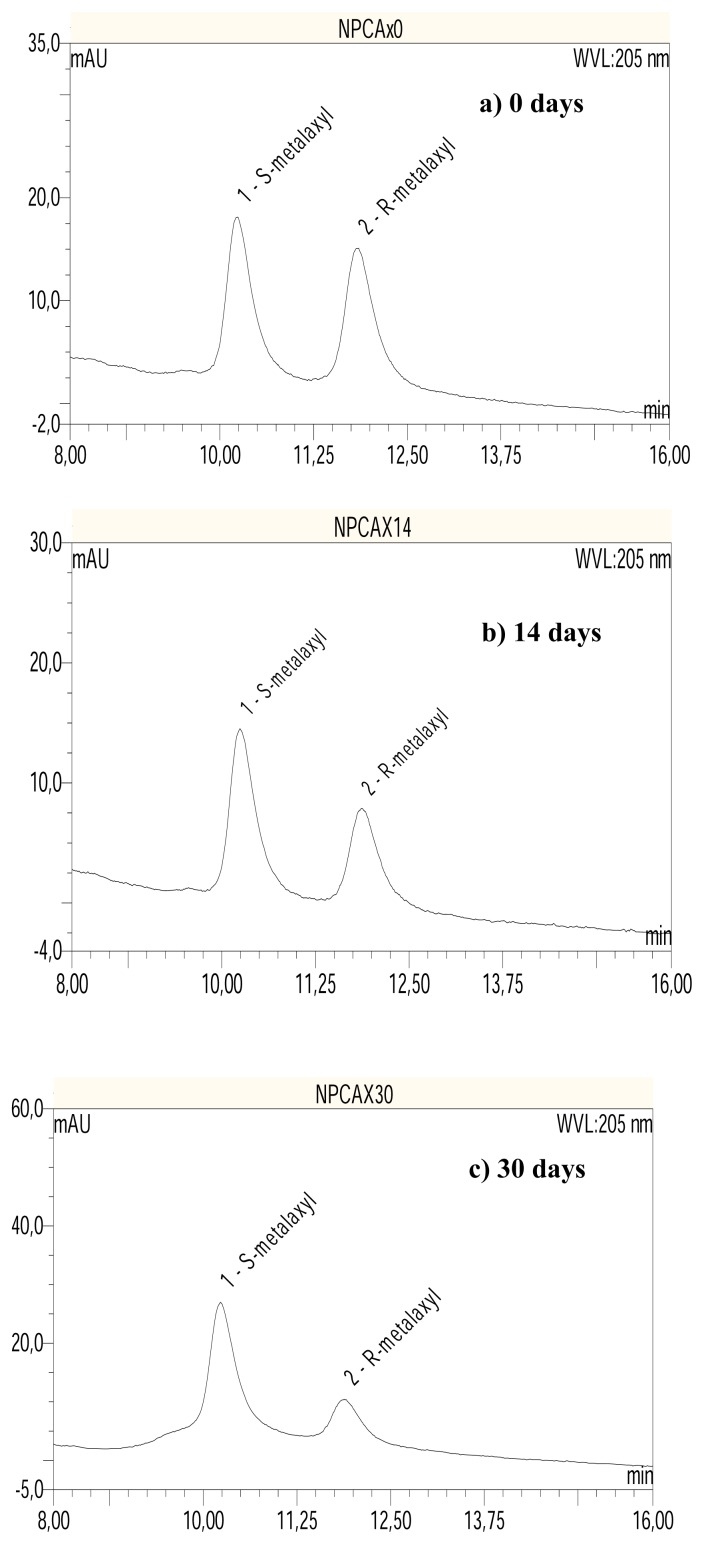
Figure 2.
HPLC-DAD chromatograms showing elution of R and S-metalaxyl in the German soil after a) 0; b) 14; and c) 30 days of incubation. Column: chiral Whelk O1; mobile phase: n-hexane:2-propanol (73:27).
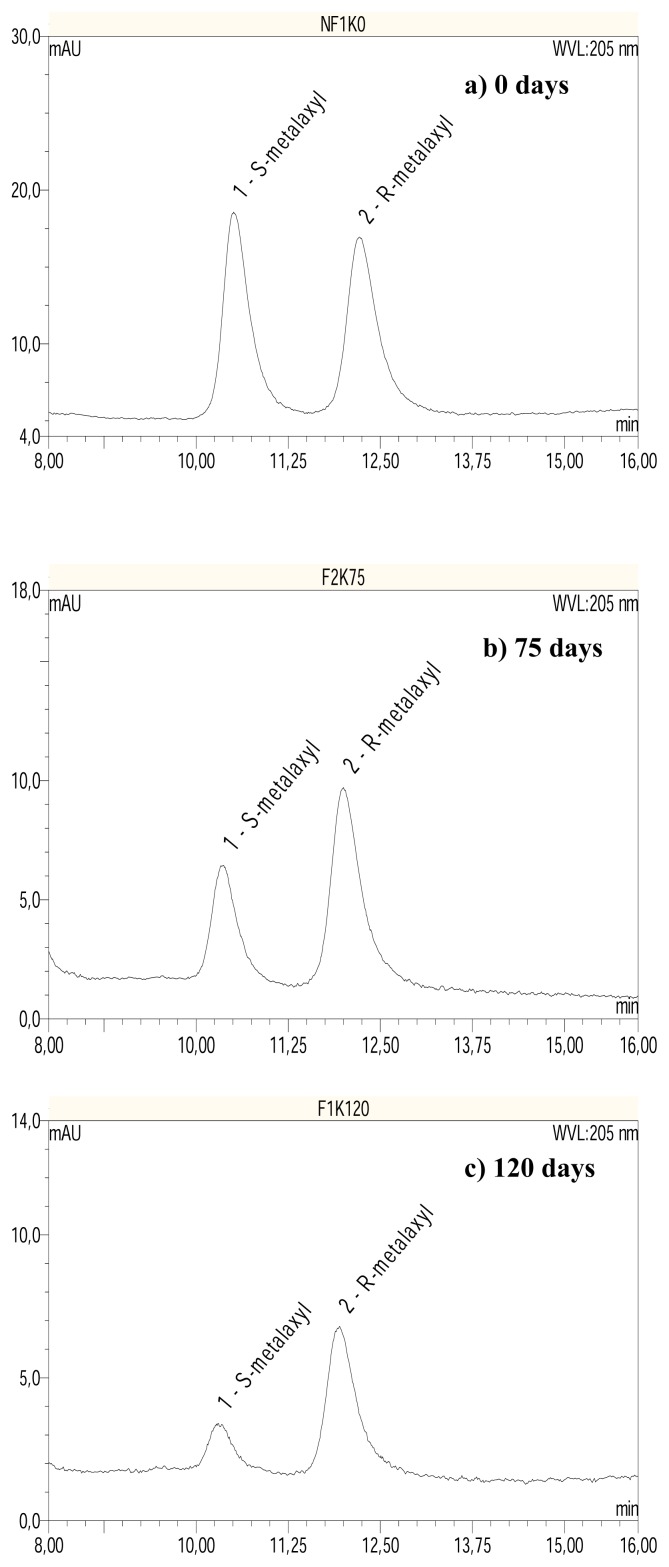
Figure 3.
HPLC-DAD chromatograms showing elution of R and S-metalaxyl in the Cameroonian soil after a) 0; b) 75; and c) 120 days of incubation. Column: chiral Whelk O1; mobile phase: n-hexane:2-propanol (73:27)
Data Calculation
First order rate constants were derived from “ln (Co/C) versus t” plots by linear regression analysis for each experiment (Excel 5.0, Microsoft, Inc.). The half-life (T1/2, days) was estimated from eq I.
| (I) |
The enantiomeric composition (EC) was used as measure of the enantioselectivity of the degradation of enantiomers of metalaxyl in soils. The EC was defined by the eq II.
| (II) |
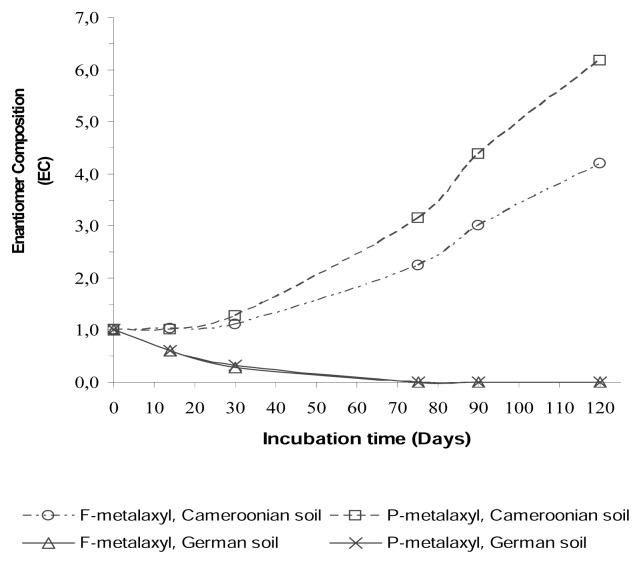
where R and S are the concentration of R- and S-enantiomers in %, respectively. The EC values thus defined range from 0 (R=0, S=100%) to >1 (R>S). The EC for racemic metalaxyl is 1 (R=S). The degradation behaviour of R-and S-enantiomers was assessed by plotting values of enantiomer composition (EC) versus time (Figure 4).
Figure 4.
Profiles of the enantiomeric composition of F-metalaxyl and P-metalaxyl in soils.
Effect on Nitrogen Transformation
Available N (NH4+ and NO3−) was extracted into 2M KCl following the procedures described in “Soil analysis handbook reference methods” [32] and quantified using a reported colorimetric procedure [33].
Effects on Enzyme Activities
Dehydrogenase activity in soil was determined following the method of reduction of 2,3,5-triphenyltrazolium chloride (TTC) [34]. Each soil sample (20g) was thoroughly mixed with CaCO3 (0.2g) and 6 g of this mixture were treated in triplicate with 3% (w/v) 2,3,5-triphenyltrazolium chloride (1ml) and incubated for 24 h at 37 ± 1°C. The triphenyl formazan (TPF) formed was extracted quantitatively from the reaction mixture with methanol and assayed at 485 nm in a Shimadzu UV 1201 UV-VIS spectrophotometer. Acid and alkaline phosphatase activities were determined according to previously described methods [35, 36], with slight modifications. Soil samples (1g) were mixed with the modified universal buffer (MUB) of pH 6.5 and pH 11 for acid and alkaline phosphatase assays respectively and 0.05M p-nitrophenyl phosphate (1 ml) and incubated for 1 h at 37 ± 1°C. Then, 0.5M CaCl2 and 0.5 M NaOH (4ml) were added and the mixture was centrifuged at 3000 rpm for 10 min. The p-nitrophenol (PNP) in the supernatant was determined colorimetrically at 400 nm. Toluene was not included in the procedure because it has been shown to increase the observed activities of both acid and alkaline phosphatases [37] and can be used as source of C by most soil micro-organisms [38].
β-Glucosidase activity was measured following a reported method [39]. Four ml of MUB (pH 6.0) and p-nitrophenyl-β-D-glucopyranoside (1ml) were added to soil (1 g) and the reaction mixture was incubated at 37 ± 1°C for 1h. The rest of the method was the same as described above for acid and alkaline phosphatase activity. No toluene was used in this assay. Results of enzyme activities are reported on an oven dry-weight basis, determined by drying the soils for 24 h at 105°C.
Statistical Analysis
The results at each sampling period and from over the total incubation period were compared using analysis of variance (ANOVA), with treatment as the independent variable. For the effects studies, results were reported as percentages of the control. When treatment responses differed significantly from controls (p<0.05), multiple comparisons were made using paired-t test procedure [40]. Effect levels for significant responses were based on the nominal test substance level at each sampling period.
Results and Discussion
Degradation Studies
Degradation/Transformation of Fungicides in Soils
The presence of metalaxyl and its acid metabolite in the soil samples were verified with HPLC-MS/MS. No other metabolites were detected, though. The concentrations of F-metalaxyl and mefenoxam as well as their metabolites residues remaining in the German and Cameroonian soils are separately presented in Tables 3 and 4. The data for duplicate incubations are summarized in these tables. The degradation kinetics in German and Cameroonian soil experiments are presented in Figure 5. The concentrations of the parent compounds were plotted against time. Metalaxyl was degraded according to first order kinetics for the first 90 days. The linear regression equations were obtained from the ln (Co/C) vs. t plots. The correlation values under different conditions are presented in Table 5. The degradation of all forms of metalaxyl in both soils complied with the first-order reaction kinetics, with correlation coefficients, R2 ranging from 0.96 to 0.98 (Table 5). This compound was degraded in soils to levels of < 3% and <17% of the initial concentration within 90 d of incubation in German and Cameroonian soils respectively (Tables 3, 4) (Figure 5). Data for the formation and subsequent degradation of the primary metabolite, the metalaxyl acid are also shown in Figure 5.
Table 3.
Concentrations of metalaxyl and its enantiomers as well as the metabolite in the German soil after the respective incubation intervals.
| Fungicide/Day | Mean Concentration (μg/100g of dry soil) | |||
|---|---|---|---|---|
|
| ||||
| Metalaxyl | R-metalaxyl | S-metalaxyl | Acid Metabolite | |
| F-Metalaxyl | ||||
|
| ||||
| 0 | 163 (100%) | 81 (100%) | 81 (100%) | 2.2 (1.3%) |
| 14 | 93 (57%) | 35 (43%) | 58 (71%) | 44 (27%) |
| 30 | 54 (53%) | 12 (15%) | 42 (51%) | 80 (49%) |
| 75 | 4.9 (3.0%) | < 0.05 (<0.05%) | 4.9 (6.1%) | 80 (49%) |
| 90 | 3.7 (2.3%) | < 0.05 (<0.05%) | 3.7 (4.6%) | 71 (44%) |
| 120 | 2.2 (1.4%) | < 0.05 (<0.05%) | 2.2 (2.7%) | 50 (31%) |
|
| ||||
| P-Metalaxyl | ||||
|
| ||||
| 0 | 160 (100%) | 81 (100%) | 81 (100%) | 0.9 (0.55%) |
| 14 | 97 (60%) | 36 (45%) | 61 (75%) | 47 (29%) |
| 30 | 60 (37%) | 15 (18%) | 45 (56%) | 93 (57%) |
| 75 | 5.3 (3.3 %) | < 0.05 (<0.05%) | 5.3 (6.6%) | 86 (53%) |
| 90 | 4.0 (2.4 %) | < 0.05 (<0.05%) | 4.0 (4.9%) | 80 (49%) |
| 120 | 2.0 (1.2%) | < 0.05 (<0.05%) | 2.0 (2.5%) | 51 (32%) |
|
| ||||
| Mefenoxam | ||||
|
| ||||
| 0 | 81 (100) | 2.01 (2.5%) | ||
| 14 | 29 (36) | 31 (38%) | ||
| 30 | 13 (16) | 53 (65%) | ||
| 75 | 1.3 (1.7) | 61 (75%) | ||
| 90 | 1.3 (1.7) | 72 (89%) | ||
| 120 | 0.60 (0.73) | 55 (67) | ||
Table 4.
Concentrations of metalaxyl and its Enantiomers as well as the metabolite in the Cameroonian soil after the respective incubation intervals
| Fungicide/Day | Mean Concentration (μG/100g Of Dry Soil) | |||
|---|---|---|---|---|
|
| ||||
| Metalaxyl | R-Metalaxyl | S-Metalaxyl | Acid Metabolite | |
| F-Metalaxyl | ||||
|
| ||||
| 0 | 170 (100%) | 86 (100%) | 86 (100%) | < 0.05 (0%) |
| 14 | 100 (59%) | 52 (60%) | 50 (58%) | 11 (6.5%) |
| 30 | 85 (49%) | 45 (52%) | 40 (47%) | 6.5 (3.8%) |
| 75 | 29 (17%) | 20 (24%) | 9.1 (10%) | 3.0 (1.8%) |
| 90 | 27 (16%) | 20 (23%) | 6.7 (7.8%) | 2.0 (1.2%) |
| 120 | 20 (12%) | 16 (19%) | 3.9 (4.5%) | 3.4 (2.0%) |
|
| ||||
| P-Metalaxyl | ||||
|
| ||||
| 0 | 170 (100%) | 87 (100%) | 87 (100%) | < 0.05 (0%) |
| 14 | 100 (60%) | 51(59%) | 51 (58%) | 7.0 (4.1%) |
| 30 | 85 (49%) | 48 (55%) | 38 (43%) | 5.5 (3.2%) |
| 75 | 28 (16%) | 21 (25%) | 6.8 (7.8%) | 2.8 (1.6%) |
| 90 | 25 (14%) | 21 (24%) | 4.7 (5.4%) | 1.6 (0.90%) |
| 120 | 18 (11%) | 16 (1 %) | 2.6 (3.0%) | 1.6 (0.92%) |
|
| ||||
| Mefenoxam | ||||
|
| ||||
| 0 | 86 (100%) | 0.55 (0.64%) | ||
| 14 | 50 (59%) | 5.6 (6.6%) | ||
| 30 | 38 (45%) | 2.5 (2.9%) | ||
| 75 | 14 (16%) | 0.12 (0.14%) | ||
| 90 | 14 (16%) | 0.12 (0.14%) | ||
| 120 | 9.5 (11%) | 0.18 (0.21%) | ||
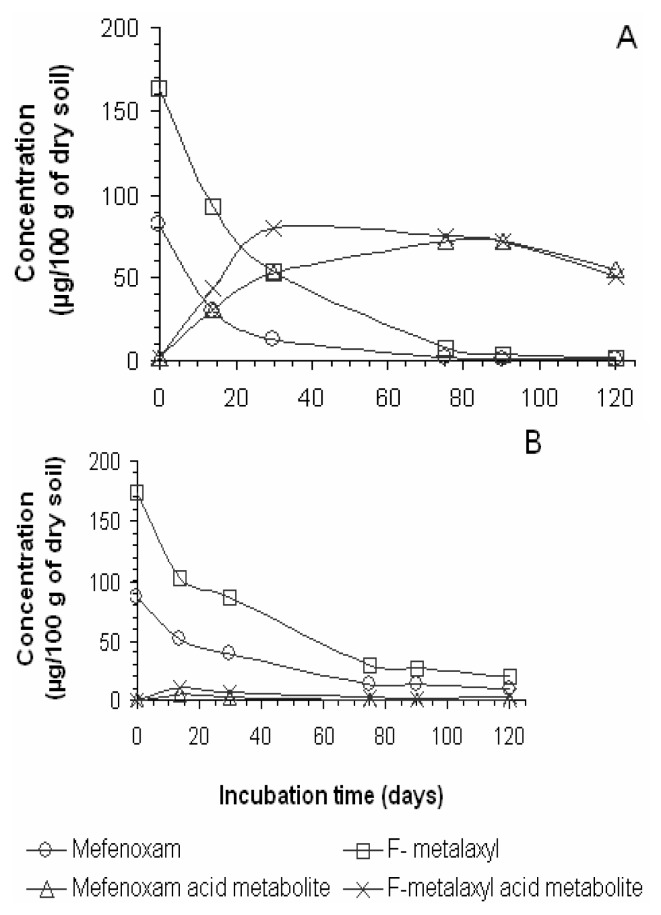
Figure 5.
Profiles of Mefenoxam and F-metalaxyl fungicides degradation in A) German soil and B) Cameroonian soil. Note the concurrent formation and degradation of the respective acid metabolite resulting from Mefenoxam and F-metalaxyl. Concentrations plotted versus incubation time (days).
Table 5.
Degradation Rate Constants (K); And Half-Lives (T1/2) as Derived from the Regression Line from the ln (Co/C)/t Plot, as Well as Correlation Coefficients (R2) Values for the Fit for the Degradation of F-metalaxyl and P-metalaxyl and Mefenoxam in German and Cameroonian Soils. Enantioselective Degradation of Racemic Metalaxyl in Soils
| Fungicides | Soil Origin | R2 | K (Day−1) | T1/2(Days) |
|---|---|---|---|---|
| F-metalaxyl | German | 0.97 | 0.039 | 18 |
| Cameroonian | 0.96 | 0.018 | 38 | |
|
| ||||
| P-metalaxyl | German | 0.98 | 0.039 | 18 |
| Cameroonian | 0.97 | 0.019 | 37 | |
|
| ||||
| Mefenoxam | German | 0.96 | 0.041 | 17 |
| Cameroonian | 0.96 | 0.018 | 38 | |
The degradation of F-metalaxyl and mefenoxam gave higher amounts of the acid metabolite in the German soil (Figure 5A) as compared to the Cameroonian soil (Figure 5B). As much as 89% of the initial concentration of mefenoxam was converted into its acid metabolite in German soil (Table 3) within 90 days of incubation compared to <1% in Cameroonian soil in the same period of incubation. Similar observation was made for F-metalaxyl, but a lower production of acid metabolite in the German soil (~49% of the initial concentration within 30 days of incubation) was obtained. The degradation profile of P-metalaxyl (data not shown) was similar to that of F-metalaxyl in soils. The maximum concentration of the acid metabolite formed from the P-metalaxyl was observed after 30 days of incubation in the German soil where ~57% of the initial concentration was converted into the acid metabolite. In the Cameroonian soil, as for the other products, the production of acid metabolite was significantly less important. Possibly the metalaxyl was transformed to other metabolites than the acid metabolite or even mineralised in the Cameroonian soil. A maximum concentration of ~4% at the 14d of incubation was observed. The degradation rate constant (k values) of these fungicides are listed in Table 5.
Enantioselective Degradation of Racemic Metalaxyl in Soils
In the chiral HPLC chromatograms obtained from the soil incubation of metalaxyl enantiomers, peak area ratios significantly different from the racemic standards were obtained. This indicated enantioselective degradation of metalaxyl in both soils (Figure 2). In the German soil the R-metalaxyl was degraded much faster (k = 0.064 day−1) than S-metalaxyl (k=0.033 day−1) when spiked with formulated racemic metalaxyl (see chromatograms in Figure 2). This is in agreement with previous reports on the behaviour of metalaxyl in temperate sandy loam soil [7, 13]. In the Cameroonian soil, the opposite was observed (see chromatograms in Figure 3). The S-enantiomer was degraded faster (k = 0.0 26 day−1) in relation to R-metalaxyl (k = 0.014 day−1) when spiked with F-metalaxyl. The EC values in this experiment changed from initially 1 to 0 after 75 d of incubation in German soil (evolution towards S), compared to changes from 1 to ~6 in the Cameroonian soil (progression towards R) (see Figure 4), indicating a more selective process by means of enantioselectivity for the degradation of metalaxyl in the German soil.
The difference in the degradation behaviour of metalaxyl in soil could be explained by the fact that the different soil types may contain different microbial populations equipped with different enzymes, which are preferential degraders of different enantiomers. This could be one reason for the different ranking order of the two enantiomers of metalaxyl in their degradation rate constants.
Effect Studies
Effect on Nitrogen Transformation
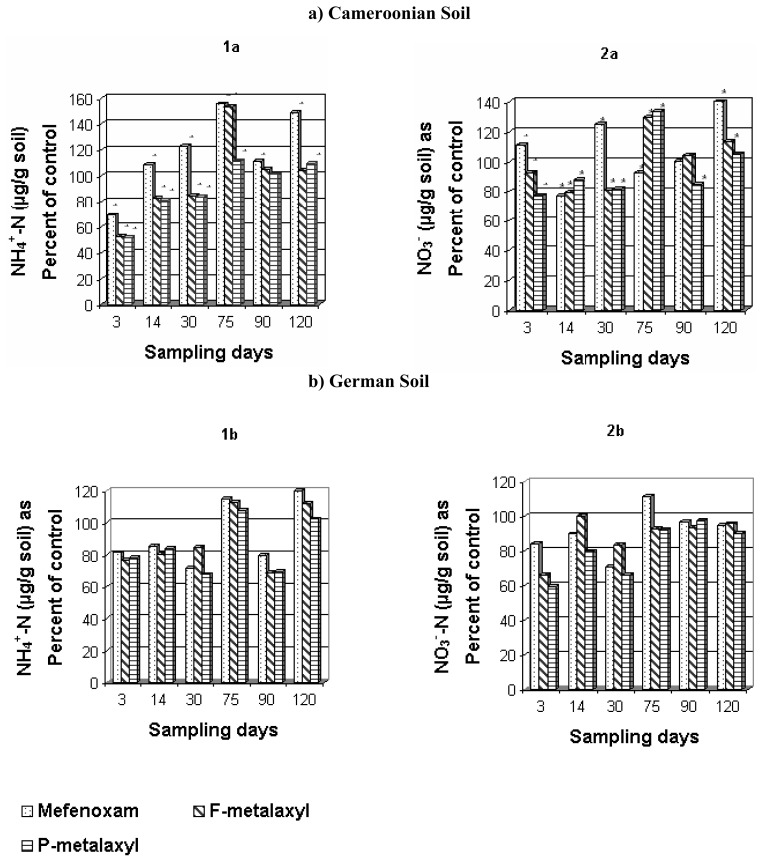
The changes of NH4+ and NO3− content in soils during the incubation are shown in Figure 6. In the shorter term (3–30 days), F-metalaxyl and P-metalaxyl caused a significant decrease in NH4+ content of both soils. Mefenoxam, while causing a significant decrease in the German soil NH4+ content, stimulated that of the Cameroonian soil as early as 14 days after application. The inhibitory effects of these fungicides on short-term exposure were reversible on long-term incubation (especially after 120 days) as evidenced by the significant increase in NH4+ content in both soils (Figure 6a, b). This increase was more pronounced with mefenoxam followed by F-metalaxyl. Probably mefenoxam is more bioavailable to bacteria than F-metalaxyl and P-metalaxyl. This assessment is supported by previous report indicating that after 21 days of incubation 78% of mefenoxam was degraded by rhizosphere microbial populations [11]. Metalaxyl has been reported to have DT50 values (the time taken for 50% active ingredient to be metabolized) in soil ranging from 3 to 8 weeks [41]. Similar trends were recorded for NO3− content in Cameroonian soil (Figure 6a). This indicated that these compounds stimulated the growth and the activities of ammonifying and nitrifying bacteria, which were mainly responsible for the mineralization of organic N to NH4+ and oxidation of NH4+ to NO3− respectively. The inhibitory effects of all these chemicals on nitrification was more pronounced in German soil, even at the 120th day of incubation (Figure 6b), but generally mefenoxam was comparatively less inhibitory and even showed a significant increase on the 75th day of incubation (Figure 6b). P-metalaxyl and F-metalaxyl effects resulted in delays of 30 days in the recovery of NH4+ and NO3− in the Cameroonian soil (Figure 6a). These effects can be considered normal according to the theoretical framework for testing the side-effects of pesticides [42]. As delays of recovery of this available N were more than 60 days in the German soil (Figure 6b), the effects of P-metalaxyl and F-metalaxyl were considered critical in this soil [42].
Figure 6.
Effect of fungicides on the availability of NH4+-N and NO3−-N in the Cameroonian (a) and German (b) soils. * P<0.05 between fungicide addition and no fungicide addition (paired Student t-test).
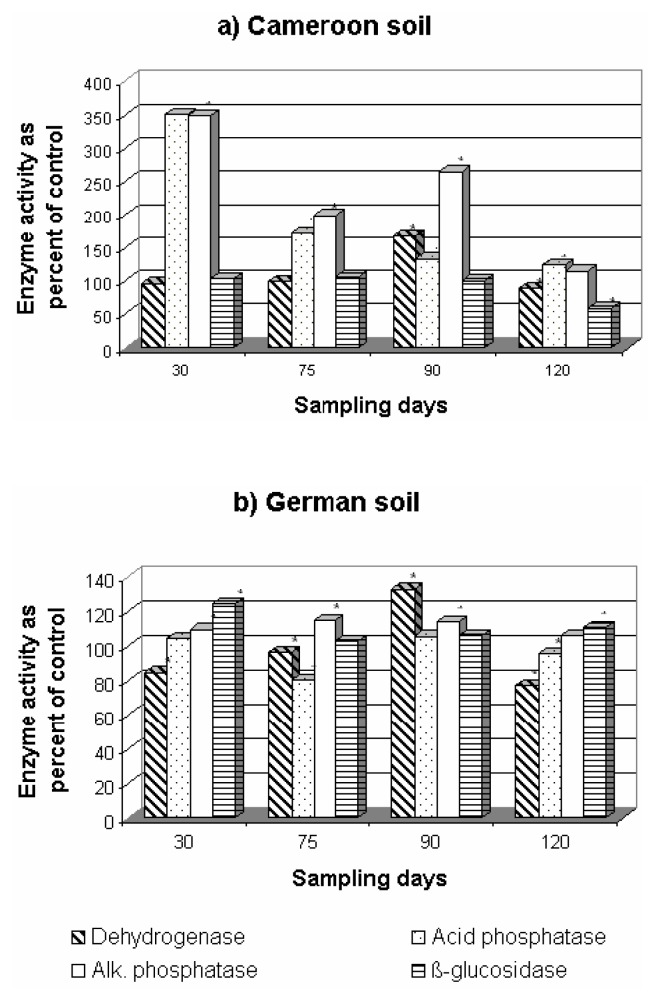
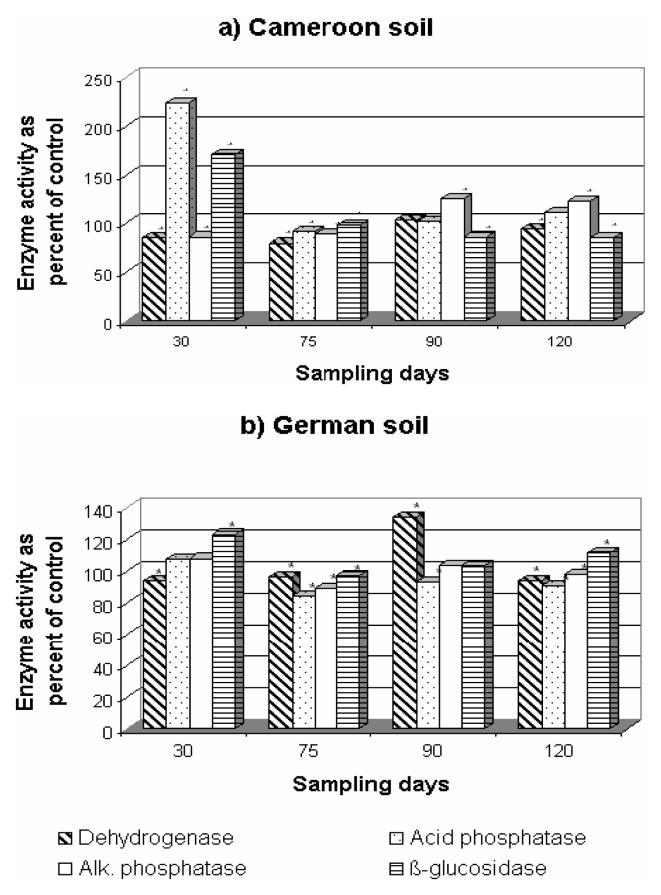
Effect on Soil Enzyme Activities
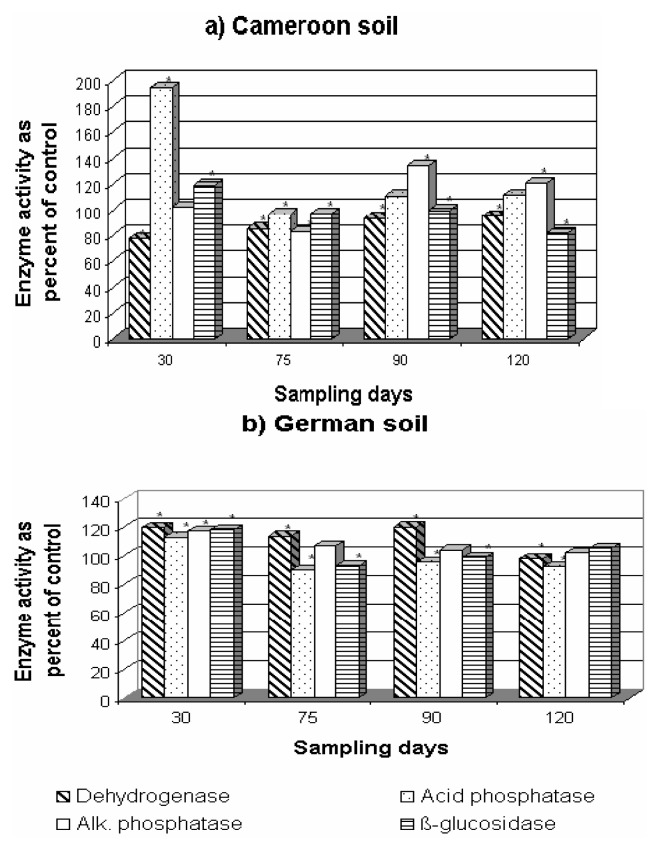
Pesticides have been shown to have direct and indirect effects on soil enzyme activity [43]. The addition of fungicides, in general, stimulated the activities of phosphatases and β-glucosidase in both soils as shown in Figures 7, 8, 9. Dehydrogenase activity was generally negatively affected, especially in the Cameroonian soil treated with F-metalaxyl and P-metalaxyl (Figures 8, 9). Mefenoxam significantly stimulated the activity of acid and alkaline phosphatases and β-glucosidase in both soils (Figure 7). Dehydrogenase activity was the most affected of all enzyme activities under mefenoxam stress. This inhibitory effect was more pronounced in the German soil. This difference in the dehydrogenase activity in the two soils may be ascribed to the difference in the decomposition rates of fungicides or their transformation to less toxic by-products in both soils as suggested earlier [44]. This adverse effect was not permanent since a significant increase in this enzyme activity was observed on the 90th day of incubation in both soils (Figure 7). A significant increase in the two phosphatase activities and in β-glucosidase activity was observed on the addition of F-metalaxyl and P-metalaxyl to both soils (Figures 8, 9). This increase was more pronounced in the Cameroonian soil. Dehydrogenase activity was stimulated at the 90th and at the 30 to 90th day of incubation by F-metalaxyl and P-metalaxyl respectively in the German soil (Figures 8b, 9b). P-metalaxyl, however, significantly decreased the activity of dehydrogenase in the Cameroonian soil (Figure 9a). As P-metalaxyl and F-metalaxyl caused delays of more than 75 days of recovery of the dehydrogenase activity in the Cameroonian soil (Figures 8a–9a), their effects on the activity of this enzyme were, thus, critical in this soil [42].
Figure 7.
Effect of mefenoxam on enzyme activities on both soils in the Cameroonian (a) and German (b) soils. * P<0.05 between fungicide addition and no fungicide addition (paired Student t-test).
Figure 8.
Effect of F-metalaxyl on enzyme activities in the Cameroonian (a) and German (b) soils. * P<0.05 between fungicide addition and no fungicide addition (paired Student t-test).
Figure 9.
Effect of P-metalaxyl on enzyme activities in the Cameroonian (a) and German (b) soils. * P<0.05 between fungicide addition and no fungicide addition (paired Student t-test).
In general, dehydrogenase activity appeared more sensitive to all fungicides in both soils, though to varying degrees. This is in agreement with many reports on the adverse effects of pesticides including fungicides on the dehydrogenase activity [45]. Dehydrogenase occurs intracellularly in all living microbial cells and it is linked with microbial respiratory processes. Its rapid degradation in soils could be followed by cell death and, thus, it does not accumulate in soils [46]. The dehydrogenase activity has been reported to reflect the microbial activity of soil [27, 47, 48].
The fungicides, in general, stimulated the activities of phosphatases and β-glucosidase. Being extracellular enzymes, they are generally protected from degradation by adsorption on clays or humic substances and, thus, may accumulate [27, 49, 50]. Moreover, the extracellular enzymes, immobilized by soil colloids, may not be as sensitive to fungicides as those associated with microbial cells [43].
Results suggest that the addition of the fungicides led to a change in the ecophysiological status of soil microbial community as expressed by the availability of NH4+-N and NO3−-N and the enzyme activities in soils. An attempt was made to correlate the concentrations of fungicides after the respective incubation intervals in the German (Table 2) and the Cameroonian (Table 3) soils and the corresponding concentrations of NH4+-N and NO3−-N (Figure 6) and the enzyme activities (Figures. 7–9) in soils. A weak relationship (data not shown) was found when fungicide residual concentrations were plotted against NH4+-N and NO3−-N levels in both German (r2 = 0.00004 to 0.2058) and Cameroonian (r2 = 0.0672–0.5430) soils, suggesting a lack of proportionality between the transformation rates of nitrogen and the degradation of metalaxyl and mefenoxam in these soils. The degradation of fungicides studied did not correlate also with the activity of dehydrogenase, Alkaline phosphatase and β-glucosidase in the German (r2 = 0.0325–0.8529) and the Cameroonian (r2 = 0.0427–0.7519) soils. However, a close positive correlation was found between P-metalaxyl degradation and the activity of Alkaline phosphatase in the German soil (r2 = 0.9294) and between that of F-metalaxyl and the activity of β-glucosidase (r2 = 0.9877) in the Cameroonian soil. A close positive relationship was also found between the activity of acid phosphatase and the degradation of all the fungicides in the Cameroonian soil (r2 = 0.9611–0.9778). In the German soil only P-metalaxyl degradation showed a high correlation (r2 = 0.9417) with the activity of this enzyme.
The almost lack of correlation found between some enzyme activities and the degradation of fungicides suggests that dehydrogenase, alkaline phosphatase and β-glucosidase in general may not be implicated in the degradation of the studied fungicides in soils, except for P-metalaxyl whose degradation may involve alkaline phosphatase in the German soil and the degradation of F-metalaxyl in the Cameroonian soil involving also β-glucosidase. The high correlation found for the acid phosphatase activity suggests that this enzyme may be closely involved in the degradation of the fungicides in the Cameroonian soil and to some extend in the degradation of F-metalaxyl and P-metalaxyl in the German soil.
Conclusions
Our findings show that the degradation of metalaxyl in soil occurs with some chiral preference. The rate of degradation of enantiomers and thus, the chiral preference depends on the type of soil and is enzymes mediated of which acid phosphatase may play an important role, especially in the tropical soil. These findings may also have some relevance to pesticide registration and approval.
The application of the investigated phenylamide fungicides at their maximum recommended field rates had positive and negative effects on soil chemical and biochemical properties. The positive effect on phosphatases and β-glucosidase activities was probably due to the microbial growth stimulated by the addition of these fungicides which served as source of energy. In general, mefenoxam like F-metalaxyl exerted a negative influence on biochemical parameters of soil as manifested by the observed decrease in available nitrogen, especially nitrate in German soil, and altered enzymatic activities, especially that of dehydrogenase. The effects of P-metalaxyl and F-metalaxyl on the content of available N were normal in the Cameroonian soil and critical in the German soil. These chemicals also exerted a critical effect on the activity of dehydrogenase in the Cameroonian soil. The stimulatory effect of mefenoxam on available nitrogen, phosphatases and β-glucosidase activities was, in general, greater than that of F-metalaxyl. F-metalaxyl and P-metalaxyl, in general, exerted similar effects on soil properties.
Acknowledgement
We are thankful to the Alexander von Humboldt Foundation for periods of research spent by the first author in Germany, under a Georg Forster Research Fellowship. We thank Novartis Agro GmbH, (Frankfurt, Germany) for kindly supplying the mefenoxam and metalaxyl employed, and Mr. A. Achermann from Novartis Crop Protection AG (Basel, Switzerland) for providing the R-metalaxyl and metalaxyl acid metabolite standards. We thank Dr. T. Pfeiffer, University of Dortmund, for recording the mass spectral data, Dr. E. Hellpointner from Bayer AG, and Dr. Kai Bester from the University of Dortmund, for their technical suggestions. We acknowledge technical assistance from Michael Schlüsener, Jörn Sickerling, Jürgen Scheen and Jürgen Storp of the University of Dortmund.
References
- 1.Urech P. A., Schwinn F., Staub T. CGA 48988, a novel fungicide for the control of late blight, downy mildew and related soil borne disease. Proceedings of the British Crop Protection Conference; Boots, Nottingham: Brigton. Nov. 21–24, 1977; pp. 623–631. [Google Scholar]
- 2.Buchenauer H. Chemistry of plant protection. In: Bowers W.S., Ebing W., Martin D., Wegler R., editors. Part 6: controlled release, biochemical effects of pesticides, inhibition of plant pathogenic fungi. Springer-Verlag; Heidelberg, Germany: 1990. p. 235. [Google Scholar]
- 3.Singh U. S., Tripathi R. K. Physico-chemical and biological properties of metalaxyl. Indian J. Mycol. Plant Pathol. 1982;12:287–294. [Google Scholar]
- 4.Kormondy E. J. Concepts of Ecology. Printice Hall; Englewood Cliffs, NJ: 1976. p. 238. [Google Scholar]
- 5.Usataya A. S., Merenyuk G. V., Katruk E. A. Biological activity of vineyard soils under the application of fungicides. Buletinul Academiei de Stiinte a Republicci Moldova Stiinte Biologice si Chimice. 1993;6:40–42. [Google Scholar]
- 6.Dvornikova T. P., Granatskaya T. A., Finkelshtein Z. I., Tolochkina S. A., Pestereva N. S., Neshinskii A. A. Behavior of Ridomil in soil and its effect on soil microflora. Agrokhimiya. 1988;11:116–118. [Google Scholar]
- 7.Buser H., Müller M.D. Environmental behaviour of acetamide pesticide stereoisomers 1: Stereo-and enantioselective determination using chiral-resolution gas chromatography and chiral HPLC. Environ. Sci. Technol. 1995;29:2023–2030. doi: 10.1021/es00008a022. [DOI] [PubMed] [Google Scholar]
- 8.Droby S., Coffey M. D. Biodegration process and the nature of metabolism of metalaxyl in soil. Ann Appl. Biol. 1991;118:543–553. [Google Scholar]
- 9.Sukul P., Spiteller M. Persistence, Fate, and metabolisme of [14C] metalaxyl in typical Indian soils. J. Agric. Food Chem. 2001;49:2352–2358. doi: 10.1021/jf001181r. [DOI] [PubMed] [Google Scholar]
- 10.Jones W. J., Anayeva N. D. Correlations between pesticide transformation rate and microbial respiration activity in soil of different ecosystems. Biol. Fertil. Soils. 2001;33:477–483. [Google Scholar]
- 11.Pai S. G., Riley M. B., Camper N. D. Microbial degradation of mefenoxam in rhizosphere of Zinnia angustifolia. Chemosphere. 2001;44:577–582. doi: 10.1016/s0045-6535(00)00368-4. [DOI] [PubMed] [Google Scholar]
- 12.Buser H. R., Müller M. D., Balmer M. E. Environmental behavior of the chiral acetamide pesticide mentality: enantioselective degradation and chiral stability in Soil. Environ Sci Technol. 2002;36:221–226. doi: 10.1021/es010134s. [DOI] [PubMed] [Google Scholar]
- 13.Müller M. D., Buser H. R. Environment behaviour of acetamide pesticide stereoisomers. 2. Stereo- and enatioselective degradation in sewage sludge and soil. Environ. Sci. Technol. 1995;29:2031–2037. doi: 10.1021/es00008a023. [DOI] [PubMed] [Google Scholar]
- 14.Regitano J. B., Tornisielo V. L., Lavorenti A., Pacovsky R. S. Transformation pathways of 14C-chlorothalonil in tropical soils. Arch. Environ. Contam. Toxicol. 2001;40:295–302. doi: 10.1007/s002440010175. [DOI] [PubMed] [Google Scholar]
- 15.Johnson S. L. Notice of voluntarily cancellation of Ciba Crop Protection Registrations on metalaxyl technical and end-use products that contain metalaxyl. Enviromental Protection Agency; 1996. Opp-66225; FRL 5364-7, FR Doc. 96-10806. [Google Scholar]
- 16.Monkiedje A., Njine T., Demanou J., Kemka N., Zebaze S. The responses of plankton communities in laboratory microcosms to Ridomil plus 72, a heavily used fungicide in Cameroon. A. J. Sci. Technol. 2000;1:13–20. [Google Scholar]
- 17.Nuninger C., Watson G., Leadbitter N., Ellgehausen H. CGA 329 351. Introduction of the enantiomer form of the fungicide metalaxyl. Proceedings of the British Crop Protection Conference: Pest and disease; Brighton. 1996; Nov 18–21, pp. 41–46. [Google Scholar]
- 18.Doran J. W., Parkin T. B. Defining and assessing soil quality. In: Doran J. W., Coleman D. C., Bezdicek D. F., Stewart B. A., editors. defining soil quality for a sustainable environment. Madison, Wisconsin, USA: 1994. pp. 3–21. SSSA special publication No. 35. [Google Scholar]
- 19.Dick R. P. Soil enzyme activities as indicators of soil quality. In: Doran J.W., Coleman D.C., Bezdicek D.F., Stewart B.A., editors. Defining soil quality for a sustainable environment. Madison, Wisconsin, USA: 1994. pp. 3–21. SSSA special publication No. 35. [Google Scholar]
- 20.Hattori T. Microbial life in the soil. Marcel Dekker; New York, NY: 1973. p. 427. [Google Scholar]
- 21.FAO. Pesticides Residues in Food. Evaluations. Rome. 1982:420. [Google Scholar]
- 22.Finkelstein Z. I., Golovleva L. A. Effects of regular application of pesticides on nitrogen bacteria. Zentrablatt für Mikrobiol. 1988;143:453–456. [Google Scholar]
- 23.Readman J. W., Albanis T. A., Barcelo D., Galassi J., Tronczynski, Gabrielides G. P. Fungicide contamination of Mediterranean estuarine waters: results from a MED POL pilot survey. Marine Pollut. Bull. 34. 1997:259–263. [Google Scholar]
- 24.Petrovic A. M., William C. B., Larson-Kovach I. M., Reid C. M., Lisk D. J. Donward migration of metalaxyl fungicide in creeping bentgrass sand lysimeters as affected by organic waste, peat and Zeolite amendments. Chemosphere. 1998;37:249–256. [Google Scholar]
- 25.Om A. S., Chung K. W., Ko Y. S. Pesticide residues in marketed sesame. Bull Environ. Contam. Toxicol. 1998;61:716–721. doi: 10.1007/s001289900820. [DOI] [PubMed] [Google Scholar]
- 26.Tu C. M. Effects of fungicides on microbial activities in sandy soil. Inter. J. Environ. Health Res. 1994;4:133–140. [Google Scholar]
- 27.Nannipieri P., Ceccanti B., Gregos S. Ecological significance of the biological activity in soil. In: Bollag J. M., Stotzky G., editors. Soil Biochemistry. Vol. 6. Marcel Dekker; New York: 1990. pp. 293–355. [Google Scholar]
- 28.Herrick J. E., Wander M. M. Relationship between soil organic carbon and soil quality in cropped and rangeland soils: the importance of distribution, composition and soil biological activity. In: Lal R., Kimble J., Follett J., Steward B.A., editors. Advances in soil sciences: soil processes and the carbon cycle. CRC Press; Boca Raton, Fl: 1998. pp. 405–425. [Google Scholar]
- 29.Monreal C. M., Dinel H., Schnitzer M., Gamble D. S., Biederbeck V. O. Impact of carbon sequestration on functional indicators of soil quality as influenced by management in sustainable agriculture. In: Lal R., Kimble J., Follett J., Steward B.A., editors. Advances in soil sciences: soil processes and the carbon cycle. CRC Press; Boca Raton, Fl: 1998. pp. 435–457. [Google Scholar]
- 30.Anonymous. Landesumweltamt. NRW; Essen: 2000. Deutscher Wetterdienst. Umwelt NRW Daten und Fakten; pp. 115–116. [Google Scholar]
- 31.Omoko M. Thèse de Doctorat de 3ième cycle. Université de Bordeaux; 1984. Dynamique de l’eau dans un sol ferralitique et etude comparée entre l’evapo-transpiration mesurée et calculée en climat equatorial. [Google Scholar]
- 32.Anonymous. Soil plant analysis council, Inc. CRC Press; Boca Raton, Florida, USA: 2000. Soil Analysis Handbook of Reference Methods; p. 245. [Google Scholar]
- 33.Tan K H. Soil sampling preparation and analysis. Marcel Dekker, Inc; New York: 1996. pp. 135–152. [Google Scholar]
- 34.Casida L. E., Jr., Klein D. A., Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98:371–376. [Google Scholar]
- 35.Tabatabai M.A., Bremmer J. M. Use of p-nitro-phenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1:301–307. [Google Scholar]
- 36.Eivazi F., Tabatabai M.A. Phosphatases in soils. Soil Biol Biochem. 1977;9:167–172. [Google Scholar]
- 37.Tabatabai M. A. Methods of soil analysis, Part 2. Chemical and microbiological properties. Agronomy Monograph N° 9. 2nd ed. Madison WS, USA: 1982. Soil Enzymes; pp. 907–927. [Google Scholar]
- 38.Kaplan D. L., Hartenstein R. Problems with toluene and the determination of extracellular enzyme activity in soils. Soil Biol. Biochem. 1979;11:335–338. [Google Scholar]
- 39.Eivazi F., Tabatabai M. A. Glucosidases and galactosidases in soils. Soil Biol Biochem. 1988;20:601–606. [Google Scholar]
- 40.Dunnett C. Multiple comparison procedure for comparing several treatments with a control. J. Am. Assoc. 1985;50:1096–1121. [Google Scholar]
- 41.Sharom M. S., Edgington L. V. The adsorption, mobility and persistence of metalaxyl in soil and queous system. Can. J. Plant. Pathol. 1982;4:334–340. [Google Scholar]
- 42.Domsch K. H., Jagnow G., Anderson T. H. An ecological concept for the assessment of side-effects of agrochemicals on soil microorganisms. Residue Rev. 1983;86:65. [Google Scholar]
- 43.Nannipieri P. The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In: Pankhurst C. E., Doube B. M., Gupta V.V.S.R., Grace P. R., editors. Soil biota: management in sustainable farming systems. CSIRO; East Melbourne: 1994. pp. 238–244. [Google Scholar]
- 44.Nannipieri P., Bollag J. M. Use of enzymes to detoxify pesticide-contaminated soils and waters. J. Environ. Quality. 1991;20:510–517. [Google Scholar]
- 45.Schuster E., Schroeder D. Side effects of sequencely and simultaneously applied pesticides on non-target soil organisms: laboratory experiments. Soil Biol. Biochem. 1990;22:375. [Google Scholar]
- 46.Somerville L., Greaves M. P., Domsch K. H., Verstracte W., Poole N. J., Van Dijk H., Anderson J.P.E. Recommended laboratory tests for assessing the side-effects of pesticides on the microflora. In: Somerville L., Greaves M.P., editors. Pesticide effects on soil microflora. Taylor & Francis; London: 1987. p. 235. [Google Scholar]
- 47.Thalman A. Zur bestmmung des dehydrogenaseaktivität im Boden mittels. Vol. 21. Triphenyltetrazomliumchlorid (TTC); Landwirt. Forsch: 1968. p. 249. [Google Scholar]
- 48.Tabatabai M. A. Soil Enzymes. In: Weaver R. M., Angle S., Bottomley P., Bezdicek D., Smith S., Tabatabai A., Wollum A., editors. Methods of soil analysis, Part2. Microbial and biochemical properties. American Society of Agronomy; Wisconsin: 1994. pp. 775–833. [Google Scholar]
- 49.Skujins J. Extracellular enzymes in soil. CRC Crit Rev. Microbiol. 1976;4:383–421. doi: 10.3109/10408417609102304. [DOI] [PubMed] [Google Scholar]
- 50.Boyd S. A., Mortland M. M. Enzyme interactions with clays and clay-organic matter complexes. In: Bollag J. M., Stotzky G., editors. Soil biochemistry. Marcel Dekker; New York, NY: 1990. pp. 325–389. [Google Scholar]